Abstract
Background
The main concern in noninvasive (NI) glucose measurement is achieving high accuracy readings, although no blood (or other fluid) is involved in the process. Using methods based on different physical properties of a measured object can ensure the independence of each of the readings and therefore improve the validity of the end result. By using a combination of (three) independent technologies—ultrasonic, electromagnetic, and thermal—GlucoTrack™ presents a unique approach for a real-time, truly NI blood glucose spot measurement.
Methods
Clinical trials were performed in two stages. Stage 1 was an initial method validation and performance verification of the device. In this stage, 50 type 1 and 2 diabetic patients, as well as healthy subjects, were evaluated with GlucoTrack against Ascensia Elite® (Bayer). In the second stage, 85 additional diabetic subjects were evaluated in half and full daytime sessions using a GlucoTrack comparison with HemoCue® (Glucose 201+).
Results
A total of 135 subjects were tested during the trial period, producing 793 data pairs. Using Clarke error grid analysis, 92% of the readings fell in the clinically acceptable zones A and B, with 50% in the A zone. Mean and median relative absolute differences were 29.9 and 19.9%, respectively.
Conclusions
Integrating several modalities for NI assessment of glucose level enables more accurate readings, while a possible aberration in one modality is bypassed by the others. The present generation of GlucoTrack gives promising results; however, further improvement of the accuracy of the device is needed.
Keywords: combination methodology, ear clip, GlucoTrack™, noninvasive glucose monitoring, self-monitoring of blood glucose
Introduction
Diabetes and its complications impose significant economic consequences on individuals, families, health systems, and countries. The annual economic cost of diabetes in 2007 in the United States was estimated to be $174 billion, attributed to both direct and indirect expenditures.1
Research has shown conclusively that improved glucose control reduces the long-term complications of diabetes.2,3 According to the American Diabetes Association, self-monitoring of blood glucose (SMBG) has a positive impact on the outcome of therapy with insulin, oral agents, and medical nutrition.4 Moreover, SMBG is useful in generating knowledge about individual glucose profiles, as well as knowledge about the effects of one's habits, including exercise and food intake on that profile, thus helping to achieve specific glycemic goals.5,6 However, the inconvenience, expense, pain, and complexity involved in SMBG lead to its underutilization, mainly in people with type 2 diabetes. Availability of an accurate, painless, and easy-to-operate device will encourage more frequent testing, leading to tighter glucose control and a delaying/decreasing of long-term complications and their associated health care costs.
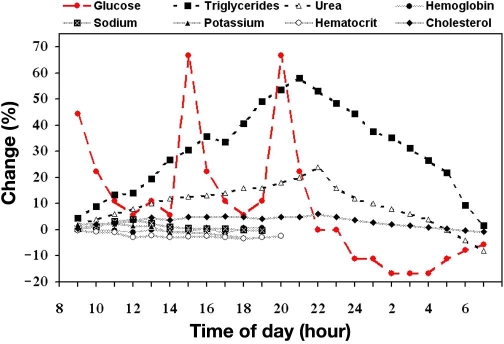
Noninvasive (NI) methods used for the determination of glucose fall into two categories. The first is based on the measurement of glucose using one or more of its intrinsic molecular properties, such as near-infrared or mid-infrared absorption coefficient, optical rotation, Raman shifts, and photoacoustic absorption, as well as others. These methods assume the ability to detect glucose in tissue or blood independently of other body components or physiological state. The second category measures the effects of glucose on the physical properties of blood and tissue.7 This category is based on an assumption that glucose is a dominant (highly fluctuating) blood analyte (as shown in Figure 1) and, as such, contributes significantly to the change in the relevant physical parameters of the tissue. Hence, measurement of such parameters can lead indirectly to evaluation of the blood glucose (BG) level. The measured parameters are evaluated relatively to calibration, performed through correlation of the NI signal to a reference BG value. Therefore, the relative change of glucose in blood or interstitial fluid (ISF) plays the major role, as other blood analytes, which are less fluctuating, are fully or at least partially eliminated through calibration. However, glucose determination in indirect and nonspecific NI measurement faces several obstacles. Depending on the particular method used, readings may vary with changes in glucose level, but may also be affected by sensor–skin interface variations, changes in microcirculation and blood supply, medications that affect fluid distribution, comorbidities, a person's metabolic rate, and so on. The main concern, consequently, is to achieve high accuracy results, despite the fact that no direct blood or ISF glucose measurement is performed.
