Abstract
Computational models of arterial growth and remodeling promise to increase our understanding of basic biological processes such as development, tissue maintenance, and aging, the biomechanics of functional adaptation, the progression and treatment of disease, responses to injuries, and even the design of improved replacement vessels and implanted medical devices. Ensuring reliability of and confidence in such models requires appropriate attention to verification and validation, including parameter sensitivity studies. In this paper, we classify different types of parameters within a constrained mixture model of arterial growth and remodeling; we then evaluate the sensitivity of model predictions to parameter values that are not known directly from experiments for cases of modest sustained alterations in blood flow and pressure as well as increased axial extension. Particular attention is directed toward complementary roles of smooth muscle vasoactivity and matrix turnover, with an emphasis on mechanosensitive changes in the rates of turnover of intramural fibrillar collagen and smooth muscle in maturity. It is shown that vasoactive changes influence the rapid change in caliber that is needed to maintain wall shear stress near its homeostatic level and the longer term changes in wall thickness that are needed to maintain circumferential wall stress near its homeostatic target. Moreover, it is shown that competing effects of intramural and wall shear stress regulated rates of turnover can develop complex coupled responses. Finally, results demonstrate that the sensitivity to parameter values depends upon the type of perturbation from normalcy, with changes in axial stretch being most sensitive consistent with empirical reports.
Keywords: vasoactivity, stress, collagen turnover, verification, experimental design
1 Introduction
Ubiquitous mechanosensitive growth and remodeling (G&R) processes are fundamental to many aspects of vascular biology and pathobiology as well as diverse arterial responses to injury and clinical intervention. Because of the complexity of such processes at molecular, cellular, and tissue levels, there is a pressing need for integrative multiscale computational models having both descriptive and predictive capability. Toward this end, there is first a need for reliable models at each of the individual scales. We have proposed a constrained mixture model for tissue-level G&R of arteries that accounts for individual material properties, natural configurations, and rates and extents of turnover of different structurally significant constituents that constitute the wall. This basic framework and illustrative constitutive relations have represented well the salient features of both normal arterial adaptations to altered pressure and flow [1] and different types of disease progression [2–5]. Moreover, by numerically testing multiple null hypotheses [6], we have shown the reasonableness of many of the fundamental hypotheses upon which the constrained mixture model is based. The goal of this paper, therefore, is to extend our previous investigations by classifying the types of material parameters that exist in constrained mixture models of arterial G&R and then assesing the sensitivity of model predictions to realistic ranges of these material parameters, particularly those that are not well known or easily determined from experiments.
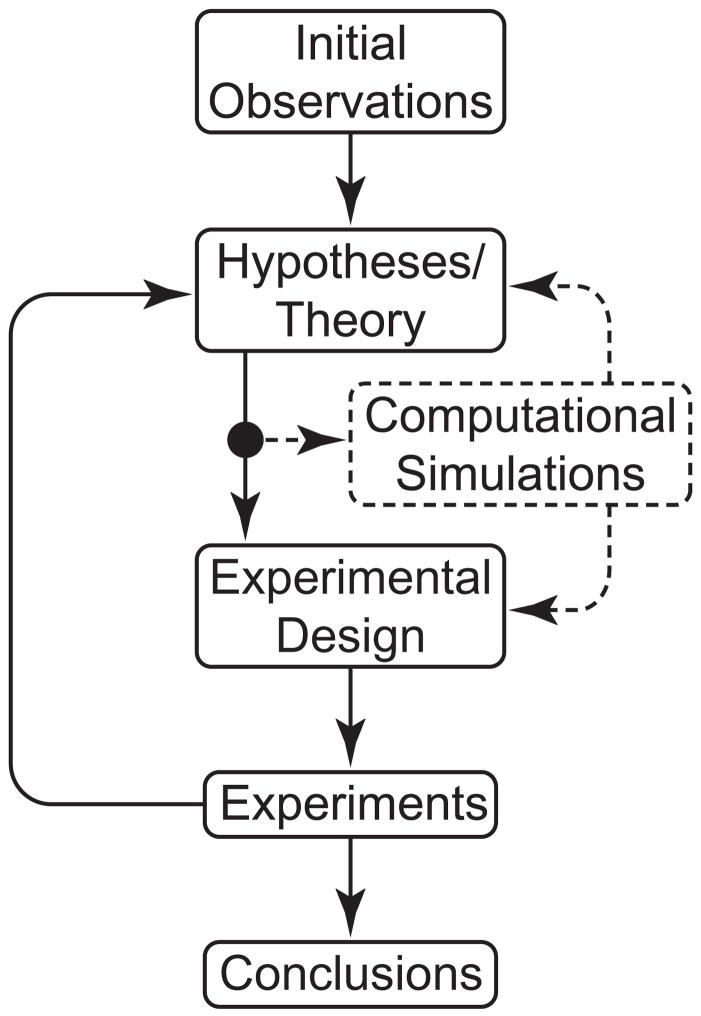
Parameter sensitivity studies based on numerical simulations can play fundamental roles within the overall verification and validation process in modeling [7]. By allowing values of parameters to approach particular limits, one can generate and test basic hypotheses in a cost- and time-efficient manner; by comparing predictions over reasonable ranges of parameter values, one can estimate the resolution needed in an experimental measurement or restrict the search space for a best-fit regression based on data; and by comparing predictions based on different sets of parameter values, one can elucidate possible complex mechanisms of coupling and thereby provide important guidance for the design and interpretation of an experiment. Indeed, given the advances in computational methods, numerical simulation has become an important addition to the traditional method of scientific inquiry based solely on theory and experiment (figure 1). In this paper, we confirm via numerical simulation that effective cell and matrix turnover require mechano-control in arterial G&R. We also confirm that vasoactivity and matrix remodeling represent complex, complementary, coupled mechanisms of arterial adaptation to altered flows, pressures, and axial extension, including those characterized by competing effects due to wall shear and intramural stress mediated turnover. Finally, sensitivity of model predictions to parameter values in particular classes of G&R suggest that vascular cells are more sensitive to perturbations in axial loading than to those in flow or pressure. Overall, the present parameter sensitivity study of a constrained mixture model of the growth and remodeling of a basilar artery suggests that current constitutive relations provide reasonable descriptions of the behavior even though there is strong motivation to identify better, more comprehensive constitutive relations for cell and matrix turnover as a function of altered mechanical stimuli.
Figure 1.
Schematic representation of the iterative process of the modern scientific method, as applied to G&R biomechanics. Computational models enable time- and cost-efficient simulations that can both serve an important role in the refinement of hypotheses/theories and motivation of experiments and their design. For example, simulations enable one to evaluate competing hypotheses, thereby focusing the experimental need.
2 Methods
2.1 General Framework
The mean Cauchy stress response for an artery, accounting for a constrained mixture of structurally significant passive constituents and active smooth muscle, was approximated using a rule of mixtures as
| (1) |
where F is the 2-D deformation gradient tensor, W = ΣkWk is the strain energy function for the mixture, with Wk representing energy stored in each structurally significant constituent k, and σact is the vasoactive contribution in the smooth muscle fiber direction em. This active contribution depends on intracellular calcium ion concentration and muscle fiber stretch λm(act). Extending the rule of mixtures approach to account for continuous cell and matrix turnover, this strain energy function was written as the sum of constituent contributions [1, 2]
| (2) |
where Mk(0) is the apparent mass density of constituent k, per reference area, at G&R time s = 0, Qk(s) are fractions for constituents deposited before time s = 0 that survive to current time s, mk(τ) are mass density production rates, qk(s, τ) are fractions for constituents deposited at time τ ∈[0, s] that survive to current G&R time s, and ρ(s) ≡ ρ (0) is the assumed constant mass density of the mixture. , where xk(s) = x(s) constrains all structurally significant constituents to deform together, but Xk(t) allows individual evolving natural configurations for each constituent k produced at time τ. See Baek et al. [2, 3] and Valentín et al. [1] for additional details of this basic framework and implementation.
