Summary
The EGF receptor is a transmembrane receptor tyrosine kinase that is enriched in lipid rafts. Subdomains I, II and III of the extracellular domain of the EGF receptor participate in ligand binding and dimer formation. However, the function of the cysteine-rich subdomain IV has not been elucidated. In this study, we analyzed the role of the membrane-proximal portion of subdomain IV in EGF binding and signal transduction. A double Cys→Ala mutation that breaks the most membrane-proximal disulfide bond (Cys600 to Cys612), ablated high affinity ligand binding and substantially reduced signal transduction. A similar mutation that breaks the overlapping Cys596 to Cys604 disulfide had little effect on receptor function. Mutation of residues within the Cys600 to Cys612 disulfide loop did not alter the ligand binding or signal transducing activities of the receptor. Despite the fact that the C600,612A EGF receptor was significantly impaired functionally, this receptor as well as all of the other receptors with mutations in the region of residues 596 to 612 localized normally to lipid rafts. These data suggest that the disulfide-bonded structure of the membrane-proximal portion of the EGF receptor, rather than its primary sequence, is important for EGF binding and signaling but is not involved in localizing the receptor to lipid rafts.
Keywords: EGF receptor, lipid raft, cholesterol, tyrosine kinase, microdomain
Introduction
The EGF receptor is a type I transmembrane protein with an extracellular domain composed of ~620 amino acids. The transmembrane domain is comprised of 24–26 amino acids that probably traverse the membrane once in the form of an alpha helix. The ~550 amino acid cytoplasmic domain harbors the tyrosine kinase enzyme activity as well as the tyrosine residues that become autophosphorylated following stimulation with EGF [1]. Under basal conditions, the EGF receptor exists as a monomer. However, upon binding EGF, the receptor dimerizes. This activates the intracellular tyrosine kinase leading to autophosphorylation of the receptor and the beginning of the process of signal transduction. [2, 3].
The extracellular domain of the EGF receptor contains four subdomains numbered I through IV. Domains I and III are homologous as are domains II and IV [4]. Domains II and IV are cysteine-rich with each ~150 amino acid domain containing ~20–25 cysteines in specific disulfide pairings [5]. Recent structural studies of the extracellular domain of the EGF receptor have provided insight into the mechanism through which EGF binds and induces receptor dimer formation. The inactive EGF receptor is held in a closed conformation by interactions between a dimerization arm in subdomain II and a tethering arm in subdomain IV [6]. EGF is bound primarily through interactions with subdomains I and III [7, 8]. Ligand binding requires a change in the relative positions of subdomains I and III from that found in the closed, inactive conformation and results in the release of the intramolecular tether that holds the receptor closed [6]. Two open, activated monomers then form a back to back dimer through interaction of their respective dimerization arms [7, 8].
While the role of subdomains I, II and III in ligand binding and dimer formation in the activated receptor are relatively well-understood, the function of subdomain IV remains unclear. This subdomain is disordered in the crystal structure [8]. Thus, the extent of its participation in ligand binding and receptor dimerization is not known although recent studies suggest that it contributes little to receptor dimerization [9]. Point mutations in the tethering arm in subdomain IV that block its interaction with the dimerization arm in subdomain II, result in the loss of high affinity EGF binding. This implicates the intramolecular tether and thus subdomain IV in ligand binding [10, 11]. However, these subdomain IV mutations do not significantly alter the maximal level of EGF-stimulated receptor autophosphorylation [10, 11] indicating that the tethering arm may not be crucial for signal transduction.
In this study, we investigated the function of the most membrane proximal module in subdomain IV of the EGF receptor, residues 596–612. The proximity of this portion of the receptor to the membrane suggested the possibility that this region might be important in signal transduction. We report here that a double Cys→Ala mutation that breaks the most membrane-proximal disulfide bond (Cys-600 to Cys-612), ablates high affinity ligand binding and markedly impairs signal transduction. Mutations in the sequence of this disulfide-bonded loop, do not substantially affect binding or signaling. These findings suggest that the disulfide-bonded structure of this juxtamembrane region, rather than its primary sequence, is important in the development of high affinity binding sites for EGF as well as in converting the structural change that results from EGF binding into the activation of the intracellular tyrosine kinase domain.
A previous study [12] implicated residues 557 to 616 of the extracellular domain of the EGF receptor in the localization of the receptor to lipid rafts. We find that mutations in the sequence between Cys-596 and Cys-612 do not affect raft localization but deletion of residues 518 to 589 leads to the exclusion of the receptor from rafts. These findings further define the region of the receptor involved in raft localization and suggest that the tethering arm, comprised of residues 561 to 585, may be the important structural feature that mediates the targeting of the EGF receptor to lipid rafts.
Experimental
Materials
The Effectene Transfection Kit and Gel Extraction Kit were from Qiagen. Opti-Prep was from Granier BioOne. The polyclonal anti-EGF receptor antibody was from Cell Signaling. The polyclonal anti-Gq antibody was from Santa Cruz. The monoclonal anti-transferrin receptor antibody was from Zymed (San Francisco, CA). The polyclonal anti-ß-COP antibody was from Sigma and the polyclonal anti-calnexin antibody was from Stressgen. The anti-EEA1 monoclonal antibody was from BD Biosciences. The polyclonal anti-VSV-G protein antibody was the generous gift of Dr. Sondra Schlessinger (Dept. of Microbiology, Washington Univ.) Horseradish peroxidase-conjugated anti-rabbit IgG was from Pierce. Horeseradish peroxidase-conjugated anti-mouse IgG and the Enhanced Chemiluminescence kit were from Amersham. 125I-EGF was prepared by the chloramine T method as described [13]. All other reagents were of analytical grade and were purchased from Sigma-Aldrich.
Mutagenesis
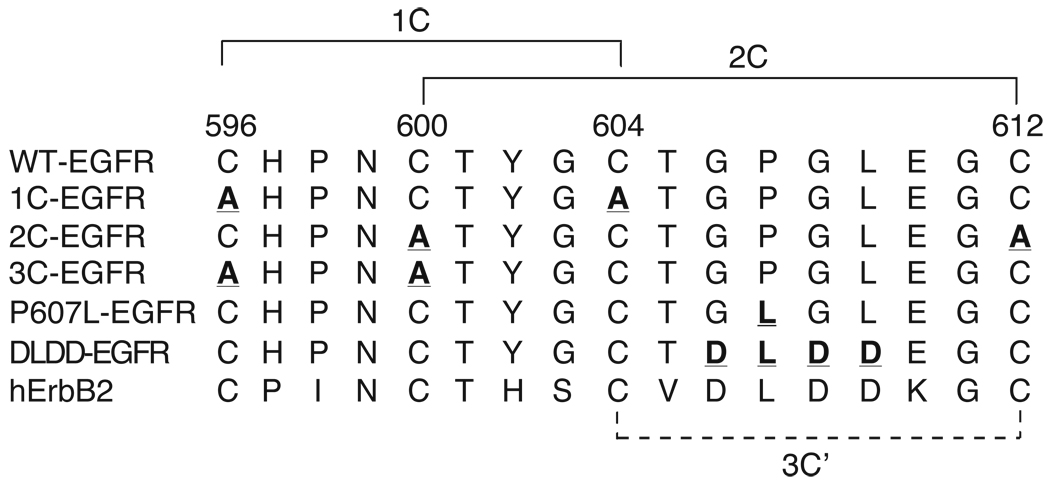
The three EGF receptor mutants containing alterations in the disulfide loops between residues 596 and 612 of the EGF receptor are referred to as: 1C- EGF receptor, containing the C596,604A mutations that break the first disulfide of the module; 2C-EGF receptor, containing the C600,612A mutations that break the second disulfide of the module; and 3C’EGF receptor, containing the C596,600A mutations that breaks both disulfides but would permit the formation of an alternative disulfide bond between C604 and C612. The mutations in the sequence of the second disulfide loop between C600 and C612 are referred to as: P607L-EGF receptor, in which the proline at position 607 has been replaced with a leucine; and, DLDD-EGF, receptor in which residues 606 to 609 of the EGF receptor have been replaced with the sequence DLDD that is present at this position in ErbB2.
