Abstract
Stem cells have the ability for prolonged self-renewal and differentiation into mature cells of various lineages, which makes them important cell sources for tissue engineering applications. Their remarkable ability to replenish and differentiate in vivo is regulated by both intrinsic and extrinsic cellular mechanisms. The anatomical location where the stem cells reside, known as the “stem cell niche or microenvironment,” provides signals conducive to the maintenance of definitive stem cell properties. Physiological condition including oxygen tension is an important component of the stem cell microenvironment and has been shown to play a role in regulating both embryonic and adult stem cells. This review focuses on oxygen as a signaling molecule and the way it regulates the stem cells' development into mesenchymal tissues in vitro. The physiological relevance of low oxygen tension as an environmental parameter that uniquely benefits stem cells' expansion and maintenance is described along with recent findings on the regulatory effects of oxygen on embryonic stem cells and adult mesenchymal stem cells. The relevance to tissue engineering is discussed in the context of the need to specifically regulate the oxygen content in the cellular microenvironment in order to optimize in vitro tissue development.
Keywords: embryonic stem cells, adult stem cells, mesenchymal stem cells, oxygen tension, hypoxia, 3D
Introduction
Stem cells are currently used in clinical applications to augment the healing of orthopedic tissue defects. Their applicability to multiple other therapeutic situations has also been investigated. One approach to eliciting the therapeutic benefits of stem cells is to inject them into the defect site in suspension or in a delivery gel.1 However, the therapeutic potential of stem cells may best be realized via tissue-engineering approaches to develop biological tissue substitutes which, through in vitro cultivation, can be functional at the time of implantation. The use of stem cell-based approaches in combination with scaffolds and bioreactor systems has resulted in engineered tissues of various mesenchymal lineages including bone,2,3 cartilage,4 fat,5 and ligament,6 among others.
Stem cells produce all multicellular tissues in the body through proliferation and differentiation in tightly controlled in vivo environments. Because of their plasticity, they are particularly sensitive to their immediate environments. In vivo they are thought to reside in specific “niches,” which maintain their pluripotent or multipotent capabilities. Consequently, differentiation along specific lineages is thought to coincide with their migration out of their specific niche into an environment that provides appropriate differentiation cues.7 The in vitro conversion of unspecialized cells to immature, but functional tissues depends on establishing the cellular microenvironment, regulated so that key in vivo stimuli, which guide cellular organization and development, are recapitulated.8 As the field of tissue engineering has matured, new technology has been employed to regulate the application of mechanical as well as biological factors to developing tissue constructs. However, one developmentally important stimulus that is still rarely accounted for during in vitro culture is the oxygen tension. The role of oxygen as a metabolic substrate for cells in three-dimensional (3D) organization has now been investigated extensively9-12 and others have reviewed techniques utilized to mitigate mass transport limitations and avoidance of anoxic regions and steep oxygen gradients in thick 3D tissue constructs.13,14 However, much less studied (but gaining increasing attention) has been of the utilization of oxygen in its role as a signaling molecule that influences stem cell survival, proliferation, and differentiation in culture.
The effect of oxygen tension on stem cell physiology has been studied for over 30 years beginning with the haematopoietic system.15-17 For haematopoietic stem cells (HSCs), it has been found that cultivation under low oxygen tensions maintained a significantly higher number of long-term colony initiating cells (LTC-ICs) relative to cultures under ambient (20%, v/v) oxygen concentrations.18-20 Recently, it has also been seen for several other stem and progenitor cell populations that cultivation under hypoxic conditions resulted in enhanced proliferation and maintenance of their naïve states.21 In vivo studies have shown that mesenchymal stem and progenitor cells home specifically to hypoxic events and function as therapeutic agents, enabling limited regeneration to damaged tissues.22-24 In particular, the stem cells have demonstrated the potential to organize themselves into vascular structures as well as secrete angiogenic growth-factors in response to hypoxic challenges. Several in vitro studies have also shown that both bone marrow- and adipose-derived stem cells upregulate VEGF expression during hypoxia,25-28 which along with other factors, may be responsible for the “cyto-protective” effects on neighboring cells.26-29 Although the identity of cellular oxygen sensor is being debated,30,31 emerging evidence indicate that some of the effects of hypoxia on stem cell function are directly regulated by hypoxia-inducible factor (HIF) proteins. The role of HIFs in regulating stem cells' response to hypoxia has been recently reviewed by Keith and Simon32 and is not included in this review.
This review focuses on the role of oxygen tension on the stem cells' development into mesenchymal tissues in vitro. As a result, oxygen's influence as a signaling molecule (rather than metabolic substrate) on the proliferation, differentiation, and tissue development is discussed. Although there have been studies on numerous types of stem and progenitor cells, we discuss only embryonic stem cells (ESCs) and adult mesenchymal stem cells (MSCs) derived either from the bone marrow or adipose tissues in terms of their expansion and terminal differentiation into tissues of mesenchymal lineage. Cells from various mammalian species are included as they share many properties. The physiological relevance of low oxygen tension as an environmental parameter that uniquely benefits stem cells' expansion and maintenance is described. This provides a context for reviewing the results of in vitro studies of stem cells cultivated under hypoxic conditions. Finally, we discuss the impact of these findings on tissue engineering approaches and the need to specifically regulate the oxygen content of the cellular microenvironment in order to optimize in vitro tissue development is discussed in the last section. It is noted that hypoxia refers to the condition when oxygen tension is below physiological level but is used in this review to describe O2 lower than 21% for consistency with conventional terminology.