2.2 Parameter classification
Previous implementations of constrained mixture models for arterial G&R involved several classes of parameters for the requisite geometry, constitutive relations, and applied loads [cf. 1, 3]. The chosen functional forms and parameter values were motivated both by reported observations and hypothesized behaviors; they were merely required to yield biologically and physically realistic predictions. Herein, however, we classify these constitutive relations and parameters by level of consensus and function (see tables 1 and 2) and study parametrically those for which only bounds are known. Quantities such as arterial geometry, volumetric flowrates, and local blood pressures are easily measured in vivo [cf. 8] and thus are well-known. Bulk mechanical behaviors are also easily quantified in vitro [9] and similarly for constituent mass fractions, given appropriate histological preparations [10, 11]. In contrast, values for quantities such as the stretch at which constituents are incorporated within extant matrix, values of shear stress-regulated vasoactive molecule production, and the changing rates of of cell and matrix turnover as a function of changes in mechanical stimuli are less well known and thus amenable to parametric study.
Table 1.
Classification of specific functional forms of constitutive relations employed in a constrained mixture model of the basilar artery. ‘Well Accepted’ relations represent those for which experimental data have established some level of consensus; references are listed as examples, not necessarily as proof of consensus. Postulated forms represent those for which experimental data are wanting; linear functions allow us to begin to explore salient behaviors and trends.
| Class | Relationship | Form | Ref. |
|---|---|---|---|
| Well Accepted | active stress-constrictor | sigmoidal | [17] |
| active force-length | inversely parabolic | [18] | |
| mass density removal-time | exponential decay | [53] | |
| energy density-strain (collagen/muscle) | Fung exponential | [78] | |
| energy density-strain (elastin) | neo-Hookean | [12] | |
| Postulated | constrictor-shear stress | linear | |
| mass density production-stress | linear | ||
| mass density production-constrictor | linear | ||
| mass density removal-tension | piecewise linear | ||
Table 2.
Classification of the requisite material parameters and their values for a representative mature basilar artery under homeostatic conditions. ‘Observed’ parameters include those reported in literature; these are readily obtained via direct measurements or fits to experimental data. ‘Bounded’ parameters are less well known, but can be chosen such that the model yields expected biomechanical behaviors. ‘Calculated’ parameters herein satisfy equilibrium for homeostatic conditions.
| Class | Role | Value | Ref. |
|---|---|---|---|
| Observed | vessel geometry | ah = 1.42 mm | [8] |
| initial loads | P = 93 mmHg, Q= 3.075 ml/s | [8] | |
| constituent mass fractions |
|
[11] | |
| physical constants | ρ = 1050 kg/m3, μ = 0.037 g/cm s | [68] | |
| homeostatic kinetics |
|
[22, 61] | |
| muscle activation parameters | Tmax = 150 kPa, λM = 1.1, λ0 = 0.4 | [18] | |
| Bounded | target stresses | σh = 100 kPa, | |
| deposition stretches |
|
||
| production kinetics |
|
||
| shear-constrictor | CB = 0.68, CS = 20 CB | ||
| elastic parameters |
|
||
| Calculated | vessel geometry | hh = 0176 mm | |
| elastic parameters | c = 588.3 kPa, | ||
2.2.1 Passive and Active Mechanical Behavior
Consistent with prior studies [1, 3], we used a neo-Hookean strain energy function for elastin [12, 13]
| (3) |
where and are constituent-specific stretches, which can be determined from arterial stretches (λθ,(s), λz(s)) and “growth-induced” prestretched [5]. Note that the latter assumes that elastin is not produced in maturity, rather it is produced during development, cross-linked, and stretched elastically during normal development/maturation [14]. We also utilized Fung exponential strain energy functions for both collagen [13, 15]
| (4) |
and passive smooth muscle [16]
| (5) |
The stretch experienced by each of these constituents depends on its deposition stretch, original orientation, and the stretch experienced by the arterial wall; as in Valentín et al. [1], we assumed four families of collagen (axial, circumferential, and symmetric diagonal). In the G&R formalism, values for the parameters and (table 2) were specified such that the artery exhibits reasonable passive behavior while the remaining parameters c, , and were computed rather than prescribed so as not to overprescribe the basal behavior given the prescription of both “deposition stretches” and “homeostatic target stresses,” which are discussed below.
Vasoactive function is a fundamental determinant of arterial mechanical behavior and thus G&R. Among others, Price et al. [17] reported constrictor dose-response curves exhibiting sigmoidal behavior and active force-length curves exhibiting inversely parabolic behavior [cf. 18]. The combined effect of these two observations was expressed as
| (6) |
where Tmax is a scaling factor with units kPa, φm is the mass fraction of active smooth muscle, λM and λ0 are the stretches at which the force generating capacity is maximum and zero, respectively, and is the current active muscle fiber stretch. C(s) is a lumped parameter ratio of constrictors to dilators, which we prescribed as a function of wall shear stress, namely
| (7) |
where τw(s) is the current shear stress acting on the endothelium and is a homeostatic target value. CB effectively defines the basal level of active stress generation and CS models the artery’s sensitivity to changing wall shear stress.
Note that equation (7) was motivated by interpretations by Rodbard [19] and Zamir [20] of Murray’s observation [21] of an optimal (target) condition: arteries constrict or dilate to maintain a target wall shear stress. For fully developed laminar flow of a Newtonian fluid through a rigid cylindrical tube, mean wall shear stress can be approximated as τw = 4 μ Q/π a3, where μ is the viscosity of blood, Q is the volumetric flow rate, and a is the luminal radius, each measurable parameters. The target value for shear stress was thus derived from empirical observations [8].
2.2.2 G&R Kinetics
It is well known that arteries can functionally adapt to changing physiological demands or hemodynamic loads, in part, by changing rates of constituent turnover [22]. Mass density production rates of collagen and smooth muscle are known to vary with changing mechanical stimuli [23–26]. Altered flow [27–29], pressure [30–33], axial extension [34, 35], and responses to clinical interventions such as balloon angioplasty [36–42] can each induce substantial changes from basal rates of turnover. For example, coarctation-induced hypertension has been observed to elicit a 15-fold increase in smooth muscle production [30] and an ~3 fold increase in collagen production [33]. Matrix metalloproteinase (MMP) levels increased by 4–5 fold in cases of hypoxia-induced hypertension [43]. Such changes are complicated by the multifunctional effects of other molecules such as nitric oxide (NO) and endothelin-1 (ET-1), which vary with imposed wall shear stress and affect cell and matrix turnover rates [28, 44–49].
For illustrative purposes, we let the production rate of constituent k be a linear function of the two primary mechanical stimuli, intramural and wall shear stresses [cf. 50, 51], namely
| (8) |
where Δσk is the difference between the current σk(s) and target value of a scalar measure of intramural stress for constituent k, as described by Baek et al. [3], and Δτw is the difference between the current τw(s) and target value of the magnitude of the wall shear stress. The relationship between wall shear stress and constrictor concentration was prescribed via equation (7), with the assumption that constrictors such as ET-1 increase smooth muscle and matrix production and dilators such as NO decrease production [52]. Rate (gain) parameters and govern stress-mediated production rates, and are basal production rates (assumed constant during maturity, but they likely change from development to maturity to aging) that maintain the artery under homeostatic conditions.
Despite the complex kinetics of smooth muscle and matrix turnover, half-lives for these structurally significant constituents appear to be well described by first order type kinetics [53–57]. We thus prescribed survival functions
| (9) |
where Kk(τ̃) are rate-type parameters for mass removal having units of days−1. These rate parameters were prescribed to be piecewise linear functions of changing fiber tension
| (10) |
with higher tensions accelerating removal via MMP activity [35, 58–60]. are basal arterial constituent rate-type parameters with values of approximately 1/80 day−1 [22, 61]. Finally, Δζ(τ̃) is the difference between the current and homeostatic tensions for a fiber deposited at time τ[1]. Note that Δζ= 0 in normalcy and that recovers a simple first order decay. Moreover, elastin is stable biologically under normal conditions in maturity, during which Qe(s) = 1 and me(τ) = 0; that is, we assumed that functional elastin does not turn over in mature, healthy arteries [62]. In the case where differences in mechanical perturbations are zero, equations (8) to (10) recover homeostatic turnover rates.
