Abstract
Objective
To investigate the mechanism of aggrecanolysis in IL-1-treated cartilage tissue by examining the time course of aggrecan cleavages and the tissue and medium content of MT4-MMP and ADAMTS4.
Methods
Articular cartilage explants were harvested from newborn bovine femoropatellar groove. The effects of IL-1 treatment with or without aggrecanase blockade were investigated by Western analysis of aggrecan fragment generation, ADAMTS4 species (p68 and p53), and MT4-MMP, as well as by realtime PCR for ADAMTS4 and 5. Aggrecanase was blocked with mannosamine, an inhibitor of glycosylphosphatidylinositol anchor synthesis, and esculetin, an inhibitor of MMP-1, MMP-3, and MMP-13 gene expression.
Results
IL-1 treatment caused a major increase in MT4-MMP abundance in the tissue and medium. ADAMTS4(p68) was abundant in fresh cartilage and this was retained in the tissue in untreated cartilage. IL-1 treatment for six days caused a marked loss of p68 from the cartilage and the appearance of p53 in the medium. Addition of either 1.35 mM mannosamine or 31μM–500μM esculetin blocked IL-1-mediated aggrecanolysis and this was accompanied by nearly complete inhibition of the MT4-MMP increase, the p68 loss and the formation of p53. IL-1 treatment increased mRNA abundance for ADAMTS4 (~3-fold) and ADAMTS5 (~10-fold) but this was not accompanied by a marked change in enzyme protein abundance.
Conclusion
These studies support a central role for MT4-MMP in IL-1-induced cartilage aggrecanolysis and are consistent with the identification of p68 as the aggrecanase that cleaves within the CS2 domain, and of p53 as the aggrecanase that generates G1-NITEGE. Since the induction by IL-1 was not accompanied by marked changes in total ADAMTS4 protein, but rather in partial conversion of p68 to p53 and release of both from the tissue, we conclude that aggrecanolysis in this model system results from MT4-MMP-mediated processing of a resident pool of ADAMTS4 and release of the p68 and p53 from their normal association with the cell surface.
Keywords: aggrecan degradation, ADAMTS4, MT4-MMP, mannosamine, esculetin
Introduction
Enhanced release of proteoglycans from the articular cartilage to the synovial fluid is a prominent feature of early and late osteoarthritis. In particular, aggrecan is the most abundant proteoglycan in cartilage tissue and provides much of the tissue’s compressive strength through electrostatic repulsion of its tightly packed glycosaminoglycan chains1. Excess degradation of aggrecan in the cartilage tissue thus contributes directly to the failure of the mechanical function of the joint.
Investigation of enzymatic degradation of aggrecan has led to the identification of specific cleavage sites generated by enzymes termed aggrecanases, which have since been identified as a sub-group (ADAMTS-1,4,5,8,9,15)2–4 of the 19-member family of ADAMTS (a disintegrin and metalloproteinase with thrombospondin type I motifs) proteinases5, 6. It is now clear that degradation of aggrecan induced by major inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor is mediated by the action of the aggrecanases and not the matrix metalloproteinases (MMPs)7–10. In addition, the evidence for aggrecanase-mediated cleavage of aggrecan in cartilage tissue and synovial fluid from patients with osteoarthritis11, 12 suggests that aggrecanase activity is predominantly or maybe exclusively responsible for the excess degradation of cartilage aggrecan which is a feature of human osteoarthritis.
Recently, the steps involved in processing of ADAMTS4 (aggrecanase-1) have been described in a human chondrosarcoma cell line stably transfected with ADAMTS4, and the aggrecanase activities of the processed forms characterized. To exhibit any aggrecanase activity, the ADAMTS4 proenzyme first requires intracellular furin-mediated removal of the N-terminal pro-domain13, which generates the p68 form. While this form can degrade the C-terminal chondroitin sulfate (CS)-bearing region, it appears that for “destructive” cleavage of aggrecan in the interglobular domain (IGD), further C-terminal truncation of ADAMTS4 is required2, 14, 15, producing the p53 and p40 forms of the proteinase. We have previously hypothesized that this C-terminal truncation requires a glycosylphosphatidylinositol (GPI)-linked MMP, since IGD cleavage is blocked by inhibitors of GPI anchor synthesis such as mannosamine (ManN) and by MMP inhibitors2, 16. This was recently supported by the finding that transfection of ADAMTS4-expressing cells with MT4-MMP (a GPI-anchored MMP17) promoted C-terminal processing of ADAMTS4 to the p53 and p40 forms and acquisition of IGD cleavage activity18.
However, there has been no detailed description of the processing of ADAMTS4 in native cartilage tissue. Initial studies of the protein forms present in normal and IL-1-stimulated cartilage have shown evidence for ADAMTS4 forms in the 30–60 kDa range9 and 37–46 kDa14, and the effect of IL-1 treatment on levels of MT4-MMP protein in cartilage has not been reported.
We therefore examined aggrecan degradation in newborn bovine cartilage explants treated with IL-1 by Western analysis of aggrecan, ADAMTS4 and MT4-MMP in the cartilage tissue and conditioned medium. In addition, we examined the effects of two aggrecanase blockers, mannosamine16, 19, 20 and esculetin21. We hypothesized that as an inhibitor of GPI anchor synthesis16, 20, 22, mannosamine would interfere with the processing of the p68 form to the p53 form. The mechanism for aggrecanase inhibition by esculetin is not clear but it appears to inhibit MMP gene expression21, 23, and thus allows investigation of the effects of aggrecanase blockade through what is likely a different mechanism than mannosamine. Finally, to investigate regulation of aggrecanase activity at the level of transcription, we measured the effect of IL-1 and mannosamine treatment on mRNA expression of ADAMTS4 and ADAMTS5 by realtime PCR analysis.
Methods
Materials
Human recombinant IL-1α was from R&D Systems, Inc., Minneapolis, MN. Unless indicated, other reagents were from Sigma Chemical Co. (St. Louis, MO).