The starting plasmid, pEGFR-WT contains the wild type human EGF receptor in the pcDNA3 plasmid. To replace Cys 596 and 604 with Ala in the 1C-EGF receptor mutant, a two-step PCR method with internal primer overlap was used. Two pairs of primers were used. The first pair of primers was: P3-835-Forward (5’ ACCCACTGCTTACTGGCTTATC) which corresponds to pcDNA3 sequence 835–856; and, EGFR- C596,604A-Reverse (5’CAGTGGCTCCGTAGGTGCAGTTTGGA TGGGCCAGGT) which corresponds to EGF receptor sequences 1781–1816 and contains the two alanine mutations (in bold). The second pair of primers was: E-2890-Reverse (5’ GGTCATCAACTCCCAAACGGTCA) which corresponds to the EGF receptor sequence at position 2912–2890 in the pEGFR-WT plasmid; and EGFR-C596,604A-Forward (5’ ACCTGGCCCATCCAAACTGCACCTACGGAGCCACTG) which corresponds to EGF receptor sequence at positions 1781–1816 in pEGFR-WT and contains the alanine mutations (in bold). PCR was carried out with each pair of primers using pEGFR-WT as the template. The PCR products were gel-purified and a second PCR reaction was performed using primers P3-835-Forward and E-2705-Reverse using the two PCR products from the first round as template. The resulting PCR product was purified and cut with Xba I and BstE II. pEGFR-WT was also digested with Xba I and BstEII and the PCR restriction fragment was ligated into the digested vector fragment to produce pEGFR- C596,604A. The final construct was confirmed by sequencing.
The 2C-EGF receptor mutant in which the cysteines at residues 600 and 612 were replaced with alanines was synthesized in a similar manner using EGFR-C600,612A-Reverse (5’GGAGCGGCCTTCAAGACCTGGCCCAGTGCATCCGTAGGTGGCGTTT) and EGFR-C600,612A-Forward (5’ AAACGCCACCTACGGATGCACTGGGCCAGGTCTTGAAGGCGCTCC instead of the EGFR-C596,604A forward and reverse primers. Similarly the 3C’-EGFR mutant in which the cysteines at positions 596 and 600 were replaced with alanines was synthesized using EGFR-C596,600A-Reverse(5’-CCCAGTGCATCCGTAGGTGGCGTTTGGATGGGCCAGGTGG -3’) and EGFR-C596,600A-Forward (5’-CCACCTGGCCCATCCAAACGCCACCTACGGATGCACTGGG-3’) as primers. The P607L-EGF receptor mutant was made using the primers EGFR-P607L-Reverse (5’TTGGACAGCCTTCAAGACCTAGCCCAGTGCATCCGTAGGTG) and EGFR-P607L-Forward (5’CACCTACGGATGCACTGGGCTAGGTCTTGAAGGCTGTCCAA). And the DLDD-EGF receptor mutant was synthesized using the primers EGFR-DLDD-Reverse (5’-TTCATCGTCTAGGTCAGTGCATCCGTAGGTG-3’) and EGFR-DLDD-Forward (5’-ATGCACTGACCTAGACGATGAAGGCTGTCCAAC -3’).
To delete the C-terminal domain of the EGF receptor, a reverse primer was designed (5’CGAAAGCTTTACTTGTCGTCATCATCTTTATAGTCGCGCCTTCGCATGAAGA) that included the EGF receptor sequence from nucleotides 2004-1985, a Flag-tag (underlined), a stop codon (bold) and a HindIII cutting site. PCR was carried out using this reverse primer as well as a forward primer which corresponded to EGF receptor nucleotides 579–605 (5’GTGATCCAAGCTGTCCCAATGGGAGCT). The PCR product was sequenced, digested with PmI I and Hind III and ligated into pEGFR-WT cut with the same restriction enzymes. The final construct was confirmed by sequencing.
For construction of the EGF receptor containing the transmembrane domain of VSV-G, pEGFR-WT was cut with PmI I to release a 1.5 kB fragment containing the EGF receptor transmembrane domain. This fragment was inserted into pBS-SK by blunt end ligation with the PmI I-cut vector to generate pBS-SK-EGFR-TMD. This vector was used as the PCR template in a reaction containing the forward primer, 5’GGGCTGATCGGGCTCTTCCTCGGCAATGCTGCTCGAAGGCGCCACATCGTTCG, where the underlined sequences represent VSV-G transmembrane domain sequence and the remaining sequence corresponds to EGF receptor sequence from nucleotides 2005 to 2024. The reverse primer was 5’GATGATGAAAAAGAAGCTGGCAATGCTGCTGGACGGGATCTTAGGCCCAT where the underlined sequence represents the VSV-G transmembrane domain sequence and the remaining sequence corresponds to EGF receptor sequence from nucleotides 1916–1935. Using this pair of primers, essentially the entire pBS-SK-EGFR-TMD vector was synthesized with the exception that the EGF receptor sequences between nucleotides 1935 and 2024 were replaced by VSV-G transmembrane domain sequence. The PCR product was purified, treated with T4 polynucleotide kinase and closed using T4 DNA ligase to generate pBS-SK-VSVG-TMD-EGFR. This vector was cut with PmI I to release a 1.5 kB fragment that contained the EGF receptor sequences plus the VSV-G transmembrane domain. The purified fragment was ligated with pEGFR-WT cut with PmI I to form pcDNA3-EGFR-VSVG-TMD. The final construct was confirmed by sequencing.
For construction of the EGF receptor mutant lacking the entire extracellular domain, the two-step PCR method with internal primer overlap described above was used. An N-terminal Flag tag was inserted to aid in identification of the protein product. The first pair of primers was used in a PCR reaction to amplify the Flag-tag plus the N-terminal signal sequence of the EGF receptor. The forward primer, PF1, 5’ACCACTGCTTACTGGCTTATC corresponded to nucleotides 835 to 856 in the pcDNA3 vector. The reverse primer, PR2, 5’CTTGTCGTCATCATCTTTATAGTCAGCCCGACTCGCCGGGCAGAGCGCAG corresponded to the Flag-tag sequence (underlined) and EGF receptor nucleotides from 72 to 47 representing the signal sequence. A second pair of primers was used in a separate PCR reaction to amplify the Flag-tag plus EGF receptor sequences C-terminal to the extracellular domain. The forward primer, PF3, 5’GACTATAAAGATGATGACGACAAGATCGCCACTGGGATGGTGGGGGCCCT corresponded to the Flag-tag sequence (underlined) plus EGF receptor sequence from nucleotides 1937–1962. The reverse primer, PR4, 5’GGTCATCAACTCCCAAACGGTCA, corresponded to EGF receptor sequences from nucleotides 2727 to 2705. The PCR products from each of these two reactions were purified and combined in a second round of PCR that utilized forward primer PF1 and reverse primer PR4. The resulting PCR product was purified and digested with Xba I and BstE II. The restriction fragment was purified and ligated into pEGFR-WT digested with the same restriction enzymes. The final construct was confirmed by sequencing.
Cells, cell culture and transfections
CHO cells were maintained in Ham’s F12 medium containing 10% fetal calf serum and were passaged or fed every two days. CHO cells expressing the receptor mutant referred to as EGFR-ΔEN [14] were obtained from Dr. David Lee (Univ. of North Carolina, Chapel Hill, NC).