2.2.3 Deposition Stretches
The hypothesis that newly produced constituents are incorporated within extant matrix at preferred mechanical states is fundamental to the basic constrained mixture model [63]. The stretch at G&R time s experienced by a fibrous constituent deposited at time τ is [2]
| (11) |
where is the homeostatic deposition stretch for the kth constituent and λ(s) and λ (t) are arterial level stretches in the fiber direction relative to a computationally convenient (original) unloaded reference configuration. Note that if λ (s) = λ (τ), then the newly deposited fiber is stretched only at the level at which it was deposited. Whereas deposition stretches were conjectured based on heuristic arguments [63], increasing cell biological evidence supports this concept. Alberts et al. [64] summarized early observations on fibroblasts stating that they “work on the collagen they have secreted, crawling over it and tugging on it–helping to compact it into sheets and draw it out into cables.” More recent works [65–67] suggest that synthetic cells exert mechanical forces during deposition and/or matrix reorganization.
It thus appears that cells can and do incorporate new constituents within extant matrix at a preferred stress or stretch. Although we do not know the precise values of these deposition stretches, they must be less than maximum values of stretch in normal tissues. Clearly, this requires deposition stretches greater than 1 and typically less than 2. Functional elastin is deposited almost exclusively during development. As such, it is likely to experience a relatively high prestretch ( ) in most arteries. Stiff collagen constantly turns over and is assumed to have a relatively low deposition stretch ( ) consistent with observations from purely collagenous tissues such as tendons and intracranial saccular aneurysms [68]. Smooth muscle is less stiff, and is likely deposited as some intermediate stretch ( ), which would place it within its normal vasoactive range at basal tone. It is important to note that these are not experimentally derived quantities. Rather, these are estimates that fall within reasonable bounds and yield expected behavior, as, for example, results consistent with the good agreement on mean homeostatic intramural biaxial stresses σh of approximately 100 kPa [11, 69].
3 Illustrative Results
3.1 Vasoactivity
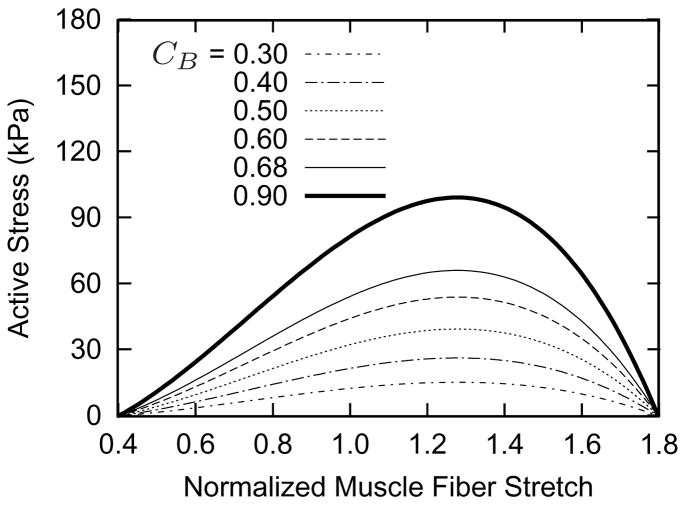
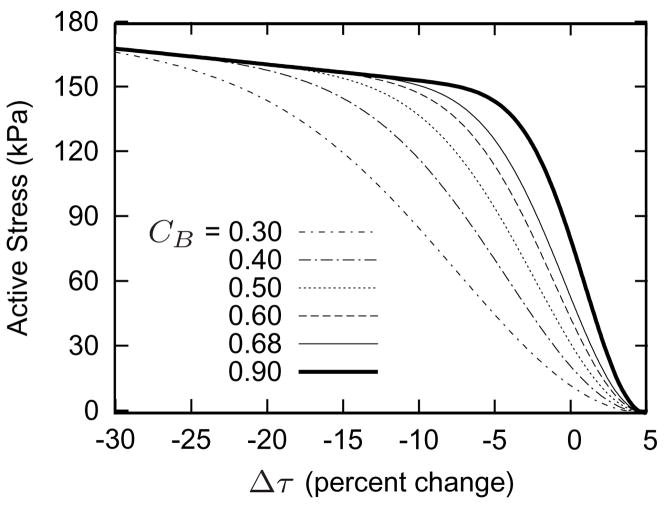
Figure 2 shows the simulated vasoactive stress response as a function of muscle fiber stretch for a range of basal constrictor to dilator ratios CB (cf. equation (6)). The homeostatic inner radius ah corresponds to a normalized muscle fiber stretch , and CB effectively defines the basal level of active stress. Simulations revealed that a lower value for CB results in a lower value for σact, thus requiring passive muscle to bear higher stresses, and necessitating a larger value of the Fung parameter to maintain a constant geometry. Thus, each curve effectively represents a different artery. The complex, coupled relationship between active muscle function and passive behavior also causes the vasoactive parameter CB to influence the vasoactive responsiveness to changes in wall shear stress τw (figure 3). This effect occurs, in part, because of the stiffer passive smooth muscle behavior for low CB; a more compliant passive muscle allows the artery to adjust its caliber more easily to achieve the target inner radius.
Figure 2.
Active stress-stretch muscle responses for indicated basal values of constrictor to dilator ratio CB at time σ = 0 (cf. equations (6) and (7)). All other parameters are as listed in table 2. Each curve represents a functionally different artery. The abscissa ‘normalized muscle fiber stretch’ is expressed as a range of values for , because no G&R is taking place. Note the inverse parabolic behavior [cf. 17].
Figure 3.
Active muscle responses for indicated basal values of constrictor to dilator ratio CB. All other parameters are as listed in table 2. Increasing values for CB result in increasing vasoactive responsiveness to changes in τw. Note the near sigmoidal behavior.
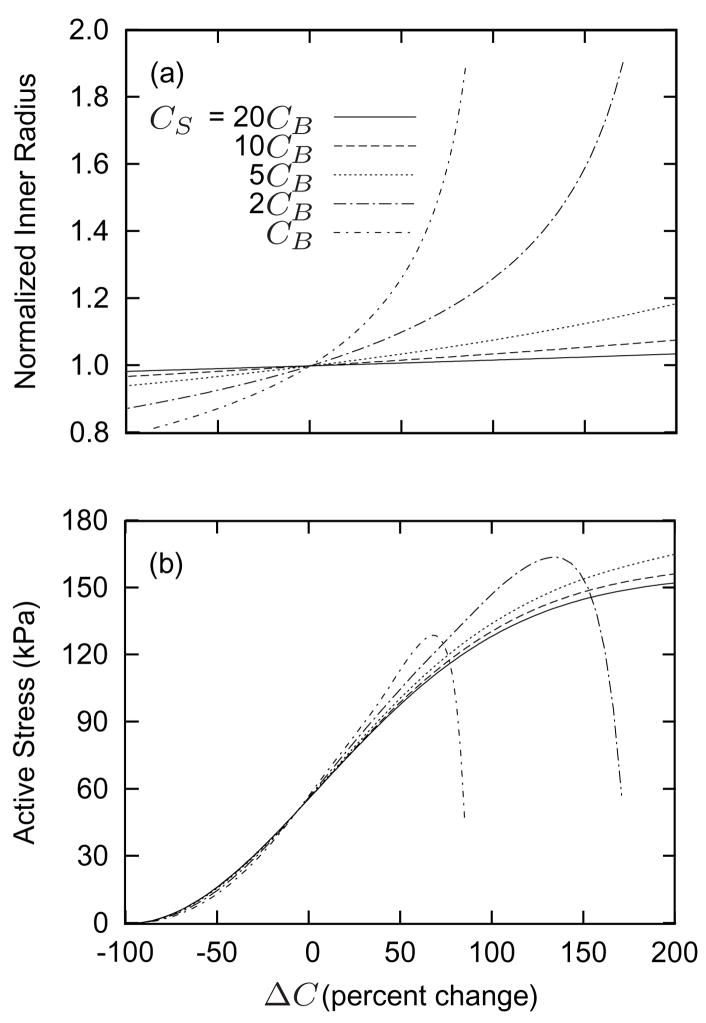
The shear stress scaling parameter CS (cf. equation (7)) plays a similarly important role in vasoactivity. Figure 4 shows effects of changing the constrictor to dilator ratio C on inner radius (panel a) and active stress (panel b) at a prescribed constant (homeostatic) flow. Higher values of CS allow the artery to maintain inner radius over a wider range of C and yield a sigmoidal behavior similar to that reported by Price et al. [17]. As the inner radius increases, ΔC increases due to shear stress regulation. For a given inner radius a, lower CS results in lower values of C. Coupled with the inversely parabolic active force-length behavior (figure 2), lower CS also results in peak active stress generation occurring at lower C. Thus, by choosing CB and CS appropriately, we can endow the simulated artery with vasoactive biases to certain ranges of C and τw. The parameters listed in table 2 yield vasoactive behavior comparable to that of basilar arteries [9].