Physiological Basis of Hypoxic Environments
In vivo microenvironments
Stem cells' unique ability to replenish themselves during the development, maintenance of tissue homeostasis, and repair of many tissues through self-renewal and differentiation are regulated by both intrinsic programming and input from their local environment, often referred to as the “stem cell niche or microenvironment.”7,33 As a basic unit of tissue architecture, the stem cell microenvironment constitutes specific molecular, cellular, and physiological components and is also subject to physical and mechanical stimuli. Replicating the essential components of the stem cell in vivo microenvironment has become an important approach in stem cell tissue engineering to develop functional constructs. Although stem cells can reside in markedly different local microenvironment and have distinctly different developmental paths, low oxygen tension seems to be a common in vivo feature shared by embryonic stem cells and many types of adult stem cells at early stages of development. Indeed, increasing evidence demonstrates that oxygen tension is not only a metabolic substrate but also a powerful signaling molecule that regulates stem cell proliferation and differentiation. In recent years, the requirements for replicating stem cell microenvironment have evolved from the initial effort of supplying sufficient oxygen to support tissue growth to understanding and utilizing oxygen in its more complex signaling role to regulate migration, differentiation, and development. The physiological basis for this is discussed in the context of the in vivo cellular microenvironment:
Embryonic microenvironment
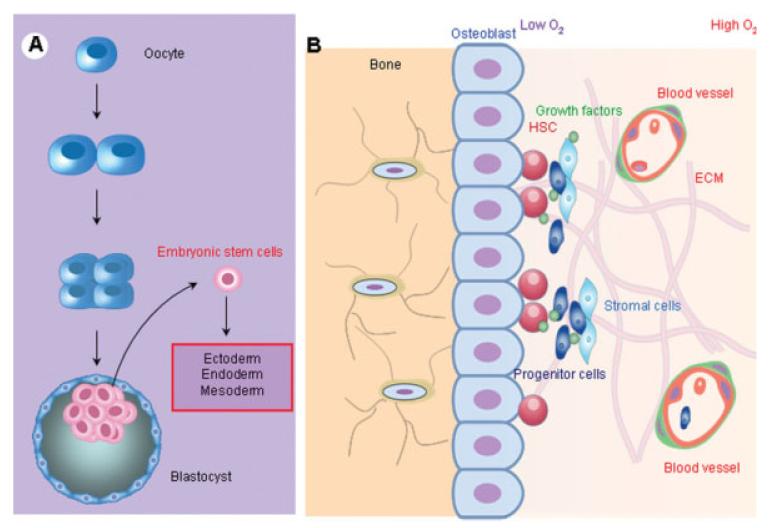
Oxygen tension in the mammalian reproductive tract is in the range of 1.5–8%.34 For example, the measured oviductal oxygen tension in rhesus monkeys was about 8%, whereas the intrauterine oxygen tension was only about 2%.34 It is to this low oxygen environment that naturally conceived embryos are exposed during the early stages of development (Figure 1A). After implantation of embryos into the uterine wall, the trophoblast shell that surrounds the embryo excludes oxygenated maternal blood and forms a hypoxic environment until the onset of vascularization. At 8–10 weeks of gestation, the mean oxygen tensions at the uterine surface and within the endometrium were estimated to be ~2.3% and 5.2%, respectively.35 The role of oxygen tension in modulating proliferation and differentiation of human placenta cells has been demonstrated using placental organ culture systems, in which 2% O2 induces cytotrophoblast mitosis and 20% O2 induces cell cycle arrest and differentiation.36 The low oxygen environment at the range of 4–5% O2 lasts throughout the fetal developmental process even after access to the maternal vasculature (normal pO2 at ~10 and ~13% in venous and arterial, respectively) with dissolved O2 in fetal circulation rarely exceeding 5%.35 Human embryonic stem cells that are derived from the inner cell mass of early stage blastocytes adapt to grow in this low oxygen environment until about the 11th week when the cytotrophoblasts traverse the arterial walls and the local partial pressure of oxygen increases from ~2.3% to 7.8% O2.35 Thus, low oxygen environment is physiologic for most mammalian embryos, and ambient oxygen tension of 20% is in fact a “nonphysiological” condition to which the embryo is never exposed.
Figure 1. Oxygen tensions in stem cell microenvironments.
Embryonic stem cells and adult bone marrow-derived stem cells reside in low oxygen environments. (A) Embryonic stem cells are derived from the inner cell mass of early stage blastocytsts and maintain a totipotent state that forms ectoderm, endoderm, and mesoderm. The intrauterine environment in which the embryonic stem cells growth and development occur has an oxygen tension ranging from 1–5% through the first trimester. (B) Oxygen tension in bone marrow is well below ambient oxygen environment in the range of 3–7% O2 with oxygen concentrations decreasing with distance away from sinuses. Recent in vivo studies provided direct experimental evidence that HSCs are distributed predominantly at the lowest end of an oxygen gradient within the bone marrow and away from sinuses.
Bone marrow microenvironment
The oxygen distribution within the bone marrow has been studied almost exclusively in the context of hematopoietic stem cells (HSCs) and to a much lesser extent in the context of MSCs. However, bone marrow has a hierarchical structure, in which the haematopoietic compartments are bound by stromal elements37—mainly mesenchymal stromal or stem cells (MSCs)—and such that the two cell types form an integral part of each other's niche (Figure 1B). The oxygen concentrations in the MSCs' in vivo microenvironment may therefore be discussed in terms of what is known for HSCs. It has long been proposed that hematopoietic progenitors exist at high concentration at the endosteal surface and release via the central venous sinus as they differentiate and mature.38 Although the importance of understanding the spatial progenitor cell distribution with respect to oxygen supply from blood vessel has long been recognized, direct, noninvasive in vivo measurement of spatial oxygen gradient in bone marrow has been a major technical hurdle. Early direct measurement revealed that bone marrow in general is hypoxic, where some regions are as low as ~1–2% O2.39,40 Results from recent in vivo studies, however, provided direct experimental evidence that long-term repopulating HSCs in the mouse reside in a hypoxic environment41 and hypoxia may in fact be an essential part of the microenvironment that maintains them in an undifferentiated state. These measurements were further supported by modeling analysis of in vivo oxygen distribution in bone marrow indicating that HSCs exist within an extremely hypoxic region within bone marrow.42 Results from in vitro studies of HSCs under hypoxic conditions strengthen this hypothesis: At 5% O2, there is enhanced production of erythroid, megakaryocytic, and granulocyticmonocytic progenitors18,43,44 and substantial increases in the number and frequency of colony-forming cells.45
Adipose stem cell microenvironment
Adipose tissue is readily available through the high abundance of elective surgeries for fat removal. This tissue is typically highly vascularized and is a rich source of adipose-derived mesenchymal stem cells (ADSCs) having the potential to differentiate into adipogenic, osteogenic, chondrogenic, myogenic, endothelial, hepatic, and neuronal lineages.46,47 ADSCs are typically harvested from the stromal vascular fraction of lipo-aspirated tissues47,48; however, the position of ADSCs relative to other cell types (adipocytes, preadipocytes, etc.) and structures (blood vessels) is still unknown. Despite the high degree of vascularity, in vivo measurements of oxygen concentrations in mouse adipose tissues have shown the oxygen concentration to be in the vicinity of 3%49 and so hypoxic cultures may still be beneficial to ADSC in vitro proliferation and differentiation characteristics. However, studies investigating the role of reduced oxygen tension on ADSC in vitro characteristics have not reported increased maintenance of an undifferentiated state or drastic changes in proliferation in an analogous manner with what has been reported with ESCs and bone marrow-derived MSCs, but hypoxia has been shown to affect the ADSC differentiation potential as discussed below.