For transfections, 4×105 CHO cells were seeded into 60 mm diameter dishes and cultured for 24 hr in standard medium. Cells were then incubated at 37° C with Effectene-DNA complexes formed according to the manufacturer’s instructions. After 24 hr, cells were trypsinized and replated in medium containing 400 µg/ml G418. Single colonies were selected after 2–3 weeks growth in G418-containing medium. Protein expression was checked by SDS-polyacrylamide gel electrophoresis and Western blotting.
125I-EGF Binding
All assays were done in triplicate. Cells were grown to confluence in 35 mm diameter plates. For the binding assay, cells were chilled on ice and transferred into Hams’ F12 medium containing 25 mM Hepes, 1 mg/ml bovine serum albumin, 25 pM 125I-EGF and increasing concentrations of unlabelled EGF. Non-specific binding was determined in replicate wells containing 50 nM unlabeled EGF. Cells were incubated for 2 hr at 4° C and subsequently washed three times in ice cold Hanks balanced salt solution. Monolayers were dissolved in 1 ml 1N NaOH and counted for 125I. Data were analyzed using GraphPad Prism 4.0.
EGF receptor phosphorylation and MAP kinase activation
CHO cells were grown to confluence in 35 mm diameter plates and transferred into Hams F12 medium containing 0.1% serum for 16 hr. For assay of receptor tyrosine phosphorylation, cells were stimulated with the indicated dose of EGF for 1 min. For assay of MAP kinase activation, cells were stimulated with EGF for 5 min. The medium was removed and the cultures washed with cold phosphate-buffered saline. Monolayers were scraped into RIPA buffer (10 mM Tris, pH 7.2, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholate and 5 mM EDTA) containing 20 mM p-nitrophenylphosphate and 100 µM sodium orthovanadate plus protease inhibitors. Equal amounts of total protein were analyzed by electrophoresis on a 10% SDS polyacrylamide gel and analyzed for phosphotyrosine and EGF receptor or phospho-MAP kinase and MAP kinase by Western blotting.
Isolation of lipid rafts
Lipid rafts were prepared according to the non-detergent method of Macdonald and Pike [15]. Briefly, the cell pellet from four 150 mm diameter plates was resuspended in lysis buffer (20 mM Tris, pH 7.8, 0.25 M sucrose, 1 mM MgCl2 and 1 mM CaCl2 plus protease inhibitors) and lysed by passage through a 22 gauge needle 20 times. A post-nuclear supernatant was collected after centrifugation of the lysate for 10 min at 1000g. The post-nuclear supernatant was mixed with an equal volume of 50% OptiPrep in lysis buffer and placed at the bottom of a 12 ml centrifuge tube. An 8 ml gradient from 0 to 20% Opti-Prep in lysis buffer without divalent cations was layered on top of the sample. After centrifuging at 52000g for 90 min, the gradient was manually fractionated into 18 equal fractions, beginning at the top of the gradient. Equal volumes (typically 100 µl) of each gradient fraction were analyzed by SDS polyacrylamide gel electrophoresis and Western blotting.
Western blotting and quantitation of proteins in lipid rafts
The proteins in SDS polyacrylamide gels were electrophoretically transferred to PVDF membranes and the membranes blocked with 10% powdered milk. After incubation with the appropriate primary antibody, proteins were detected using a horseradish peroxidase-linked secondary antibody and chemiluminescence reagents.
The amount of EGF receptor present in each OptiPrep gradient fraction was quantitated by densitometry using Image J software. For purposes of quantitation, the top four fractions of the OptiPrep gradient were defined as the raft fractions. To determine the proportion of EGF receptor present in lipid rafts, the amount of EGF receptor in the top four fractions of the OptiPrep gradient was divided by the total amount of the EGF receptor in all 18 gradient fractions. A similar procedure was used to calculate the fraction of Gq, a control raft marker, present in the lipid raft fraction. To control for experimental variations among gradients run on different days, the fraction of the EGF receptor recovered in rafts was normalized to the fraction of Gq recovered in rafts in that same gradient. Since the ratio of the fraction of wild type EGF receptor in rafts and the fraction of Gq present in rafts ratio is close to 1, any change in the propensity of a mutant EGF receptor to localize to rafts is indicated by a value of this ratio that is substantially less than 1.
Results
EGF binding to receptor mutants
Subdomain IV of the extracellular domain of the EGF receptor is comprised of three repeated disulfide-bonded modules, with the basic element consisting of eight cysteines paired in a 1–3, 2–4, 5–6, and 7–8 pattern [5]. The third module is truncated compared to the first two and contains only 4 cysteines. Figure 1 shows the sequence and bonding pattern of these four cysteines. This sequence contains two overlapping disulfide bonds linking Cys596 with Cys604 and Cys600 with Cys612. The disulfide bonding pattern has been determined by sequencing using mass spectrometry [5] and is confirmed in the crystal structure of the extracellular domain of the EGF receptor [6, 8]. To investigate the role of these most membrane proximal disulfides in ligand binding and signal transduction, both cysteines in a given disulfide were mutated to alanine to preclude formation of a disulfide bond at that position. The 1C-EGF receptor lacks the first disulfide bond whereas the 2C-EGFR lacks the second disulfide bond.
Figure 1. Sequence and disulfide bonds in the third disulfide module of subdomain IV of the human EGF receptor.
The sequence of the wild type EGF receptor is given in the first line. Confirmed disulfide bonds are indicated by solid lines. Lines 2 through 6 give the sequence of the mutations analyzed in the study with the mutated residues in bold and underlined. The dotted line indicates the position of a disulfide bond that could form in the 3C’-EGF receptor. The last line shows the homologous sequence from human ErbB2.
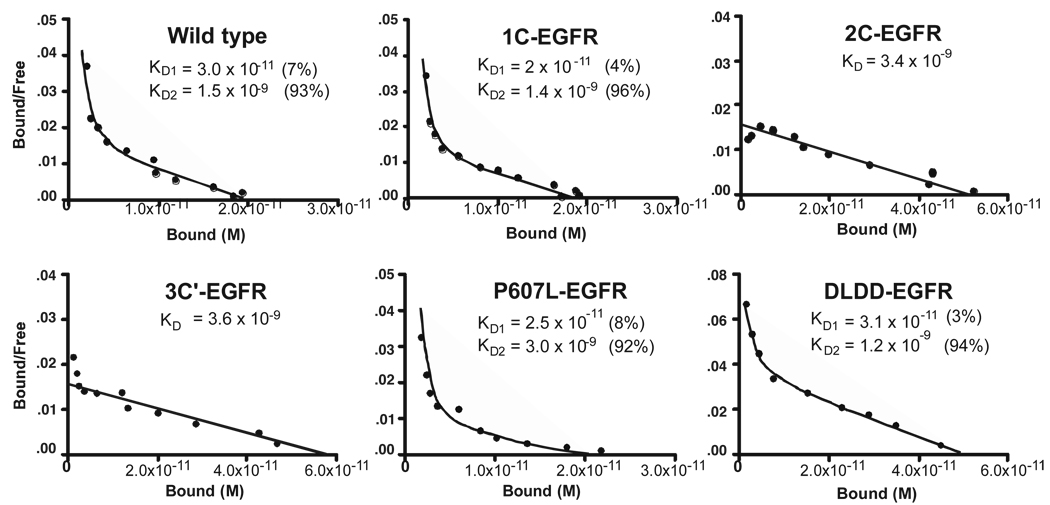
These EGF receptor constructs were stably expressed in CHO cells that lack endogenous EGF receptors. Scatchard analyses of the binding of 125I-EGF to CHO cells expressing wild type, 1C- or 2C-EGF receptors are shown in Figure 2. The wild type EGF receptor exhibited an upwardly concave Scatchard plot, indicative of 2 classes of sites. The high affinity site had a Kd of approximately 30 pM and accounted for ~7% of the total number of EGF binding sites. The low affinity site exhibited a Kd of ~1.5 nM. Removal of the disulfide bond between Cys596 and Cys604 in the 1C-EGF receptor resulted in relatively little change in the binding of EGF to the receptor. The high and low affinity Kd’s were similar to those of wild type receptor and approximately 4% of the binding sites were of high affinity. By contrast, the binding of EGF to the 2C-EGF receptor was markedly altered. These receptors exhibited only low affinity EGF binding with a Kd similar to that of the low affinity site of the wild type EGF receptor. These data suggest that the membrane proximal disulfide bond between Cys600 and Cys612 plays a significant role in the generation of high affinity EGF binding sites.