Figure 4.
Normalized target inner radius (panel a) and active muscle responses (panel b) as functions of changing constrictor to dilator ratio C (shown here as the percentage of ΔC = C − CB) for indicated values of CS. Flowrate Q is constant at the homeostatic value. Higher values of CS allow the artery to better maintain the target inner radius over a wider range of C. Lower values of CS cause the artery to generate peak active stresses for smaller changes in C. By increasing CS, the artery is able to accommodate larger changes in constrictor concentration (see equation (6)). Note the generally sigmoidal behavior [cf. 17].
3.2 Mass Production
In addition to instantaneous passive and active behavior, the constrained mixture G&R framework relates mechanical stimuli to constituent mass density production rates. For example, an increase in blood pressure at a constant flow results initially in passive dilation due to wall distensibility. This dilation, along with isochoric thinning, elevates stresses in circumferentially aligned constituents while decreasing τw, which in turn leads to increases in C. Thus a sustained increase in pressure increases “circumferential” mass production for two reasons (cf. equation (8)), increased intramural stress and increased shear mediated constrictor concentration. Hence, the mechanical stimuli work in unison in this case; there are no competing effects. It proves useful, however, to consider cases in which changing mechanical stimuli induce competing effects. Consider, therefore, the case of a step decrease in flow while pressure remains constant. Following the reduction in flow, the artery constricts to restore τw to . This initial vasoactivity unloads circumferential collagen, and the lower intramural stress serves as a negative input for mass production, while the increased constrictor to dilator ratio C, which is a function of τw, serves as a positive input for mass production.
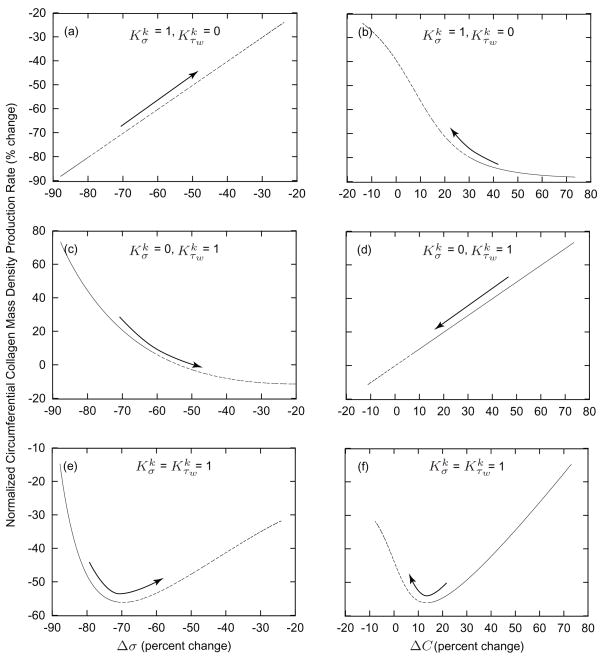
Figure 5 illustrates relationships between changing mechanical and mechanically induced stimuli (Δσ and ΔC) and the mass density production rate of circumferential collagen for a sustained 30% decrease in flow. Recalling equation (8), consider cases in which and (panels a and b), and (panels c and d), and (panels e and f). As prescribed, setting and yields a direct relationship between Δσ and mk (figure 5, panel a). Similarly, setting and yields a direct relationship between ΔC and mk (figure 5, panel d). In contrast, results for the case in which suggest a biphasic G&R response (figure 5, panels e and f). From days 0 to 30, mass production is related inversely to σ while related directly to C; after day 30, mass production is related directly to σ while related inversely to C. An inverse correlation of one stimulus plus a direct correlation with the other stimulus reveals strong competing effects. Specifically, the relationship between mass production and ΔC from days 0 to 30 suggests that early flow induced G&R is dominated by shear induced stimulation of vasoactive molecule production whereas that between mass production and Δσ from days 30 to 1000 suggest a second phase dominated by changes in wall stress. This finding is consistent with flow-induced changes in caliber preceding changes in thickness as reported in the literature and extends the earlier speculation of Rodbard [19] with regard to a “two-phase” response by to a sustrained alteration in flow.
Figure 5.
Comparison of intramural and wall shear stress regulation of circumferential collagen production rates. Values given as functions of changes in the scalar measure of stress borne by circumferential collagen (panels a, c, and e) and changes in constrictor to dilator ratio C (panels b, d, and f) for G&R in response to a sustained 30% decrease in flow from days 0 to 30 (solid) and from days 30 to 1000 (dashed). Arrows indicate advancing time. Note the direct relationship between changes in σ and mass production when and (panel a) and the direct relationship between changes in C and mass production when and (panel d), each linear as postulated separately (table 1). In contrast, note the biphasic progression of G&R when (panels e and f), that is, when production rates depend on changes in both intramural and wall shear stresses, which reveals potentially competing effects.
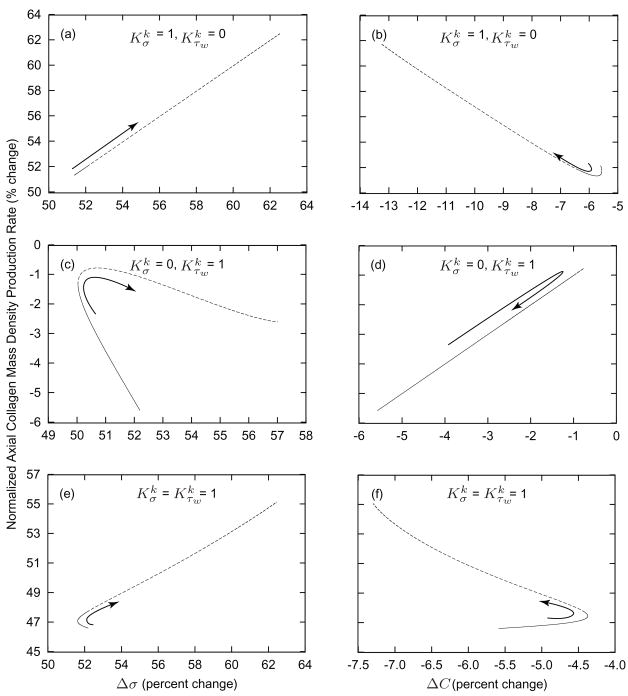
Changing axial length elicits similar competing effects. Figure 6 illustrates relationships between changing Δσ and ΔC and the mass density production rate of axially aligned collagen for a 2% increase in in vivo axial length at a constant pressure and flow. This increase in axial length reduces slightly the inner radius and the wall thickness, due to an initial isochoric response. As the inner radius decreases, τw increases and causes the vessel to dilate, thus restoring τw to . These factors serve to decrease C and increase stresses in the axially aligned constituents. As in the case of a step decrease in flow, setting and results in a direct relationship between Δσ and mk (figure 6, panel a) whereas and results in a direct relationship between ΔC and mk (figure 6, panel d). Setting again reveals a biphasic behavior: ΔC has a weak influence from days 0 to 40 whereas Δσ dominates mk from approximately day 20 to 1000. Comparatively large changes in σ override the competing effects of ΔC, indicated by the nearly direct relationship with respect to Δσ and inverse relationship with respect to ΔC.
Figure 6.