Cartilage explant and culture
Articular cartilage disks were obtained from the femoropatellar groove of one-to-two-week-old calves, a model system that has been widely studied to examine the biological response pathways relevant to cartilage degradation in human osteoarthritis24, using methods similar to those described in detail previously25. In brief, 9-mm-diameter cylinders of full-thickness cartilage and bone were cored from the articular cartilage. After slicing off sufficient superficial cartilage to create a flat surface (usually less than 500 μm), the next two sequential 0.5-mm-thick slices were cut with a sledge microtome. From each of these slices, four cartilage disks (3 mm in diameter and 0.5 mm in thickness) were cut out with a dermal punch. Groups of cartilage disks were incubated at 37°C in an atmosphere of 5% CO2, in wells containing 0.25 ml/disk of a serum-free culture medium (low-glucose DMEM [Gibco, Grand Island, NY] and 10 mM HEPES buffer, with 100 U/ml penicillin G, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B). Experimental treatments with IL-1 and aggrecanase blockers were begun on the day of harvest. With the exception of the kinetic analysis, these cultures were incubated without medium replacement for up to six days. Each experiment had four-to-eight treatment groups, allowing disks among different groups to be matched for their original location along the surface of the joint.
Biochemical Analysis of Proteoglycans
Tissue and medium were analyzed for composition of aggrecan fragments by Western blotting as previously described26. Aggrecan remaining in the cartilage tissue was extracted in 4 M guanidine (1.2 ml per 75 mg wet weight) for 48 hr at 4°C in the presence of proteinase inhibitors. Aggrecan fragments in tissue extracts and conditioned medium were isolated by ethanol precipitation and deglycosylated. Portions of medium and tissue extracts (corresponding to 10 μg GAG in each case) were loaded on 4–12% polyacrylamide gels (Novex) for Western analysis and probed with polyclonal antisera to the bovine G1 domain (G1–2), the G3 domain (antibody LEC7, provided by Dr. Kurt Doege) and the C-terminal neoepitopes TFKEEE1706 (antiserum provided by Merck), TAGELE1520 (antiserum provided by DuPont), and NITEGE392 (antibody JSCNIT). Tissue (digested overnight with proteinase K) and conditioned medium were assayed for sulfated glycosaminoglycan (GAG) content by reaction with dimethylmethylene blue (DMMB) dye.
Western analysis of ADAMTS4 (aggrecanase-1) and MT4-MMP
Pilot studies were done to optimize the extraction conditions for ADAMTS4 in cartilage. Cartilage (15 mg wet weight) was freeze-milled in a Biopulverizer (Biospec Products, Bartlesville, OK) and the powder was extracted for 20 hrs. at 4°C in 3 volumes (μl/mg wet weight) of a) 50 mM Tris HCl, pH 7.0; b) 50 mM Tris HCl, 100 mM NaCl, pH 7.0; c) 50 mM Tris HCl, 100 mM NaCl, 0.5% Nonidet P-40, pH 7.0; or d) 4 M guanidine, 50 mM Tris HCl, pH 7.0. Macromolecules in the 4 M guanidine extract were purified by Sephadex G50 and DE 52 as previously described for aggrecan fragments27 and the unbound (protein) and bound (proteoglycan) fractions were analyzed. Other cartilage samples were treated at 100°C for 10 minutes in 50 mM Tris HCl and 2.5% SDS. The results of the comparative extraction experiments (not shown) indicated that the highest yield of immunoreactive product was consistently obtained with extractant c). Poor recoveries were seen with 4M guanidine extracts and boiling in SDS. Therefore, all data shown in the present paper were obtained using extraction with 0.5% Nonidet P-40, 50 mM Tris, and 100 mM NaCl at pH 7.0, and dilution of 5–10 μl of the extracts in gel-loading buffer before SDS-PAGE. Conditioned medium from cartilage explants was concentrated by lyophilization, dialyzed, dried and dissolved in gel loading buffer.
Western analysis of ADAMTS4 was done with two affinity-purified antibodies that exhibit different reactivities for the molecular forms of ADAMTS42. JSCVMA was raised to a peptide (Val394–Pro403) in the catalytic domain, and it preferentially reacts with the p53 form in tissue extracts. JSCYNH was raised to a peptide (Tyr590–Pro603) within the Cys-rich region and it reacts preferentially with the p68 form. All soluble samples were loaded on an equivalent tissue weight basis and separate blots were probed with JSCYNH or JSCVMA. For Western analysis of MT4-MMP (MMP-17), blots were probed with antibodies to MT4-MMP from Sigma (catalog #M3684).
Realtime PCR analysis
RNA was extracted from cartilage disks after 24 hours of treatment and analyzed by realtime PCR. Frozen cartilage was pulverized and then homogenized in Trizol reagent (Invitrogen, Carlsbad, CA). After addition of 10% v/v chloroform, the samples were centrifuged at 13,000g for 10 min. in phase-lock gel tubes (Eppendorf AG, Hamburg, Germany). The aqueous supernatant was collected, and RNA was isolated with the RNeasy Mini Kit (Qiagen, Valencia, CA). Extracted RNA concentration and purity was measured by optical density at 260 nm and 280 nm. Equal quantities of RNA from each sample were reverse transcribed with the Amplitaq-Gold RT kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed with the Applied Biosystems 7700 instrument and SYBR Green Master Mix (Applied Biosystems). Primers were designed as described previously28.
For statistical analysis of mRNA expression levels, mRNA copy number was first log-transformed to normalize the distribution of the data. Since the experiment was designed as a factorial experiment, linear regression was performed to test for the effects of the two factors (mannosamine and IL-1) as well as for an interaction between the effects of mannosamine and IL-1. Two indicator variables were also included to adjust for proportional changes in baseline between experiments.
Results
Kinetics of aggrecan degradation in calf explants treated with IL-1
In preliminary experiments to describe the kinetics of IL-1-induced aggrecan degradation in the newborn bovine cartilage tissue, we obtained cartilage disks with a defined cylindrical geometry and treated them with 1, 10 and 100 ng/ml IL-1α. At 2, 4, and 6 days the cultures were terminated and the proportion of total GAG remaining in the tissue as well as the equilibrium and dynamic compressive moduli were measured. This analysis (data not shown) revealed that for explants cut to 0.5 mm in thickness, the addition of IL-1 at 10 ng/ml generated a gradual catabolic response which was most appropriate for kinetic analysis over 6 days.
