Abstract
Background
Hypoglycemia is often cited as a barrier to achieving inpatient glycemic targets. We sought to characterize hypoglycemic events in our institution by work-shift cycle and by specific treatment area.
Methods
Capillary (bedside) and blood (laboratory) glucose values of <70 mg/dl for patients with either a known diagnosis of diabetes or with evidence of hyperglycemia were abstracted from our laboratory database for hospitalizations between October 1, 2007, and February 3, 2008. Hypoglycemic events were analyzed by 12 h nursing work-shift cycles (day shift, 07:00 to 18:59; night shift, 19:00 to 06:59) and by the six medical, surgical, and intensive care areas in the hospital (designated areas 1 to 6).
Results
We identified 206 individual patients with either diabetes or hyperglycemia (mean age, 67 years; 56% men; 83% white) who had 423 hypoglycemic events. There were 78% more hypoglycemic events during the night shift (n = 271 events in 128 individual patients) than during the day shift (n = 152 events in 96 individual patients). Most of the night-shift hypoglycemic measurements were detected between 04:00 and 04:59 or 06:00 and 06:59. The mean hypoglycemic level was comparable between shifts (p = .79) and across the six inpatient areas. The number of hypoglycemic events per person increased with lengths of hospital stay >5 days. The prevalence of hypoglycemia varied across patient care areas within the hospital, with most (28%) detected in one area of the hospital.
Conclusion
There are temporal and geographic patterns in the occurrence of hypoglycemia among patients with diabetes or hyperglycemia in our hospital. Further study should focus on the reasons underlying these variations so that specific interventions can address the risk of hypoglycemia during peak times and places.
Keywords: diabetes mellitus, glucose, hospitalizations, hypoglycemia
Introduction
Hospitalized persons are at risk for the development of hypoglycemia. Hypoglycemia in nondiabetes patients is often associated with severe underlying illness, such as renal failure or advanced liver disease.1,2 Patients with diabetes or hyperglycemia, however, are the inpatients in whom hypoglycemia is most often observed, and it is frequently related to the use of therapies for the management of high glucose levels or to the disruption of nutritional intake while receiving hyperglycemic therapy.1,3–5 Severe hypoglycemia, which various studies define as glucose values of <50 or <40 mg/dl, has been associated with higher mortality in persons with and without diabetes, although it is still unclear whether hypoglycemia is only a marker for poor outcome or is itself the principal cause of death.1,2,6–9
The prevalence of hypoglycemia in inpatients with diabetes is difficult to establish, because reports use different biochemical criteria to define an event;1,4,6–10 however, the overall and per person prevalence of hypoglycemia is much lower compared with that of hyperglycemia.11–13 With a biochemical cutoff of <70 mg/dl (the definition of hypoglycemia used by our institution), the per person prevalence of hypoglycemic events among patients with diabetes or hyperglycemia in our facility is two per person, and the frequency of severe hypoglycemic events is even lower.12 A low frequency of hypoglycemia may be common in hospitals in general.13
Practitioner fear of hypoglycemia is often cited as the main obstacle to controlling hospital hyperglycemia.14 In recent surveys, hypoglycemia was ranked highly by clinicians as a barrier to the management of inpatient hyperglycemia, but it was not the number one obstacle listed. However, in these surveys, practitioners also reported not feeling comfortable in managing hypoglycemia in the hospital, and most were not very familiar with institutional policies guiding its treatment.15–17
As hospitals begin to implement tight glycemic control programs, there is a risk that hypoglycemia will increase; hence the prevention of hypoglycemia remains a justifiable inpatient goal.9 Developing effective hypoglycemia prevention programs requires greater understanding of event patterns in the hospital. Specifically, determining if there is any diurnal or geographic variation in hypo-glycemic events could help focus quality improvement efforts that are geared toward reducing the risk of occurrence. To better elucidate hypoglycemia patterns in our facility, we conducted a cross-sectional analysis of hypoglycemia detected in inpatients with diabetes or hyperglycemia to search for any temporal or geographic variation of events during their hospital stays.
