Abstract
Objectives
To determine the risk factors for recurrent lupus nephritis, allograft loss, and survival among systemic lupus erythematosus (SLE) kidney transplant patients.
Methods
The archival records of all SLE kidney transplant recipients (American College of Rheumatology criteria) from June 1977 – June 2007 were reviewed. Patients who died or lost the allograft within 90 days of the transplant were excluded. Time-to-event (above outcomes) was examined by univariable and multivariable proportional Cox regressions.
Results
Two hundred-twenty of nearly 7000 renal transplantations were performed in 202 SLE patients during the 30-year interval; 177 patients met the entry criteria. These patients were predominantly women (80%) and African American (57%); mean age was 35.6 years and mean disease duration 11.2 years. Recurrent lupus nephritis ensued in 20 patients (11.3%), allograft loss in 39.0% and death in 20.3%. In all three regressions, African American ethnicity was found to be associated with a shorter time-to-the-event (HR=4.63; 95% CI 1.29 – 16.65 for recurrent lupus nephritis, HR=2.71; 95% CI 1.35 – 5.44 for allograft loss and HR=3.14; 95% CI 1.21 – 8.14 for survival). Recurrent lupus nephritis was not a significant factor for allograft loss or survival. In patients with recurrent lupus nephritis, the lesion in the engrafted kidney was predominantly mesangial (WHO Class II) as opposed to proliferative or membranous (WHO Class III, IV, V) in the native kidney.
Conclusions
African American ethnicity was independently associated with poorer outcomes, but recurrent lupus nephritis is not a risk factor for either allograft loss or survival. Recurrent lupus nephritis is infrequent and benign.
Introduction
Lupus nephritis continues to be a major cause of morbidity and mortality in patients with systemic lupus erythematosus (SLE). Renal involvement affects up to 60% depending on the ethnic composition and geographic origin of those studied (1–4). Despite advances in the understanding of the pathogenesis of SLE and the use of aggressive treatments for lupus nephritis, 5-22% of these patients will progress to end-stage renal disease (ESRD) (5;6).
Although successful transplantation with deceased-donor renal allografts has been performed since 1959, there has been the concern that lupus patients may have poor outcomes as compared to other patient populations. In 1975, however, the American College of Surgeons/National Institutes of Health (ACS/NIH) Transplant Registry (7) reported that lupus patients undergoing renal transplantation had outcomes comparable to those of non-lupus patients, opening the doors to transplantation for lupus patients (8–10). To date there are relatively limited data about the factors that may account for renal and patient outcomes for lupus patients undergoing renal engraftment. Risk factors for allograft loss in the lupus patient include thrombotic events (11;12), donor type (8) and earlier rejection (8;13–15). The exact role of recurrent disease in the engrafted kidney, however, remains unknown.
Recurrent lupus nephritis, a major concern in the early transplant years, occurs at variable rates ranging from 0 to 30% (16–21). Differences in patient characteristics and in the indications for renal biopsy, to a certain extent, may account for this broad range. The factors associated with recurrence have not been identified in the studies published to date. Furthermore, whether recurrent lupus nephritis in the allograft may influence either allograft loss or patient survival has not been determined.
Utilizing a single US center renal-transplant registry, we identified all transplanted SLE patients to: (1) assess the rate of recurrence of lupus nephritis and the factors predisposing to it, (2) characterize the features of recurrent lupus nephritis, and (3) ascertain the risk factors for allograft and patient survival with special emphasis on the impact of recurrent lupus nephritis on these outcomes. The results of this study provide the information necessary to close the gaps in the existing literature regarding the safety of renal transplantation for lupus nephritis.
Patients and Methods
Patients
Between June 1977 and June 2007, a total of 6922 kidney transplantation were performed at the University of Alabama at Birmingham, a large tertiary academic medical center serving Alabama, southern Tennessee, western Georgia, Mississippi and the Florida panhandle. Two hundred-twenty of the procedures (3.7%) were done in 202 SLE patients with ESRD secondary to lupus nephritis. All patients met the criteria for SLE as established over the years by the American College of Rheumatology (ACR), formerly known as the American Rheumatism Association, for the classification of patients with SLE (22–24). This study was conducted with the approval of the Institutional Review Board at The University of Alabama at Birmingham. About five years ago, a significant effort was placed in compiling clinical, immunological, and therapeutic variables in these patients as well as data for their donors (25).
For the purpose of this study, this database was expanded by review of all available archival records, including both hospital and outpatient encounters (Nephrology, Renal Transplant and Rheumatology). Patients who lost the allograft or died within 90 days of engraftment were excluded from these analyses as they may not have time to develop recurrent disease. For recipients who had undergone renal transplantation on more than one occasion, the first procedure was considered the target transplantation, except for one patient in whom the second transplantation was analyzed because recurrent lupus nephritis occurred in this graft rather than in the first. At our institution, biopsies were not routinely performed in all transplant patients; they were prompted by the detection of unexplained hematuria, proteinuria, or decline in renal function.
Outcome variables
The outcome variables examined in this study were recurrent lupus nephritis defined histopathologically according to the World Health Organization (WHO) classifications of lupus nephritis introduced in 1974 and revised in 1982 and 1995 (26), allograft loss (loss of renal function requiring renal replacement therapy), and patient survival.