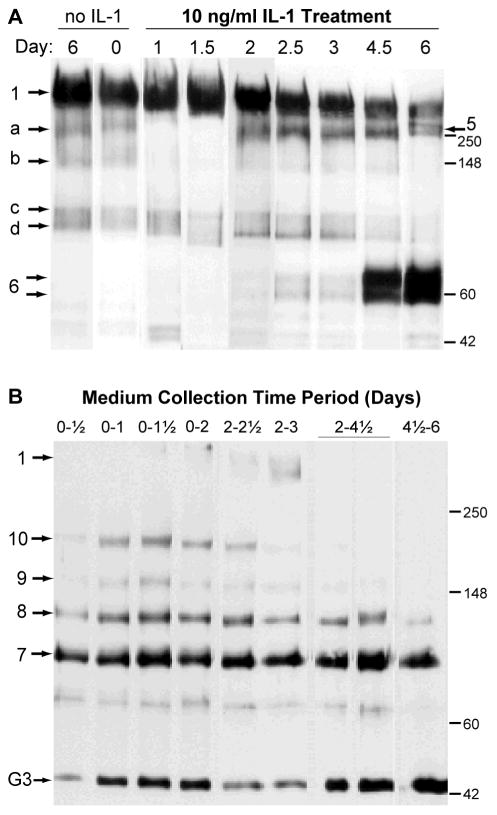
We therefore treated cartilage explants with 10 ng/ml for 6 days and terminated cultures at 8 different times (0, 0.5, 1, 1.5, 2, 2.5, 3, 4.5, and 6 days), with removal of medium and replacement with fresh medium and IL-1 at days 2 and 4.5. To examine the composition of aggrecan remaining in the tissue at these times we performed a Western analysis with anti-aggrecan G1 domain on the tissue extracts (Fig. 1A). The composition of the control tissue (maintained in culture without IL-1 for 6 days) and fresh tissue (maintained for zero days) was primarily core species 1 (full-length aggrecan) with a low abundance of species a, b, c and d, which are not generated by aggrecanase action (see Fig. 2 for peptide notations). Early in the IL-1 treatment period (days 1 and 1.5), species a and b appeared to be eliminated from the tissue, whereas from days 2–6 there was an accumulation in the tissue of species 5 (G1-TAGELE) at about 250 kDa. Western analysis of these samples with anti-TAGELE and anti-TFKEEE confirmed that the abundance of these aggrecanase-generated species (at about 200–250 kDa) was increased during days 2–6, in parallel with the appearance of the G1-reactive band(s) in that region of the gel (data not shown). Interestingly, the N-terminal product G1-NITEGE (species 6 doublet) was not observed until day 4.5 of culture, consistent with the common finding that IL-1-promoted aggrecanase-mediated aggrecan degradation occurs in a stepwise fashion, with the C-terminal region being degraded before cleavage of the interglobular domain9, 16, 29.
Figure 1.
Kinetics of aggrecanolysis by IL-1 treatment in 0.5-mm-thick newborn bovine articular cartilage explants. Cartilage cultures treated with10 ng/ml were terminated at various times during a 6-day experiment. (A) Aggrecan remaining in the tissue was extracted and Western analysis performed with an antibody to aggrecan G1 domain (G1–2). (B) Western analysis of aggrecan fragments released to the corresponding conditioned medium was performed with an antibody to aggrecan G3 domain (LEC-7). Portions of tissue and medium were loaded on an equal-GAG basis. See Figure 2 for peptide identifications.
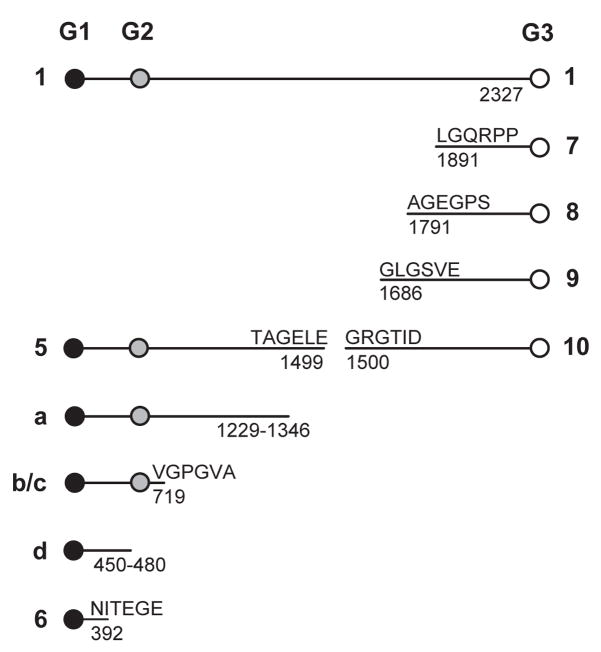
Figure 2.
Schematic of the structures for bovine aggrecan core protein peptides The diagram shows the currently accepted structures for aggrecan core protein peptides derived from bovine cartilage explants before and after treatment with IL-1 (see Figure 1). The sequences and residue numbers shown are taken from data in Hering et al.50 for bovine aggrecan (accession number AAB38524), and the identifications are taken from Oshita et al.31 and references therein. Note that these residue numbers differ from previous reports11, 29 by a constant offset of 19 to conform with genome-based numbering of the leader sequence. Peptides identified with the numbers 5–10 represent aggrecanase-generated aggrecan species.
This interpretation was supported by Western analysis of the medium collected over various periods during this culture with an antibody to the G3 domain (Fig. 1B). The “ladder” of G3-reactive species labelled as 7,8,9,10 are characteristic of aggrecanase-mediated C-terminal processing during the early period (days 0–3), whereas the relative paucity of these products in medium collected after day 4.5 is consistent with aggrecanase cleavage now being targeted to the interglobular domain. This was verified by Western analysis of the medium for the G1 domain, which showed nearly undetectable G1-NITEGE release until day 4.5, and no evidence for higher-molecular-weight G1-bearing fragments (data not shown), as observed in this system previously30. An unidentified G3-reactive fragment at about 44 kDa was a major medium product at all time periods, and this migration behaviour is consistent with the isolated globular region of the G3 domain of bovine aggrecan. The proteinase responsible for the generation of this species is unknown; however, the same or similar product is readily generated from bovine aggrecan by m-calpain digestion31. Analysis of the tissue extracts with the anti-G3 antibody detected only the full-length core protein (data not shown), suggesting that these G3-bearing fragments diffuse rapidly into the medium once formed.