We included patients without a definite diabetes diagnosis but with evidence of hyperglycemia for three reasons. First, these patients may require treatment (and therefore be at risk for hypoglycemia). Second, patients with hyperglycemia may represent individuals with undiagnosed diabetes. Third, there could be a coding error (someone with diabetes may be coded as having hyperglycemia rather than diabetes or vice versa). Finally, the pathophysiology of glucose damage (e.g., antioxidant formation) is a pathway that likely does not differ on the basis of whether someone has a preexisting diagnosis of diabetes.
Methods
Description of Facility
Our tertiary care academic teaching hospital is a facility with 200+ beds in metropolitan Phoenix, Arizona, that offers all general medical and surgical specialties for adult patients but does not provide pediatric care or obstetric care. The hospital also has a level II trauma center and an inpatient rehabilitation unit. Inpatient care is provided by resident physicians, students in allied health and medical schools, physician assistants, nurse practitioners, and faculty of the College of Medicine, Mayo Clinic. Inpatient areas typically house both surgical and medical cases. There are six inpatient care areas (excluding the emergency department and preanesthesia and post-anesthesia recovery areas). Although some inpatient areas may represent specific specialties (e.g., cardiac; transplant; hematology or oncology; or intensive care), patients are often placed where there is bed availability, and thus wards may have a patient population with mixed diagnoses and severity of illness.
In our hospital, patients not receiving an insulin infusion (which is only provided in the intermediate and intensive care areas) generally have bedside glucose readings obtained before each meal (typically between 07:00 and 08:00, 11:00 and 12:00, or 16:00 and 17:00) and at bedtime (around 21:00); for persons being treated with an insulin drip, hourly values are measured. Laboratory glucose levels are generally obtained between 04:00 and 04:59 but are not necessarily ordered on a daily basis for noncritically ill patients. Bedside glucose monitoring is conducted using an Accu-Chek® Inform (Roche Diagnostics, Indianapolis, IN), an instrument that scans and records patient identification from a bar code, followed by direct downloading to our laboratory database. Commercial software (RALS®-Plus, Medical Automation Systems, Charlottesville, Virginia) facilitates the interfacing of glucometer data with the electronic laboratory file.13
Case Selection
Between October 1, 2007, and February 3, 2008, electronic laboratory records were searched weekly to identify all laboratory and bedside glucose values of <70 mg/dl occurring in the six inpatient care areas. Patient admission and discharge dates were determined, and electronic medical records were examined to abstract demographic data, including any diagnosis of diabetes or hyperglycemia. Data from patients without diabetes or hyperglycemia whose hypoglycemia resulted from other causes were excluded from analysis.
Data Analysis
Management of hypoglycemia in our hospital follows a written protocol involving treatment of the hypoglycemic event followed by a recheck of the patient's glucose, typically within 15 min after attempted correction. This cycle is repeated until the hypoglycemia resolves. Thus all hypoglycemic values in the laboratory database do not necessarily represent unique occurrences but may instead be a continuation of a signal event.5 Consequently, when we identified hypoglycemic values recorded during the first hour of an index event for a specific patient, they were not counted, because they most likely represented persistently low values rather than unique episodes.
Identified hypoglycemic events were then analyzed by 12 h nursing work-shift cycles (day shift, 07:00 to 18:59; night shift, 19:00 to 06:59). For each of the two shifts, the number of hypoglycemic episodes was counted separately for laboratory glucose and bedside glucose values and then for both combined. The 24 h pattern of events was then plotted. Additionally, we calculated the number of hypoglycemic readings per patient by shift.
The number of hypoglycemic episodes was counted separately for each of the six medical, surgical, and intensive care wards (designated areas 1 to 6 in this analysis) within the hospital and were ranked according to the areas that had the highest prevalence of hypo-glycemia. Area 1 typically accommodates patients with cardiovascular diseases; area 2, persons with neurologic diseases; area 3, transplant patients; area 4, intermediate/intensive care; area 5, hematology/oncology/bone marrow transplant patients; and area 6, orthopedic/urologic patients.