SLE patients’ variables
Variables from the socioeconomic-demographic domain include age, gender, ethnicity, weight, and health-related behaviors (alcohol intake and smoking) as obtained at the time of the engraftment. Where available, information on clinical manifestations of SLE, auto-antibodies (anti-nuclear, anti-Smith and anti-double stranded DNA), complement levels, viral serologies, and immunosuppressant drugs taken before transplantation were compiled. The histopathologic diagnosis of the native-kidney disease according to the WHO classification (26) and the type of renal replacement therapy (hemodialysis or peritoneal dialysis) were documented. The post-transplant histopathologic findings were classified as normal, recurrent lupus nephritis, acute or chronic rejection, thrombotic changes, acute tubular necrosis, calcineurin inhibitor toxicity or other. Allograft survival or loss as well as death and cause of death were rigorously researched using all available records. The Social Security Administration and the National Death Index databases, however, were not accessed.
Interval Variables
To perform our analyses, the following intervals were computed: time to ESRD defined from the date of the diagnosis of lupus to ESRD onset; dialysis duration defined from the date of ESRD to the date of transplantation; duration of follow-up defined from the date of transplantation to the date of death, allograft loss or last follow-up visit; and disease duration defined from the date of the diagnosis of lupus to the date of transplantation. Intervals to recurrent lupus nephritis, allograft loss, and death were computed from the date of transplantation.
Donor variables
The following donor variables were examined: age, gender, ethnicity, donor type (living-related, living-unrelated or deceased), and HLA-antigen match. Occurrence of delayed graft function (defined as the need for dialysis within the first week of engraftment) was also recorded.
Transplant medications
The immunosuppressive medications and the standard induction therapy have changed during the 30-year study period. Azathioprine was introduced in the late 1960’s, but the immunosuppressive protocol was modified in 1984 when cyclosporine, a calcineurin inhibitor was added. Tacrolimus and mycophenolate mofetil were not introduced in the standard protocol until the mid-1990’s. The use of antibodies (OKT3, anti-IL2 and thymoglobulin) as components of the induction regimen and the use of glucocorticoids were also recorded. Cumulative dosage and treatment duration for each of these compounds, however, could not be precisely defined from the available records.
Statistical analyses
For the outcomes of interest (recurrent lupus nephritis, allograft loss and survival), time-to-the-event analyses were performed. In a small proportion of patients (less than 10%), important dates were missing in a non-systematic manner that would have precluded their inclusion in the study. These missing dates were therefore imputed based on the available data for either diagnosis, ESRD, or transplantation dates for all the other patients by calculating the proportions for these intervals; these proportions were then used to impute the missing dates. Next the variables of interest were examined by univariable Cox regression analyses in relation to all three outcomes of interest. Variables with p≤0.10 in these univariable regressions were entered into multivariable Cox regressions. Recurrent lupus nephritis was entered into the allograft loss regression analyses, whereas both recurrent lupus nephritis and allograft loss were included in the survival regression. Variables with p≤0.05 in these analyses were considered to be statistically significant. The data are presented as hazard ratios (HRs) with their 95% confidence intervals (CIs). A HR <1 indicates a longer time-to-the event and a HR >1 indicates a shorter time. In addition, the subset of patients who developed recurrent lupus nephritis was examined using descriptive statistical analyses. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Patients Characteristics
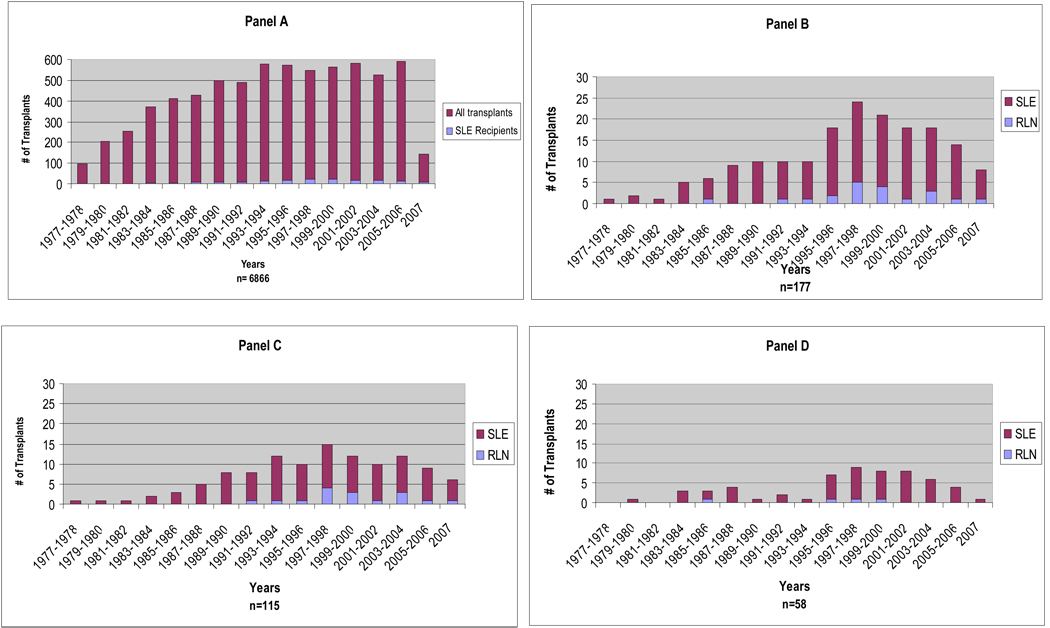