Figure 2. Scatchard analysis of the binding of 125I-EGF to wild type and mutant EGF receptors.
Cells were grown to confluence in 35 mm dishes and analyzed for 125I-EGF binding as outlined in Experimental Procedures. All points were done in triplicate and experiments were repeated a minimum of two times. Data were analyzed using GraphPad Prism 4.0. High and low affinity Kd’s as well as the percent each site represents in the total receptor population is given in the insets.
To determine whether any disulfide-bonded structure in this region could restore high affinity binding to the EGF receptor, an additional mutant was generated in which cysteines 596 and 600 were converted to alanines. This breaks both the first and the second disulfide bonds. However, the crystal structure of this region indicates that the remaining Cys604 and Cys612 are close enough to form an alternative disulfide bond (see dotted line in Figure 1). This mutant was named 3C’-EGF receptor and was stably expressed in CHO cells. As shown in Figure 2, the binding of 125I-EGF to the 3C’-EGF receptor resulted in a linear Scatchard plot indicative of a single class of sites that exhibited a low affinity Kd of ~4 nM. Thus, like the 2C-EGF receptor, this mutation does not support high affinity binding.
To determine whether it is the disulfide bond itself that is important in high affinity EGF binding or whether sequences within the disulfide-bonded loop also contribute to the establishment of high affinity binding, two additional EGF receptor mutants were constructed. The proline at position 607 is conserved in EGF receptors from Drosophila to humans. Since this proline might be involved in the adoption of a specific conformation in this loop, this residue was mutated to leucine (P607L-EGF receptor). This substitution does not change the hydrophobic nature of this residue but prevents the introduction of a kink into the loop by the proline. As shown in Figure 2, this mutation did not significantly change the binding properties of the EGF receptor. The affinity of both the low and high affinity sites was similar to that of wild type EGF receptor and the fraction of high affinity sites was also unchanged.
The sequence GPGL from residues 606 to 609 is evolutionarily conserved among EGF receptors and the GP dyad is also present in hErbB3 and hErbB4. However, in hErbB2, this sequence is replaced by the sequence DLDD (Figure 1). To determine whether this sequence within the membrane-proximal disulfide loop was important for high affinity EGF binding, the DLDD sequence of hErbB2 was substituted into the EGF receptor at position 606 to 609 to generate the DLDD-EGF receptor mutant. As can be seen in Figure 2, this substitution resulted in a receptor that showed two classes of sites with essentially wild type affinity for each. The fraction of high affinity sites, though somewhat low, was not significantly different from that seen in wild type receptors. These data suggest that it is mainly the disulfide bond between cysteines 600 and 612, rather than specific sequences within the disulfide loop, that is the important contributor to the generation of high affinity binding sites for EGF.
Signaling of EGF receptor mutants
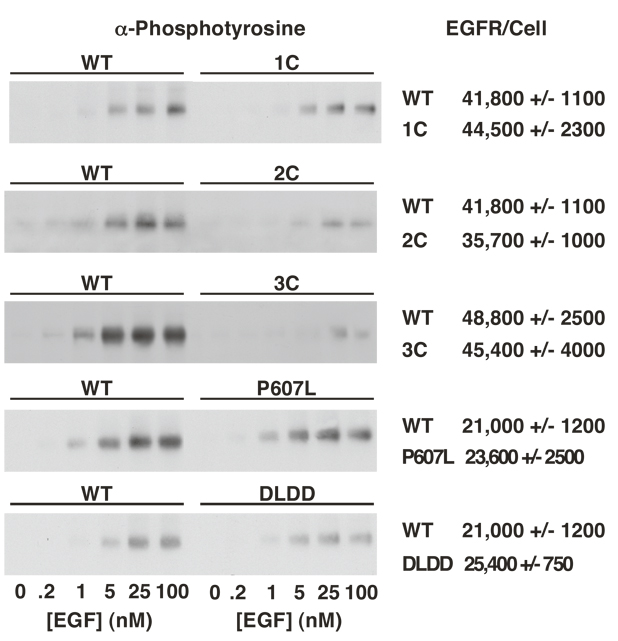
The ability of these EGF receptor mutants to mediate EGF-stimulated receptor tyrosine phosphorylation was next examined. For these experiments, each mutant was paired with a CHO cell line expressing a similar level of wild type EGF receptors as determined by Scatchard analysis of 125I-EGF binding. Figure 3 compares the ability of increasing doses of EGF to stimulate receptor autophosphorylation in each of these mutants. The number of cell surface EGF receptors in each line is given to the right. These differences are quantitated in Figure 4 in which the activity of the mutant is calculated as the percent of the activity of a matched wild type control line that contains a similar number of cell surface EGF receptors.
Figure 3. EGF-stimulated receptor autophosphorylation in wild type and mutant EGF receptors.
Cells expressing the EGF receptor mutants were stimulated with the indicated concentration of EGF for 1 min at 37° C and the monolayers solubilized and analyzed as outlined in Experimental Procedures. Receptor tyrosine phosphorylation (left panels) in each mutant was compared directly with a clone of CHO cells expressing a similar number of cell surface EGF receptors as determined by Scatchard analysis of 125I-EGF binding (right).
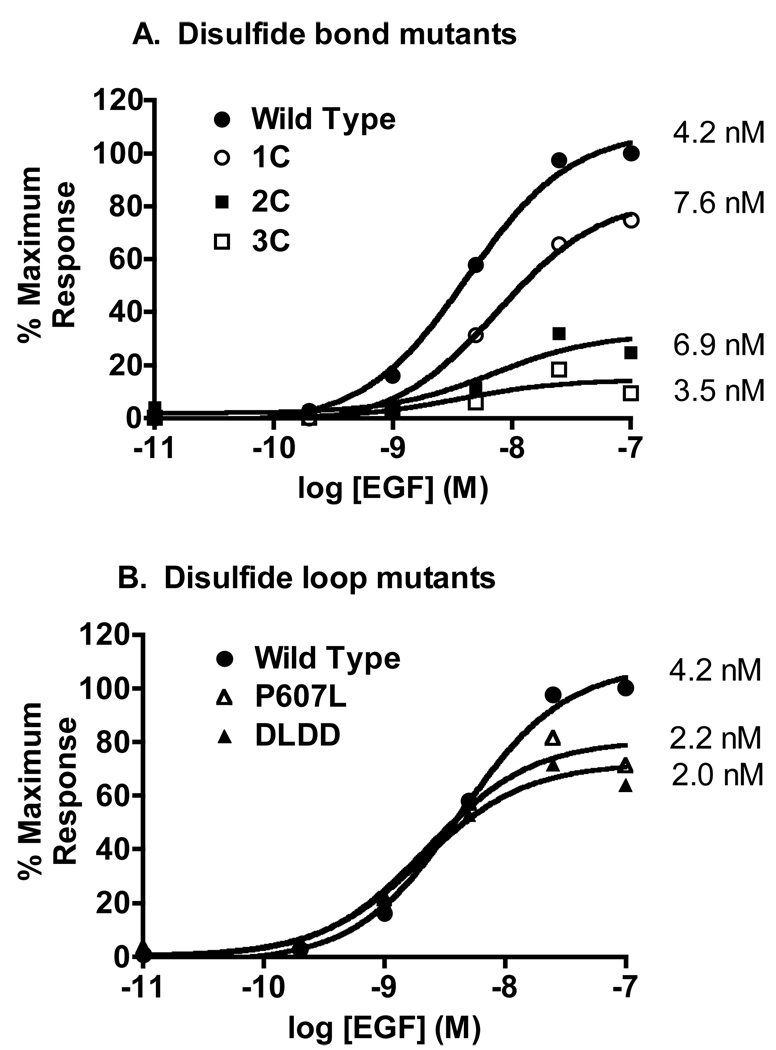
Figure 4. Dose response to EGF for the stimulation of receptor autophosphorylation.