Comparison of intramural and wall shear stress regulation of axial collagen production rates. Values given as functions of changes in the scalar measure of stress borne by circumferential collagen (panels a, c, and e) and changes in constrictor to dilator ratio C (panels b, d, and f) for G&R in response to a sustained 2% increase in axial length from days 0 to 40 (solid) and from days 40 to 1000 (dashed). Arrows indicate advancing time. Note the biphasic progression when (panels e and f). From days 0 to 40, mass production is inversely related to σ while directly related to C. After day 40, mass production is directly related to σ while inversely related to C.
3.3 Evolving Geometry
Time courses of evolving radius, thickness, unloaded inner radius, and unloaded axial length depend greatly on mechanical stimuli and the associated mass density production parameters [1]. To appreciate better the complex coupled roles of changing wall shear stress and intramural constituent stresses, we analyzed the observable evolving geometric quantities as functions of the kinetic parameters and for cases of increased transmural pressure, decreased luminal flow, and increased axial length. These parameter sensitivity studies over multiple orders of magnitude (from 0.1 to 10) provide important insight and intuition regarding the differing modes of interaction among similarly involved mechanisms.
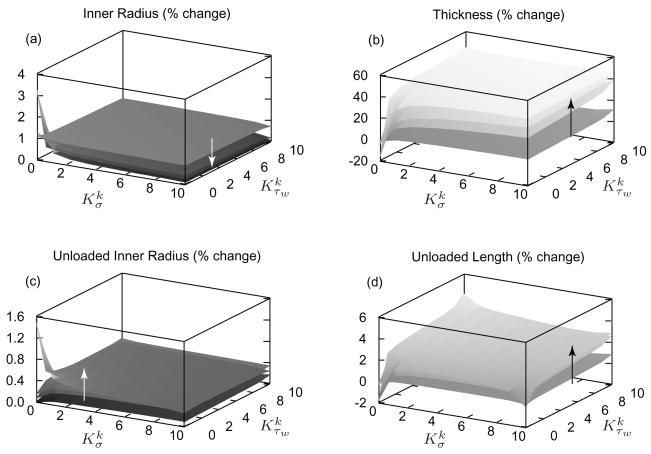
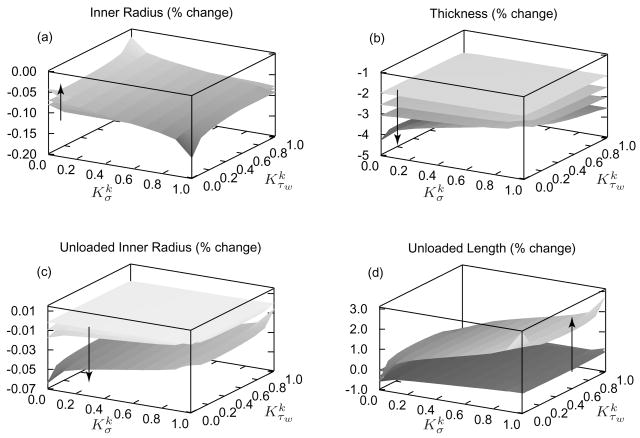
Figure 7 illustrates time varying consequences of a sustained 50% increase in pressure, at a constant flow and length, as functions of and . In vivo geometries generally approached their targets more rapidly with larger values of both and , but important differences surfaced. Note that the “singular” behavior at , which models constant mass density production, is not biologically relevant [6]. Inner radius (figure 7, panel a) shifts toward its target rapidly and is least sensitive to and . This finding suggests that inner radius is mostly regulated by an early vasoactive behavior as expected. In contrast, and exert a greater influence on the evolution of wall thickness (figure 7, panel b); larger values of and accelerate evolution. In the limiting case of , mass density production rates are constant while mass removal remains a function of fiber tension (see equation (9)). After 100 days, the artery atrophies appreciably as degradation outpaces production, resulting in a dilation of 3% and a reduction in thickness of ~40%. Such a loss of stiff collagen and muscle requires a higher level of muscle activation to maintain inner radius constant, which would seem to be energetically unfavorable. Figure 7 (panel c) shows that after 100 days, the artery’s unloaded inner radius (without vasoactivity) increases when . For or , unloaded inner radius remains nearly constant as it should in response to constant flow.
Figure 7.
Percent changes in inner radius (panel a), thickness (panel b), unloaded inner radius (panel c), and unloaded length (panel d) for a sustained 50% increase in pressure; results shown at days 1, 7, 14, and 100 of G&R with the arrows denoting advancing time. The model predicts small changes in inner radius as the vessel restores τw toward . Wall thickness increased by 50% as it should, with higher values of accelerating the process. Unloaded length increases with increased collagen deposition, thus resisting the recoiling effects of elastin. Note the near singular behavior at , consistent with prior findings that such values are unrealistic biologically [6].
Evolution of the unloaded axial length is more complex (figure 7, panel d). For the limiting case of , the unloaded axial length decreases (which implies a larger in vivo axial stretch), as decreased axial and helical collagen allow highly prestretched (unchanging) elastin to recoil the artery further. Focus, however, on values of and and recall that each fiber family’s mass production rate was defined individually as a function of its unique scalar measure of stress but a common τw (equation (8)). Moreover, note that helically and axially oriented collagen fiber families greatly influence the unloaded axial length. As pressure increases, C and σk increase for all constituents. Due to the prescribed constant in vivo axial length, however, circumferentially oriented constituents experience greater increases in σ than do those aligned helically and axially. Hence, as time progresses and the wall thickens, σk can decrease for helical and axial collagen, thereby resulting in a competition of influences between and , but an overall increase in unloaded length and consequently a lower in vivo axial stretch similar to experimental observations.
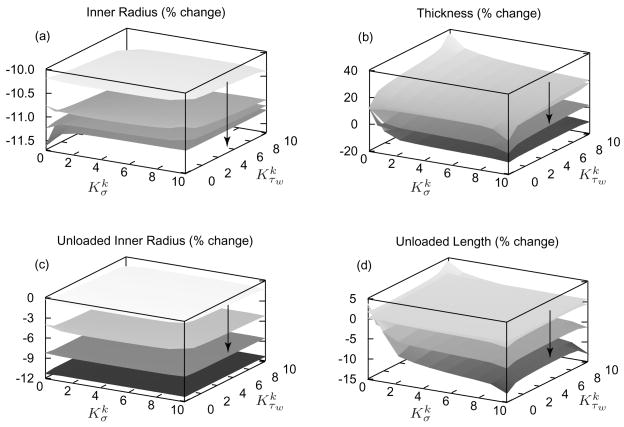
Evolving inner radius in response to a 30% reduction in luminal flow (figure 8, panel a) is nearly insensitive to changing and . Similarly, evolution of the unloaded inner radius (figure 8, panel c) is remarkably insensitive to these kinetic parameters. The unloaded inner radius follows in vivo inner radius as the artery remodels around its new vasoconstricted state. Evolving thickness (figure 8, panel b) is a function of isochoric motion and mass kinetics, with the initial vasoconstriction resulting in an ~10% isochoric increase in wall thickness. Note that in the limiting case when , wall thickness does not change because mass production rates remain constant. Setting or results in gradual thinning in response to reduced intramural constituent stresses, with diminishing rates of evolution beyond or . Long-term evolution of wall thickness is largely insensitive to because the initial vasoconstriction nearly restores τw to . Also, a decrease in intramural constituent stress results in reduced mass density production below , with a minimum production rate of zero. Any Δσ such that mass production is zero will yield the same wall thickness evolution, regardless of . For these same reasons, evolving unloaded axial length is less sensitive to , with diminishing sensitivity beyond (figure 8, panel d).
Figure 8.
Percent changes in inner radius (panel a), thickness (panel b), unloaded inner radius (panel c), and unloaded length (panel d) for a sustained 30% decrease in flow; results shown at days 1, 30, 100, and 1000 of G&R with the arrows denoting advancing time. The inner radius ultimately decreases by the predicted amount (0.71/3 = 0.88 [79]) as it should. Note the large changes in unloaded inner radius as the artery remodels around its new constricted state. Unloaded axial length decreases as the artery’s collagen to elastin ratio decreases and the thickness decreases, thereby allowing the elastin to retract the artery further when unloaded.