One hundred and seventy-seven patients of the 202 transplanted lupus patients met the inclusion criteria and are the subject of this study. The number of renal transplantations conducted at UAB has gradually increased over the years (Figure 1, Panel A) whereas the number of transplantations in SLE patients peaked in the late 1990’s (3.73%) and has decreased somewhat in the current decade (3.01%) compared to 1.12% in the late 1970’s-early 1980’s and 1.95% in the late 1980’s and 1990’s (p<0.0001) (Figure 1, Panel B). Patients were predominantly women (80%) and 65% were African American. The mean (standard deviation, SD) age was 35.6 (10.0) years (range 13–82); the mean (SD) disease duration at the time of the transplantation was 11.2 (8.7) years whereas the mean (SD) interval from SLE diagnosis to ESRD was 8.1 (7.5), years the mean (SD) dialysis duration was 3.1 (2.9) years, and the mean (SD) follow-up interval after transplantation was 7.1 (5.5) years (range 0.3 – 26.8). Renal replacement therapy before engraftment was required in nearly all patients (97%); most patients received hemodialysis (76% vs. 24% for peritoneal dialysis). The induction therapy protocols changed throughout the years, as have post-transplantation medication regimens. Patients received azathioprine (n=68, 38%), cyclosporine (n=135, 76%), mycophenolate mofetil (n=131, 74%) or tacrolimus (n=66, 37%). In some cases, more than one medication was used. All patients received glucocorticoids.
Figure 1.
Bargraft depicting the renal transplantation experience at the University of Alabama at Birmingham. Except from the last bar which represents the data from January to June 2007, all others depict the data for 2-year periods. Panel A. Number of renal transplantations performed in SLE recipients and all transplant recipients over the study period. Panels B–D. Proportion of recipients transplanted for lupus nephritis who developed recurrent disease. Panel B – All patients. Panel C – African Americans. Panel D– Caucasians.
Donor Characteristics
Sixty percent of the donors were male and 66% African American; 104 (59%) grafts were obtained from deceased donors, 60 (34%) from living-related donors, and 12 (7%) from living-unrelated donors. For one donor, this information was not available.
Recurrent Lupus Nephritis
Among the 177 patients who met inclusion criteria, a total of 20 patients (11.3%) developed recurrent lupus nephritis. The rate of recurrence has varied over the years from a low of 5.6% for transplantations performed in 2001–2002 to a high of 20.8% for those performed in 1997–98 (Figure 1, panel B). The overall rates for African Americans (13.9%, Panel C) and for Caucasians (6.9%, Panel D) did not significantly differ (p=0.2674). The univariable and multivariable analyses of the predictors of time to the occurrence of recurrent lupus nephritis are shown in Table 1. Age and gender were not associated with either a shorter or a longer interval. African American ethnicity, on the other hand, was associated with a shorter interval albeit statistical significance was only reached in the multivariable analysis (HR=4.63; 95% CI 1.29–16.65).
Table 1.
Univariable and Multivariable Cox Regression Analyses for Time-to-the-Occurrence of Recurrent Lupus Nephritis
| Variable | Hazard ratio |
Univariable 95% Confidence interval |
p value | Hazard ratio |
Multivariable 95% Confidence interval |
p value |
|---|---|---|---|---|---|---|
| Age | 0.99 | 0.94 | 0.4313 | 0.97 | 0.92 – 1.02 | 0.1729 |
| Gender (male) | 1.49 | 0.54 | 0.4425 | 1.36 | 0.43 – 4.34 | 0.6058 |
| Ethnicity (African American) | 2.78 | 0.93 | 0.0683 | 4.63 | 1.29 – 16.65 | 0.0190 |
| Post-transplantation medication | ||||||
| Azathioprine | 0.17 | 0.05–0.54 | 0.0028 | 0.28 | 0.08 – 1.02 | 0.0534 |
| Cyclosporine A | 0.71 | 0.23–2.16 | 0.5395 | |||
| Mycophenolate mofetil | 7.06 | 1.01–57.13 | 0.0487 | 3.21 | 0.35 – 29.19 | 0.3003 |
| Tacrolimus | 2.75 | 1.13–6.68 | 0.0256 | 2.99 | 1.12 – 7.97 | 0.0288 |
The roles of various post-transplant medications are shown in Table 1. Both cyclosporine and mycophenolate mofetil were not associated with either a longer or a shorter interval to recurrence. Azathioprine was associated with a longer interval in the univariable analyses (HR=0.17, 95% CI 0.05–0.54) but it did not quite reach statistical significance in the multivariate analysis (HR=0.28; 95% CI 0.08–1.02). Tacrolimus, on the other hand, had an opposite effect exhibiting a shorter interval in both univariable and multivariable analyses.