The Western blots shown in Figure 3 were quantitated by densitometry. Phosphorylation of the mutant EGF receptors was scaled according to the level of phosphorylation observed in a line of CHO cells expressing a similar number of EGF receptors. A) EGF dose response curves for receptors containing disulfide bond mutations. B) EGF dose response curves for receptors containing mutations in the Cys600-Cys612 disulfide loop sequence.
The 1C-EGF receptor that showed essentially normal EGF binding, also exhibited a nearly normal ability to stimulate receptor autophosphorylation. By contrast, the 2C-EGF receptor that showed only a single class of low affinity sites in binding experiments, was relatively weak in its ability to mediate EGF-stimulated receptor autophosphorylation. Likewise, the 3C’-EGF that exhibited only low affinity binding, showed only limited receptor autophosphorylation compared to control receptors. Despite these differences in the maximal level of EGF receptor autophosphorylation, the EC50’s for EGF in each of these mutants differed by less than two-fold from that of the wild type receptor. Mutation of the amino acid sequences in the second disulfide loop did not significantly affect EGF-stimulated receptor autophosphorylation (Figure 3 and Figure 4B). Consistent with their relatively normal EGF binding behavior, the P607L-EGF receptor and the DLDD-EGF receptor showed essentially wild type activity in the receptor autophosphorylation assay.
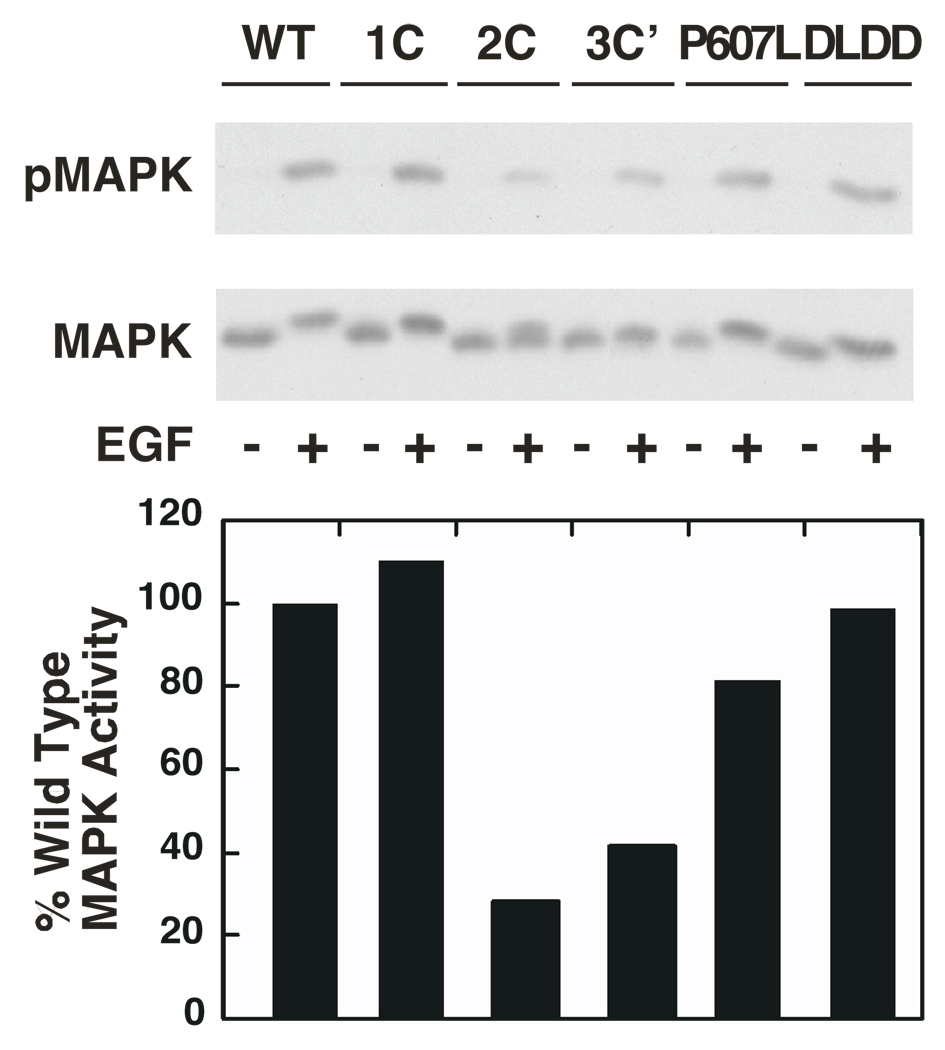
The ability of the mutant EGF receptors to stimulate downstream signaling was next assessed by measuring EGF-stimulated MAP kinase activation. The data in Figure 5 demonstrate that, as was the case for agonist-induced receptor autophosphorylation, the 1C-EGF receptor showed wild type levels of EGF-stimulated MAP kinase activity. Consistent with their decreased maximal level of receptor autophosphorylation, both the 2C-EGF receptor and the 3C’-EGF receptor showed a significantly reduced ability to mediate MAP kinase activation. The P607L- and DLDD-EGF receptor mutants showed normal levels of ligand-stimulated MAP kinase activation.
Figure 5. EGF-stimulated activation of MAP kinase by wild type and mutant EGF receptors.
Cultures were stimulated with 25 nM EGF for 5 min at 37° C and the monolayers solubilized and analyzed for MAP kinase activation as described in Experimental Procedures. Upper panel) Western blot for activated phospho-MAP kinase. Middle panel) Western blot for MAP kinase as a loading control. Lower panel) The phospho-MAP kinase Western blots were quantitated by densitometry. The level of MAP kinase activation mediated by the wild type EGF receptor was set to 100% and activation of MAP kinase by the receptor mutants is shown relative to this wild type control.
Raft localization of EGF receptor mutants
The EGF receptor has been shown to partition into lipid rafts [16–19] and previous studies have suggested that a raft targeting signal for the EGF receptor lies within the most membrane-proximal 60 amino acids of the extracellular domain [12]. As our disulfide loop mutations reside within this region, we tested the ability of these mutant receptors, along with several others, to localize to lipid rafts.
