Abstract
Background
Control of hypertension is paramount in treating chronic kidney disease. The relationship between kidney function and blood pressure (BP) components has been studied in persons with diagnosed CKD, diabetes, or hypertension. Whether kidney function in the normal range is associated with systolic BP (SBP), diastolic BP (DBP), and pulse pressure is unclear.
Methods
We evaluated the association between kidney function and each BP component using cystatin C and 24-h creatinine clearance (CrCl) among 906 participants in the Heart and Soul Study.
Results
We observed that SBP was linearly associated with cystatin C concentrations (1.19 ± 0.55 mm Hg increase per 0.4 mg/L cystatin C, P = .03) across the range of kidney functions. In contrast, using CrCl, SBP was significantly associated with kidney function only in subjects with CrCl <60 mL/min (6.4 ± 2.13 mm Hg increase per 28 mL/min, P = .003) but not >60 mL/min (0.36 ± 0.77 mm Hg per 28 mL/min, P = .64). Slopes differed significantly (for spline term P = .001). We found that DBP was not associated with cystatin C (0.34 ± 0.40 mm Hg per 0.4 mg/L cystatin, P = .39) or CrCl (0.62 ± 0.44 mm Hg per 28 mL/min clearance, P = .16). Pulse pressure was linearly associated with cystatin C (1.28 ± 0.55 mm Hg per 0.4 mg/L cystatin, P = .02) and with CrCl <60 mL/min (7.27 ± 2.16 mm Hg per 28 mL/min, P = .001).
Conclusions
Both SBP and pulse pressure were significantly associated with kidney function across a wide range of cystatin C concentrations, even in subjects with presumably normal kidney function, by creatinine-based measures. Cystatin C may provide new insights into the association of CKD and hypertension, a relationship that may be an underappreciated barrier to hypertension control.
Keywords: Kidney, hypertension, cystatin C, systolic blood pressure
Hypertension is an important risk factor for the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD), and adequate control of blood pressure (BP) has been shown to attenuate decline in glomerular filtration rate (GFR).1 Hypertension is prevalent in persons with CKD, and rates of control are poor in the setting of CKD.2 Knowledge of the association between kidney function with each of the BP components (systolic, diastolic, or pulse pressure) has been mostly limited to persons with known CKD, diabetes, or hypertension.3–5 A recent study found a strong association between renal function and pulse pressure in elderly persons with isolated systolic hypertension.6 Another study found that systolic BP (SBP) was a stronger predictor of serum creatinine increases than diastolic BP (DBP), pulse pressure, or mean arterial pressure in a prospective cohort of older persons with isolated systolic hypertension.7 A meta-analysis of clinical trials of persons with clinical CKD showed that higher levels of SBP increased the risk of kidney disease progression.4 In addition, systolic hypertension and wide pulse pressure characterized most of the participants with CKD who had uncontrolled hypertension according to the fourth National Health and Nutrition Examination Survey.2 Although SBP, DBP, and pulse pressure are known to confer different degrees of cardiovascular risk,8–10 their relative associations with the full range of kidney function are unknown.
Because creatinine-based measures are insensitive markers of renal disease in persons with glomerular filtration rates (GFR) >60 mL/min,11 we used cystatin C to investigate the association between kidney function and each BP component. Cystatin C appears to be more sensitive for detecting reduced GFR than either creatinine or estimated GFR.12,13 Although both creatinine and cystatin C are freely filtered at the glomerulus, a major difference between them is that creatinine is secreted by renal tubules, whereas cystatin C is metabolized by the proximal tubule and only a small fraction appears in the urine.14 Cystatin C has also been shown to be a stronger predictor of adverse outcomes than serum creatinine.15,16 Based on the more linear relationship of cystatin C with GFR, we hypothesized that cystatin C would have a stronger association with SBP than conventional measures of kidney function, but that DBP would be less strongly associated with kidney function. For this purpose, we studied 906 participants in the Heart and Soul Study and compared SBP, DBP, and pulse pressure among participants across a wide range of kidney functions.