Description of Patients
The overall features of the 20 patients who developed recurrent lupus nephritis are depicted in Table 2. These patients were predominantly women (75%) and the mean (SD) age was 33.5 (9.6) years, features that were comparable to those of the patients who did not develop recurrent disease. The proportion of African Americans among those who developed recurrent lupus nephritis (75%) did not statistically differ from those who did not (64%). Histopathological data were not available for the native kidneys of three of the 20 patients. It is noteworthy that, as shown in Table 3, the predominant glomerular lesion in the native kidneys was either proliferative (10) or membranous (6), whereas in the majority of the allografts it was a mesangial lesion; this difference in pattern is statistically significant (Yates Chi square: 5.02; p=0.0251). The relatively small number of patients precluded performing additional analyses to determine factors associated with a proliferative lesion in the engrafted kidneys. Even though nine of these 20 patients (45%) eventually lost the allograft, this proportion is comparable to that in those patients who did not develop recurrent disease (38%) (p=0.558). Pre-transplant serologic markers and clinical manifestations were unavailable in the large majority of the patients’ archival records at our institution. Thus, we could not include any of these pre-transplant variables as potential risk factors for the examined outcomes.
Table 2.
Selected Sociodemographic and Clinical Features of Patients in Whom Lupus Nephritis Recurred (n=20)
| Features | |
|---|---|
| Age, years (SD) | 33.5 (9.6) |
| Gender, % women | 75 |
| Ethnicity, % | |
| African American | 80 |
| Caucasian | 20 |
| Smoker, % | 5 |
| Alcohol use, % | 5 |
| Weight, kg (SD) | 65.4 (7.6) |
| Died, % | 10 |
| Intervals [years, mean (SD)] | |
| Diagnosis duration (SLE-ESRD)* | 11.3 (10.9) |
| Dialysis duration, years ESRD-transplant) | 3.4 (3.7) |
| Time to recurrence (transplant-RLN)† | 4.5 (3.7) |
| Follow up time (transplant-last visit) | 6.2 (4.1) |
| Disease duration (SLE-last visit) | 14.1 (4.1) |
| Serologic Features, Post Transplant [# positive/ total tested, (%)] | |
| Anti-DNA antibodies | 3 / 13 (23) |
| Anti-nuclear antibodies | 12 / 12 (100) |
| Low complement | 6 / 15 (40) |
Systemic lupus erythematosus; end-stage renal disease
recurrent lupus nephritis.
Table 3.
Histopathological Findings in the Native and Transplanted Kidneys in Patients in Whom Lupus Nephritis Recurred (n=20)*
| Histopathology | Native* | Transplanted* |
|---|---|---|
| Mesangial glomerulonephritis (WHO† II) | 1 | 12 |
| Focal proliferative glomerulonephritis (WHO III) | 2 | 2 |
| Diffuse proliferative glomerulonephritis (WHO IV) | 8 | 1 |
| Membranous glomerulonephritis (WHO V) | 6 | 5 |
| Unknown | 3 | 0 |
p=0.0251 (Yates Chi square = 5.02). Three patients for whom this information was not available were excluded from this computation (two patients in whom a biopsy had not been obtained and one in whom this information was missing; these three patients had mesangial glomerulonephritis in the transplanted kidneys)
World Health Organization.
Allograft loss
At the time of the analysis 69 patients (39%) had lost the allograft. In nearly 74% of these patients (n=51) a kidney biopsy had been performed (mean interval from transplantation to biopsy 4.7 years); nine of the 51 patients in whom biopsies had been performed exhibited changes of recurrent lupus nephritis whereas in the remaining 42 histological features were those of chronic rejection. Of all the variables examined, only African American ethnicity was associated with a shorter interval to allograft loss in the univariable and multivariable analyses (HR=2.24-2.71; 95% CIs 1.28 to 1.35 – 3.93 to 5.44). Smoking was not associated with a shorter interval to allograft loss in the multivariable analysis. Recurrent lupus nephritis was not associated with either a shorter or a longer interval to allograft loss in the univariable and multivariable analyses. The same was the case for donor source and post-transplant medications with the exception of mycophenolate mofetil that appeared to be associated with a longer interval to allograft loss but statistical significance was borderline in the univariable and multivariable analyses. These data are depicted in Table 4.
Table. 4.
Univariable and Multivariable Cox Regression Analyses for Time-to-Allograft-Loss in Patients with Lupus Nephritis who had Undergone Kidney Transplantation
| Variable | Hazard ratio |
Univariable 95% Confidence interval |
p value | Hazard ratio |
Multivariable 95% Confidence interval |
p value |
|---|---|---|---|---|---|---|
| Age | 0.98 | 0.96–1.01 | 0.1495 | 1.00 | 0.97 – 1.04 | 0.8067 |
| Gender (male) | 0.89 | 0.46–1.69 | 0.7128 | 0.96 | 0.40 – 2.27 | 0.9228 |
| Ethnicity (African American) | 2.24 | 1.28–3.93 | 0.0050 | 2.71 | 1.35 – 5.44 | 0.0049 |
| Smoker | 1.24 | 0.69–2.22 | 0.4663 | 2.04 | 0.98 – 4.25 | 0.0575 |
| Recurrent lupus nephritis | 1.52 | 0.75–3.10 | 0.2418 | 1.76 | 0.78 – 4.00 | 0.1756 |
| Living-related donor* | 0.79 | 0.46–1.35 | 0.3804 | |||
| Living-unrelated donor* | 0.53 | 0.13–2.18 | 0.3785 | |||
| Post-transplant medications | ||||||
| Azathioprine | 1.52 | 0.88–2.62 | 0.1345 | |||
| Cyclosporine A | 1.64 | 0.77–3.49 | 0.1986 | |||
| Mycophenolate mofetil | 0.60 | 0.36–1.02 | 0.0552 | 0.59 | 0.32 – 1.07 | 0.0814 |
| Tacrolimus | 1.35 | 0.77–2.35 | 0.2918 | |||
Deceased donor is the reference group.