Increased axial stretching, at constant pressure and flow, results in a decreased inner radius and decreased thickness due to an initial isochoric motion. Yet, a 2% increase in axial length (figure 9) causes negligible changes in inner radius (panel a), and thus unloaded inner radius (panel c), as the artery vasodilates to restore . For low values of and , the unloaded inner radius decreases more appreciably as the wall atrophies. Evolution of wall thickness (figure 9, panel b) clearly reveals a competition between the effects of shear- and stress-mediated mass production: because luminal flow is constant, the reduced inner radius elevates τw, which works to diminish mass production, while a decreased wall thickness increases intramural constituent stresses, which heightens mass production. For these reasons, the wall thickens the most when and at any given G&R time s. Similarly, deposition rates for axial and helical fiber increase with increasing , thereby increasing the unloaded axial length. This change in unloaded length is largely independent of (figure 9, panel d).
Figure 9.
Percent changes in inner radius (panel a), thickness (panel b), unloaded inner radius (panel c), and unloaded length (panel d) for a sustained 2% increase in axial length; results shown at days 1, 7, 14, and 100 of G&R with the arrows denoting advancing time. As in the case of increasing pressure, the inner radius remains nearly constant, given the constant flow. Unloaded length increases, as expected, due to increasing deposition of axial collagen at its preferred value.
4 Discussion
Truesdell and Noll [70] articulated well the complementary roles of theory and experiment:
The task of the theorist is to bring order into the chaos of the phenomena of nature, to invent a language by which a class of these phenomena can be described efficiently and simply. Here is the place for “intuition,” and here the old preconception, common among natural philosophers, that nature is simple and elegant, has led to many great successes. Of course, physical theory must be based on experience, but experiment comes after, not before, theory. Without theoretical concepts one would neither know what experiments to perform nor be able to interpret their outcome.
As theories and experiments have become more detailed and complex, numerical simulations have emerged as a third pillar of scientific research. Numerical simulations allow researchers to generate, test, and refine hypotheses and theoretical concepts with much greater efficiency in terms of both time and expense. This refinement, in turn, permits the design of more rational and fruitful experiments, as called for (albeit differently) by Truesdell and Noll. The emergence of the need for multiscale models to integrate mechanobiological information and increase understanding from the genome to medical or surgical treatment at tissue and organ levels only highlights further the need for iterative observational, theoretical, experimental, and computational studies. Of these, our focus herein was limited to the role of parameter sensitivity studies within numerical simulation.
Predicted vasoactive behaviors were consistent with observed trends [17] based on the prescribed material behavior of equation (6) and associated parameters listed in table 2. In particular, active behavior was sensitive to both the initial level of the constrictor to dilator ratio CB and the shear stress scaling factor CS. Coupled length dependent active behaviors and constrictor dose responses greatly affected vasoactive efficiency. For low values of CS, smooth muscle developed peak stresses for relatively low increases in C. That is, the artery was not able to compensate well for large decreases in flow without shear stress mediated regulation, consistent with experimental observations [71, 72]. Along with appropriate passive behavior, this coupling was crucial to obtaining realistic vasoactive behavior for the artery of interest. The associated muscle activity parameters CS, CB, λM, and λ0 can be tailored to describe a particular artery by stipulating that peak active stresses are generated at appropriate ratios of constrictors to dilators, thereby yielding realistic relationships between wall shear stress and the active response [73].
Vasoactive behavior influences long term G&R by controlling the state in which turnover occurs, thereby affecting changes in wall thickness and unloaded length, which in turn affect intramural stresses [cf. 74, 75]. The competing effects of intramural stress- and wall shear stress-regulated turnover resulted in complex G&R based on our model. This was most evident in cases of decreased flow or increased axial length; decreased (increased) wall shear stress and decreased (increased) intramural stresses provide opposite inputs to mass production. Even for the case of increased transmural pressure, where all mechanical stimuli tend to accelerate turnover, there was a competition between and in the evolution of unloaded axial length. This finding further emphasizes the complex interactions possible even with linear production rates.
Simulations revealed that progression of G&R for some perturbations involved the dominance of one mechanical stimulus during one phase but the emergence of another dominant stimulus during a subsequent phase. For example, reductions in flow and increases in axial extension elicit such responses. Increased MMP activity can precede mass production [37], thus resulting in initial atrophy followed by eventual compensatory hypertrophy and maintenance. These predicted time courses suggest provocative possibilities for designing intervention, as, for example, timed drug delivery. Similarly, such time courses could aid in the decision process when choosing time intervals at which to collect samples and/or use appropriate immunohistological stains or other markers, for example. Finally, predictive models promise to aid in the refinement of tissue engineering strategies to build in desirable properties via appropriately timed stimuli.
The model predicted differing modes of G&R and degrees of sensitivity to parameter values depending upon the type of perturbation, despite the similarly involved mechanisms. Most notably, the model was most sensitive to increases in axial stretch beyond the homeostatic. This extreme sensitivity is similar to the observations reported by Jackson et al. [35], wherein they noted “unprecedented” rates of change in vivo when arterial length was increased. Our numerical implementation predicted upper and lower bounds (saturation points) in shear-induced active stress generation and values for beyond which the system was no longer sensitive to a particular stimulus. For example, the model predicted little sensitivity beyond with respect to evolving thickness for cases of reduced flow for this drove mass density productions to zero.
Predicted geometric consequences of G&R, like evolving passive and active behaviors, can be compared to experimentally observed behaviors. Such comparisons will assist in formulating improved constitutive relations and determining best-fit values of the associated parameters. Nevertheless, mixture models require mechanical response parameters for each individual constituent, which increases the overall number of parameters and thereby raises concerns by some that there are too many parameters. We suggest, however, that one advantage of structurally motivated models is that many of the parameter values can be prescribed independently and in many cases prescribed directly, based on experimental data. In this way, one reduces the need to perform nonlinear regressions based on large numbers of unknown parameters, which would otherwise raise issues of non-uniqueness. Moreover, once good estimates are determined for the parameters, one can perform nonlinear regressions based on restricted (physically meaningful) parameter search spaces. We did not study the sensitivity of the constrained mixture model to ranges of observed parameters or certain bounded parameters because they are experimentally available. Indeed, noting that functional forms appear to be preserved across species, whereas parameter values vary with species and to some degree individuals, Stålhand and Klarbring [76] and Masson et al. [77] showed that parameter values can be estimated in individual patients in part because of known bounds on many of the parameters and prior experience in modeling [68]. There is, however, a need for better experimental data where possible to refine further the values of many of the parameters.
Constituent turnover (production and removal) as a function of mechanical stimuli remains the least well-understood aspect of arterial growth and remodeling. That is, there remains a pressing need for a better understanding of cellular responses to mechanical stimuli and how these responses manifest at the tissue and organ levels. To that end, we hope that continuum based constrained mixture models will motivate experimentalists and theorists alike to elucidate these intricately linked behaviors. Although we anticipate the need for a more rigorous analysis, hypothesis testing [6] and parameter sensitivity studies represent an important first step toward verification [7]. While no framework or numerical model can ever be strictly correct, the ultimate measure of a model’s utility is to what extent it can describe and predict what is physically reasonable. Rational theories founded upon realistic fundamental cellular behavior and continuum mechanics promise to help us develop intuition, understand complex biomechanical systems, and design better experiments and ultimately clinical interventions.
Acknowledgments
This work was supported, in part, NIH grants (HL-64372, 80415, 86418, and EB-08366).
References
- 1.Valentín A, Cardamone L, Baek S, Humphrey JD. Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J R Soc Interface. 2008 doi: 10.1098/rsif.2008.0254. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek S, Rajagopal KR, Humphrey JD. A theoretical model of enlarging intracranial fusiform aneurysms. J Biomech Eng. 2006;128(1):142–9. doi: 10.1115/1.2132374. [DOI] [PubMed] [Google Scholar]
- 3.Baek S, Valentín A, Humphrey JD. Biochemomechanics of cerebral vasospasm and its resolution: II. constitutive relations and model simulations. Ann Biomed Eng. 2007;35(9):1498–1509. doi: 10.1007/s10439-007-9322-x. [DOI] [PubMed] [Google Scholar]
- 4.Baek S, Rajagopal KR, Humphrey JD. Competition between radial expansion and thickening in the enlargement of an intracranial saccular aneurysm. J Elasticity. 2005;80(19):13–31. URL http://www.ingentaconnect.com/content/klu/elas/2005/00000080/F0030001/00009004.