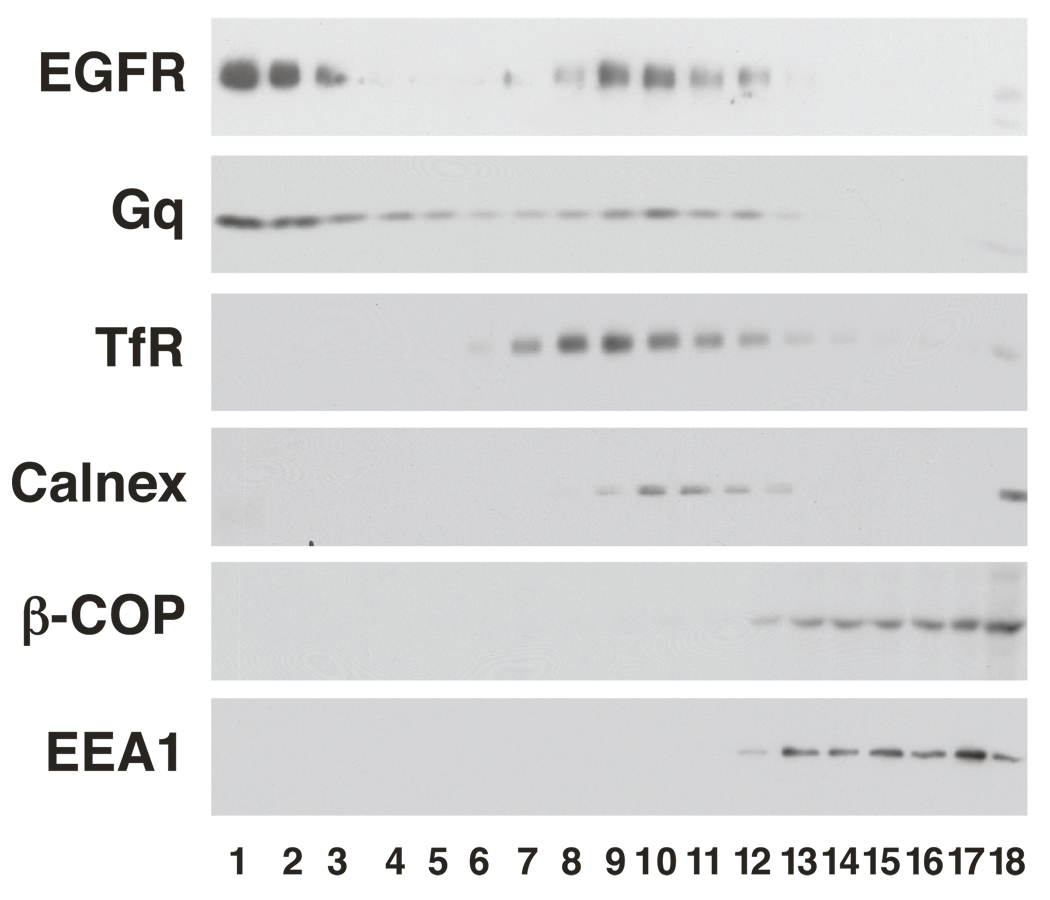
Lipid rafts were prepared from cells expressing wild type and mutant EGF receptors using a detergent-free method [15]. As shown in Figure 6, wild type EGF receptors were found distributed throughout the OptiPrep gradient but were largely localized in the low density, lipid raft fractions at the top of the gradient. Similarly, the raft marker protein, Gq was found primarily in the low density region of the gradient. In the experiment shown, 59% of the EGF receptor and 61% of Gq were recovered in the lipid raft fraction. This ratio of EGFR/Gq partitioning into lipid rafts was consistently ~1, enabling us to use Gq as an internal reference against which to measure changes in the distribution of the EGF receptor (see below). In contrast to the EGF receptor and Gq, the transferrin receptor, a plasma membrane protein that does not localize to rafts, was found exclusively in the mid-portion of the gradient, well-separated from the raft proteins. Calnexin, a marker for the endoplasmic reticulum, was also recovered in the middle fractions of the gradient. ß-COP, a Golgi marker and EEA1, a marker for early endosomes, were found in the bottom third of the gradient.
Figure 6. Localization of the wild type EGF receptor to lipid rafts.
Detergent-free lipid rafts were prepared from CHO cells expressing the wild type EGF receptor as described [15]. Equal aliquots from each gradient fraction were analyzed by SDS polyacrylamide gel electrophoresis and Western blotting with the indicated antibodies. Lipid raft fractions are indicated by the presence of the EGF receptor and Gq. TfR, transferrin receptor. Calnex, calnexin.
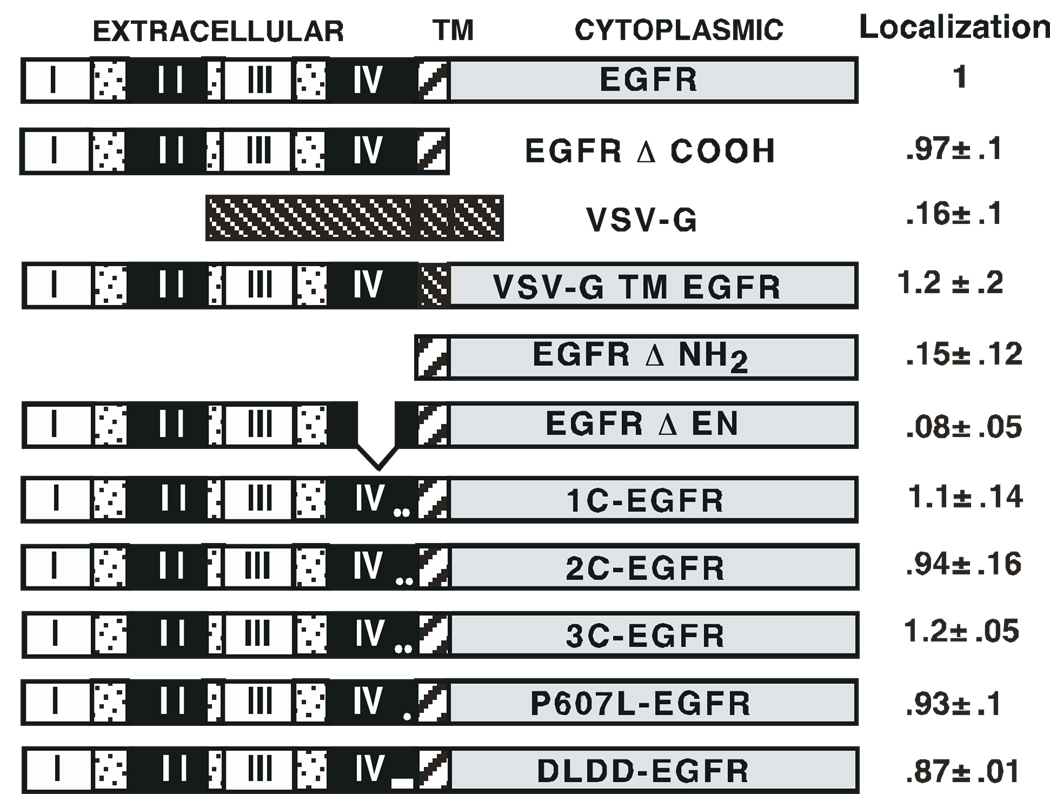
The structures and raft partitioning of the mutant EGF receptors are shown in Figure 7. For purposes of calculating the fraction of EGF receptors localized to lipid rafts, the top four fractions of the gradient were defined as rafts and receptor localization was calculated as the amount of EGF receptor present in these fractions divided by the total amount of EGF receptor recovered throughout the gradient. The ratio of wild type EGFR/Gq partitioning into rafts in a given experiment was set to 1 and the localization of the mutant receptors is reported relative to that of the intra-experiment wild type EGF receptor control.
Figure 7. Localization of mutant EGF receptors to lipid rafts.
Detergent-free lipid rafts were prepared from CHO cells expressing the various EGF receptor mutants as described[15]. An equal volume (100 µl) of each gradient fraction was analyzed by SDS polyacrylamide gel electrophoresis followed by Western blotting for the EGF receptor, Gq and transferrin receptor (as a non-raft control). EGF receptors containing the cytoplasmic domain were identified using a polyclonal anti-EGF receptor antibody. Mutants lacking the C-terminus were Flag-tagged on their amino terminus and were identified using anti-Flag tag antibodies. Raft localization was quantitated as described in Experimental Procedures. The localization of the mutants reported in the figure was calculated by dividing the EGFR/Gq ratio for the mutant by the EGFR/Gq ratio for wild type EGF receptor internal control in a that experiment. Localization was determined a minimum of twice for each receptor variant.