Analysis of explant system for ADAMTS4 protein
Since it was clear that an active aggrecanase was present in this system and since it has been suggested that ADAMTS4 (rather than TS-1, 5, 8, 9 or 15) is mostly responsible for this activity4, 9, 32–35, we analyzed both tissue and medium for ADAMTS4 in control cultures, IL-1-treated cultures, and IL-1-treated cultures with the addition of 1.35 mM mannosamine. Consistent with our previous findings29, in this experiment GAG released from the cartilage to the medium increased from 24 ± 2 μg/disk in controls to 115 ± 6 μg/disk in IL-1-treated cultures, and this aggrecanase-mediated degradation was blocked by the addition of mannosamine (40 ± 3 μg/disk; all N = 4 disks per group).
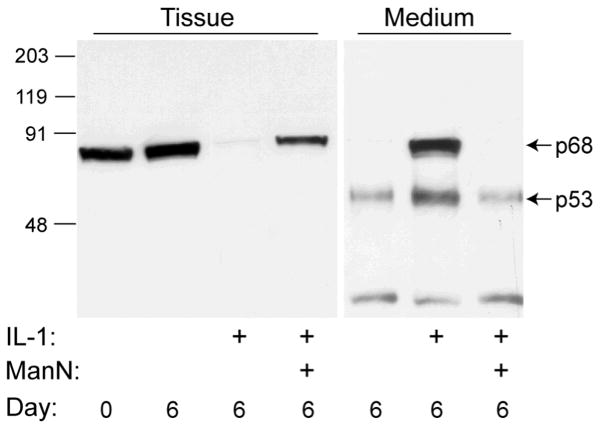
Extracts of untreated control tissue at days 0 and 6, probed with JSCYNH (Fig. 3, tissue), contained a single major immunoreactive protein which was identified as the p68 form. Since this antibody reacts with the proenzyme in cell culture experiments, the absence of immunoreactive proenzyme here suggests that either the proenzyme does not accumulate in the tissue or it is poorly reactive in these extracts, perhaps due to the presence of masking proteins. After 6 days of IL-1 treatment, the p68 form was essentially eliminated from the tissue, a process which was markedly blocked by the inclusion of mannosamine. Analysis of the medium showed that under control conditions, some of the p53 form (and an unidentified fragment of about 30 kDa) was released from the tissue, unlike the p68 form, which remained associated with the tissue. In marked contrast, abundant p68 and p53 were released into the medium after treatment with IL-1 for 6 days; however, the inclusion of mannosamine returned this process to control levels.
Figure 3.
Western analysis of ADAMTS4 in cartilage tissue and conditioned medium with JSCYNH. Cartilage was analyzed immediately after harvest (Day 0) or incubated for 6 days in serum-free medium with no treatment, 10 ng/ml IL-1, or IL-1 plus 1.35 mM ManN. Conditioned medium was concentrated and loaded on an equal volume basis (500 μl). IL-1 treatment resulted in a loss of tissue p68 to the medium and an increase in abundance of the p53 in the medium that was blocked by addition of ManN.
Analysis of explant system for MT4-MMP protein
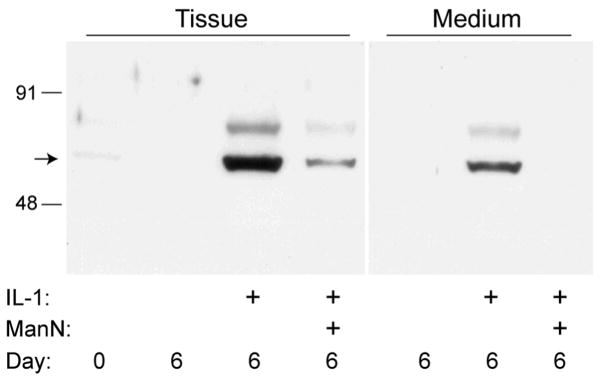
To examine the possibility that GPI-anchored MT4-MMP is involved in processing of ADAMTS4 in bovine cartilage, the tissue and conditioned medium from this experiment were further examined by Western analysis for MT4-MMP (MMP-17) protein (Fig. 4). In fresh tissue (day 0), a trace amount of MT4-MMP protein was detected, whereas in control tissue and medium at 6 days it was not detectable. In contrast, analysis of tissue treated with IL-1 showed a marked increase in MT4-MMP abundance at ~65 kDa (a size consistent with previous observations in cells and tissues18, 36). This increase in protein abundance was largely reversed on addition of mannosamine. Similarly, analysis of the collected conditioned medium showed an increased abundance of MT4-MMP protein in IL-1-treated cultures that was blocked by inclusion of mannosamine. The nature of the minor immunoreactive product at ~80 kDa is unknown.
Figure 4.
Western analysis of MT4-MMP in cartilage tissue and conditioned medium. Cartilage was analyzed immediately after harvest (Day 0) or incubated for 6 days in serum-free medium with no treatment, 10 ng/ml IL-1, or IL-1 plus 1.35 mM ManN. IL-1 treatment resulted in a strong increase in MT4-MMP abundance that was blocked by addition of ManN.