Survival
At the time of the analysis 36 of the 177 (20%) patients had died. In these patients biopsies had been performed in 63% (n=23) (mean interval from transplantation to biopsy 4 years) but in only two of them was there evidence of recurrent lupus nephritis. Despite intensive efforts it was not possible to ascertain the cause of death in ~31% of these patients. Deceased patients showed age and gender distribution comparable to those of the surviving patients, but African Americans were overrepresented among them (81% vs. 62%, p=0.0483).
Even though azathioprine seemed to be associated with diminished survival and mycophenolate mofetil appeared to be protective, only African American ethnicity remained an independent and significant predictor of survival in the multivariable analysis (Table 5). Of note, lupus nephritis in the engrafted kidney did not have a negative impact on survival. Because allograft loss had occurred among only the deceased patients, a precise estimate (HR and 95% CI) could not be computed for the impact of allograft loss on patient survival. Nevertheless, allograft loss should be considered a strong predictor of early demise among SLE patients undergoing renal engraftment.
Table. 5.
Univariable and Multivariable Cox Regression Analyses for Time-to-Allograft-Loss in Patients with Lupus Nephritis who had Undergone Kidney Transplantation*
| Variable | Hazard ratio |
Univariable 95% Confidence interval |
p value | Hazard ratio |
Multivariable 95% Confidence interval |
p value |
|---|---|---|---|---|---|---|
| Age | 0.98 | 0.95 – 1.02 | 0.3030 | 1.00 | 0.96 – 1.04 | 0.9556 |
| Gender (male) | 1.29 | 0.59 – 2.85 | 0.5185 | 1.75 | 0.69 – 4.42 | 0.2402 |
| Ethnicity (African American) | 2.48 | 1.19 – 6.28 | 0.0172 | 3.14 | 1.21 – 8.14 | 0.0187 |
| Smoker | 1.63 | 0.76 – 3.52 | 0.2135 | |||
| Living-related transplant † | 0.57 | 0.26 – 1.27 | 0.1706 | |||
| Living-unrelated transplant† | 0.45 | 0.06 – 0.31 | 0.4304 | |||
| Recurrent lupus nephritis | 0.58 | 0.14 – 2.45 | 0.4610 | 0.58 | 0.14 –2.50 | 0.4646 |
| Azathioprine | 2.41 | 1.10 – 5.27 | 0.0277 | 1.20 | 0.47 – 3.06 | 0.7014 |
| Cyclosporine | 1.94 | 0.67 – 5.62 | 0.2210 | |||
| Mycophenolate | 0.29 | 0.14 – 0.58 | 0.0005 | 0.55 | 0.24– 1.26 | 0.1566 |
| Tacrolimus | 0.68 | 0.29 – 1.59 | 0.3730 |
Estimates for allograft loss could not be obtained because none of the patients who retained the allograft had died by the time the study was conducted.
Deceased donor is the reference group.
Discussion
We have examined the largest single-center population of its kind for the risk factors that could influence outcomes in lupus nephritis patients undergoing renal transplantation. We have shown for the first time that African American ethnicity is independently associated with recurrent lupus nephritis, allograft loss, and diminished patient survival. Furthermore, we have shown that lupus nephritis in the transplanted kidney is relatively milder than the original disease and that it does not seem to have a negative effect on either allograft loss or patient survival.
The overall rate of recurrent lupus nephritis in our registry (11%) falls within the rates from the published literature (9;16;18–21;27;28); however, even within our own registry significant variability was observed (~6 to 21%) that could be due, in part, to the variable ethnic composition of the patients being transplanted at our institution over the span of 30 years. The true rate of recurrence can be ascertained only if all patients with lupus nephritis undergoing renal engraftment have a renal biopsy at specified intervals, which was not the case in our study. Furthermore, immunosuppressive therapy may have ameliorated the signs of active nephritis as well as other non-renal lupus manifestations, leading to diminished recognition of recurrent lupus nephritis. Nevertheless, given that our data are derived from a single institution in which two of the senior authors (GSA, BAJ) have been on the faculty for almost as long as the study period, less variability in the indication for a biopsy would be expected in this study as compared with data generated from more than one center.
We have also characterized the disease in the allograft as relatively benign (WHO Class II, predominantly) as compared to that in the native kidney. These findings are consistent with the data reported by Goral et al (10;19) and probably account for why recurrent lupus nephritis per se does not lead to early allograft loss or patient death. The newer immunosuppressive regimens administered pre- and post-transplantation may explain the fact that proliferative lesions rarely develop in the transplanted kidneys or that recurrent lupus nephritis is less likely to occur. Thus, we could have expected a sustained decrease in the occurrence of recurrent lupus nephritis (or the severity of the lesions) over time but that was not observed. In terms of allograft loss, deceased-donor allografts have, by and large, worse outcomes than living-donor allografts (8;29–31), but we did not observe such a relationship.