Per patient differences in mean hypoglycemia levels were compared between night and day shifts and across inpatient areas using nonparametric tests. We also analyzed the number of hypoglycemic events per patient according to length of hospital stay. Length of stay was determined for those patients who had an admission and discharge date within the time frame of the data collection period. We evaluated the distribution of hypoglycemic values by determining the percentage of values that were <40, 40 to 49, 50 to 59, and 60 to 69 mg/dl. The number of hypoglycemic events per person and the severity of hypoglycemia were compared between intermediate/intensive care units and nonintermediate/intensive care units of the hospital.
Results
Patient Characteristics
We identified 251 individual patients with unique hypo-glycemic events in the hospital between October 1, 2007, and February 3, 2008. Of these 251 inpatients, 45 (18%) had hypoglycemia due to causes other than diabetes (e.g., insulinoma, factitious insulin use, severe illness, or drug overdose). The final analytic dataset focused on the remaining 206 patients who had a diagnosis of diabetes or hyperglycemia. The mean (SD) age of these patients was 67 years (14); 56% were men, and 83% were white. The 206 patients had a total of 423 hypoglycemic episodes (laboratory glucose and bedside glucose values combined).
Temporal Hypoglycemia Patterns
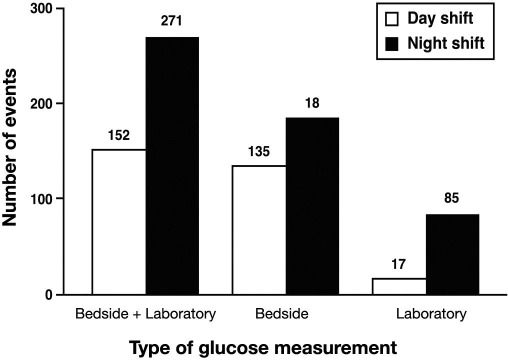
Regardless of whether our analysis focused on laboratory glucose values or bedside glucose values, we detected more hypoglycemic events during the night shift than during the day shift (Figure 1). An examination of combined (laboratory plus bedside) glucose values showed that 78% more hypoglycemic occurrences were recorded during the night shift than during the day shift; the night shift had 271 episodes of hypoglycemia, and the day shift had 152 episodes (Figure 1).
Figure 1.
More hypoglycemic events (defined as <70 mg/dl) were detected in hospital patients in a 24 h period during the 12 h night shift between 19:00 and 06:59 than during the 12 h day shift between 07:00 and 18:59.
When bedside glucose values were analyzed separately, there were 186 events for the night shift and 135 for the day shift (Figure 1). There were 85 laboratory glucose values <70 mg/dl for the night shift but only 17 for the day shift (Figure 1). In our facility, insulin infusion is provided only in the intensive care setting. Hence the hypoglycemia patterns observed may be biased by the contribution of more aggressive use of insulin or more frequent monitoring from the intensive care setting. However, this pattern of more hypoglycemic readings during the night shift persisted even after the exclusion of events that occurred in the intensive care areas of the hospital (not shown).
There were 98 patients in the night shift and 86 in the day shift who had hypoglycemic bedside readings, and 40 patients had low bedside values in both shifts. Sixty-one patients in the night shift and 16 in the day shift had laboratory glucose readings that were hypoglycemic, with 8 having hypoglycemia detected in both shifts. Forty patients experienced low bedside readings in the night and day shifts, and 8 had low laboratory readings in both shifts.