We first tested the effect of deletion or replacement of the three major domains of the EGF receptor—intracellular, transmembrane and extracellular--on its localization to rafts. The localization of similar mutants has been reported previously [12] so these represent controls to establish the ability of the system to detect differences in raft localization. Deletion of the entire cytoplasmic domain of the EGF receptor yielded a mutant (EGFR-ΔCOOH) that continued to be effectively enriched in lipid rafts, indicating that the cytoplasmic domain is not required for localization of the receptor in lipid rafts. The VSV-G protein is an integral membrane protein that has been shown not to partition into lipid rafts [20]. In cells expressing the wild type VSV-G protein, this protein was not enriched in lipid rafts relative to plasma membranes. The transmembrane domain of the EGF receptor was replaced with that of the VSV-G protein yielding the mutant, VSV-G TM-EGFR. This mutant continued to localize to lipid rafts demonstrating that the transmembrane domain of the EGF receptor does not target the EGF receptor to lipid rafts. By contrast, when the entire extracellular domain of the EGF receptor was deleted, the resulting mutant EGF receptor failed to localize to the raft fraction. These data indicate that the extracellular domain contains information necessary to target the EGF receptor to lipid rafts.
The EGF receptor mutant EGFR-ΔEN contains a deletion from residues 518 to 589 within the second cysteine-rich region [14]. This receptor has been shown to be well-expressed on the cell surface but to have impaired EGF binding [14]. As shown in Figure 7, deletion of this region resulted in a receptor that failed to localize to lipid rafts, consistent with the involvement of subdomain IV in raft targeting of the receptor. Removal of the disulfide bonds in the juxtamembrane portion of the second cysteine-rich region (1C-, 2C and 3C’-EGF receptors) did not alter the ability of these receptors to localize to rafts. Similarly, mutation of the sequences in the Cys600-Cys612 loop (P607L- and DLDD-EGF receptors) did not affect raft localization. Thus, the most membrane-proximal portion of subdomain IV of the EGF receptor does not appear to be involved in its localization to lipid rafts.
Discussion
In this study, we examined the role of the cysteine residues in the most membrane proximal, disulfide module of the extracellular domain of the EGF receptor in EGF binding and signal transduction. In this module, Cys-596 is paired with Cys-604 and Cys-600 is paired with Cys-612. These disulfides represent the last two disulfides in the extracellular domain of the EGF receptor, prior to the entry of the polypeptide chain into the membrane at ~residue 621.
Our data show that breaking the disulfide bond between Cys-596 and Cys-604 (1C-EGF receptor) does not alter either high affinity EGF binding or signal transduction. Thus, this disulfide does not appear to be critical to receptor function. By contrast, breaking the most membrane-proximal disulfide bond between Cys-600 and Cys-612 (2C-EGF receptor) ablated high affinity EGF binding. This phenotype is similar to that exhibited by receptors that contain mutations in other portions of subdomain IV. Mutation of residues in the tethering arm of the EGF receptor (amino acids ~561–585) results in receptors that exhibit only low affinity binding sites for EGF [10, 11], indicating that this more membrane distal region of the receptor is also important for high affinity binding. Together with our findings, these data suggest that a large portion of subdomain IV is involved in the establishment of high affinity EGF binding. Thus, while this subdomain is not directly involved in ligand binding, it clearly contributes to the ability of the EGF receptor to produce a high affinity binding site for its ligand. Whether this is due to the interaction of subdomain IV with other structures within the receptor itself or with distinct macromolecules [21] remains to be determined.
Although the 2C- and 3C-EGF receptors exhibited a loss of high affinity EGF binding similar to that observed in receptors harboring mutations in the tethering arm of subdomain IV, the phenotype of the 2C- and 3C-EGF receptor mutants was distinct from that of the other subdomain IV mutants. Specifically, the 2C- and 3C-EGF receptors showed a significantly impaired ability to transduce the signal of EGF binding to the intracellular tyrosine kinase domain. Receptors with mutations in the tethering arm of subdomain IV showed wild type levels of receptor autophosphorylation at saturating concentrations of EGF [10, 11]. However, even at very high doses of EGF, autophosphorylation of the 2C- and 3C-EGF receptors was minimal and their ability to stimulate MAP kinase activation was markedly reduced. Thus, breaking the disulfide bond between Cys-600 and Cys-612 appears to uncouple EGF binding from signal transduction. This suggests that, in addition to its role in supporting high affinity binding, the membrane-proximal region of the EGF receptor is specifically involved signal transduction.
Mutation of the sequence within the Cys-600/Cys-612 disulfide loop had little to no effect on EGF receptor binding or signaling. These findings imply that it is the stabilized loop structure maintained by this disulfide bond, rather than the primary sequence within this loop, that is crucial for normal ligand binding and signal transduction.
Together, our data suggest that the bond between Cys- 600 and Cys-612 is a key structural element in the generation of high affinity binding sites for EGF and in signal transduction. The fact that these cysteines are part of a subdomain that contains a total of 10 disulfide bonds within 130 residues suggests that a rigid structure may be important to the function of this domain. Subdomain IV has not been shown to be directly involved in receptor dimerization. However, models based on the structure of this domain in the inactive monomer, suggest that in the active dimer, residues in subdomain IV are likely to make contacts across the dimer interface [6]. Interestingly, peptidomimetics based on this juxtamembrane region in the homologous ErbB2 protein have been shown to inhibit heterodimerization of EGF receptor family members [22]. Thus, the rigid, disulfide-bonded structure of this region, stabilized in part by the Cys-600/Cys-612 bond, may be required for receptor-receptor interactions that are induced by EGF binding and that are necessary to transduce the signal across the membrane.
Like many other signaling proteins [17, 23–28], the EGF receptor has been shown to partition into lipid rafts [16–19, 29, 30]. Previous studies have suggested that residues 557 to 616 (in our numbering system which does not count the signal peptide) contain information that is sufficient to target the EGF receptor to lipid rafts [12]. Because our disulfide mutations were within this region, we examined the effect of these mutations on the localization of the EGF receptor. Consistent with the findings of Yamabhai and Anderson [12], we found that deletion of the intracellular domain or replacement of the transmembrane domain of the EGF receptor did not affect raft localization of the receptor while deletion of the extracellular domain resulted in a receptor that no longer partitioned into lipid rafts. These findings implicate the extracellular domain in targeting of the EGF receptor to lipid rafts. The ΔEN-EGF receptor mutant contains a deletion from residue 518 to 589 which encompasses a small portion of the first and all of the second disulfide module in subdomain IV. Though well-expressed on the surface of cells, this receptor neither binds EGF nor transduces a signal [14]. This mutant was excluded from rafts, implying that subdomain IV sequences play a role in the localization of the receptor to lipid rafts. None of the cysteine mutations or mutations of the amino acids within the membrane proximal disulfide loops altered the ability of the EGF receptor to localize to rafts. This suggests that residues 596 to 612 are unlikely to be involved in targeting of the EGF receptor to rafts. Together with published data, these findings implicate residues 557–595 in raft localization.
The loop responsible for tethering the EGF receptor in the closed configuration lies between residues 561 and 585 [6]. Since EGF binding untethers the receptor and causes a change in the conformation of this region, it is possible that the accessibility of the targeting sequence is sensitive to the addition of EGF. However, using detergent-free raft preparations, we do not see a significant alteration in the localization of the EGF receptor to lipid rafts following EGF treatment (unpublished observations). This is consistent with the findings of Puri et al. [31] and Ringerike et al. [29] who used electron microscopy to show that binding of EGF to its receptor did not alter the localization of the receptor to rafts. These data suggest that the targeting signal sequence is accessible both in the absence and presence of EGF and thus may not be part of the tethering loop.