Effect of esculetin on IL-1-induced changes
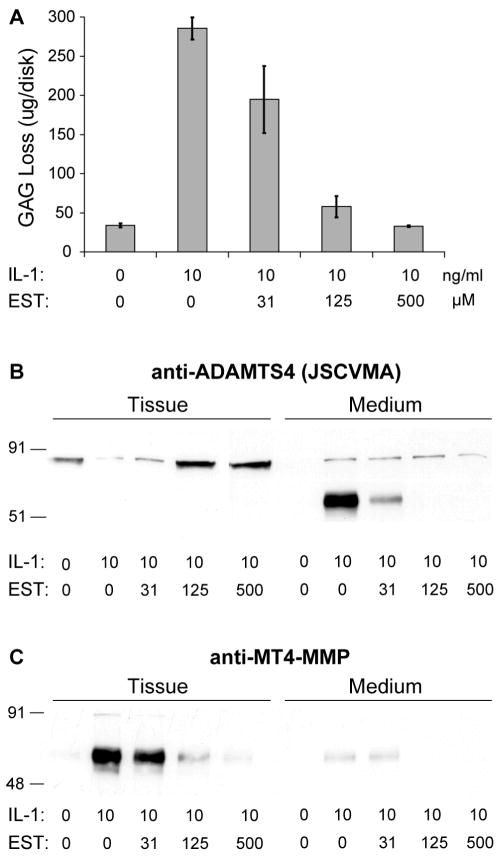
Esculetin (dihydroxycoumarin) has previously been shown to inhibit cartilage degradation induced by the combination of IL-1 and oncostatin M, but the mechanism is not clear21, 23, 37. We therefore performed a parallel set of experiments, first demonstrating inhibition of IL-1-induced aggrecanase activity by esculetin, and then testing whether esculetin also reversed IL-1-induced changes in ADAMTS4 and MT4-MMP protein. Cartilage explants were incubated in the presence or absence of 10 ng/ml IL-1 and esculetin for six days, with doses from 31 μM to 500 μM esculetin. Esculetin was solubilized in dimethylsulfoxide (DMSO) at a concentration sufficient to ensure that the final concentration of DMSO did not exceed 0.5% in any culture medium.
Esculetin inhibited GAG loss from tissue in a dose-dependent manner (Fig. 5A), and had marked effects on both the abundance and distribution of ADAMTS4 (Fig. 5B) and MT4-MMP (Fig. 5C). In this experiment, the control cartilage contained the p68 form of ADAMTS4, and no detectable ADAMTS4 was released during culture for six days. In the presence of IL-1, there was a loss of p68 from the tissue and appearance of p53 in the medium. While the Western analysis suggests an increase in total immunoreactive ADAMTS4, this may be misleading since the antibody used (JSCVMA) reacts preferentially with the p53 form (see Methods). Esculetin markedly blocked this IL-1-mediated conversion and release process at 31 μM and completely blocked it at 125 μM. In keeping with these profound effects of esculetin on ADAMTS4, the abundance of MT4-MMP in the tissue (very little was found in the medium) was markedly (more than 10-fold) enhanced by IL-1 treatment and this effect was blocked by esculetin in a dose-dependent manner over the concentration range tested.
Figure 5.
Esculetin (EST) inhibits IL-1-induced changes in ADAMTS4 and MT4-MMP proteins. Cartilage tissue was treated for 6 days with 10 ng/ml IL-1 and EST over a range of doses up to 500 μM. (A) The GAG released from the tissue to the conditioned medium showed dose-dependent inhibition of IL-1-induced GAG loss by EST (bars indicate mean ± SEM for 6 replicates per group). Linear regression analysis of rank-transformed EST dose vs. GAG loss showed a significant trend (p = 0.02 for N = 4 doses). (B) Western analysis for ADAMTS4 was performed on cartilage tissue extracts and conditioned medium by probing with JSCVMA. Treatment with IL-1 caused a prominent increase in medium abundance of p53 that was blocked by addition of 125 μM EST. (C) Western analysis for MT4-MMP in cartilage tissue and conditioned medium showed that IL-1 treatment resulted in a strong increase in MT4-MMP abundance, and this increase was blocked by addition of 125 μM EST.
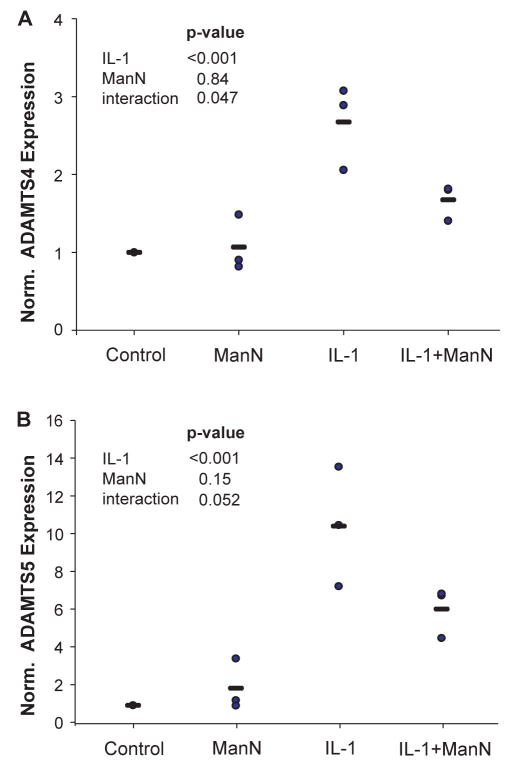
Realtime PCR Analysis
To examine the possibility that the mannosamine blockade of IL-1 mediated aggrecanase activity in bovine cartilage explants could be due to transcriptional inhibition we carried out realtime PCR of ADAMTS4 and ADAMTS5 in this system. The data (Fig. 6) shows that for both aggrecanases there was a marked (~3-fold for ADAMTS4 and ~10-fold for ADAMTS5) increase in transcription one day after addition of 10 ng/ml IL-1 (p < 0.001 for both). There was no significant effect of mannosamine treatment alone, but mannosamine significantly inhibited the IL-1-induced increase in ADAMTS4 (p = 0.047) and showed the same trend for ADAMTS5 (p = 0.052).
Figure 6.
Realtime PCR analysis of ADAMTS4 and ADAMTS5. Cartilage disks were incubated with no additives (control), 10 ng/ml IL-1 (IL-1), 1.35 mM mannosamine (ManN), or the combination (IL-1 + ManN). After one day of culture, mRNA was extracted and realtime PCR was performed. Expression levels for (A) ADAMTS4 and (B) ADAMTS5 are shown relative to untreated control disks for three experiments as individual data points (circles) and mean (bars). The effect of ManN, IL-1, and the interaction between ManN and IL-1 was tested for significance by linear regression.