The mean number of bedside hypoglycemic readings was 1.9 (1.4) per patient in the night shift and 0.7 (0.4) in the day shift (p < .0001 by Mann–Whitney test). The mean number of laboratory hypoglycemic values was 1.4 (0.8) per patient for night shift and 1.1 (0.3) in day shift (p = .25). The per patient number of bedside hypoglycemic readings was similar (p = .43) for the intermediate/intensive care and nonintermediate/intensive care areas. The number of laboratory hypoglycemic events per patient was also similar (p = .16) for the intermediate/intensive care and nonintermediate/intensive care areas. The mean hypoglycemic value for bedside readings was comparable between the night shift and the day shift (mean hypoglycemia value, 56 mg/dl (10) and 56 mg/dl (12), respectively; p = .27 by the Mann–Whitney test), and there were no statistically significant differences in severity of laboratory hypoglycemia between the night shift and the day shift (mean hypoglycemia value, 58 mg/dl (9) and 54 mg/dl (14), respectively; p = .26 by Mann–Whitney test). The mean bedside hypoglycemic values were similar (p = .22) for the intermediate/intensive care and nonintermediate/intensive care areas. The mean laboratory hypoglycemic values were also similar (p = .15) for the intermediate/intensive care and nonintermediate/intensive care areas. The overall distribution of bedside hypoglycemic values was as follows: 6%, <40 mg/dl; 20%, 40 to 49 mg/dl; 25%, 50 to 59 mg/dl; and 49%, 60 to 69 mg/dl, with no differences in distribution based on shift (p = .34 by Chi square). The distribution of laboratory glucose values were as follows: 5%, <40 mg/dl; 16%, 40 to 49 mg/dl; 24%, 50 to 59 mg/dl; and 55%, 60 to 69 mg/dl, with no differences between shifts (p = .09 by Chi square).
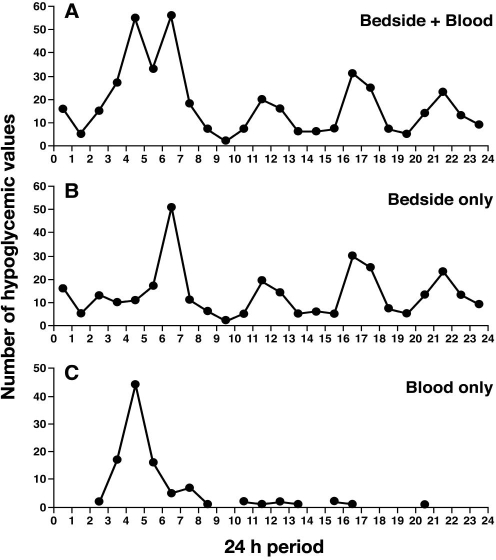
When hourly hypoglycemic values were assessed, we found that most events occurred between 04:00 and 04:59 and between 06:00 and 06:59, with other peaks occurring between 11:00 and 11:59, 16:00 and 16:59, and 21:00 and 21:59 hours (Figure 2A). Bedside glucose data contributed to the peaks found between 06:00 and 06:59, 11:00 and 11:59, 16:00 and 16:59, and 21:00 and 21:59 hours (Figure 2B), whereas laboratory glucose data contributed to the peak found between 04:00 and 04:59 hours (Figure 2C).
Figure 2.
In a 24 h period, most hypoglycemic events in hospital patients occurred (A) between 04:00 and 04:59 or between 06:00 and 06:59; other peaks occurred between 11:00 and 11:59, 16:00 and 16:59, and 21:00 and 21:59 hours. (B) bedside glucose data contributed to the peaks occurring between 06:00 and 07:00, 11:00 and 11:59, 16:00 and 16:59, and 21:00 and 21:59 hours. Whereas, (C) laboratory glucose data contributed to the peak that occurred between 04:00 and 05:00 hours.