Hypoxia Influences Stem Cells' In Vitro Characteristics
Embryonic stem cells
The exciting potential of embryonic stem cells (ESCs) in tissue repair and regeneration depends on the ability to maintain their pluripotency during expansion in culture and to direct their differentiation under controlled conditions. Recapitulating the physiological conditions that regulate these cellular events in vivo has emerged as an important criterion to achieve this goal. One challenge inherent in ESC culture is their tendency to differentiate spontaneously. Low oxygen tension has been explored as a strategy to maintain human ESC pluripotency and minimize spontaneous differentiation.50-52
Low O2 environments have long been used by embryologists to culture embryos53,54 and the blastocytes produced under low O2 have significantly more inner cell mass compared with those generated under higher O2.55 The benefits have been demonstrated for rabbit,56 mouse,57 sheep,53 cow,53 and human58 embryos. Physiological oxygen tension of 5% instead of 20% was also found to improve the establishment of mouse embryonic stem cell line by reducing oxidative stress.59 A low oxygen tension of 2% resulted in enhanced human ESC (hESC) clonal recovery and reduced chromosomal abnormalities without inducing hESCs to a more differentiated phenotype.60 When maintained at 1 or 3% O2, hESCs showed less signs of overt morphological differentiation than the normoxic colonies (Figures 2A–D) and produced lower amounts of chorionic gonadotrophin (hCG) and progesterone.50 The hypoxic condition also enhanced hESC formation of embryoid bodies and did not prevent subsequent differentiation of hESCs when they were exposed to biochemical cues. Manipulating oxygen tension has also been shown to direct ESC differentiation.62 Systemic studies are required to provide in-depth understanding of the dynamic interactions between oxygen tension and ESC fate and to facilitate the development of robust stem cell expansion strategies.
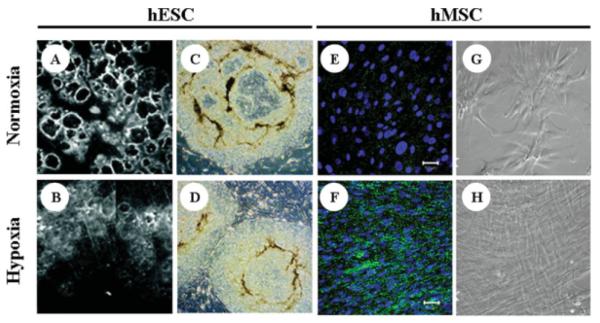
Figure 2. Effect of hypoxia on in vitro stem cell phenotype.
(A and B) Human ESCs (H1) cultured on MEF feeder layers under normoxic (21% O2) and hypoxic (5% O2) conditions. Normoxic cells display extensive regions of differentiation (dark circles surrounded by bright rings) compared with hypoxic cultures. (C and D) Further comparison of H1 hESCs grown at 21% O2 and 3% O2, respectively, showing fewer differentiated (darker, nonuniform) regions under hypoxic conditions. Images adapted from Ref. 50, with permission from National Academy of Sciences. (E–H) Comparisons of bone marrow-derived hMSCs grown under normoxic (21% O2) or hypoxic (2% O2) conditions: (E and F) Significantly higher connexin-43 expression is observed in hypoxic hMSCs grown in monolayer culture for 11 days using expansion medium. (G and H) Phase contrast images demonstrate the maintenance of uniform spindle morphology at late stages of passage under hypoxic conditions. Images adapted from Ref. 61, with permission from Academic Press.
Bone marrow-derived mesenchymal stem cells
Low oxygen tension studies have been carried out with mesenchymal stem cells (MSCs) from a variety of mammalian species. In general, MSCs exhibited greater colony-forming potential,63,64 proliferated faster,65,66 and longer,64,61,67,68 and maintained their undifferentiated characteristics better under low oxygen conditions.61,69 Our group demonstrated that hMSCs grown in 3D scaffolds under extended hypoxic conditions (2% O2) increased their expression of pluripotent genes Oct-4 and Rex-1 and had elevated CFU-F ability while maintaining their ability to differentiate along osteogenic or adipogenic lineages.64 In two-dimensional (2D) culture studies, hMSCs under hypoxic conditions exhibited 30-fold greater expansion potential over a 6-week period than normoxic cells, homogenously maintained their spindle morphology, and formed multiple cell-layers with high expression of connexin-4361 (Figures 2E–H). Hypoxia (3% O2) has also been used to isolate a population of marrow-isolated adult multilineage inducible (MIAMI) cells from human bone marrow with pluripotent characteristics.70 Hypoxia affects the differentiation characteristics of MSCs in ways that may be correlated with the physiological oxygen requirements of the differentiated cells (e.g., chondrocytes in avascular cartilage have low oxygen requirements while osteoblasts in vascular bone require 20% O2 for optimal differentiation). However, it is very difficult to arrive at a consensus even after collating and analyzing the results of published studies: Differences in cell isolation methods, experimental parameters, growth factors, oxygen tensions, and specific evaluation techniques highlight the challenges in determining the role of oxygen in stem cell differentiation. Therefore, it has been reported that hypoxia enhanced the in vitro and in vivo bone-forming potential of rat MSCs63 but inhibited the in vitro osteogenic potential of MIAMI cells69 and induced down-regulation of osteoblastic genes in hMSCs in vitro.25 Likewise, late-passage murine MSCs grown in Matrigel under 2% O2 tension exhibited increased angiogenic properties and formed tube-like structures71 while immortalized murine mesenchymal cells incubated in 1% oxygen expressed increased levels of chondrogenic markers Sox-9, aggrecan, and Col IIa.72 Other studies involving hMSC have found that hypoxia (1% O2) induced lipid-droplet formation within 1 day of exposure to a hypoxic atmosphere but without actually upregulating the expression of adipocytic genes.73 Table 1 is provided to give a more detailed (though not exhaustive) summary of the findings for the effects of in vitro hypoxia on the stem cells of interest.
Table 1.