- 5.Figueroa CA, Baek S, Taylor CA, Humphrey JD. A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput Meth Appl Mech Eng. 2009 doi: 10.1016/j.cma.2008.09.013. URL http://www.sciencedirect.com/science/article/B6V29-4TPPDXD-1/2/469bea93997eede01638392fc8fc8157in press. [DOI] [PMC free article] [PubMed]
- 6.Valentín A, Humphrey JD. Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodeling. Phil Trans R Soc Lond A. 2009 doi: 10.1098/rsta.2009.0113. accepted, pending revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson AE, Ellis BJ, Weiss JA. Verification, validation and sensitivity studies in computational biomechanics. Comput Methods Biomech Biomed Engin. 2007;10(3):171–184. doi: 10.1080/10255840601160484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodi CA, Ursino M. Hemodynamic effect of cerebral vasospasm in humans: a modeling study. Ann Biomed Eng. 1999;27(2):257–73. doi: 10.1114/1.168. [DOI] [PubMed] [Google Scholar]
- 9.Wicker BK, Hutchens HP, Wu Q, Yeh AT, Humphrey JD. Normal basilar artery structure and biaxial mechanical behaviour. Comput Meth Biomech Biomed Eng. 2008;11(5):539–551. doi: 10.1080/10255840801949793. URL http://www.informaworld.com/10.1080/10255840801949793. [DOI] [PubMed]
- 10.Fischer GM, Llaurado JG. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966;19(2):394–399. doi: 10.1161/01.res.19.2.394. [DOI] [PubMed] [Google Scholar]
- 11.Walmsley JG, Campling MR, Chertkow HM. Interrelationships among wall structure, smooth muscle orientation, and contraction in human major cerebral arteries. Stroke. 1983;14(5):781–790. doi: 10.1161/01.str.14.5.781. [DOI] [PubMed] [Google Scholar]
- 12.Dorrington KL, McCrum NG. Elastin as a rubber. Biopolymers. 1977;16(6):1201–1222. doi: 10.1002/bip.1977.360160604. [DOI] [PubMed] [Google Scholar]
- 13.Holzapfel GA, Gasser TC, Ogden RW. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elasticity. 2000;61(1–3):1–48. [Google Scholar]
- 14.Cardamone L, Valentín A, Eberth JF, Humphrey JD. Origin of axial prestretch and residual stress in arteries. Biomech Model Mechanobiol. 2009 doi: 10.1007/s10237-008-0146-x. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanir Y. Constitutive equations for fibrous connective tissues. J Biomech. 1983;16(1):1–12. doi: 10.1016/0021-9290(83)90041-6. [DOI] [PubMed] [Google Scholar]
- 16.Gleason RL, Taber LA, Humphrey JD. A 2-d model of flow-induced alterations in the geometry, structure, and properties of carotid arteries. J Biomech Eng. 2004;126(3):371–81. doi: 10.1115/1.1762899. [DOI] [PubMed] [Google Scholar]
- 17.Price JM, Davis DL, Knauss EB. Length-dependent sensitivity in vascular smooth muscle. Am J Physiol. 1981;241(4):H557–63. doi: 10.1152/ajpheart.1981.241.4.H557. [DOI] [PubMed] [Google Scholar]
- 18.Rachev A, Hayashi K. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng. 1999;27(4):459–468. doi: 10.1114/1.191. [DOI] [PubMed] [Google Scholar]
- 19.Rodbard S. Vascular caliber. Cardiology. 1975;60(1):4–49. doi: 10.1159/000169701. [DOI] [PubMed] [Google Scholar]
- 20.Zamir M. Shear forces and blood vessel radii in the cardiovascular system. J Gen Physiol. 1977;69(4):449–461. doi: 10.1085/jgp.69.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray CD. The physiological principle of minimum work: I. the vascular system and the cost of blood volume. Proc Natl Acad Sci USA. 1926;12(3):207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- 23.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191(4226):475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- 24.Wilson E, Mai Q, Sudhir K, Weiss RH, Ives HE. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993;123(3):741–7. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-β. J Vasc Res. 1998;35(2):93–103. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 26.O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-β1. Hypertension. 2000;36(3):319–24. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 27.Sluijter JPG, Smeets MB, Velema E, Pasterkamp G, de Kleijn DPV. Increase in collagen turnover but not in collagen fiber content is associated with flow-induced arterial remodeling. J Vasc Res. 2004;41(6):546–55. doi: 10.1159/000081972. [DOI] [PubMed] [Google Scholar]
- 28.Mondy JS, Lindner V, Miyashiro JK, Berk BC, Dean RH, Geary RL. Platelet-derived growth factor ligand and receptor expression in response to altered blood flow in vivo. Circ Res. 1997;81(3):320–7. doi: 10.1161/01.res.81.3.320. [DOI] [PubMed] [Google Scholar]
- 29.Cho A, Mitchell L, Koopmans D, Langille BL. Effects of changes in blood flow rate on cell death and cell proliferation in carotid arteries of immature rabbits. Circ Res. 1997;81(3):328–37. doi: 10.1161/01.res.81.3.328. [DOI] [PubMed] [Google Scholar]
- 30.Xu C, Lee S, Singh TM, Sho E, Li X, Sho M, Masuda H, Zarins CK. Molecular mechanisms of aortic wall remodeling in response to hypertension. J Vasc Surg. 2001;33(3):570–8. doi: 10.1067/mva.2001.112231. [DOI] [PubMed] [Google Scholar]
- 31.Nissen R, Cardinale GJ, Udenfriend S. Increased turnover of arterial collagen in hypertensive rats. Proc Natl Acad Sci USA. 1978;75(1):451–453. doi: 10.1073/pnas.75.1.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamal A, Bendeck M, Langille BL. Structural changes and recovery of function after arterial injury. Arterioscler Thromb. 1992;12(3):307–17. doi: 10.1161/01.atv.12.3.307. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, Zarins CK, Bassiouny HS, Briggs WH, Reardon C, Glagov S. Differential transmural distribution of gene expression for collagen types I and III proximal to aortic coarctation in the rabbit. J Vasc Res. 2000;37(3):170–82. doi: 10.1159/000025728. [DOI] [PubMed] [Google Scholar]
- 34.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009 doi: 10.1016/j.jbiomech.2008.11.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90(8):918–25. doi: 10.1161/01.RES.0000016481.87703.CC. [DOI] [PubMed] [Google Scholar]
- 36.Sluijter JPG, Smeets MB, Velema E, Pasterkamp G, de Kleijn DPV. Increased collagen turnover is only partly associated with collagen fiber deposition in the arterial response to injury. Cardiovasc Res. 2004;61(1):186–95. doi: 10.1016/j.cardiores.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Strauss BH, Robinson R, Batchelor WB, Chisholm RJ, Ravi G, Natarajan MK, Logan RA, Mehta SR, Levy DE, Ezrin AM, Keeley FW. In vivo collagen turnover following experimental balloon angioplasty injury and the role of matrix metalloproteinases. Circ Res. 1996;79(3):541–50. doi: 10.1161/01.res.79.3.541. [DOI] [PubMed] [Google Scholar]
- 38.Strauss BH, Chisholm RJ, Keeley FW, Gotlieb AI, Logan RA, Armstrong PW. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994;75(4):650–8. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- 39.Karim MA, Miller DD, Farrar MA, Eleftheriades E, Reddy BH, Breland CM, Samarel AM. Histomorphometric and biochemical correlates of arterial procollagen gene expression during vascular repair after experimental angioplasty. Circ. 1995;91(7):2049–57. doi: 10.1161/01.cir.91.7.2049. [DOI] [PubMed] [Google Scholar]
- 40.Bendeck MP, Irvin C, Reidy M, Smith L, Mulholland D, Horton M, Giachelli CM. Smooth muscle cell matrix metalloproteinase production is stimulated via αVβ3 integrin. Arterioscler Thromb Vasc Biol. 2000;20(6):1467–72. doi: 10.1161/01.atv.20.6.1467. [DOI] [PubMed] [Google Scholar]