Figure 1.
Approximated profile of diurnal variations (pooled data8–12) in serum constituents of healthy individuals expressed as a relative change in percentage from the fasting state. Meals are taken around 8:30, 13:30, and 18:30, denoting breakfast, lunch, and dinner, respectively.
Scientific Background
Measurement Concept
An influx of sodium ion into the cellular compartment and an efflux of potassium accompany the movement of water into the interstitium and plasma, induced by changes in the glucose concentration.13–15 This response to glucose variations results in changes of physical properties such as electric and acoustic impedance, as well as heat transfer (HT) characteristics of the cellular, interstitial, and plasma compartments, due to changes in ion concentration, density, compressibility, and hydration of both compartments.
In order to achieve reliable readings, random errors that have no consistent effect and contribute to the variability of data, as well as systematic errors that introduce constant bias to the measurement, must be minimized. In general, averaging over a number of N measurements (of the same technology) decreases the standard deviation by a factor of . Therefore, the resultant precision is improved by the repetition of measurements.
The use of methods based on different physical properties of the measured object may improve the accuracy level when the different methods are independent and do not share the same systematic errors.
Therefore, simultaneous evaluation of the mentioned physiological changes through the measurement of different sets of tissue perturbations, induced by changes in glucose concentration, is expected to increase the validity of the end result.
Device Description
GlucoTrack™ presents a novel approach using a combination methodology, where three independent, real-time NI technologies are integrated: ultrasonic, electromagnetic, and thermal. Each technology measures different tissue parameters that are affected by the same change in glucose concentration. Independency is assumed due to noninterference between disturbing variables of any pair of the device's measurement channels, to the extent that they allow effective averaging over a set of measurements.
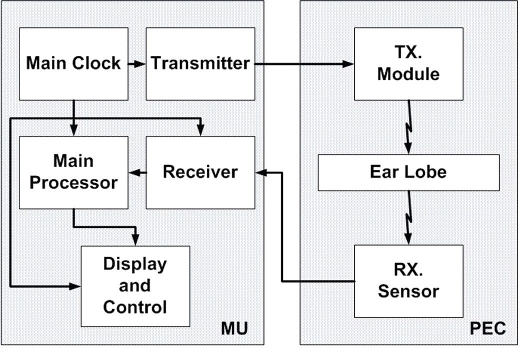
The device comprises a main unit (MU), which drives three different sensor pairs (one per technology), located at the tip of a personal ear clip (PEC) (Figure 2).
Figure 2.
GlucoTrack conceptual block diagram.
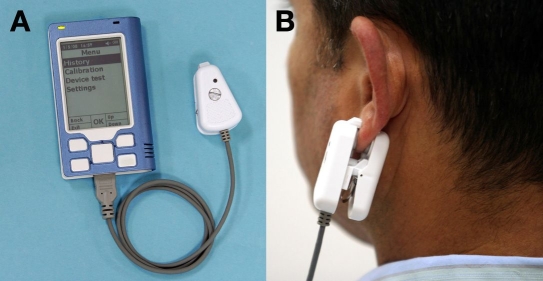
In order to perform a spot measurement, the PEC is clipped externally to the user's earlobe for the duration of the measurement and is removed afterward (Figure 3B). This is an easily approachable site, which has a large blood supply and does not interfere with routine activities. Moreover, ear and finger capillary BG concentrations are well correlated, and the two sites can be used interchangeably, yielding similar results in an individual patient.16
Figure 3.