Effect of Hypoxia on Proliferation and Differentiation Properties of ESC, hMSC, and ADSC
| Cell Type | Species | Oxygen Tension | Cultivation Conditions | Expansion (compared to 20% O2) | Differentiation (compared to 20% O2) | Reference |
|---|---|---|---|---|---|---|
| ESC | Human | 1%, 3%, 5% | Monolayer culture of ES cells and embryoid body formation | 3 and 5% : ○ proliferation rate of ES colonies 1% : Slightly ↓ proliferation of ES colonies | Better maintenance of undifferentiated state ↑ EB formation, ↑ SSEA-4, ↑Oct-4, ↓SSEA-1 | Ezashi et al.50 |
| 2% | Monolayer culture of ES cells | ↑ (Increase in clone recovery) | Smaller and less granular cells ○ SSEA4, SSEA1, TRA-1-60, or TRA-1-81 ↓ chromosomal aberrations | Forsyth et al.60 | ||
| Mouse | 1% | EB formation | No changes in first 5 days | ↑ mRNA levels of Aldolase A and VEGF | Gassman et al.52 | |
| 5%, 20%, 40% | Monolayer culture of ES cells and EB formation | Suppressed proliferation of ES cells at 5% in comparison to 20 and 40% O2 | 5% and 20%: ↓ specific AP activity in ES cells 40%: ○ AP activity in ES cells, ↑ Oct-4 expression in comparison to 5 and 20% (ES cells as well as EBs) | Kurosawa et al.51 | ||
| Adipose-derived MSC | Human | 5% | Expansion in monolayer or/and alginate beads in chondrogenic medium: up to 14 days | ○ proliferation in expansion medium ↓ proliferation in chondrogenic cultures | Chondrogenesis: ↑ GAG synthesis at certain time points; ○ collagen type II ↑ protein content, ↑ total collagen synthesis | Wang et al.74 |
| 2% | Monolayer; osteogenic and adipogenic differentiation | N/A | Adipogenesis: No intracellular lipid droplets Osteogenesis: No mineralization | Lee and Kemp75 | ||
| 5% | Cell aggregate culture in chondrogenic medium, 14 days. | ↓ cell proliferation | Chondrogenesis: ↑ GAG content, ↑collagen type II, ↑IX, ↑XI, ↑ SOX6, ↑ SOX5, ↑ SOX9, ↑ HIF2α not HIF1α | Khan et al.76 | ||
| 5% | Elastin-like polypeptide scaffold; 7 days DMEM/F-12 supplemented with ascorbate 2-phosphate | N/A | Chondrogenesis: ↓ expression of Collagen type II, ↑ expression of SOX9, ↓ expression of Collagen X | Betre et al.77 | ||
| Mouse | 2% | Micromass culture in chondrogenic medium; up to 12 days | ↑ cell proliferation | Chondrogenesis: ↓ collagen type II, ↓ GAG content | Malladi et al.78 | |
| Monolayer culture in osteogenic medium; up to 3 weeks | Osteogenesis: ↓ AP activity, ↓ extent of mineralization | |||||
| Monolayer in basal medium Micromass culture in chondrogenic medium in normoxia; up to 6 days |
↑ cell proliferation | Chondrogenesis: ↑ PG nodule formation, Micromasses rapidly condensed | Xu et al.79 | |||
| Monolayer; Osteogenic differentiation in normoxia; up to 3 weeks | Osteogenesis: ↓ AP activity, ↓ extent of mineralization | |||||
| BM-derived MSC | Human | 2% | 3D PET Scaffolds (4 weeks) Osteogenic and adipogenic differentiation at 20 % O2 | Slower proliferation during initial period but maintained expansion phase longer | ↑ Oct-4, ↑ Rex-1 expression, ↑ CFU-F Osteogenesis: ↑ Mineralization (von Kossa) ↑ AP activity (max. levels) ○ Osteonectin Adipogenesis: ↑ LPL expression | Grayson et al.64 |
| Monolayer; Osteogenic and adipogenic differentiation at 20 % O2 | ↑ (30 times greater expansion over 6 passages) | ↑ Oct-4, ↑ HIF-2α, ↑ connexin-34 expression Osteogenesis: ○ mineralization (von Kossa), ○ AP activity Adipogenesis: ○ lipid vacuole formation | Grayson et al.61 | |||
| 3% | Long term monolayer culturesfollowed by osteogenic, adipogenic differentiation. | ↑ proliferative lifespan over 100 days. Delayed onset of senescent phenotype (↓ AGE) | ○ expression of hypoxia related genes: HIF-1α, PH-4, HIF1AN, VHL, Hyou1, HIG1, HIG2. Osteogenesis: ↓ calcium, ↓ AP mRNA, ↓ IBSP mRNA. Adipogenesis: ↓ lipid-droplet containing cells, ↓ FABP4, ↓ LPL Osteogenic and adipogenic potential regained when cells induced in normoxia. | Fehrer et al.67 | ||
| 1.5% | Monolayer cultures exposed to hypoxia for 24 h, followed by osteogenic (14 days), adipogenic (21 days) and chondrogenic (21 day-pellets) differentiation in normoxia | ↑ rate of cell division and proliferation | ↑HIF1α, ↑BNIP3, ↑VEGFA, ↑AK3L1/L2, ↑STC1, ↑EPHA3, ↓EDG1expression Osteogenesis: ○ mineralization, ○ RUNX2 expression Adipogenesis: ○ number of lipid droplets, ○ (PPAR)-α Chondrogenesis: ↑ pellet size, ↑SOX9 expression | Martin-Rendon et al.65 | ||
| 4% | Monolayer cultures exposed to hypoxia for 48 h, followed by osteogenic differentiation (up to 28 days) | Cell survival not affected when lasted up to 72 h. Cell death (35%) occurred when cultured for 120 h. | ↑ bFGF mRNA but ↓bFGF protein secretion, ↑VEGF mRNA, ↑VEGF protein secretion, ○ IL-6, MCP-1, TIMP-1, TIMP-2 Osteogenesis: ○ AP expression, ○ BMP-2, ○ BSP, slightly ↓ expression of cbfa-1/Runx2 and Osteocalcin (day 0 and 14); no changes at day 28, ↑ Osteopontin, ↓ Collagen type I expression | Potier et al.25 | ||
| Mouse | 8% | Monolayer cultures in expansion medium (IMDM) and adipogenic differentiation | ↑ cell proliferation (2.8 fold increase in cell number after 8 days) | ↑ in number of AP positive, flattened cells, ↑HIF-1α, ↑ H-2Dd, ↑ CD44, ○ IA-d and CD13, ↓Oct4, ○ VEGF expression Adipogenesis: ↑ in number of lipid droplets (5.6 fold) | Ren et al.66 | |
| Rat | 5% | Monolayer culture, osteogenic differentiation; in parallel ceramic scaffolds implanted in vivo (up to 6 weeks) | ↑ number of colonies | Osteogenesis in vitro: ↑ AP activity, ↑ calcium content, ↑ mineralization (von Kossa) Osteogenesis in vivo: ↑ bone formation | Lennon et al.63 |
AGE, advanced glycation endproducts; SSEA, stage-specific embryonic antigen; ES, embryonic stem; EB, embryoid body; VEGF, vascular endothelial growth factor; AP, alkaline phosphatase; GAG, glycosaminoglycans; PG, proteoglycan; HIF, hypoxia-inducible factor; BSP, bone sialoprotein; PPAR, peroxisome proliferator-activated receptor; BMP, bone morphogenetic protein; TIMP, tissue inhibitor of metalloproteinase; MCP, monocyte chemoattractant protein; IL, interleukin; bFGF, basic fibroblast growth factor; EDG, endothelial differentiation gene; STC, stanniocalcin; LPL, lipoprotein lipase; FABP4, fatty acid–binding protein; HIG, hypoxia-inducible gene; Hyou, hypoxia upregulated.