Methods
Participants
The Heart and Soul Study is a prospective cohort designed to investigate the influence of psychosocial factors on coronary artery disease. Methods have previously been described.17 Briefly, participants were recruited from clinics in the San Francisco Bay Area if they met one of the following inclusion criteria: history of myocardial infarction; angiographic evidence of >50% stenosis in one or more coronary vessels; evidence of exercise-induced ischemia by treadmill or nuclear testing; history of coronary revascularization; or documented diagnosis of coronary artery disease by an internist or cardiologist. Participants were excluded if they could not walk one block or were moving out of the area within 3 years. The study protocol was approved by the appropriate Institutional Review Boards, and all participants provided informed consent. Between September 2000 and December 2002, a total of 1024 participants were recruited. Of these, 118 were either unable to provide a serum sample or 24-h urine collection, leaving 906 participants for this cross-sectional analysis.
Measurements
Primary Predictor Variables
Kidney function was evaluated by measuring serum cystatin C using a BNII nephelometer (Dade Behring Inc., Deerfield, IL) that used a particle-enhanced immunonephelometric assay (N Latex Cystatin-C). The assay range is 0.195 to 7.330 mg/L, with the reference range for young healthy individuals reported as 0.53 to 0.95 mg/L. The intraindividual coefficient of variation was 7.7%, reflecting long-term stability of the measurement.
In addition, kidney function was evaluated using a 24-h urine collection for creatinine clearance. Details of collection method have been published.18 The 24-h creatinine clearance (CrCl) was calculated using the following formula: urine creatinine (mg/dL) * 24-h urine volume (dL)/serum creatinine (mg/dL) * 1440 (min/day).
Secondary Predictors
Age, ethnicity, smoking status, income, education, and history of diabetes, myocardial infarction, and coronary revascularization were determined by self-report. Height and weight were measured at intake, and body mass index (kg/m2) was calculated. Medication use was determined by having participants bring bottles to the study appointment, and study personnel recorded all medications. After a 12-h fast, samples were obtained for measurement of creatinine, total cholesterol, HDL, and LDL. All participants underwent resting echocardiography using an Acuson Sequoia Ultrasound System (Mountainview, CA) with a 3.2-MHz transducer to evaluate left ventricular ejection fraction (LVEF). Urine albumin and creatinine were measured by nephelometry and the rate Jaffe method, respectively. Urine albumin-to-creatinine ratios (milligrams of albumin per gram of creatinine) were calculated from the 24-h urine samples. Microalbuminuria was defined as a ratio ≥30 mg/g. CRP was measured from fasting blood that was frozen at –70°C until the time of the CRP assay. We used the Roche Integra high sensitivity assay or the Beckman Extended Range high-sensitivity CRP assay.
Outcome Variables
Both SBP and DBP were measured by sphygmomanometer at the intake appointment by a trained member of the research personnel. Measurements were recorded according to guidelines in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of Hypertension.19 Participants were instructed to take their BP medications on the morning of the intake appointment and not to smoke or consume caffeine 5 h before the visit. Pulse pressure was calculated as the difference between SBP and DBP.
Statistical Analysis
We compared differences in baseline characteristics between participants with SBP levels of <120 mm Hg, 120 to 140 mm Hg, and >140 mm Hg, using χ2 tests for dichotomous variables and analysis of variance for continuous variables. We divided the cohort into deciles of cystatin C and computed mean SBP, DBP, and pulse pressure for each decile (approximately 90 participants per decile). Using multivariable linear regression we studied the association between kidney function (using either cystatin C or 24-h creatinine clearance) and each BP component (in separate models for SBP, DBP, and pulse pressures). In these analyses, each measure was evaluated as a continuous variable per standard deviation (0.4 mg/dL for cystatin C and 28 mL/min for 24-h CrCl). We controlled for secondary variables of interest (age, sex, ethnicity, income, education, smoking status, body mass index, LVEF, history of myocardial infarction, diabetes, angioplasty, coronary artery bypass grafting (CABG), or congestive heart failure, use of HMG-CoA reductase inhibitors (statins), angiotensin-converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), β-blockers, diuretics, and calcium channel blockers, presence of microalbumunuria, and C-reactive protein levels). A two-tailed P value < .05 was considered significant.
To determine whether the association of kidney function with SBP appeared to be linear throughout the distribution of each measurement, we used a single-knot linear spline model. This method tests whether the slope of the predictor (kidney function) and the outcome (SBP) differs significantly above or below the chosen cutpoint. Therefore, a P value < .05 rejects the null hypothesis that the slopes are the same above and below the specified cutpoint. We initially chose a cutpoint of 60 mL/min for CrCl, per guidelines.20 We repeated the analyses with cutpoints of 50 and 70 mL/min. We repeated the spline analyses for cystatin C, choosing an initial cutpoint of 1.0 mg/L, with subsequent analyses testing cutpoints of 0.92 mg/L (25th percentile) and 1.07 mg/L (median).