(A) The MU with PEC connected; (B) PEC attached to an earlobe during measurement.
The Principle behind Ultrasonic Technology
The acoustic velocity (c) in fluids and soft tissue depends on the compressibility (β), which is determined by the intermolecular bonding forces, and the density (ρ) of the medium, according to the following equation:
Breaking the water structure by hydrogen bonding of the glucose molecules causes a less-bonded water to form a closer-packed and less compressible structure.17 Hence, glucose concentration changes in the extracellular fluid affect both density and adiabatic compressibility and therefore directly affect the acoustic velocity by a linear relationship.18,19 Furthermore, the acoustic impedance in live tissue is modulated through the water balance. In the hyperglycemic state, because of water shifts from the cellular to the extracellular compartment, tissue becomes less hydrated. This affects tissue density as well as compressibility:20 the tissue behaves more like a dense solid rather than a fluid, increasing the acoustic velocity through the tissue. Thus, changes in glucose can be evaluated indirectly by measurement of the sound velocity through the tissue.
An ultrasound wave, which is produced by a transmitter located at one side of the subject's earlobe, travels through the earlobe with characteristic velocity and is received on the opposite side. The measured signal is corrected for temperature changes, as propagation velocity is temperature dependent.18,19
The Principle behind Electromagnetic Technology
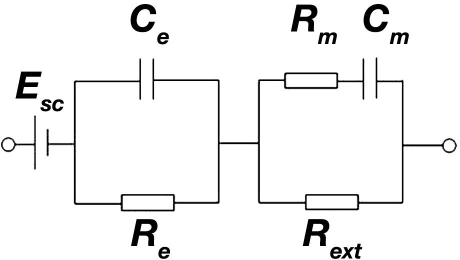
Disruption of the transmembrane electrolyte balance by a glucose-induced fluid shift causes changes in the cell membrane potential.21 Moreover, it has been reported previously that variations of the metabolically active enantiomer D-glucose affect the permittivity and conductivity of the cellular membranes.22 Hence, glucose-induced water and ion transport across the cellular membrane leads to changes in the electrical properties of the cellular and consequently extracellular compartments. These alterations are reflected in changes of the tissue impedance represented by the circuit shown in Figure 4.23
Figure 4.
Approximated equivalent circuit of the epidermis and underlying tissue.23 Esc, Ce, Re, Rm, Cm, and Rext denote skin surface potential, epidermal capacitance, epidermal resistance, tissue cellular membrane capacitance, tissue cellular membrane resistance, and extracellular resistance, respectively.
To measure the electrical impedance, the earlobe is positioned between two electrodes and is connected to an electrical oscillating circuit, inducing voltage upon the earlobe skin and underlying tissue. The earlobe temperature is also considered in the measurement, as tissue impedance is temperature dependent.24
The Principle behind Thermal Technology
Changes in thermal properties within the earlobe can be described using Penne's bioheat equation25:
where ρt, ct, and kt are the density, specific heat, and thermal conductivity of the tissue, respectively. U denotes the temperature developed at the measurement point in the earlobe. Metabolic heat generation, heat transfer between tissue and blood, and any degree of external heating are denoted by hm, hb, and hext, respectively. BG variations affect HT characteristics through changes of ρt, ct, and kt due to water/electrolytes shifts. Therefore, glucose can be evaluated indirectly through the measurement of changes in HT characteristics in response to a specific amount of energy supplied to the tissue for a predetermined duration of time.
However, the HT process is also affected by blood velocity and metabolic heat generation. Hence it may also be sensitive to changes in perfusion and circulatory problems, which result from diabetes complications, as well as to the level of patient's physical activity prior to the measurement.