↑, increase;
↓, decrease;
○, unchanged (or change not quantified).
Adipose-derived stem cells
Much less investigations have been carried out concerning the effects of hypoxia on adipose-derived stem cells. The first published study done by Wang et al.74 on human ADSCs found that when they were grown in alginate beads at 5% O2 there was no effect on their proliferation when cultured in normal expansion medium, but that they had lower proliferation rates at 5% O2 compared with 20% O2 when cultured in chondrogenic medium. However, under these conditions they exhibited enhanced chondrogenic differentiation markers including collagen II, glucosaminoglycan, and chondroitin-4-sulfate production.74 The influence of hypoxia on ADSCs is strongly dependent on cultivation conditions. For this reason, there are conflicting reports in the literature regarding chondrogenic gene expression during induction under hypoxic conditions.76,77 In other studies of human ADSCs, it was found that hypoxia also affected their potential to differentiate along muscle lineages.75 The effect of hypoxia on the differentiation potential of ADSCs was also reported for murine cells. It was found that murine ADSCs exhibited decreased chondrogenic and osteogenic potential when differentiated at 2% O2 compared with normoxic conditions.78 However, a subsequent study by this group reported that if the murine ADSCs were first expanded at 2% O2 and then differentiated under normoxic conditions to either osteoblasts or chondrocytes, they still exhibited decreased osteogenesis, but increased their expression of chondrogenic markers relative to cells that were both expanded and differentiated under normoxic conditions.79 This occurred even if cells were reoxygenated and cultured at 21% O2 prior to chondrogenic differentiation, suggesting that hypoxic culture may have selected specifically for chondroprogenitors. The interpretation of the combined results is complicated by other studies from the same group demonstrating that HIF-1α deletion inhibited chondrogenesis in murine ADSCs80 suggesting that upregulation of HIF-1α under hypoxic conditions should in theory enhance chondrogenic differentiation. In combination, these studies underscore the importance of understanding the effects of oxygen signaling in the context of the culture conditions and other growth factors present.
Optimizing Oxygen Tension for Stem Cell-Based Tissue Engineering
Oxygen consumption characteristics may be distinct in 2D vs. 3D
The creation of 3D functional tissue constructs using stem cells and 3D scaffolds depends on stem cells' innate ability to proliferate and differentiate in response to environmental cues. Hence, these processes are strongly influenced by the in vitro cultivation conditions and the scaffold properties for stem cell maintenance or differentiation. The localized physiological microenvironment is determined by the interplay between supply and demand. Oxygen supply to cells in 3D constructs is more complex than that of 2D cultures as it is influenced by the presence of scaffolding materials and extracellular substances as well as by the mechanism(s) of transport, i.e., diffusion or convection. To this end, modeling has served as a powerful tool to provide insight into the oxygen profile of the in vivo tissue and the 3D constructs (see a recent review by Sengers et al.81). Sophisticated modeling approaches have been developed to account not only for matrix formation and cell proliferation and differentiation but also variations of scaffold geometry and culture environment.
An important barrier to estimating the oxygen profile in the 3D stem cell constructs, however, is the reliability of input data—particularly, the rates of oxygen consumption at various developmental stages. The Michaelis-Menten-like constitutive equations for oxygen consumption are based on the assumption that the rate constants are independent of time and material, and the variation of oxygen profiles are primarily the results of cell density.82,83 However, these assumptions are being challenged. Recent studies have shown that there may be a strong dependence of cellular metabolic activity and oxygen consumption rate on the scaffolding material and geometry, presumably due to the altered cell-material interactions in the scaffold.84,85 In fact, the changes in regulatory effects of 3D cellular organization on the morphology, gene expression, growth, morphogenesis, and differentiation of cells are being established, and the signaling mechanisms are being identified.86,87 The role of 3D scaffolds may be more pronounced for the stem cells that not only exhibit plasticity but also display an extensive ability to modulate their microenvironment. For example, hMSCs in 3D scaffolds developed an extensive ECM network and changed their integrin profile and cell adhesion apparatus compared with 2D counterparts.88 Thus, the role of oxygen tension in stem cell development needs to be addressed in the context of the 3D microenvironment that includes matrix properties, cell–cell and cell-matrix interactions, distribution of growth factors as well as the local physiological and biomechanical properties.
It is important to not only recognize the dynamic interplay between the developing stem cell microenvironment in the 3D construct and the biosynthetic activity that actively modulates the microenvironment, but also the varying developmental requirements over an extended period. For example, per cell oxygen demand decreased over a 40-day period when hMSC were grown in a perfusion bioreactor due to the variation in cellular microenvironment.83 As a result, oxygen depletion that would otherwise occur due to high cell density was not observed at the later stage of the construct development. These results highlight the importance of obtaining temporal and spatial measurements of oxygen concentration in developing constructs in order to validate oxygen distribution profiles. Furthermore, these findings underscore the complexity associated with regulating the microenvironment and the need to further integrate experimental-computational approaches to understand the developmental dynamics and to identify the key regulatory mechanisms.
Regulating O2 tension in developing tissue constructs
As a result of stem cells' natural adaptation to the “hypoxic conditions” and sensitivity to ambient oxygen concentrations, there is a need to carefully consider the role of oxygen in the microenvironment of stem cell-based tissue-engineered grafts. In contrast to cultures of terminally differentiated cellular phenotypes, maintaining hypoxic regions throughout stem cell-based constructs may be desirable if it allows cells to proliferate longer and give rise to constructs with higher cell densities. Furthermore, hypoxia appears to either support or “select” a more primitive population of cells, which maintain the potential to be directed along various lineage pathways.62,64 Directing subsequent stem cell differentiation along particular lineage pathways may require modulation of the prevailing oxygen concentrations in conjunction with providing signaling stimuli from appropriate growth factors.