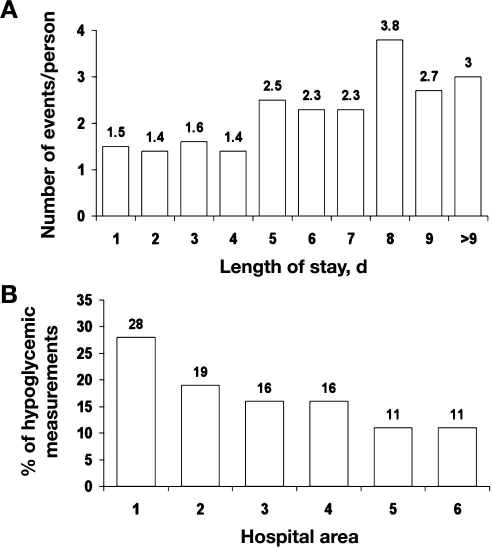
The median length of stay was 6 days, and the mean was 9 days (11) (range of 1 to 97 days). Most patients (72%) had lengths of stay that were 9 days or less. Therefore we analyzed the number of hypoglycemic events per person for lengths of stay up to 9 days. The number of hypoglycemic events (combined bedside and laboratory glucose values) per patient varied in relation to hospital length of stay. Fewer hypoglycemic events occurred with lengths of stay of 4 days or less (Figure 3A) and then increased after lengths of stay were >5 days.
Figure 3.
(A) More hypoglycemic events were seen with longer lengths of stay. (B) Prevalence of hypoglycemia by inpatient care area.
In-Hospital Distribution of Hypoglycemia
Some areas in the hospital experienced more hypoglycemia than others. The percentage of hypoglycemic values ranged from a high of 28% in area 1 to lows of 11% in areas 5 and 6 (Figure 3B). Although these areas would have had mixed medical/surgical patient populations, they were generally designated for persons with specific diagnoses. Hence area 1 typically accommodated patients with cardiovascular diseases; area 2, persons with neurologic diseases; area 3, transplant patients; area 4 was intermediate/intensive care; area 5, hematology/oncology/bone marrow transplant patients; and area 6, orthopedic/urologic patients.
No one patient care area had worse hypoglycemia than any other (i.e., mean hypoglycemic values were not statistically significant among areas; p = .88 by the Kruskall–Wallis test).
Discussion
The risk of hypoglycemia remains a concern for hospitalized patients with diabetes.14 As might be expected, tight glycemic control programs are associated with more hypoglycemia.9,18 Identifying the times of highest risk for hypoglycemic events and the areas within the hospital where most of these events occur can help hospitals focus on identifying the contributing factors and developing targeted interventions to reduce the frequency and severity of low glucose values among diabetes inpatients.
We found that most of the hypoglycemic events in our facility occurred during the night shift, particularly during the early morning hours. The increased frequency of events occurring overnight persisted even after excluding the measurements taken in intensive care areas, where glucose values are typically obtained more frequently; hence the higher overnight values would not represent a measurement bias introduced by the more frequent glucose monitoring of intensive care patients. The greater frequency of bedside readings occurring overnight were due both to more patients having events as well as more events occurring per patient, while the higher night-shift laboratory hypoglycemic readings were from more patients having low values.
We observed that the number of hypoglycemic events occurring per person was lower with shorter hospital stays but increased with longer hospitalizations. One explanation for this finding could be that longer stays in the hospital allowed practitioners more time to aggressively intensify insulin therapy, which led to more hypoglycemia. The other possibility is that efforts to control hyperglycemia earlier in the hospital stay finally caught up with the patient (in the form of more hypoglycemia) as the hospitalization progressed.
This was not an analysis of diabetes prevalence by hospital area (which would require the number of all patients by hospital area as the denominator) or a prevalence study of hypoglycemia among diabetes patients, which we have reported on previously.12 It should be noted that this article is also not an analysis of changes in glucose control over time, an assessment of glycemic severity, or an assessment of therapeutic strategies. We have previously described mean glucose values, changes in glucose over the first and last 24 h of the hospital stay at our facility, and patterns of insulin use.12,19
There is no consensus about how best to summarize and
report glycemic control in the hospital (i.e., “glucometrics”); correlation between laboratory versus bedside glucose values can be imprecise, particularly in intensive care settings.20–22 We have previously used bedside glucose measurements to evaluate the status of inpatient glucose control.11–13 The limits of bedside glucose testing must be recognized when deciding on inpatient glycemic goals and how to measure success at achieving those goals. However, bedside glucose measurements remain the mainstay of how practitioners judge the daily (if not hourly, in intensive care settings) status of inpatient hyperglycemia and make therapeutic decisions about its management. In our facility, the number of bedside glucose measurements averages approximately four per day, whereas mean laboratory glucose measurements are less than one per day (too few to effectively monitor for hypoglycemia).12
In this study, a substantial portion of hypoglycemic events were detected through the use of routine bedside glucose monitoring; these might have been missed by relying on less frequently monitored laboratory glucose. It is possible that many hypoglycemic events were either asymptomatic, occurred in patients who had altered mental status and hence were unable to respond appropriately to hypoglycemia, or happened in patients who were not familiar regarding the symptoms of low laboratory glucose.