Materials and Methods
Applied Technologies Integration: Algorithm
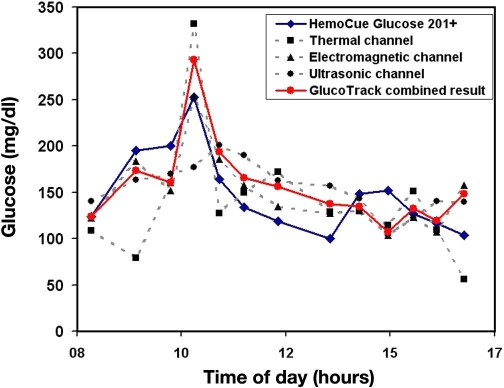
Each method per se is indicative of glucose but is confined by the impact of the interfering factors due to a lack of specificity. The behavior of each measured parameter is different as a function of glucose and interfering factors, affecting the sensitivity of each measurement channel distinctively. We believe that a combination of several modalities decreases the errors derived from each method separately, thereby increasing the accuracy of the final result (Figure 5). Figure 6 shows implementation of the same concept in a single subject.
Figure 5.
Data collected as a confirmation for the concept of using combined technologies. (A) Raw glucose readings per each technology [(•) thermal, (•) electroconductivity, and (•) ultrasonic channels with mean ARD values of 23.2%, 19.9%, and 22.1%, respectively]. (B) Final results after algorithm activation (mean ARD 15.8%).
Figure 6.
Example of correlation of separate results per technology vs combined result against invasive glucose reference of an 80-year-old type 2 diabetic male.
Independent results from each technology are analyzed and compared using a unique algorithm.26 For each glucose reading, achieved by one method, a tolerance window (TW) of ±20% is opened around the original value. The three windows are then correlated and compared; overlapping is expected. In case of full correlation, meaning three overlaps are achieved, the final result is a weighted average of the three measured values. If only two overlaps occur, the TWs are shrunken to ±15% and overlapping between the two closest readings is then checked. Overlap failure produces a reading error and leads to repeat measurement.
Calibration
Calibration is required to be performed prior to glucose measurements so that the influence of individual quasistable factors, such as tissue structure, can be minimized. The process consists of correlating invasive basal and postprandial BG data, taken from finger capillary blood, with six sequential measurements with the NI instrument, generating a calibration curve that is exclusive to each individual.
The first measurement pair is taken in a fasting state, followed by food and drink consumption to increase the BG value by at least 30 mg/dl. Twenty minutes later, a set of five sequential measurement pairs, with a time interval of about 10 minutes in between, is taken. The calibration process takes about 1.5 hours.
It should be noted that the PEC is clipped to the earlobe only for the duration of a spot measurement (about a minute) and is removed afterward.
A calibration model is established by fitting measured NI data to invasively determined glucose concentrations using linear least squares fitting:
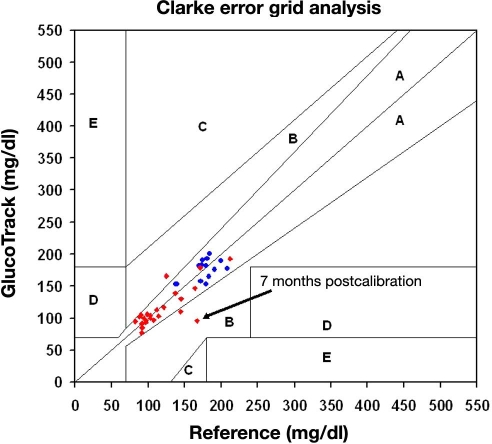
where n, g, and T are inner noise, net glucose contribution, and temperature, respectively. This calibration requires a periodical update. Based on our experiments and analyses, a calibration is valid for a few months, as shown in Figure 7. However, until sufficient data are collected from a wide range of subjects, we advise monthly calibration.
Figure 7.
Examples of long-term calibration validity: (·) 30-year-old healthy male and (·) 49-year-old type 2 diabetic male; readings were taken over 7 and 3 months, correspondingly, using a single calibration process per each individual.