We conducted a sensitivity analysis using the Modified Diet in Renal Disease equation to estimate GFR, and defined CKD as eGFR <60 mL/min/1.73 m2. In addition, we looked for interactions with African American ethnicity, diabetes, older age (>75 years), and use of ACE/ARB and diuretics to evaluate whether these factors modify the association between kidney function and SBP. All analyses were performed using STATA software, version 8.0 (StataCorp., College Station, TX).
Results
Baseline Characteristics
Among 906 participants, the average age was 66 years (SD 11) and 82% were male. The mean CrCl was 81 mL/min (SD 28 mL/min) and mean cystatin C level was 1.17 mg/L (SD 0.42 mg/L). Mean SBP was 133 mm Hg (SD 22 mm Hg) and mean DBP 75 mm Hg (SD 11 mm Hg). Characteristics of participants by SBP are listed in Table 1. On average, subjects with higher SBP were older, more likely to be African American, and more likely to have diabetes.
Table 1.
Characteristics of Heart and Soul Study participants by systolic blood pressure (SBP) (N = 906)
| Characteristic | SBP <120 mm Hg | SBP 120–140 mm Hg | SBP >140 mm Hg | P |
|---|---|---|---|---|
| Age (y) | 65 ± 12 | 66 ± 11 | 66 ± 910 | <.001 |
| Sex (% male) | 186 (89) | 364 (83) | 194 (76) | .002 |
| Ethnicity | .002 | |||
| Non-Hispanic white | 144 (69) | 272 (62) | 135 (53) | |
| Hispanic white | 19 (9) | 40 (9) | 24 (9) | |
| African American | 13 (6) | 74 (17) | 50 (20) | |
| Asian | 27 (13) | 47 (11) | 33 (13) | |
| Other | 6 (3) | 8 (2) | 13 (5) | |
| Current smoker | 44 (21) | 96 (22) | 36 (14) | .04 |
| Income <$20,000 | 95 (45) | 201 (46) | 133 (52) | .18 |
| Less than high school education | 190 (90) | 387 (88) | 212 (83) | .05 |
| Medical history | ||||
| Myocardial infarction | 126 (60) | 239 (55) | 122 (48) | .03 |
| Diabetes | 44 (21) | 103 (23) | 90 (35) | <.001 |
| Prior angioplasty | .89 (42) | 167 (38) | 100 (40) | .56 |
| Prior CABG | 87 (42) | 162 (37) | 79 (31) | .06 |
| Heart failure | 45 (21) | 68 (16) | 41 (16) | .16 |
| Measurement | ||||
| BMI | 28 ± 5 | 29 ± 5.5 | 29 ± 5.5 | .11 |
| Total cholesterol | 174 ± 42 | 17 ± 640 | 183 ± 45 | .05 |
| LDL | 103 ± 33 | 103 ± 31 | 107 ± 37 | .28 |
| HDL | 44 ± 12 | 46 ± 14 | 46 ± 14 | .25 |
| LVEF | 0.60 ± 12 | 0.62 ± .09 | 0.62 ± .09 | .006 |
| Microalbuminuria | 81 (27) | 99 (28) | 85 (33) | .24 |
| Medication | ||||
| ACE/ARB | 115 (55) | 204 (46) | 149 (58) | .005 |
| Statin | 136 (65) | 283 (64) | 163 (64) | .98 |
| Aspirin | 169 (80) | 331 (75) | 203 (80) | .20 |
| β-blockers | 118 (56) | 247 (56) | 154 (60) | .50 |
| Diuretics | 51 (24) | 128 (29) | 88 (35) | .05 |
| Calcium blocker | 36 (17) | 95 (22) | 85 (33) | <.001 |
ACE/ARB = angiotensin converting enzyme inhibitors or angiotensin receptor blockers; BMI = body mass index; CABG = previous coronary artery bypass grafting; LVEF = left ventricular ejection fraction.
Data for characteristics are given as mean ± SD or N (%).