Our data most likely do not represent a true physiologic circadian pattern of nocturnal hypoglycemia, as has been proposed for hyperglycemia in the intensive care setting.23,24 The diurnal and preprandial patterns we detected are mostly incidental findings resulting from routine glucose monitoring, and they suggest that the hypoglycemia is caused by extrinsic factors rather than some innate rhythm. One likely explanation is inaccurate application of insulin therapy. Sliding scale insulin therapy has been associated with more hypoglycemia,10,25 and if there is a predominant use of sliding scale insulin therapy at bedtime to cover high glucose readings, for example, this may predispose to more nighttime hypo-glycemic events. We have previously shown that there is a heavy use of bolus (short-acting insulin therapy), despite the presence of persistent hyperglycemia.12 Another possibility is that patients must often fast overnight for morning procedures, which places them at risk for hypoglycemia, particularly if there is concomitant use of insulin therapy. Lastly, the higher occurrence of nocturnal hypoglycemia may be due to poorer symptom detection during sleep on the part of the patient.26
The temporal patterns that we observed may also be specific to our hospital. In addition, our findings are limited in that we did not evaluate the possible extrinsic reasons that might account for the observed diurnal hypoglycemic patterns. However, determining the etiology of hypoglycemia is challenging. The antecedent factors to a hypoglycemic event are likely a result of a chain of events (e.g., making a patient fast for a procedure then not adjusting the insulin dose) rather than from a single predisposing cause and may be different for separate episodes of hypoglycemia due to the dynamic nature of dietary intake, medication use, and patient status. Understanding the cause of a hypoglycemic event often requires gathering information from the patient, the nurse, and the chart. Because of this complexity, retrospective chart reviews are probably not the best means of examining contributing causes of inpatient hypoglycemia. One approach to this issue that our institution has taken since completing this analysis that may allow more “real-time assessment” is introducing into the electronic chart a list from which a nurse can choose the most probable contributing cause of a hypoglycemic event at the time of the occurrence.
We also did not collect data on the morbidities associated with hypoglycemic events. Accurate information is difficult to obtain from retrospective chart reviews about the clinical sequela of hypoglycemia. In our institution, hypoglycemia intervention is typically conducted by nursing staff according to written policy guidelines and an algorithm. Grading of the severity of hypoglycemia is not part of our policy requirements, but it could be incorporated into future quality improvement initiatives. From a biochemical standpoint, however, the majority of hypoglycemic values were moderate to mild in severity, with most falling into the 50 to 69 mg/dl range.
Besides identifying temporal patterns in hypoglycemic events, our findings also demonstrated variation across different care areas within our hospital. Prior studies on hypoglycemia in hospital patients have typically focused on the overall institutional prevalence of the problem or on its prevalence within specific treatment settings such as intensive care units.1,4,6–13 Glycemic targets are different in ward patients versus intensive care unit patients, severity of illness is different, and there are very different methods used to achieve control. Because of these factors, the reason, severity, and mechanism for an intensive care patient being hypoglycemic may be very different than for a non-intensive care patient.
Our data indicate that hypoglycemia does not occur uniformly throughout our hospital. The reasons for this geographic variation within our hospital cannot be ascertained from this analysis, but they warrant further investigation. It is possible that some hospital areas are using more aggressive measures to control hyperglycemia or perhaps that some areas have more severely ill diabetes patients who have a greater predisposition to hypoglycemia.