- 41.Weiser-Evans MC, Quinn BE, Burkard MR, Stenmark KR. Transient reexpression of an embryonic autonomous growth phenotype by adult carotid artery smooth muscle cells after vascular injury. J Cell Physiol. 2000;182(1):12–23. doi: 10.1002/(SICI)1097-4652(200001)182:1<12::AID-JCP2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circ. 1996;93(2):340–8. doi: 10.1161/01.cir.93.2.340. [DOI] [PubMed] [Google Scholar]
- 43.Thakker-Varia S, Tozzi CA, Poiani GJ, Babiarz JP, Tatem L, Wilson FJ, Riley DJ. Expression of matrix-degrading enzymes in pulmonary vascular remodeling in the rat. Am J Physiol. 1998;275(2 Pt 1):L398–406. doi: 10.1152/ajplung.1998.275.2.L398. [DOI] [PubMed] [Google Scholar]
- 44.Rizvi MAD, Katwa L, Spadone DP, Myers PR. The effects of endothelin-1 on collagen type I and type III synthesis in cultured porcine coronary artery vascular smooth muscle cells. J Mol Cell Cardiol. 1996;28(2):243–252. doi: 10.1006/jmcc.1996.0023. [DOI] [PubMed] [Google Scholar]
- 45.Rizvi MAD, Myers PR. Nitric oxide modulates basal and endothelin-induced coronary artery vascular smooth muscle cell proliferation and collagen levels. J Mol Cell Cardiol. 1997;29(7):1779–1789. doi: 10.1006/jmcc.1996.0480. [DOI] [PubMed] [Google Scholar]
- 46.Dooley A, Gao B, Shi-Wen X, Abraham DJ, Black CM, Jacobs M, Bruckdorfer KR. Effect of nitric oxide and peroxynitrite on type I collagen synthesis in normal and scleroderma dermal fibroblasts. Free Radic Biol Med. 2007;43(2):253–264. doi: 10.1016/j.freeradbiomed.2007. 04.017. [DOI] [PubMed] [Google Scholar]
- 47.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269(6 Pt 1):C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 48.Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol. 1992;263(2 Pt 1):C389–96. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- 49.Singh TM, Abe KY, Sasaki T, Zhuang YJ, Masuda H, Zarins CK. Basic fibroblast growth factor expression precedes flow-induced arterial enlargement. J Surg Res. 1998;77(2):165–73. doi: 10.1006/jsre.1998.5376. [DOI] [PubMed] [Google Scholar]
- 50.Taber LA. A model for aortic growth based on fluid shear and fiber stresses. J Biomech Eng. 1998;120(3):348–54. doi: 10.1115/1.2798001. [DOI] [PubMed] [Google Scholar]
- 51.Rachev A. A model of arterial adaptation to alterations in blood flow. J Elasticity. 2000;61(1–3):83–111. doi: 10.1023/A:1010800703478. [DOI] [Google Scholar]
- 52.Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza JJ, Egido J. Endothelin-1, via ETA receptor and independently of transforming growth factor-β, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res. 2005;97(2):125–134. doi: 10.1161/01.RES.0000174614.74469.83. URL http://circres.ahajournals.org/cgi/content/abstract/97/2/125. [DOI] [PubMed]
- 53.Niedermüller H, Skalicky M, Hofecker G, Kment A. Investigations on the kinetics of collagen-metabolism in young and old rats. Exp Gerontol. 1977;12(5–6):159–68. doi: 10.1016/0531-5565(77)90001-8. [DOI] [PubMed] [Google Scholar]
- 54.Cho A, Courtman DW, Langille BL. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ Res. 1995;76(2):168–75. doi: 10.1161/01.res.76.2.168. [DOI] [PubMed] [Google Scholar]
- 55.Gelman RA, Williams BR, Piez KA. Collagen fibril formation. evidence for a multistep process. J Biol Chem. 1979;254(1):180–6. [PubMed] [Google Scholar]
- 56.Gelman RA, Poppke DC, Piez KA. Collagen fibril formation in vitro. the role of the nonhelical terminal regions. J Biol Chem. 1979;254(22):11741–11745. [PubMed] [Google Scholar]
- 57.Kao WW, Berg RA, Prockop DJ. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977;252(23):8391–7. [PubMed] [Google Scholar]
- 58.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25(5):957–962. doi: 10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- 59.Ellsmere JC, Khanna RA, Lee JM. Mechanical loading of bovine pericardium accelerates enzymatic degradation. Biomaterials. 1999;20(12):1143–1150. doi: 10.1016/s0142-9612(99)00013-7. URL http://www.sciencedirect.com/science/article/B6TWB-3WJFKHR-9/2/659df41763b1881a533c48bf87d60e5d. [DOI] [PubMed]
- 60.Ruberti JW, Hallab NJ. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun. 2005;336(2):483–489. doi: 10.1016/j.bbrc. 2005.08.128. [DOI] [PubMed] [Google Scholar]
- 61.Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21:S11–7. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- 62.Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol. 1996;74(7):834–41. [PubMed] [Google Scholar]
- 63.Humphrey JD, Rajagopal KR. A constrained mixture model for growth and remodeling of soft tissues. Math Models Methods Appl Sci. 2002;12(3):407–30. doi: 10.1142/S0218202502001714. [DOI] [Google Scholar]
- 64.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Garland Science. 4. 2002. The Molecular Biology of the Cell. [Google Scholar]
- 65.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–88. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- 66.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7(2):157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 67.Kozel BA, Rongish BJ, Czirok A, Zach J, Little CD, Davis EC, Knutsen RH, Wagenseil JE, Levy MA, Mecham RP. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2006;207(1):87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- 68.Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer-Verlag; New York: 2002. [Google Scholar]
- 69.Hu JJ, Baek S, Humphrey JD. Stress-strain behavior of the passive basilar artery in normotension and hypertension. J Biomech. 2007;40(11):2559–2563. doi: 10.1016/j.jbiomech.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Truesdell CA, Noll W. The Non-Linear Field Theories of Mechanics, volume III of Handbuch der Physik. Springer; Berlin: 1965. [Google Scholar]
- 71.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. 11 . doi: 10.1038/288373a0. URL http://dx.doi.org/10.1038/288373a0. [DOI] [PubMed]
- 72.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986 Jan;8(1):37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 73.Dajnowiec D, Langille BL. Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodelling. Clin Sci (Lond) 2007;113(1):15–23. doi: 10.1042/CS20060337. [DOI] [PubMed] [Google Scholar]
- 74.Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5(3):413–420. [PubMed] [Google Scholar]
- 75.Lehman RM, Owens GK, Kassell NF, Hongo K. Mechanism of enlargement of major cerebral collateral arteries in rabbits. Stroke. 1991;22(4):499–504. doi: 10.1161/01.str.22.4.499. [DOI] [PubMed] [Google Scholar]
- 76.Stålhand J, Klarbring A. Aorta in vivo parameter identification using an axial force constraint. Biomech Model Mechanobiol. 2005;3(4):191–199. doi: 10.1007/s10237-004-0057-4. URL http://dx.doi.org/10.1007/s10237-004-0057-4. [DOI] [PubMed]
- 77.Masson I, Boutouyrie P, Laurent S, Humphrey JD, Zidi M. Characterization of arterial wall mechanical behavior and stresses from human clinical data. J Biomech. 2008 Aug 28;41(12):2618–2627. doi: 10.1016/j.jbiomech.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nevo E, Lanir Y. Structural finite deformation model of the left ventricle during diastole and systole. J Biomech. 1989;111(4):342–349. doi: 10.1115/1.3168389. [DOI] [PubMed] [Google Scholar]
- 79.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys. 2008;50(2):53–78. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]