Discussion
Implications for ADAMTS4 processing and the mechanism of aggrecanolysis
The data provided both confirm and extend current models for the synthesis, processing and mode of action of ADAMTS4 in aggrecan degradation. The importance of the present work is that it provides evidence that the model established in recombinant cell expression systems2, 14, 18 is supportable when examined in live cartilage tissue in explant culture.
The central features of this model include a secreted furin-cleaved p68 protein2, 13 which cannot cleave the interglobular domain of aggrecan but which has high activity toward the CS-attachment region14, 18. This p68 form can be presented on the cell surface in association with MT4-MMP18 where it is C-terminally cleaved by MT4-MMP to generate the p53 form, which can be found in association with syndecan-118, and which readily cleaves at the interglobular domain site of aggrecan to destroy the biomechanical properties of the tissue29. The MT4-MMP-mediated cleavage sites are the same as those shown to occur during autoproteolysis of ADAMTS415, 18.
In the present work we have shown that in a cartilage explant, the IL-1-promoted and aggrecanase-mediated sequential cleavage of the C-terminal and interglobular domain of aggrecan (Figs. 1A and 1B) is not accompanied by a marked change in the amount of ADAMTS4 protein but, in agreement with the studies of Pratta et al7, it appears to be due to activation of an existing pool of enzyme (Fig. 3 and 5B). The changes induced by IL-1 were in the p68 form and the p53 form (Fig. 3), both of which were found in the medium compartment after 6 days. In time-course experiments (not shown) the p68 form was found to be abundant at day 2 whereas the p53 form was only apparent after 6 days. Given that the p68 form is confined to C-terminal cleavage of aggrecan14, the kinetics of appearance of the p68 and p53 seen here are consistent with the finding that C-terminal cleavage occurred early in the process (Fig. 1B) and this was followed after about 4 days by IGD cleavage (Fig. 1A). These data on detergent extracts of cartilage tissue show some differences from previous observations of increased abundance of guanidine-extractable ADAMTS4 at 46 kDa14 or at ~60 and 30 kDa9, which may be explained by variable recovery of ADAMTS4 forms depending on the extractant.
Of particular interest is the finding that both the p68 form and the p53 form are released into the culture medium, suggesting that their proposed cell surface associations with MT4-MMP and syndecan-1 respectively18 are saturable and/or reversible. Regarding the association of p68 with MT4-MMP, it is clear that MT4-MMP also increases in abundance in the presence of IL-1 (Fig. 4) and that a proportion of this newly synthesized GPI-anchored protein is released from the cell and matrix under IL-1 stimulation. While the p68-MT4-MMP complex can be released intact from human chondrosarcoma cells by treatment with phosphatidylinositol-specific phospholipase C18, it is unknown whether the two proteins remain associated after MT4-MMP release from the cell surface in the present system. Interestingly, Itoh et al. have observed in COS cells that the release of MT4-MMP from the cell surface is partially inhibited by a metalloproteinase inhibitor17, suggesting that a member of the ADAM family of sheddases38 might also be involved in the aggrecanase cascade. Alternatively, membrane vesiculation during IL-1 treatment39 might provide an explanation for the release of the MT4-MMP.
While the p68 form is found in both the tissue and the medium, it appears that the p53 form is only abundant in the medium, suggesting that all of the newly formed p53 dissociates from the cell surface syndecan-1 at some time in the process. Again, while the association of p53 with the GAG chains of syndecan-1 in chondrosarcoma cell culture is stable to bi-directional immunoprecipitation protocols18, it is not known whether the p53 in the cartilage explant is released into the medium in association with syndecan-1 and/or aggrecan products. Elucidation of the binding partners for the p68 and p53 forms in cartilage explants, along with immunohistochemical characterization of the spatial and temporal aspects of proteinase location and substrate cleavage will be required to fully understand this complex pathway.
Inhibition of the aggrecanolysis pathway
The data reported also provide novel insight into the mechanism by which inhibitors of the aggrecanase pathway may operate. The findings that MMP inhibitors (such as TIMP-1, peptide hydroxamates, etc.) can partially block aggrecanolysis in tissue explants suggested early that either MMP inhibitors can directly inhibit the ADAMTS4 catalytic domain, or alternatively that an MMP might be involved in activation of aggrecanase30, 40–42.
In support of this, in the present study we have shown that esculetin, an inhibitor of MMP1, 3, and 13 gene transcription21, 23 is also an inhibitor of aggrecanolysis (Fig. 5A), p53 ADAMTS4 formation (Fig. 5B) and MT4-MMP protein accumulation (Fig. 5C). Since the IC50 is about 50 μM for each of these three effects, it seems likely that the esculetin effect operates primarily by blocking induction of MT4-MMP synthesis by IL-1, which results in a lack of conversion of p68 to p53, and a consequent loss of destructive aggrecanolysis. The concentrations of esculetin required to block cartilage resorption were not due to cytotoxic effects. It has previously been reported that esculetin concentrations of up to 500 μM had no effect on chondrocyte viability by measurement of LDH release21. In addition, we have shown in preliminary experiments that 500 μM esculetin does not affect expression of TIMP-1 and GAPDH mRNAs as measured by realtime PCR, indicating that transcription had not been universally altered, and suggesting that the inhibition by esculetin was not based on cytotoxic activity.
The other inhibitor studied here was mannosamine, which we have suggested16, 18 exerts its effect by inhibition of the GPI anchor formation required for membrane insertion of MT4-MMP. The present data is consistent with this idea since 1.35 mM mannosamine markedly (~80%) inhibited the IL-1-induced production of MT4-MMP (Fig. 4) in the cartilage. This predictably blocked the IL-1-induced accumulation of p68 and p53 ADAMTS4 in the medium (Fig. 3). Interestingly, in the presence of mannosamine there is a complete blockade of interglobular domain cleavage by p53; however, some C-terminal cleavage to G1-TAGELE is observed (data not shown). This is consistent with the idea that the pre-existing p68 would likely be active in the presence of mannosamine, since it would not require further GPI anchor formation.