Association of Kidney Function With SBP
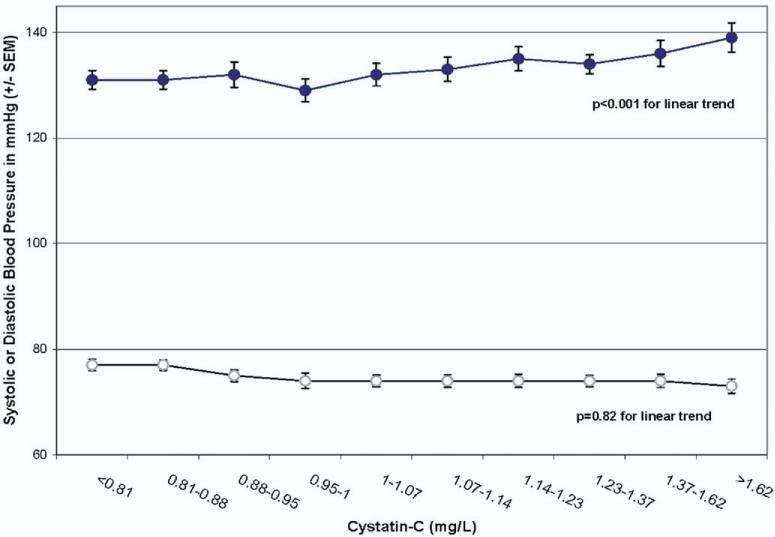
Progressively reduced kidney function was associated with higher mean SBP across the full range of kidney functions. When participants were grouped by decile of cystatin C, mean SBP increased linearly with each increasing decile (Fig. 1). In a multivariable model, SBP was significantly associated with cystatin C (Table 2). Systolic BP increased by 1.19 ± 0.55 mm Hg per 0.4 mg/L increase in cystatin C (P = .03) We found similar results when we used 1/cystatin C in place of cystatin C, although the associations were less strong. In contrast, when using CrCl as the measure of kidney function, there was no significant association between CrCl and SBP (Table 2).
FIG. 1.
Mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) by decile of kidney function measured as cystatin C.
Table 2.
Linear regression of systolic blood pressure by kidney function (N = 906)
| Age-adjusted |
Multivariable adjusted* |
||||
|---|---|---|---|---|---|
| Measure | N | β coefficient | P | β coefficient | P |
| Cystatin-C (per 0.4 mg/L [SD] increase) | 1.75 ± 0.72 | .01 | 1.19 ± 0.55 | .03 | |
| Overall | |||||
| >1.0 | 551 | 2.23 ± 0.07 | .03 | 1.23 ± 0.03 | .04 |
| <1.0 | 355 | 1.59 ± 0.04 | .71 | 0.54 ± 0.01 | .87 |
| Spline P value for difference in slopes | .85 | ||||
| 24-h CrCl (per 28 mL/min [SD] decrease) | |||||
| Overall | 1.96 ± 0.76 | .01 | 0.91 ± 0.61 | .14 | |
| <60 | 222 | 11.20 ± 2.74 | <.001 | 6.40 ± 2.13 | .003 |
| >60 | 684 | 0.31 ± 0.99 | .42 | 0.36 ± 0.77 | .64 |
| Spline P-value for difference in slopes | .01 | ||||
Adjusted for age, ethnicity, income, education, prior myocardial infarction, diabetes, prior angioplasty, prior coronary artery bypass grafting, heart failure, body mass index, cholesterol, low-density lipoprotein, high-density lipoprotein, diastolic blood pressure, left ventricular ejection fraction, and use of angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statin, aspirin, β-blocker, diuretic, calcium channel blocker, microalbuminuria, C-reactive protein.
We tested whether the association of CrCl and SBP varied >60 mL/min or <60 mL/min. We found that >60 mL/min there was no association between kidney function and SBP (0.36 ± 0.77 mm Hg increase per 28 mL/min decrease in CrCl, P = .64) but that for participants with CrCl <60 mL/min there was a significant association between kidney function and SBP (6.40 ± 2.13 mm Hg increase per 28 mL/min, P = .003). The slopes of the lines above and below this cutoff were significantly different (for spline term P = .01) (Table 2). Similar results were observed using cutpoints of 70 mL/min (1.0 ± 0.87 mm Hg for >70 mL/min and 5.41 ± 1.54 mm Hg per 28 mL/min CrCl for <70 mL/min respectively; for difference in slopes P = .002) and 50 mL/min (0.17 ± 0.71 mm Hg and 7.70 ± 3.31 mm Hg respectively; for difference in slopes P = .03).