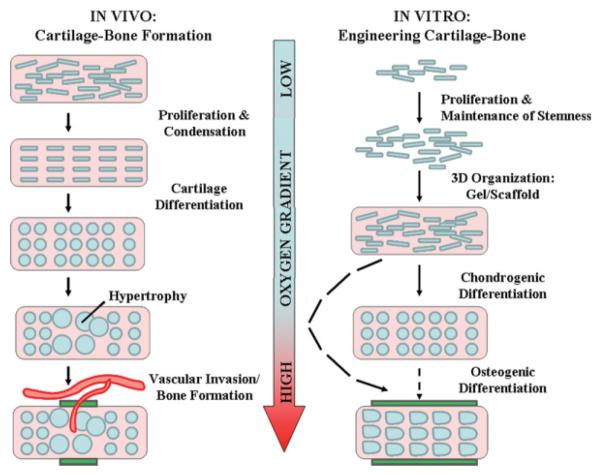
Given the strong dependence of stem cell behavior on oxygen tension, it is conceivable to employ a strategy of varying oxygen tension over the course of construct development to stimulate rapid cell proliferation and direct functional differentiation. Engineering bone and cartilage tissues for example may require distinctly different conditions of oxygen tension for optimal differentiation into either lineage. Empirical clues to validate this may even be based on the physiological example of endochondral ossification (Figure 3). During this process, undifferentiated mesenchymal cells condense and undergo chondrogenic differentiation in hypoxic, avascular environments. Similar conditions may stimulate hMSC chondrocytic differentiation within 3D scaffolds or gels: both human chondrocytes and ADSCs74 maintained in chondrogenic media at 5% O2 exhibited improved chondrogenic gene expression and phenotypic characteristics. However, subsequent endochondral bone formation is preceded by vascular invasion with a consequent increase in oxygen concentrations. In vitro bone formation also appears to require higher oxygen tensions to stimulate proper osteoblast differentiation and function.
Figure 3. Learning from nature: The influence of oxygen tension during bone-cartilage tissue formation.
In vivo: During development, cartilage forms via the processes of mesenchymal condensation and carefully regulated chondrogenic differentiation in very hypoxic environments. Long bone formation occurs via endochondral ossification where hypertrophic chondrocytes upregulate angiogenic genes triggering vascular invasion (with consequent increases in oxygen tension) followed by new bone formation (green rectangles). Images adapted from Ref. 89, with permission from Nature Publishing Group. In vitro: Hypoxia enables the expansion of stem cells while maintaining their undifferentiated states. Cells are then seeded into 3D organizations to facilitate functional tissue development. Chondrogenic differentiation is enhanced under hypoxic conditions relative to 20% O2. Differentiation into osteogenic lineage and subsequent bone formation (green rectangles) in vitro is optimized at ambient (20%) O2 tensions and inhibited under hypoxic conditions.
Another scenario of varying oxygen tension to aid construct development is in the creation of vascularized tissue constructs. One approach to vascularize the implanted construct is to allow the native blood vessels to infiltrate the construct and establish a vascular network.90 Although the delivery of multiple growth factors showed promising results,91 controlled release at physiological dosage remains a major technical hurdle for regenerating clinically relevant thick constructs. MSCs are a known source of angiogenic factors that promote blood vessel development. A recent in vitro study has shown that the conditioned media from hypoxic MSCs are more effective than normoxic MSCs in inhibiting endothelial cell apoptosis, increasing their survival, and enhancing tube formation.92 Thus, a strategically controlled oxygen gradient in the 3D construct may potentiate MSC angiogenic activity and enable MSC to function as a factory of growth factors that promote blood vessel invasion and construct vascularization. These strategies, however, depend on the precise knowledge of oxygen demand and the ability to regulate oxygen microenvironment in 3D constructs.
Technological requirements for regulating the in vitro oxygen microenvironments
New technologies are being developed to directly measure local oxygen concentration in 3D constructs. An optical fiber-based oxygen sensing system was developed for in situ, real-time oxygen monitoring for human dermal fibroblasts in 3D collagen constructs.93 Needle microelectrodes have been used to monitor local O2 and cellular activity in several tissues and have potential for oxygen monitoring in regenerating tissue.94 A novel microsystem was also developed that was able to generate and impose 1D or 2D oxidative gradients on tissue in culture.95 Although these technologies are important in controlling in vitro oxygen microenvironment, challenges remain in monitoring oxygen tension in the developing 3D constructs with high spatial resolution over the course of construct development. To this end, noninvasive imaging technology allows repetitious measurement in both in vitro and in vivo settings and offers many advantages. Local oxygen tension in the vicinity of implants was measured by EPR oximetry using the microparticles of an acta-n-butoxy derivative of naphthalocyanine neural radicals as implantable oxygen sensors. The oxygen sensor microparticular compounds can be implanted in the extravascular space or internalized by cells and thereby provide high accuracy, noninvasive local oxygen measurement of the implants for up to 10 weeks.96 The ability to measure local oxygen tension also eliminates the disparity between ambient and pericellular oxygen tension that has been reported.73 Significant disparities exist between ambient and pericellular oxygen concentrations, thus increasing oxygen concentrations by 3% (say from 2–5% O2) does not correspond to a 3% increase in the oxygen concentrations in the cellular region. The use of bioreactors may be of considerable interest in overcoming these challenges. Innovations in bioreactor design that address mass transfer limitations can be extended to improve control of oxygen profiles in the 3D constructs. The effort to determine the optimal mass transfer conditions for the developing 3D constructs can also be supported by simulation methods. Several recent reviews have summarized the development of bioreactors for stem cell expansion97 and modeling of tissue regeneration in 3D scaffolds.81
Summary
Oxygen concentration is an important component of stem cell “niche,” where it plays a fundamental role in maintaining the stem cells' proliferation and plasticity. Recapitulating the critical environmental components that control stem cell proliferation and differentiation in 3D constructs is therefore an important aspect of stem cell-based tissue engineering strategies. Recent progress has provided invaluable insight into the regulatory mechanisms of oxygen tension on stem cells' phenotype. Given the sensitivity of stem cells to their physiological microenvironment, it is imperative to evaluate the role of oxygen tension (and oxygen delivery strategies) in developing stem cell-based 3D constructs in the context of its roles as a signaling molecule as well as a metabolic substrate. Future challenges include getting more insight into the oxygen-related complex cell behavior and the interactions between the cells and their evolving environment, through integrating experimental and modeling approaches. Novel 3D culture systems coupled with advanced monitoring technology will help uncover the dynamic interplay between the stem cells in the developing tissue constructs and their microenvironment. Computational modeling is expected to help delineate the contributions of various factors and to identify the key regulatory mechanisms. Integrated experimental and modeling approach will be necessary for the optimization of tissue engineering protocols.
Acknowledgment
This work was supported by DOD Peer Reviewed Medical Research Program (TM); Contract Number: W81XWH-07-1-0363. The authors are grateful to Mr. J. D. Adams of FAMU-FSU College of Engineering for his help in the illustrations.