African American ethnicity has been shown to be a risk factor for allograft loss in SLE patients (30). We found that this ethnicity had an independent and negative effect not only on allograft loss but also on patient survival and recurrent lupus nephritis; this result underscores the well-known fact that patients from this ethnic group tend to exhibit more severe disease manifestations and less favorable outcomes (32–35). As compared to Caucasians, African Americans develop lupus nephritis more frequently (60% vs. 25%), respond less favorably to treatment, and thus progress to ESRD at higher rates (36); they also die at increased rates albeit when socioeconomic factors are adjusted for, this is no longer the case (33;37–39). Thus the findings reported now are not totally unexpected and were probably related to both genetic (40–42) and socioeconomic factors (poverty, years of education, compliance), which unfortunately could not be examined because these data elements were not part of the registry. Although we and others have also shown that Hispanic lupus patients, particularly those of Amerindian background, also experience severe disease with less than favorable outcomes (1;43–45), this registry include very few Hispanics, who until recently have been relatively underrepresented in our referral area (46).
Our study also demonstrates a protective effect of azathioprine on recurrent lupus nephritis and possibly mycophenolate mofetil on patients survival; however, we did not demonstrate a previously reported protective effect of the latter on allograft survival (8). Conversely, tacrolimus may exert a deleterious effect in relation to recurrent lupus nephritis but not on allograft loss or patient survival. The effect of glucocorticoids could not be properly examined as this medication had been included in all of the immunosuppressive regimens, albeit in a non-uniform manner.
Although smoking did not quite reach statistical significance as a factor for allograft loss, the deleterious impact of smoking for the kidney, particularly in lupus patients has been previously documented (47). Furthermore, smoking exerts negative effects on the vascular endothelium (48) and limits the efficacy of hydroxychloroquine (49), a medication commonly used for lupus patients to improve outcomes including retarding the occurrence of renal damage {add abstract} (50;51). Thus, we recommend that smoking cessation constitutes part of the pre-transplant preparation.
Some limitations of our study are worth noting. Firstly, details relative to SLE itself had not been captured uniformly for all patients and thus could not be included in these analyses; that was the case for the extent of non-renal lupus manifestations, autoantibody profile, and complement levels. Moreover, important data regarding histopathological lesions in the native kidneys were inconsistently recorded. Secondly not all patients received renal biopsies at specified times; thus, as already noted, it is conceivable that the actual incidence of lupus nephritis recurrence may be higher than the 11% we are reporting. Thirdly, the precise doses and duration of the different medications used for induction and maintenance were not available. Fourthly, the precise cause of death could not be properly examined, as we were unable to obtain this information in about one third of the patients. Finally, there was no provision to store biological specimens in all registry patients and thus genetic studies aimed at identifying potential markers of renal disease progression could not be conducted (41;42).
Despite these limitations, we have presented solid data relative to the outcome of patients with lupus nephritis who undergo kidney transplantation. First, African American ethnicity is a consistent marker of worse outcomes in these patients. Second, the occurrence of recurrent lupus nephritis does not portend a poor prognosis in terms of either allograft loss or patient survival. Lastly, azathioprine exerts a beneficial effect in these patients, in terms of recurrent lupus nephritis. A structured protocol that includes a more complete ascertainment of clinical, immunologic, and socioeconomic-demographic parameters coupled with the establishment of a biological repository would enhance the findings presented now and those in the literature. A different question worth answering is why only some lupus nephritis patients progress to ESRD (36;52). In short, much work remains to be done to advance this field for the betterment of patients afflicted with SLE.
Supplementary Material
Acknowledgements
Supported by grant P01AR49084 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (GSA, JL) and the STELLAR (Supporting Training Efforts in Lupus for Latin American Rheumatologists) Program (PIB, GJPE) funded by Rheuminations, Inc.
The authors thank Jan Scott and her staff for their assistance with all aspects of medical records review and Maria A. Tyson, AA for her expert assistance in the preparation of this manuscript. We are also indebted to the numerous Faculty, Fellows and Residents of the Departments of Surgery and Medicine who over the years collected data on these patients and to the nephrologists who referred their patients to UAB for renal transplantation and to Dr. William J. Koopman for his valuable comments to a previous version of this manuscript.