Recent data showing an increased risk of hypoglycemia in hospital patients with attempts to better control hyperglycemia may deter practitioners and hospitals from implementing programs to better control inpatient glucose levels.9,18 An analogy can be drawn with the similar challenge faced in outpatient diabetes management. Achieving desired glycemic targets among patients with diabetes is often limited in the outpatient setting by concern of causing hypoglycemia.27 Clinicians are always trying to strike a balance between tight glycemic control and the risk of hypoglycemia in diabetes outpatients as they attempt to achieve the best individual glycemic control possible.
Diabetes outpatients typically have control over factors (e.g., amount and timing of meals and exercise) that might contribute to or reduce the risk of hypoglycemia, and they typically can recognize symptoms and institute corrective measures. For many reasons, in the inpatient setting, however, patients generally must surrender control of their diabetes management to the medical staff and may not be able to participate in their own diabetes care. Since diabetes self-management is often not an option in the hospital, tight glycemic control initiatives for inpatients cannot be instituted unless they are coupled with efforts to understand and correct system-based problems that increase the risk of hypoglycemia. An example would be insulin-infusion algorithms with built-in expert rules designed to avoid hypoglycemia.28 Rather than abandon efforts to improve inpatient hyperglycemia in order to avoid hypoglycemia, hospitals should examine their hypoglycemia policies—which are typically just reactive—and alter them to incorporate preventive strategies.
Despite the limitations of our analysis, this study provided insights on the patterns of hypoglycemia in our hospital. Further investigation is needed to determine why most episodes occur overnight and in specific areas of the hospital. With more information, interventions can be developed to specifically reduce the frequency of low glucose levels at these high-risk times and places.
References
- 1.Fischer KF, Lees JA, Newman JH. Hypoglycemia in hospitalized patients. Causes and outcomes. N Engl J Med. 1986;315(20):1245–1250. doi: 10.1056/NEJM198611133152002. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza A, Kim YN, Chernoff A. Hypoglycemia in hospitalized adult patients without diabetes. Endocr Pract. 2005;11(2):91–96. doi: 10.4158/EP.11.2.91. [DOI] [PubMed] [Google Scholar]
- 3.Kresevic DM, Slavin SM. Incidence of hypoglycemia and nutritional intake in patients on a general medical unit. Nursingconnections. 1989;2(4):33–40. [PubMed] [Google Scholar]
- 4.Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, Hoekstra JB. Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med. 2006;34(1):96–101. doi: 10.1097/01.ccm.0000194536.89694.06. [DOI] [PubMed] [Google Scholar]
- 5.Varghese P, Gleason V, Sorokin R, Senholzi C, Jabbour S, Gottlieb JE. Hypoglycemia in hospitalized patients treated with antihyperglycemic agents. J Hosp Med. 2007;2(4):234–240. doi: 10.1002/jhm.212. [DOI] [PubMed] [Google Scholar]
- 6.Stagnaro-Green A, Barton MK, Linekin PL, Corkery E, deBeer K, Roman SH. Mortality in hospitalized patients with hypoglycemia and severe hyperglycemia. Mt Sinai J Med. 1995;62(6):422–426. [PubMed] [Google Scholar]
- 7.Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80(12):1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 8.Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB, Schultz MJ, Rosendaal FR, Hoekstra JB. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med. 2006;34(11):2714–2718. doi: 10.1097/01.CCM.0000241155.36689.91. [DOI] [PubMed] [Google Scholar]
- 9.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 10.Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545–552. [PubMed] [Google Scholar]