Clinical Trials
Clinical trials were performed in the diabetes unit of the Soroka University Medical Center, Beer-Sheva, Israel. The study was approved by the local ethics committee, and all participants gave written informed consent prior to participation.
The trial was performed in two stages. For the first stage, types 1 and 2, as well as healthy subjects, were recruited, whereas the second stage included only people with diabetes. In both stages, subjects were over 18 years of age with at least one anatomically suitable earlobe (above minimal size). Pregnant or breast-feeding women, subjects undergoing dialysis, or subjects with any skin conditions on the earlobe were ineligible to participate in the study.
The first stage included 50 subjects (11 type 1, 29 type 2, and 10 healthy subjects). In this stage, calibration was performed using the Ascensia Elite® (Bayer) glucose meter and was subsequently followed by at least one measurement pair, Ascensia Elite and GlucoTrack. The purpose of this first stage was initial validation of the GlucoTrack method and verification of the correlation of the three technologies with glucose levels.
The second stage included 85 subjects (16 type 1 and 69 type 2 subjects). This stage was performed for further validation of the method, evaluating GlucoTrack performance in different glycemic ranges. In order to ensure realistic conditions, measurements were performed in various random ambient conditions and in different preprandial and postprandial states. Readings were taken during different seasons of the year, under varying conditions of temperature and humidity, while fasting, after meals, and so on. This stage was performed in two steps.
Step 1: Twenty-four subjects underwent calibration using capillary glucose measurements determined by HemoCue® (Glucose 201+); following calibration, four to six simultaneous measurement pairs with HemoCue and GlucoTrack (20-minute intervals between measurements) were made.
Step 2: Sixty-one subjects were evaluated in this step. The subjects arrived at the clinic on two different days. On the first day, subjects performed individual calibration against HemoCue. During the second day, a “full daytime” session of 8 to 10 hours took place, where 9–13 simultaneous measurement pairs with GlucoTrack and HemoCue (30-minute intervals between pairs) were taken. During these 8–10 hours, the patient received breakfast, lunch, and a fruit snack in order to produce variability in the glucose profile. The interval between calibration and measurement days was 8 ± 6 days, according to the availability of the subjects.
In both steps of the second stage, subjects took their usual chronic medications as they would in real life. In both stages, all GlucoTrack and invasive device-related actions (calibration and measurements) were performed by a proficient medical staff of the clinic dedicated to the trial.
Results
A total of 135 subjects were tested during both steps of the clinical trial period, producing 793 data pairs. Subjects included 27 type 1 (16 females, 11 males), 98 type 2 (33 females, 65 males), and 10 healthy (7 females, 3 males) subjects, age 55.0 ± 28.8 years, with a body mass index of 28.0 ± 12.3 kg/m2. GlucoTrack readings were compared with invasive finger capillary BG values according to the calibration device: Ascensia Elite in the first stage (83 paired readings) and HemoCue (710 paired readings) in the second stage of the study.
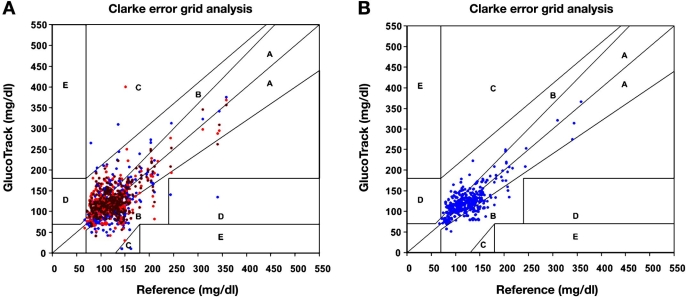
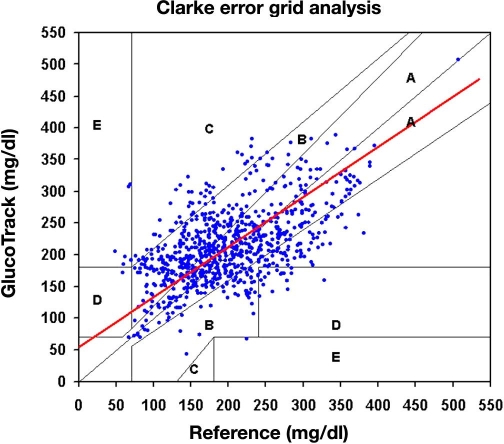
Our intention is to present data mainly from a clinical perspective (with no statistical implication); hence no assumptions on the distribution of data are necessary. Using Clarke error grid (CEG) analysis,27 92% of the readings fell in the clinically acceptable zones A and B (Figure 8). The absolute relative difference (ARD) yielded mean and median ARD values of 29.9% and 19.9%, respectively.