References
- 1.Bastian HM, Roseman JM, McGwin G, Jr, Alarcón GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups: XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–160. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine. 1999;78:167–175. doi: 10.1097/00005792-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10(2):413–424. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DJ, Podell TE, Weiner JM, Cox MB, Klinenberg JR, Forouzesh S. Lupus nephritis. Experience with 230 patients in a private practice from 1950 to 1980. Am J Med. 1982;72:209–220. doi: 10.1016/0002-9343(82)90812-9. [DOI] [PubMed] [Google Scholar]
- 5.Mojcik CF, Klippel JH. End-stage renal disease and systemic lupus erythematosus. Am J Med. 1996;101:100–107. doi: 10.1016/s0002-9343(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 6.Cheigh JS, Stenzel KH. End-stage renal disease in systemic lupus erythematosus. Am J Kidney Dis. 1993;21:2–8. doi: 10.1016/s0272-6386(12)80712-8. [DOI] [PubMed] [Google Scholar]
- 7.Renal transplantation in congenital and metabolic diseases. A report from the ASC/NIH renal transplant registry. J Am Med Assoc. 1975;232(2):148–153. doi: 10.1001/jama.1975.03250020022018. [DOI] [PubMed] [Google Scholar]
- 8.Bunnapradist S, Chung P, Peng A, Hong A, Chung P, Lee B, et al. Outcomes of renal transplantation for recipients with lupus nephritis: analysis of the Organ Procurement and Transplantation Network database. Transplantation. 2006;82(5):612–618. doi: 10.1097/01.tp.0000235740.56573.c6. [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C, Moroni G. Renal transplantation in lupus nephritis. Lupus. 2005;14:95–98. doi: 10.1191/0961203305lu2067oa. [DOI] [PubMed] [Google Scholar]
- 10.Stone JH, Amend WJC, Criswell LA. Outcome of renal transplantation in ninety-seven cyclosporine-era patients with systemic lupus erythematosus and matched controls. Arthritis Rheum. 1998;41:1438–1445. doi: 10.1002/1529-0131(199808)41:8<1438::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Moroni G, Tantardini F, Gallelli B, Quaglini S, Banfi G, Poli F, et al. The long-term prognosis of renal transplantation in patients with lupus nephritis. Am J Kidney Dis. 2005;45(5):903–911. doi: 10.1053/j.ajkd.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Stone JH, Amend WJ, Criswell LA. Antiphospholipid antibody syndrome in renal transplantation: occurrence of clinical events in 96 consecutive patients with systemic lupus erythematosus. Am J Kidney Dis. 1999;34(6):1040–1047. doi: 10.1016/S0272-6386(99)70009-0. [DOI] [PubMed] [Google Scholar]
- 13.Bartosh SM, Fine RN, Sullivan EK. Outcome after transplantation of young patients with systemic lupus erythematosus: A report of the North American pediatric renal transplant cooperative study. Transplantation. 2001;72(5):973–978. doi: 10.1097/00007890-200109150-00047. [DOI] [PubMed] [Google Scholar]
- 14.Nyberg G, Karlberg I, Svalander C, Hedman L, Blohme I. Renal transplantation in patients with systemic lupus erythematosus: increased risk of early graft loss. Scand J Urol Nephrol. 1990;24(4):307–313. [PubMed] [Google Scholar]
- 15.Stone JH, Amend WJ, Criswell LA. Outcome of renal transplantation in systemic lupus erythematosus. Semin Arthritis Rheum. 1997;27(1):17–26. doi: 10.1016/s0049-0172(97)80033-9. [DOI] [PubMed] [Google Scholar]
- 16.Bumgardner GL, Mauer SM, Payne W, Dunn DL, Sutherland DE, Fryd DS, et al. Single-center 1-15-year results of renal transplantation in patients with systemic lupus erythematosus. Transplantation. 1988;46(5):703–709. doi: 10.1097/00007890-198811000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Stone JH. End-stage renal disease in lupus: disease activity, dialysis, and the outcome of transplantation. Lupus. 1998;7(9):654–659. doi: 10.1191/096120398678920811. [DOI] [PubMed] [Google Scholar]
- 18.Azevedo LS, Romao JE, Jr, Malheiros D, Saldanha LB, Ianhez LE, Sabbaga E. Renal transplantation in systemic lupus erythematosus. A case control study of 45 patients. Nephrol Dial Transplant. 1998;13(11):2894–2898. doi: 10.1093/ndt/13.11.2894. [DOI] [PubMed] [Google Scholar]
- 19.Goral S, Ynares C, Shappell SB, Snyder S, Feurer ID, Kazancioglu R, et al. Recurrent lupus nephritis in renal transplant recipients revisited: It is not rare. Transplantation. 2003;75(5):651–656. doi: 10.1097/01.TP.0000053750.59630.83. [DOI] [PubMed] [Google Scholar]
- 20.Yu TM, Chen YH, Lan JL, Cheng CH, Chen CH, Wu MJ, et al. Renal outcome and evolution of disease activity in Chinese lupus patients after renal transplantation. Lupus. 2008;17(7):687–694. doi: 10.1177/0961203308089439. [DOI] [PubMed] [Google Scholar]
- 21.Lionaki S, Kapitsinou PP, Iniotaki A, Kostakis A, Moutsopoulos HM, Boletis JN. Kidney transplantation in lupus patients: a case-control study from a single centre. Lupus. 2008;17(7):670–675. doi: 10.1177/0961203308089430. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AS, Reynolds WE, Franklin EC, Kulka JP, Ropes MW, Shulman L, et al. Preliminary criteria for the classification of systemic lupus erythematosus. Bull Rheum Dis. 1971;21:643–648. [Google Scholar]
- 23.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 25.Kendrick SA, Kendrick WT, Cook WJ, Julian BA, Kew CE., II Recurrent lupus nephritis after renal transplantation: A single center experience. Amer Transp Congress. 2008 [Google Scholar]