Literature Cited
- 1.Muschler GE, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am A. 2004;86:1541–1558. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Meinel L, Betz O, Fajardo R, Hofmann S, Nazarian A, Cory E, Hilbe M, McCool J, Langer R, Vunjak-Novakovic G, Merkle HP, Rechenberg B, Kaplan DL, Kirker-Head C. Silk based bio-materials to heal critical sized femur defects. Bone. 2006;39:922–931. doi: 10.1016/j.bone.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, Kaplan D. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–698. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003;5:235–238. doi: 10.1186/ar991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauney JR, Volloch V, Kaplan DL. Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials. 2005;26:6167–6175. doi: 10.1016/j.biomaterials.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131–156. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- 7.Watt FM, Hogan BLM. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 8.Grayson W, Chao PHG, Marolt D, Kaplan D, Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. Trends Biotechnol. 2008;26:181–189. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malda J, Woodfield TBF, van der Vloodt F, Kooy FK, Martens DE, Tramper J, van Blitterswijk CA, Riesle J. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials. 2004;25:5773–5780. doi: 10.1016/j.biomaterials.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Obradovic B, Carrier RL, Vunjak-Novakovic G, Freed LE. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol Bioeng. 1999;63:197–205. doi: 10.1002/(sici)1097-0290(19990420)63:2<197::aid-bit8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 12.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 13.Malda J, Klein TJ, Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13:2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- 14.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Toya RE, Barrett JV, Davis ML. Hypoxia induced proliferation of hematopoietic stem-cells in bone-marrow of mice. Radiat Res. 1976;67:591–591. [Google Scholar]
- 16.Murphy MJ, Lord BI. Hematopoietic stem-cell regulation. I. Acute effects of hypoxic-hypoxia on cfu kinetics. Blood. 1973;42:81–87. [PubMed] [Google Scholar]
- 17.Lord BI, Murphy MJ. Hematopoietic stem-cell regulation. II. Chronic effects of hypoxic-hypoxia on cfu kinetics. Blood. 1973;42:89–98. [PubMed] [Google Scholar]
- 18.Ivanovic Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40:1482–1488. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 19.Cipolleschi MG, Rovida E, Ivanovic Z, Praloran V, Olivotto M, Dello Sbarba P. The expansion of murine bone marrow cells preincubated in hypoxia as an In vitro indicator of their marrow-repopulating ability. Leukemia. 2000;14:735–739. doi: 10.1038/sj.leu.2401744. [DOI] [PubMed] [Google Scholar]
- 20.Ivanovic Z, Bartolozzi B, Bernabei PA, Cipolleschi MG, Rovida E, Milenkovic P, Praloran V, Dello Sbarba P. Incubation of murine bone marrow cells in hypoxia ensures the maintenance of marrow-repopulating ability together with the expansion of committed progenitors. Br J Haematol. 2000;108:424–429. doi: 10.1046/j.1365-2141.2000.01842.x. [DOI] [PubMed] [Google Scholar]
- 21.Csete M. Oxygen in the cultivation of stem cells. In: Ourednik J, Sakaguchi D, Nilsen-Hamilton M, editors. Stem Cell Biology: Development and Plasticity. Wiley-Blackwell; 2005. pp. 1–8. [Google Scholar]
- 22.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 24.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 25.Potier E, Ferreira E, Andriamanalijaona R, Pujol JP, Oudina K, Logeart-Avramoglou D, Petite H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Sadat S, Gehmert S, Song YH, Yen YS, Bai XW, Gaiser S, Klein H, Alt E. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-1 and VEGF. Biochem Biophys Res Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 27.Xu MF, Uemura R, Dai Y, Wang YG, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gnecchi M, He HM, Noiseux N, Liang OD, Zhang LM, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 29.Patel KM, Crisostomo P, Lahm T, Markel T, Herring C, Wang M, Meldrum KK, Lillemoe KD, Meldrum DR. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res. 2007;143:281–285. doi: 10.1016/j.jss.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metabol. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metabol. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schofield R. Relationship between spleen colony-forming cell and hematopoietic stem-cell—hypothesis. Blood Cell. 1978;4:7–25. [PubMed] [Google Scholar]
- 34.Fischer B, Bavister BD. Oxygen-tension in the oviduct and uterus of Rhesus-monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 35.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early-preganancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 36.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 37.Weiss L. Hematopoietic microenvironment of bone-marrow— ultrastructural-study of stroma in rats. Anat Rec. 1976;186:161–184. doi: 10.1002/ar.1091860204. [DOI] [PubMed] [Google Scholar]
- 38.Lord BI. The architecture of bone-marrow cell-populations. Int J Cell Cloning. 1990;8:317–331. doi: 10.1002/stem.5530080501. [DOI] [PubMed] [Google Scholar]
- 39.Cipolleschi MG, Dellosbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem-cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- 40.Kofoed H, Sjontoft E, Siemssen SO, Olesen HP. Bone-marrow circulation after osteotomy—blood-flow, pO2, pCO2, and pressure studied in dogs. Acta Orthop Scand. 1985;56:400–403. doi: 10.3109/17453678508994357. [DOI] [PubMed] [Google Scholar]
- 41.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradley TR, Hodgson GS, Rosendaal M. Effect of oxygen-tension on hematopoietic and fibroblast cell-proliferation in vitro. J Cell Physiol. 1978;97:517–522. doi: 10.1002/jcp.1040970327. [DOI] [PubMed] [Google Scholar]
- 44.Katahira J, Mizoguchi H. Improvement of culture conditions for human megakaryocytic and pluripotent progenitor cells by low oxygen-tension. Int J Cell Cloning. 1987;5:412–420. doi: 10.1002/stem.5530050506. [DOI] [PubMed] [Google Scholar]
- 45.Koller MR, Bender JG, Miller WM, Papoutsakis ET. Reduced oxygen-tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone-marrow. Exp Hematol. 1992;20:264–270. [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 47.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Scherberich A, Galli R, Jaquiery C, Farhadi J, Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cell. 2007;25:1823–1829. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto A, Matsumoto S, Sowers AL, Koscielniak JW, Trigg NJ, Kuppusamy P, Mitchell JB, Subramanian S, Krishna MC, Matsumoto K. Absolute oxygen tension (pO2) muscle tissue as determined by in murine fatty and EPR. Magn Reson Med. 2005;54:1530–1535. doi: 10.1002/mrm.20714. [DOI] [PubMed] [Google Scholar]
- 50.Ezashi T, Das P, Roberts RM. Low O-2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurosawa H, Kimura M, Noda T, Amano Y. Effect of oxygen on in vitro differentiation of mouse embryonic stem cells. J Biosci Bioeng. 2006;101:26–30. doi: 10.1263/jbb.101.26. [DOI] [PubMed] [Google Scholar]