In contrast, the relationship between cystatin C and SBP remained linear across the wide range of cystatin C concentrations. Using a cutoff of 1.0 mg/L of cystatin C we found no evidence that the slope differed significantly above and below this cutoff (for spline term P = .85) (Table 2). Similar results were seen at cutoffs of 0.92 mg/L and 1.07 mg/L of cystatin C (for spline terms P = .74 and P = .80 respectively).
We tested for an independent association between microalbuminuria and SBP in a multivariable model adjusting for cystatin C. There was no significant independent relationship between microalbuminuria and SBP (1.0 ± 1.17 mm Hg increase in SBP for subjects with microalbuminuria compared with subjects without microalbuminuria, P = .41).
When we used eGFR (MDRD equation), results were similar to those using 24-h CrCl. In a multivariable model, eGFR was not linearly associated with SBP (0.57 mm Hg increase in SBP per SD of eGFR [22 mL/min/1.73 m2], P = .35). However, there was a significant linear relationship in subjects with eGFR <60 mL/min/1.73 m2 (4.79 mm Hg increase per SD of eGFR, P = .01). There was no significant linear relationship observed for subjects with eGFR >60 mL/min/173 m2 (0.50 mm Hg increase per SD eGFR, P = .51), and these slopes were significantly different (for spline term P = .03).
We also tested for interactions with African American ethnicity, diabetes, and use of ACE-I/ARB and diuretics. The relationship between cystatin C and SBP was not modified by African American ethnicity, diabetic status, advanced age, or use of ACE-I/ARB or diuretics. (For interaction, P = .38 for African American ethnicity, P =.68 for diabetes, P =.74 for advanced age, P = .89 for use of ACE-I/ARB, and P =.50 for use of diuretics).
Association of Kidney Function With DBP and Pulse Pressure
In contrast to SBP, cystatin C was not significantly associated with DBP (Fig. 1 and Table 3). The CrCl was not directly associated with DBP in unadjusted and adjusted models (Table 3).
Table 3.
Linear regression of diastolic blood pressure by kidney function (N = 906)
| Age-adjusted |
Multivariable adjusted* |
|||
|---|---|---|---|---|
| Measure | β coefficient | P | β coefficient | P value |
| Cystatin-C (per 0.4 mg/L increase) | 0.09 ± 0.38 | .81 | 0.34 ± 0.40 | .39 |
| 24-h CrCl (per 28 mL/min decrease) | 0.55 ± 0.41 | .18 | 0.62 ± 0.44 | .16 |
Adjusted for age, ethnicity, income, education, prior myocardial infarction, diabetes, prior angioplasty, prior coronary artery bypass grafting, heart failure, body mass index, cholesterol, low- density lipoprotein, high-density lipoprotein, left ventricular ejection fraction, and use of angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statin, aspirin, β-blocker, diuretic, calcium channel blocker, microalbuminuria, C-reactive protein.
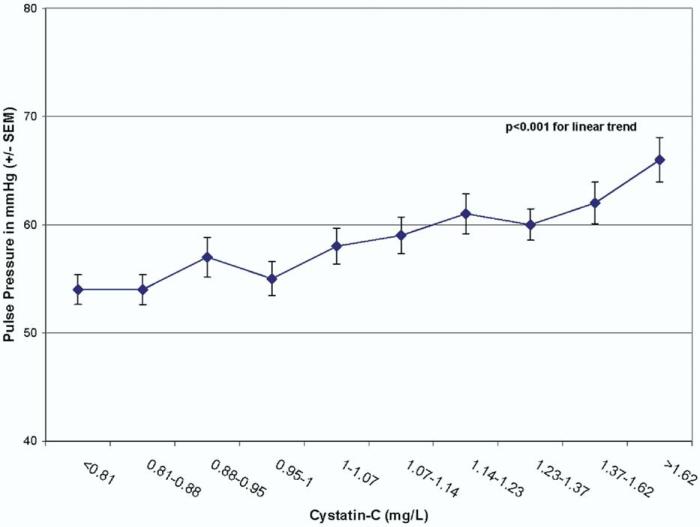
Pulse pressure, however, appeared to be linearly associated with kidney function, and mean pulse pressure increased by decile of cystatin C (Fig. 2). In age-adjusted analyses, pulse pressure was strongly associated with kidney function (1.64 ± 0.54 mm Hg increase per 0.4 mg/L cystatin C increase, P = .002), and this persisted after multivariable analysis (1.28 ± 0.55 mm Hg increase per 0.4 mg/L cystatin C increase, P = .02). Pulse pressure was not linearly associated with CrCl (1.1 ± 0.63 mm Hg increase per 28 mL/min CrCl decrease, P = .10) in a multivariable model. Using spline terms to evaluate this relationship with CrCl >60 or <60 mL/min, pulse pressure was associated with kidney function in subjects with CrCl <60 mL/min (7.27 ± 2.16 mm Hg increase per 28 mL/min, P = .001) but not in those with CrCl >60 mL/min (0.35 ± 0.79 mm Hg increase per 28 mL/min, P = .66), and these slopes were significantly different (for spline term P = .003).