- 26.Churg J, Bernstein J, Glasock RJ. Renal disease: classification and atlas of glomerular disease. In: Igaku-Shoin, editor. New York: 1995. pp. 151–179. [Google Scholar]
- 27.Grimbert P, Frappier J, Bedrossian J, Legendre C, Antoine C, Hiesse C, et al. Long-term outcome of kidney transplantation in patients with systemic lupus erythematosus: a multicenter study. Groupe Cooperatif de Transplantation d'ile de France. Transplantation. 1998;66(8):1000–1003. doi: 10.1097/00007890-199810270-00006. [DOI] [PubMed] [Google Scholar]
- 28.Ansari A, Larson PH, Bates HD. Vascular manifestations of systemic lupus erythematosus. Angiology. 1986;37(6):423–432. doi: 10.1177/000331978603700601. [DOI] [PubMed] [Google Scholar]
- 29.Lochhead KM, Pirsch JD, D'Alessandro AM, Knechtle SJ, Kalayoglu M, Sollinger HW, et al. Risk factors for renal allograft loss in patients with systemic lupus erythematosus. Kidney Int. 1996;49(2):512–517. doi: 10.1038/ki.1996.73. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Chelamcharla M, Baird BC, Shihab FS, Koford JK, Goldfarb-Rumyantzev AS. Factors affecting kidney-transplant outcome in recipients with lupus nephritis. Clin Transplant. 2008;22(3):263–272. doi: 10.1111/j.1399-0012.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 31.Chelamcharla M, Javaid B, Baird BC, Goldfarb-Rumyantzev AS. The outcome of renal transplantation among systemic lupus erythematosus patients. Nephrol Dial Transplant. 2007;22(12):3623–3630. doi: 10.1093/ndt/gfm459. [DOI] [PubMed] [Google Scholar]
- 32.Alarcon GS, McGwin G, Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort PROFILE. Lupus. 2002;11(2):95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez M, Alarcon GS, Calvo-Alen J, Andrade R, McGwin G, Jr, Vila LM, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57(4):576–584. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 34.Alarcon GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8:197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 35.Walsh SJ, Algert C, Rothfield NF. Racial aspects of comorbidity in systemic lupus erythematosus. Arthritis Care Res. 1996;9(6):509–516. doi: 10.1002/art.1790090613. [DOI] [PubMed] [Google Scholar]
- 36.Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51:1188–1195. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 37.Duran S, Apte M, Alarcon GS. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc. 2007;99(10):1196–1198. [PMC free article] [PubMed] [Google Scholar]
- 38.Sacks JJ, Helmick CG, Langmaid G, Sniezek JE. Trends in deaths from systemic lupus erythematosus-United States, 1979–1998. MMWR. 2002;51:371–374. [PubMed] [Google Scholar]
- 39.Walsh SJ, Algert C, Gregorio DI, Reisine ST, Rothfield NF. Divergent racial trends in mortality from systemic lupus erythematosus. J Rheumatol. 1995;22(9):1663–1668. [PubMed] [Google Scholar]
- 40.Alarcon GS, Bastian HM, Beasley TM, Roseman JM, Tan FK, Fessler BJ, et al. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA): contributions of admixture and socioeconomic status to renal involvement. Lupus. 2006;15(1):26–31. doi: 10.1191/0961203306lu2260oa. [DOI] [PubMed] [Google Scholar]
- 41.Alarcon GS, McGwin G, Jr, Petri M, Ramsey-Goldman R, Fessler BJ, Vila LM, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 2006;3(10):e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastian HM. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XLII: Factors predictive of new and worsening proteinuria. Rheumatology. 2007;(46):683–689. doi: 10.1093/rheumatology/kel347. [DOI] [PubMed] [Google Scholar]
- 43.Alarcon GS, McGwin G, Jr, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797–2806. doi: 10.1002/1529-0131(200112)44:12<2797::aid-art467>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Vilá LM, Alarcón GS, McGwin G, Jr, Friedman AW, Baethge BA, Bastian HM, et al. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology. 2004;43:358–363. doi: 10.1093/rheumatology/keh048. [DOI] [PubMed] [Google Scholar]
- 45.Pons-Estel GJ, Danila MI, McGwin G, Jr, Zhang S, Vila LM, Reveille JD, et al. Protective effect of Hydroxychloroquine (HCQ) on renal damage in patients with systemic lupus erythematosus (SLE): Data from a multiethnic cohort. Arthris Rheum. 2008;58(9):S924–S925. [Google Scholar]
- 46.Ramirez RR, de la Cruz GP. The Hispanic population in the United States. Current population reports. U S Census Bureau. 2002 March; www.census.gov/prod/2003pubs.
- 47.Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–2088. [PubMed] [Google Scholar]
- 48.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 49.Rahman P, Gladman DD, Urowitz MB. Smoking interferes with efficacy of antimalarial therapy in cutaneous lupus. J Rheumatol. 1998;25(9):1716–1719. [PubMed] [Google Scholar]
- 50.Alarcon GS, McGwin G, Jr, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine in the survival of patients with systemic lupus erythematosus. Data from LUMINA, a multiethnic us cohort (LUMINA L) Ann Rheum Dis. 2007;66:1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace DJ. Antimalarials---the 'rea' advance in lupus. Lupus. 2001;10:385–387. doi: 10.1191/096120301678646092. [DOI] [PubMed] [Google Scholar]
- 52.Korbet SM, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis: Racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18(1):244–254. doi: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.