Finally, we show here (Fig. 6) that IL-1 treatment of bovine chondrocytes in cartilage explants results in major upregulation of mRNA for both ADAMTS4 (3-fold) and ADAMTS5 (10-fold). This marked increase in transcriptional activity for these proteinases was not accompanied by major changes in the abundance of these proteins (data not shown for ADAMTS5). This is consistent with the general view that control of aggrecanolysis in cartilage, by inflammatory mediators such as IL-1, is initiated by proteinase processing on the cell surface and release of active enzyme into the matrix. Presumably prolonged exposure to such mediators in joint diseases, with an attendant upregulation of transcription, generates a continuous supply of newly synthesized enzyme which will maintain the activity of this degradative cascade.
The upregulation of ADAMTS5 mRNA observed here is not necessarily indicative of an increase in ADAMTS5 activity, but emphasizes that while the data presented here has focused on activation of ADAMTS4, other family members may also play a role in aggrecanolysis. In particular, the recent findings in mice that the IL-1-induced aggrecanase pathway to G1-NITEGE formation is apparently not blocked by gene deletion of ADAMTS143 or ADAMTS444 suggests that further analysis of the expression and activation of the individual aggrecanases (ADAMTS1,4,5,8,9,15) will be needed to fully define the role of MT4MMP in the aggrecanolysis associated with human osteoarthritis.
As a general comment, it is important to note that the effectiveness of mannosamine in this process should not be interpreted as suggesting the use of mannosamine in place of glucosamine for therapy of human osteoarthritis. Mannosamine, unlike glucosamine, is a potent inhibitor of GPI-anchor synthesis16, which is necessary for the anchoring and function of multiple essential cell-surface proteins45, 46. Mannosamine ingestion is therefore likely to have profound side effects which should preclude its use as a therapeutic.
Our evidence for the involvement of MT4-MMP in aggrecanolysis in cartilage tissue culture also has implications for the interpretation of MMP inhibitor studies. For example, some “broad-spectrum” MMP inhibitors reportedly do not inhibit aggrecanase activity40. However, it is not clear whether all broad-spectrum MMP inhibitors are active against MT-MMPs, which were not characterized until 1999, and may have some unique features in the structure of their catalytic domains47. It is therefore intriguing that CGS20723A, a broad-spectrum MMP inhibitor that potently inhibits the catalytic domain of MT4-MMP in solution48, does not appear to inhibit overall IL-1-induced GAG loss in tissue culture, but has been reported to protect against loss of pericellular GAG staining49. This could be explained if loss of pericellular GAG staining is due to p53 generated by MT4-MMP, whereas more generalized aggrecanolysis in the intercellular matrix is due to p68 and therefore confined to the C-terminal of aggrecan. Thus, the requirement for MT4-MMP for aggrecanolysis suggested by the present data may not extend to all experimental situations, and the development of specific small-molecule MT-MMP inhibitors may in the future be useful for testing these hypotheses.
Acknowledgments
The authors would like to thank Vivian P. Thompson for her help and technical expertise.
This work was supported by grants from the Shriners of North America and the Florida Chapter of the Arthritis Foundation (to J. D. S.), NIH grant AR33236 (to A. J. G.), the Orthopaedic Research and Education Foundation (to G. G.), and a fellowship from the National Science Foundation (J. H. L.)
References
- 1.Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179–92. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- 2.Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–41. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]
- 3.Yamaji N, Nishimura K, Abe K, Ohara O, Nagase T, Nomura N, inventors; Yamanouchi Pharmaceutical Co., Ltd., Tokyo, Japan, and Kazusa DNA Research Institute, Chiba, Japan, assignee. Novel Metalloprotease Having Aggrecanase Activity. US patent 6716613. April 6, 2004.
- 4.Collins-Racie LA, Flannery CR, Zeng W, Corcoran C, Annis-Freeman B, Agostino MJ, et al. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004;23:219–30. doi: 10.1016/j.matbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–5. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Llamazares M, Cal S, Quesada V, Lopez-Otin C. Identification and characterization of ADAMTS-20 defines a novel subfamily of metalloproteinases-disintegrins with multiple thrombospondin-1 repeats and a unique GON domain. J Biol Chem. 2003;278:13382–9. doi: 10.1074/jbc.M211900200. [DOI] [PubMed] [Google Scholar]
- 7.Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003;48:119–33. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- 8.Arner EC, Hughes CE, Decicco CP, Caterson B, Tortorella MD. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998;6:214–28. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- 9.Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–52. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 10.Westling J, Fosang AJ, Last K, Thompson VP, Tomkinson KN, Hebert T, et al. ADAMTS4 cleaves at the aggrecanase site (Glu373-Ala374) and secondarily at the matrix metalloproteinase site (Asn341-Phe342) in the aggrecan interglobular domain. J Biol Chem. 2002;277:16059–66. doi: 10.1074/jbc.M108607200. [DOI] [PubMed] [Google Scholar]
- 11.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–6. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615–26. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner L, et al. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (aggrecanase-1) in the trans-Golgi network. J Biol Chem. 2004 doi: 10.1074/jbc.M312797200. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, et al. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–19. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- 15.Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, et al. Autocatalytic cleavage of ADAMTS-4 (aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–80. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- 16.Sandy JD, Thompson V, Verscharen C, Gamett D. Chondrocyte-mediated catabolism of aggrecan: evidence for a glycosylphosphatidylinositol-linked protein in the aggrecanase response to interleukin-1 or retinoic acid. Arch Biochem Biophys. 1999;367:258–64. doi: 10.1006/abbi.1999.1234. [DOI] [PubMed] [Google Scholar]
- 17.Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J Biol Chem. 1999;274:34260–6. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- 18.Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–51. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- 19.Sabatini M, Thomas M, Deschamps C, Lesur C, Rolland G, de Nanteuil G, et al. Effects of ceramide on aggrecanase activity in rabbit articular cartilage. Biochem Biophys Res Commun. 2001;283:1105–10. doi: 10.1006/bbrc.2001.4920. [DOI] [PubMed] [Google Scholar]