Figure 8.
Pooled CEG analysis for both stages of the clinical trial: A, 50%, and B, 42%.
Evaluation by means of Deming regression (Figure 8) with a variance ratio of 0.86 provided an intercept of 53.6 mg/dl and a slope of 0.8 [95% confidence interval for the slope is (0.71 to 0.88)].
Discussion
Results achieved so far show fair correlation between the readings of GlucoTrack and the reference invasive device. No conclusion can be made thus far regarding glucose levels below 100 mg/dl, as relatively few points were obtained in this region.
Based on preliminary data derived from further examination, we assume that measurement errors in each technology may be caused by sensor–tissue interaction (contact quality and pressure consistency of the PEC), as well as the state of the epidermis. Furthermore, additional errors may be caused by the lag time between the interstitial and blood glucose, as measurements performed on the whole tissue are largely driven by the effects of glucose upon the interstitial and intracellular compartments.
These errors impact the accuracy level of the measurements and therefore should be addressed. A possible way to consider is increasing the sensitivity of each one of the technologies and reducing the contribution of the disturbing factors (noise). Based on the proposed integration algorithm, improvement in each technology may lead to a significant enhancement in the integrated result. Although further work is needed in order to improve the accuracy level of the readings, it seems that the proposed unique concept of combining several noninvasive modalities presents a promising approach.
Abbreviations
- ARD
absolute relative difference
- BG
blood glucose
- CEG
Clarke error grid
- HT
heat transfer
- ISF
interstitial fluid
- MU
main unit
- NI
noninvasive
- PEC
personal ear clip
- SMBG
self-monitoring of blood glucose
- TW
tolerance window
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):1–20. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in subjects with type 2 diabetes (UKPDS33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 4.Clinical Practice Recommendations. Standards of medical care in diabetes–2006 American Diabetes Association. Diabetes Care. 2006;29:S4–S42. [PubMed] [Google Scholar]
- 5.Florence JA, Yeager BF. Treatment of type 2 diabetes mellitus. Am Fam Physician. 1999;59(10):2835–2844. 2849-50. [PubMed] [Google Scholar]
- 6.Karter AJ, Ackerson LM, Darbinian JA, D'Agostino RB, Ferrara A, Liu J, Selbyv JV. Self-monitoring of blood glucose levels and glycemic control: the northern California Kaiser Permanente diabetes registry. Am J Med. 2001;111(1):1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 7.Khalil OS. Non-invasive glucose measurement technologies: an update from 1999 to the dawn of the new millennium. Diabetes Technol Ther. 2004;6(5):660–697. doi: 10.1089/dia.2004.6.660. [DOI] [PubMed] [Google Scholar]
- 8.Pocock SJ, Ashby D, Shaper AG, Walker M, Broughton G. Diurnal variations in serum biochemical and haematological measurements. J Clin Pathol. 1989;42(2):172–179. doi: 10.1136/jcp.42.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Wijk J, Cabezas MC, Halkes CJ, Erkelens DW. Effects of different nutrient intakes on daytime triacylglycerolemia in healthy, normolipemic, free-living men. Am J Clin Nutrition. 2001;74(2):171–178. doi: 10.1093/ajcn/74.2.171. [DOI] [PubMed] [Google Scholar]