- 52.Gassmann M, Fandrey J, Bichet S, Wartenberg M, Marti HH, Bauer C, Wenger RH, Acker H. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci USA. 1996;93:2867–2872. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson JGE, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil. 1990;89:573–578. doi: 10.1530/jrf.0.0890573. [DOI] [PubMed] [Google Scholar]
- 54.Nakao H, Nakatsuji N. Effects of coculture, medium components and gas-phase on in vitro culture of in vitro matured and in vitro fertilized bovine embryos. Theriogenology. 1990;33:591–600. doi: 10.1016/0093-691x(90)90536-3. [DOI] [PubMed] [Google Scholar]
- 55.Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71:1108–1119. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- 56.Li JM, Foote RH. Culture of rabbit zygotes into blastocysts in protein-free medium with one to 20 per cent oxygen. J Reprod Fertil. 1993;98:163–167. doi: 10.1530/jrf.0.0980163. [DOI] [PubMed] [Google Scholar]
- 57.Umaoka Y, Noda Y, Narimoto K, Mori T. Effects of oxygen-toxicity on early development of mouse embryos. Mol Reprod Dev. 1992;31:28–33. doi: 10.1002/mrd.1080310106. [DOI] [PubMed] [Google Scholar]
- 58.Catt JW, Henman M. Toxic effects of oxygen on human embryo development. Hum Reprod. 2000;15(Suppl 2):199–206. doi: 10.1093/humrep/15.suppl_2.199. [DOI] [PubMed] [Google Scholar]
- 59.Wang FN, Thirumangalathu S, Loeken MR. Establishment of new mouse embryonic stem cell lines is improved by physiological glucose and oxygen. Cloning Stem Cell. 2006;8:108–116. doi: 10.1089/clo.2006.8.108. [DOI] [PubMed] [Google Scholar]
- 60.Forsyth NR, Musio A, Vezzoni P, Simpson A, Noble BS, McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cell. 2006;8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 61.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 62.Bauwens C, Yin T, Dang S, Peerani R, Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 63.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–355. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 64.Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 65.Martin-Rendon E, Hale SJM, Ryan D, Baban D, Forde SP, Roubelakis M, Sweeney D, Moukayed M, Harris AL, Davies K, Watt SM. Transcriptional profiling of human cord blood CD133(+) and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cell. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 66.Ren HY, Cao Y, Zhao QJ, Li J, Zhou CX, Liao LM, Jia MY, Zhao Q, Cai HG, Han ZC, Yang RC, Chen GQ, Zhao RCH. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 67.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 68.Buravkova LB, Anokhina EB. Effect of hypoxia on stromal precursors from rat bone marrow at the early stage of culturing. Bull Exp Biol Med. 2007;143:411–413. doi: 10.1007/s10517-007-0143-6. [DOI] [PubMed] [Google Scholar]
- 69.D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 70.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 71.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Beliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cell. 2003;21:337–347. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 72.Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 73.Fink T, Abildtrup L, Fogd K, Abdallah BM, Kassem M, Ebbesen P, Zachar V. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cell. 2004;22:1346–1355. doi: 10.1634/stemcells.2004-0038. [DOI] [PubMed] [Google Scholar]
- 74.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 75.Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 76.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2 alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–99. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 78.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–C1145. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y, Malladi P, Chiou M, Bekerman E, Giaccia AJ, Longaker MT. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 80.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Hypoxia inducible factor-1 alpha deficiency affects chondrogenesis of adi-pose-derived adult stromal cells. Tissue Eng. 2007;13:1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- 81.Sengers BG, Taylor M, Please CP, Oreffo ROC. Computational modeling of cell spreading and tissue regeneration in porous scaffolds. Biomaterials. 2007;13:1926–1940. doi: 10.1016/j.biomaterials.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 82.Galban CJ, Locke BR. Analysis of cell growth kinetics and substrate diffusion in a polymer scaffold. Biotechnol Bioeng. 1999;65:121–132. doi: 10.1002/(sici)1097-0290(19991020)65:2<121::aid-bit1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 83.Zhao F, Pathi P, Grayson W, Xing Q, Locke BR, Ma T. Effects of oxygen transport on 3-D human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog. 2005;21:1269–1280. doi: 10.1021/bp0500664. [DOI] [PubMed] [Google Scholar]
- 84.Guaccio A, Borselli C, Oliviero O, Netti PA. Oxygen con sumption of chondrocytes in agarose and collagen gels: a comparative analysis. Biomaterials. 2008;29:1484–1493. doi: 10.1016/j.biomaterials.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 85.Malda J, Rouwkema J, Martens DE, le Comte EP, Kooy FK, Tramper J, van Blitterswijk CA, Riesle J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng. 2004;86:9–18. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 86.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 87.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Grayson WL, Ma T, Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol Prog. 2004;20:905–912. doi: 10.1021/bp034296z. [DOI] [PubMed] [Google Scholar]
- 89.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 90.Rivron NC, Liu JJ, Rouwkema J, de Boer J, van Blitterswijk CA. Engineering vascularised tissues in vitro. Eur Cell Mater. 2008;15:27–40. doi: 10.22203/ecm.v015a03. [DOI] [PubMed] [Google Scholar]
- 91.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 92.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the P13K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cell. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 93.Cheema U, Brown RA, Alp B, MacRobert AJ. Spatially defined oxygen gradients and vascular endothelial growth factor expression in an engineered 3D cell model. Cell Mol Life Sci. 2008;65:177–186. doi: 10.1007/s00018-007-7356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matthews TJW, Smith SR, Peach CA, Rees JL, Urban JPG, Carr AJ. In vivo measurement of tissue metabolism in tendons of the rotator cuff - implications for surgical management. J Bone Joint Surg Br. 2007;89B:633–638. doi: 10.1302/0301-620X.89B5.18905. [DOI] [PubMed] [Google Scholar]
- 95.Park J, Bansal T, Pinelis M, Maharbiz MM. A microsystem for sensing and patterning oxidative microgradients during cell culture. Lab on a Chip. 2006;6:611–622. doi: 10.1039/b516483d. [DOI] [PubMed] [Google Scholar]
- 96.Butt OI, Carruth R, Kutala VK, Kuppusamy P, Moldovan NI. Stimulation of peri-implant vascularization with bone marrow-derived progenitor cells: monitoring by in vivo EPR oximetry. Tissue Eng. 2007;13:2053–2061. doi: 10.1089/ten.2006.0225. [DOI] [PubMed] [Google Scholar]
- 97.King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]