- 11.Knecht LA, Gauthier SM, Castro JC, Schmidt RE, Whitaker MD, Zimmerman RS, Mishark KJ, Cook CB. Diabetes care in the hospital: is there clinical inertia? J Hosp Med. 2006;1(3):151–160. doi: 10.1002/jhm.94. [DOI] [PubMed] [Google Scholar]
- 12.Cook CB, Castro JC, Schmidt RE, Gauthier SM, Whitaker MD, Roust LR, Argueta R, Hull BP, Zimmerman RS. Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211. doi: 10.1002/jhm.188. [DOI] [PubMed] [Google Scholar]
- 13.Cook CB, Moghissi E, Joshi R, Kongable GL, Abad VJ. Inpatient point-of-care bedside glucose testing: preliminary data on use of connectivity informatics to measure hospital glycemic control. Diabetes Technol Ther. 2007;9(6):493–500. doi: 10.1089/dia.2007.0232. [DOI] [PubMed] [Google Scholar]
- 14.Braithwaite SS, Buie MM, Thompson CL, Baldwin DF, Oertel MD, Robertson BA, Mehrotra HP. Hospital hypoglycemia: not only treatment but also prevention. Endocr Pract. 2004;10(Suppl 2):89–99. doi: 10.4158/EP.10.S2.89. [DOI] [PubMed] [Google Scholar]
- 15.Cook CB, McNaughton DA, Braddy CM, Jameson KA, Roust LR, Smith SA, Roberts DL, Thomas SL, Hull BP. Management of inpatient hyperglycemia: assessing perceptions and barriers to care among resident physicians. Endocr Pract. 2007;13(2):117–124. doi: 10.4158/EP.13.2.117. [DOI] [PubMed] [Google Scholar]
- 16.Cook CB, Jameson KA, Hartsell ZC, Boyle ME, Leonhardi BJ, Farquhar-Snow M, Beer KA. Beliefs about hospital diabetes and perceived barriers to glucose management among inpatient midlevel practitioners. Diabetes Educ. 2008;34(1):75–83. doi: 10.1177/0145721707311957. [DOI] [PubMed] [Google Scholar]
- 17.Cheekati V, Osburne RC, Jameson KA, Cook CB. Perceptions of resident physicians about management of inpatient hyperglycemia in an urban hospital. J Hosp Med. 2009;4(1):E1–E8. doi: 10.1002/jhm.383. [DOI] [PubMed] [Google Scholar]
- 18.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 19.Cook CB, Zimmerman RS, Gauthier SM, Castro JC, Jameson KA, Littman SD, Magallanez JM. Understanding and improving management of inpatient diabetes mellitus: the Mayo Clinic Arizona experience. J Diabetes Sci Technol. 2008;2(6):925–931. doi: 10.1177/193229680800200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg PA, Bozzo JE, Thomas PG, Mesmer MM, Sakharova OV, Radford MJ, Inzucchi SE. “Glucometrics”: assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–569. doi: 10.1089/dia.2006.8.560. [DOI] [PubMed] [Google Scholar]
- 21.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Herbert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 22.Desachy A, Vuagnat AC, Ghazali AD, Baudin OT, Longuet OH, Calvat SN, Gissot V. Accuracy of bedside glucometry in critically ill patients: influence of clinical characteristics and perfusion index. Mayo Clin Proc. 2008;83(4):400–405. doi: 10.4065/83.4.400. [DOI] [PubMed] [Google Scholar]
- 23.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35(2):416–421. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 24.Vogelzang M, van der Horst IC, Zijlstra F, Nijsten MW. Circadian variation of glucose levels: biology or timing of measurements? Crit Care Med. 2007;35(7):1800–1802. doi: 10.1097/01.CCM.0000269406.40845.B5. [DOI] [PubMed] [Google Scholar]
- 25.Gearhart JG, Duncan JL, III, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313–322. [PubMed] [Google Scholar]
- 26.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52(5):1195–1203. doi: 10.2337/diabetes.52.5.1195. [DOI] [PubMed] [Google Scholar]
- 27.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 28.Stockton L, Baird M, Cook CB, Osburne RC, Reid J, McGowan K, Jarvis S. Development and implementation of evidence-based guidelines for IV insula statewide collaborative approach. Insulin. 2008;3(2):67–77. [Google Scholar]