In summary, the most membrane proximal disulfide of the extracellular domain of the EGF receptor appears to be involved in both high affinity EGF binding and signal transduction. This suggests that subdomain IV is thermodynamically coupled to the ligand-binding domains (subdomains I and III) and that it may act as a sensor of structural changes in those domains and a mediator of signal transduction. Sequences in this subdomain also appear to be important in localizing the EGF receptor to lipid rafts suggesting that this region plays multiple functional roles in EGF receptor-mediated signaling.
Acknowledgments
This work was supported by NIH grant GM64491 to LJP
Abbreviations used are
- CHO cells
Chinese hamster ovary cells
- EGF
epidermal growth factor
- PCR
polymerase chain reaction
References
- 1.Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, Downward J, Mayes ELV, Whittle N, Waterfield ND, Seeburg PH. Human Epidermal Growth Factor Receptor cDNA Sequence and Aberrant Expression of the Amplified Gene in A431 Epidermoid Carcinoma Cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y, Schlessinger J. Self-Phosphorylation of Epidermal Growth Factor Receptor: Evidence for a Model of Intermolecular Allosteric Activation. Biochem. 1987;26:1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y, Schlessinger J. Epidermal Growth Factor Induces Rapid, Reversible Aggregation of the Purified Epidermal Growth Factor Receptor. Biochem. 1987;26:1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj M, Waterfield MD, Schlessinger J, Taylor WR, Blundell T. On the Tertiary Structure of the Extracellular Domains of the Epidermal Growth Factor and Insulin Receptors. Biochim. Biophys. Acta. 1987;916:220–226. doi: 10.1016/0167-4838(87)90112-9. [DOI] [PubMed] [Google Scholar]
- 5.Abe Y, Odaka M, Inagaki F, Lax I, Schlessinger J, Kohda D. Disulfide Bond Structure of Human Epidermal Growth Factor Receptor. J. Biol. Chem. 1998;273:11150–11157. doi: 10.1074/jbc.273.18.11150. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson KM, Berger MB, Mendrola JM, Cho H-S, Leahy DJ, Lemmon MA. EGF Activates Its Receptor by Removing Interactions that Autoinhibit Ectodomain Dimerization. Mol. Cell. 2003;11:507–517. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 7.Garrett TPJ, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu H-J, Walker F, Frenkel MJ, Hoyne PA, Jorissen RN, Nice EC, Burgess AW, Ward CW. Crystal Structure of a Truncated Epidermal Growth Factor Receptor Extracellular Domain Bound to Transforming Growth Factor α. Cell. 2002;110:763–773. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 8.Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim J-H, Saito K, Sakamoto A, Inoue M, Shirouzu M, Yokoyama S. Crystal Structure of the Complex of Human Epidermal Growth Factor and Receptor Extracellular Domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 9.Dawson JP, Berger MB, Lin C-C, Schlessinger J, Lemmon MA, Ferguson KM. Epidermal Growth Factor Receptor Dimerization and Activation Require Ligand-Induced Conformational Changes in the Dimer Interface. Mol. Cell. Biol. 2005;25:7734–7742. doi: 10.1128/MCB.25.17.7734-7742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattoon D, Klein P, Lemmon MA, Lax I, Schlessinger J. The Tethered Configuration of the EGF Receptor Extracellular Domain Exerts Only a Limited Control of Receptor Function. Proc. Natl. Acad. Sci. U.S.A. 2004;101:923–928. doi: 10.1073/pnas.0307286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker F, Orchard SG, Jorissen RN, Hall NE, Zhang H-H, Hoyne PA, Adams TE, Johns TG, Ward CW, Nice EC, Burgess AW. CR1/CR2 Interactions Modulate the Functions of the Cell Surface Epidermal Growth Factor Receptor. J. Biol. Chem. 2004;279:22387–22398. doi: 10.1074/jbc.M401244200. [DOI] [PubMed] [Google Scholar]
- 12.Yamabhai M, Anderson RGW. Second Cysteine-Rich Region of EGFR Contains Targeting information for Caveolae/Rafts. J. Biol. Chem. 2002;277:24843–24846. doi: 10.1074/jbc.C200277200. [DOI] [PubMed] [Google Scholar]
- 13.McFarthing K. In: Receptor-Ligand Interactions: A Practical Approach. Hulme EC, editor. New York: IRL Press; 1992. pp. 1–18. [Google Scholar]
- 14.Saxon ML, Lee DC. Mutagenesis Reveals a Role for Epidermal Growth Factor Receptor Extracellular Subdomain IV in Ligand Binding. J. Biol. Chem. 1999;274:28356–28362. doi: 10.1074/jbc.274.40.28356. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald JL, Pike LJ. A Simplified Method for the Preparation of Detergent-free Lipid Rafts. J. Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Smart EJ, Ying Y-S, Mineo C, Anderson RGW. A Detergent-Free Method for Purifying Caveolae Membrane from Tissue Culture Cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mineo C, James GL, Smart EJ, Anderson RGW. Localization of Epidermal Growth Factor-stimulated Ras/Raf-1 Interaction to Caveolae Membrane. J. Biol. Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- 18.Pike LJ, Miller JM. Cholesterol Depletion De-localizes PIP2 and Inhibits Hormone-Stimulated Phosphatidylinositol Turnover. J. Biol. Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 19.Waugh MG, Lawson D, Hsuan JJ. Epidermal Growth Factor Receptor Activation is Localized within Low-Buoyant Density, Non-Caveolar Membrane Domains. Biochem. J. 1999;337:591–597. [PMC free article] [PubMed] [Google Scholar]
- 20.Scheiffele P, Roth MG, Simons K. Interaction of Influenza Virus Haemagglutinin with Sphingolipid-Cholesterol Membrane Domains via its Transmembrane Domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein P, Mattoon D, Lemmon MA, Schlessinger J. A Structure-Based Model for Ligand Binding and Dimerization of EGF Receptors. Proc. Natl. Acad. Sci. U.S.A. 2004;101:929–934. doi: 10.1073/pnas.0307285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berezov A, Chen J, Liu Q, Zhang H-T, Greene MI. Disabling Receptor Ensembles with Rationally Designed Interface Peptidomimetics. J. Biol. Chem. 2002;277:28330–28339. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- 23.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, Liquid-Ordered Domains, and Signal Transduction. Mol. Cell. Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal Transducing Molecules and Glycosyl-Phosphatidylinositol-Linked Proteins Form a Caveolin-rich Insoluble Complex in MDCK Cells. J. Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu Y-H, Cook RF, Sargiacomo M. Characterization of Caveolin-rich Membrane Domains Isolated from an Endothelial-rich Source: Implications for Human Disease. J. Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun M, Liyanage UK, Lisanti MP, Lodish HF. Signal Transduction of a G Protein-Coupled Receptor in Caveolae: Colocalization of Endothelin and its Receptor with Caveolin. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and Direct Interaction of Ras with Caveolin, an Integral Membrane Protein of Caveolae Microdomains. J. Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 28.Liu P, Ying Y, Ko Y-G, Anderson RGW. Localization of Platelet-derived Growth Factor-Stimulated Phosphorylation Cascade to Caveolae. J. Biol. Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- 29.Ringerike T, Glystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is Important in Control of EGF Receptor Kinase Activity but EGF Receptors are Not Concentrated in Caveolae. J. Cell Sci. 2002;115:1331–1340. doi: 10.1242/jcs.115.6.1331. [DOI] [PubMed] [Google Scholar]
- 30.Roepstorff K, Thomsen P, Sandvig K, van Deurs B. Sequestration of EGF Receptors in Non-caveolar Lipid Rafts Inhibits Ligand Binding. J. Biol. Chem. 2002;277:18954–18960. doi: 10.1074/jbc.M201422200. [DOI] [PubMed] [Google Scholar]
- 31.Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F, Luzzi P, Di Fiore PP, Tacchetti C. Relationships Between EGFR-Signaling-competent and Endocytosis-competent Membrane Microdomains. Mol. Biol. Cell. 16;2005:2704–2718. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]