FIG. 2.
Mean pulse pressure by decile of kidney function measured as cystatin C.
Discussion
Our results demonstrated a strong association between kidney function and both SBP and pulse pressure in this cohort of patients with coronary artery disease throughout a wide range of kidney function levels. In contrast, kidney function was not associated with DBP. When 24-h urine creatinine clearance was used to measure kidney function, its association with SBP and pulse pressure was limited to those participants who met the criteria for established kidney disease (CrCl <60). However, by using cystatin C, we detected that small changes in kidney function within the normal range (GFR >60 mL/min) were also associated with SBP and pulse pressure, even after adjustment for inflammatory markers. Thus, our study results imply that kidney function may be a more important determinant of adequate SBP control than previously appreciated, even among persons without clinical CKD.
The finding that kidney function is associated with SBP but not with DBP warrants further exploration. Isolated systolic hypertension has been shown to be a marker of cardiovascular risk.21,22 Systolic hypertension and wide pulse pressure have been associated with vascular stiffness,23,24 which may provide a clue to the association of kidney disease and cardiovascular disease. Previous findings showed that SBP was a stronger predictor of a rise in serum creatinine than DBP for patients with significantly reduced GFR, those with diabetes, and those with isolated systolic hypertension.3–5,7 In addition, a recent study found that pulse pressure was associated with renal function in an elderly cohort with untreated isolated systolic hypertension.6 Although the target SBP for those with CKD is still uncertain, our study extends the findings of the strong association between kidney function and SBP with a wider range of kidney function and SBP levels than previously known.
By using a more sensitive marker (cystatin C) to determine kidney function, our analysis found an association of kidney function with SBP even in those without clinical CKD by the use of traditional creatinine-based methods. Cystatin C has been shown to be a more sensitive marker of GFR than either creatinine or eGFR, and it is independent of body mass, height, and weight.12,13,25,26 Using cystatin C, we found a significant linear relationship between kidney function and SBP even among persons with presumably normal renal function. These results are in accordance with findings in a small cohort of subjects with essential hypertension,27 and they suggest the presence of factors that may affect the vasculature before clinical CKD is diagnosed. Therefore, cystatin C concentrations appear to offer new insights into the importance of the relationship between kidney function and SBP, which may have been underestimated in previous studies.
These findings have important implications. Studies of large national samples still show an enormous burden of hypertension in the United States, and at best approximately 45% of persons with hypertension meet targets.28–31 Mildly reduced kidney function may be an important but underappreciated factor that contributes to the lack of hypertension control in the United States, especially in those with isolated systolic hypertension. Further research should be conducted to design new antihypertensive agents that are able to reduce SBP without lowering DBP in the setting of kidney dysfunction to improve hypertension control.
A particular strength of our study is the measurement of renal function with 24-h urine collections in juxtaposition with a novel filtration marker of GFR, cystatin C. Most studies of BP and kidney function have only used serum creatinine measures or estimated GFR.3,4,7,23 We believe, therefore, that our study more accurately quantifies the association between kidney disease and SBP.
Certain limitations should also be considered in interpreting our results. Because this is a cross-sectional study, we cannot determine the direction of the association or determine causality. Specifically, we cannot discern the extent to which kidney dysfunction leads to elevated SBP, or elevated SBP leads to kidney dysfunction. Blood pressure levels were limited to one measurement, which may affect the precision of our measures. Our study included participants taking antihypertensive medications, and thus BP levels may be lower than in those without treatment. However, the fact that the association between renal function and SBP persists despite this factor supports our hypothesis that mildly reduced kidney function may be an underappreciated contributor to the lack of systolic hypertension control in the United States. Furthermore, our study participants were mostly men of non-Hispanic white ethnicity and all had known coronary disease, which limits the generalization of our findings. Thus, our findings should be confirmed in other cohorts.