- 10.Statland BE, Winkel P, Bokelund H. Factors contributing to inta-individual variation of serum constituents. 1. Within-day variation of serum constituents in healthy subjects. Clin Chem. 1973;19(12):1374–1379. [PubMed] [Google Scholar]
- 11.Rivera-Coll A, Fuentes-Arderiu X, Diez-Noguera A. Circadian rhythmic variations in serum concentrations of clinically important lipids. Clin Chem. 1994;40(8):1549–1553. [PubMed] [Google Scholar]
- 12.MacKay EM, MacKay LL. The concentration of urea in the blood of normal individuals. J Clin Invest. 1927;4(2):295–306. doi: 10.1172/JCI100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillier TA, Abbot RD, Barret EJ. Hyponatremia: evaluating a correction factor for hyperglycemia. Am J Med. 1999;106(4):399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 14.Moran SM, Jamison RL. The variable hyponatremic response to hyperglycemia. West J Med. 1985;142(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- 15.McNair P, Madsbad S, Christiansen C, Christensen MS, Transbøl I. Hyponatremia and hyperkalemia in relation to hyperglycemia in insulin-treated diabetic out-subjects. Clin Chim Acta. 1982;120(2):243–250. doi: 10.1016/0009-8981(82)90161-9. [DOI] [PubMed] [Google Scholar]
- 16.Stahl M, Brandslund I. Measurement of glucose content in plasma from capillary blood in diagnosis of diabetes mellitus. Scand J Clin Lab Invest. 2003;63(6):431–440. doi: 10.1080/00365510310002590. [DOI] [PubMed] [Google Scholar]
- 17.Banipal PK, Banipal TS, Lark BS, Ahluwalia JC. Partial molar heat capacities and volumes of some mono-, di- and tri-saccharides in water at 298.15, 308.15 and 318.15 K. J Chem Soc Faraday Trans. 1997;93(1):81–87. [Google Scholar]
- 18.Zips A, Faust U. Determination of biomass by ultrasonic measurements. Appl Environ Microbiol. 1989;55(7):1801–1807. doi: 10.1128/aem.55.7.1801-1807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Patent 5,119,819. Method and apparatus for non-invasive monitoring of blood glucose. 1992 Jun 9; [Google Scholar]
- 20.Toubal M, Asmani M, Radziszewski E, Nongaillard B. Acoustic measurement of compressibility and thermal expansion coefficient of erythrocytes. Phys Med Biol. 1999;44(5):1277–1287. doi: 10.1088/0031-9155/44/5/313. [DOI] [PubMed] [Google Scholar]
- 21.Genet S, Costalat R, Burger J. The influence of plasma membrane electrostatic properties on the stability of cell ionic composition. Biophys J. 2001;81(5):2442–2457. doi: 10.1016/S0006-3495(01)75891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y, Livshits L, Caduff A, Feldman Y. Dielectric spectroscopy study of specific glucose influence on human erythrocyte membranes. J Phys D Appl Phys. 2003;36:369–374. [Google Scholar]
- 23.McAdams ET, Jossinet J. Tissue impedance: a historical overview. Physiol Meas. 1995;16(3A):A1–A13. doi: 10.1088/0967-3334/16/3a/001. [DOI] [PubMed] [Google Scholar]
- 24.Gudivaka R, Schoeller D, Kushner RF. Effect of skin temperature on multifrequency bioelectrical impedance analysis. Appl Physiol. 1996;81(2):838–845. doi: 10.1152/jappl.1996.81.2.838. [DOI] [PubMed] [Google Scholar]
- 25.Wissler EH. Pennes' 1948? paper revisited. J Appl Physiol. 1998;85(1):35–41. doi: 10.1152/jappl.1998.85.1.35. [DOI] [PubMed] [Google Scholar]
- 26.US Patent 6,954,662. A method of monitoring glucose level. 2005 Oct 11; [Google Scholar]
- 27.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]