- 20.Ilic MZ, Martinac B, Handley CJ. Effects of long-term exposure to glucosamine and mannosamine on aggrecan degradation in articular cartilage. Osteoarthritis Cartilage. 2003;11:613–22. doi: 10.1016/s1063-4584(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 21.Elliott S, Rowan AD, Carrere S, Koshy P, Catterall JB, Cawston TE. Esculetin inhibits cartilage resorption induced by interleukin 1alpha in combination with oncostatin M. Ann Rheum Dis. 2001;60:158–65. doi: 10.1136/ard.60.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisanti MP, Field MC, Caras IW, Menon AK, Rodriguez-Boulan E. Mannosamine, a novel inhibitor of glycosylphosphatidylinositol incorporation into proteins. Embo J. 1991;10:1969–77. doi: 10.1002/j.1460-2075.1991.tb07726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe K, Ito A, Sato T, Saito T, Hayashi H, Niitani Y. Esculetin suppresses proteoglycan metabolism by inhibiting the production of matrix metalloproteinases in rabbit chondrocytes. Eur J Pharmacol. 1999;370:297–305. doi: 10.1016/s0014-2999(99)00143-0. [DOI] [PubMed] [Google Scholar]
- 24.Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–5. [PubMed] [Google Scholar]
- 25.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–36. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 26.Sandy JD, Gamett D, Thompson V, Verscharen C. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J. 1998;335 ( Pt 1):59–66. doi: 10.1042/bj3350059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandy JD. Proteoglycan core proteins and catabolic fragments present in tissues and fluids. Methods Mol Biol. 2001;171:335–45. doi: 10.1385/1-59259-209-0:335. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004 doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 29.Patwari P, Kurz B, Sandy JD, Grodzinsky AJ. Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374:79–85. doi: 10.1006/abbi.1999.1538. [DOI] [PubMed] [Google Scholar]
- 30.Bonassar LJ, Sandy JD, Lark MW, Plaas AH, Frank EH, Grodzinsky AJ. Inhibition of cartilage degradation and changes in physical properties induced by IL-1beta and retinoic acid using matrix metalloproteinase inhibitors. Arch Biochem Biophys. 1997;344:404–12. doi: 10.1006/abbi.1997.0205. [DOI] [PubMed] [Google Scholar]
- 31.Oshita H, Sandy JD, Suzuki K, Akaike A, Bai Y, Sasaki T, et al. Mature bovine articular cartilage contains abundant aggrecan that is C-terminally truncated at Ala719-Ala720, a site which is readily cleaved by m-calpain. Biochem J. 2004;382:253–9. doi: 10.1042/BJ20040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21:499–511. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 33.Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–8. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- 34.Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, et al. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000;478:241–5. doi: 10.1016/s0014-5793(00)01854-8. [DOI] [PubMed] [Google Scholar]
- 35.Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–13. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- 36.Dong Z, Katar M, Alousi S, Berk RS. Expression of membrane-type matrix metalloproteinases 4, 5, and 6 in mouse corneas infected with P. aeruginosa. Invest Ophthalmol Vis Sci. 2001;42:3223–7. [PubMed] [Google Scholar]
- 37.Yamada H, Watanabe K, Saito T, Hayashi H, Niitani Y, Kikuchi T, et al. Esculetin (dihydroxycoumarin) inhibits the production of matrix metalloproteinases in cartilage explants, and oral administration of its prodrug, CPA-926, suppresses cartilage destruction in rabbit experimental osteoarthritis. J Rheumatol. 1999;26:654–62. [PubMed] [Google Scholar]
- 38.Blobel CP. Functional and biochemical characterization of ADAMs and their predicted role in protein ectodomain shedding. Inflamm Res. 2002;51:83–4. doi: 10.1007/BF02684007. [DOI] [PubMed] [Google Scholar]
- 39.Smith RJ, Speziale SC, Bowman BJ. Properties of interleukin-1 as a complete secretagogue for human neutrophils. Biochem Biophys Res Commun. 1985;130:1233–40. doi: 10.1016/0006-291x(85)91746-2. [DOI] [PubMed] [Google Scholar]
- 40.Arner EC, Pratta MA, Trzaskos JM, Decicco CP, Tortorella MD. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999;274:6594–601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- 41.Buttle DJ, Handley CJ, Ilic MZ, Saklatvala J, Murata M, Barrett AJ. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases. Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis Rheum. 1993;36:1709–17. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- 42.Bottomley KM, Borkakoti N, Bradshaw D, Brown PA, Broadhurst MJ, Budd JM, et al. Inhibition of bovine nasal cartilage degradation by selective matrix metalloproteinase inhibitors. Biochem J. 1997;323 ( Pt 2):483–8. doi: 10.1042/bj3230483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Little CB, Mittaz L, Meeker CT, Pritchard M, Fosang AJ. ADAMTS-1 knockout mice do not exhibit abnormalities in aggrecan turnover in-vivo or in-vitro. Transactions of the Orthopaedic Research Society. 2004;29:608. [Google Scholar]
- 44.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma H-K, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–58. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 45.Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–11. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 46.Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, et al. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–58. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- 47.Lang R, Braun M, Sounni NE, Noel A, Frankenne F, Foidart JM, et al. Crystal structure of the catalytic domain of MMP-16/MT3-MMP: characterization of MT-MMP specific features. J Mol Biol. 2004;336:213–25. doi: 10.1016/j.jmb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Johnson AR, Ye QZ, Dyer RD. Catalytic activities and substrate specificity of the human membrane type 4 matrix metalloproteinase catalytic domain. J Biol Chem. 1999;274:33043–9. doi: 10.1074/jbc.274.46.33043. [DOI] [PubMed] [Google Scholar]
- 49.Ganu V, Melton R, Wang W, Roberts D. Matrix metalloproteinase inhibitor CGS 27023A protects COMP and proteoglycan in the bovine articular cartilage but not the release of their fragments from cartilage after prolonged stimulation in vitro with IL-1 alpha. Ann N Y Acad Sci. 1999;878:607–11. doi: 10.1111/j.1749-6632.1999.tb07740.x. [DOI] [PubMed] [Google Scholar]
- 50.Hering TM, Kollar J, Huynh TD. Complete coding sequence of bovine aggrecan: comparative structural analysis. Arch Biochem Biophys. 1997;345:259–70. doi: 10.1006/abbi.1997.0261. [DOI] [PubMed] [Google Scholar]