In summary, cystatin C was significantly and linearly associated with SBP and pulse pressure but not with DBP across a wide range of kidney functions. This association may have important implications in the treatment of hypertension, as mildly reduced kidney function may be an underappreciated barrier in the achievement of BP targets. Further studies are required to evaluate the pathways involved, as new therapies are urgently needed to reduce SBP in persons with either clinical or preclinical kidney disease.
Acknowledgments
The Heart and Soul Study is supported by grants from the Department of Veterans Affairs, the American Federation for Aging Research (Paul Beeson Scholars Program), the Robert Wood Johnson Foundation (Faculty Scholars Program), and the Ischemia Research and Education Foundation. Dr. Peralta was supported by the General Medicine Fellowship at University of California–San Francisco (UCSF). Dr. Ix was supported by the UCSF Academic Senate Individual Investigator Program. Dr. Shlipak was supported by the American Federation for Aging Research and National Institute on Aging (Paul Beeson Scholars Program) and the Robert Wood Johnson Foundation (Generalist Faculty Scholars Program).
References
- 1.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Peralta CA, Hicks Leroi S, Chertow Glenn M, Ayanian John Z, Vittinghoff Eric, Feng Lin, Shlipak Michael G. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45:1–6. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 3.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 4.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 5.Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int. 1995;48:851–859. doi: 10.1038/ki.1995.361. [DOI] [PubMed] [Google Scholar]
- 6.Verhave JC, Fesler P, du Cailar G, Ribstein J, Safar ME, Mimran A. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension. 2005;45:586–591. doi: 10.1161/01.HYP.0000158843.60830.cf. [DOI] [PubMed] [Google Scholar]
- 7.Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP). J Am Soc Nephrol. 2002;13:2776–2782. doi: 10.1097/01.asn.0000031805.09178.37. [DOI] [PubMed] [Google Scholar]
- 8.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 9.Mattace-Raso FU, van der Cammen TJ, van Popele NM, van der Kuip DA, Schalekamp MA, Hofman A, Breteler MM, Witteman JC. Blood pressure components and cardiovascular events in older adults: the Rotterdam study. J Am Geriatr Soc. 2004;52:1538–1542. doi: 10.1111/j.1532-5415.2004.52419.x. [DOI] [PubMed] [Google Scholar]
- 10.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 12.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 14.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 15.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Sarnak MJ, Katz R, Fried L, Seliger S, Newman A, Siscovick D, Stehman-Breen C. Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005;45:268–271. doi: 10.1016/j.jacc.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 17.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. J Am Med Assoc. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the Heart and Soul Study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 21.Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. J Am Med Assoc. 2004;292:1074–1080. doi: 10.1001/jama.292.9.1074. [DOI] [PubMed] [Google Scholar]
- 22.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). J Am Med Assoc. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 23.Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. 2004;43:163–168. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- 24.Bielak LF, Turner ST, Franklin SS, Sheedy PF, 2nd, Peyser PA. Age-dependent associations between blood pressure and coronary artery calcification in asymptomatic adults. J Hypertens. 2004;22:719–725. doi: 10.1097/00004872-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C—a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 26.O'Riordan SE, Webb MC, Stowe HJ, Simpson DE, Kandarpa M, Coakley AJ, Newman DJ, Saunders JA, Lamb EJ. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40:648–655. doi: 10.1258/000456303770367243. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe S, Okura T, Liu J, Miyoshi K, Fukuoka T, Hiwada K, Higaki J. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003;26:895–899. doi: 10.1291/hypres.26.895. [DOI] [PubMed] [Google Scholar]
- 28.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–486. doi: 10.1056/NEJMoa010273. [DOI] [PubMed] [Google Scholar]
- 29.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Hypertension control: how well are we doing? Arch Intern Med. 2003;163:2705–2711. doi: 10.1001/archinte.163.22.2705. [DOI] [PubMed] [Google Scholar]
- 30.He J, Muntner P, Chen J, Roccella EJ, Streiffer RH, Whelton PK. Factors associated with hypertension control in the general population of the United States. Arch Intern Med. 2002;162:1051–1058. doi: 10.1001/archinte.162.9.1051. [DOI] [PubMed] [Google Scholar]
- 31.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]