Abstract
Objective:
To study the role of anti-platelet antibodies in the thrombocytopenia of murine WAS.
Methods:
A flow cytometric method was developed for detection of serum anti-platelet antibodies via their binding to intact target platelets lacking surface antibodies. Platelets were labeled with CMFDA in order to track their clearance from the circulation. WASP(−)μMT(−/−) mice were generated by standard breeding methods.
Results:
Serum anti-platelet antibodies were detected in approximately 40% of WASP(−) males. The mean level of reticulated platelets is significantly increased in these antibody(+) males. While WASP(−) males show an approximately 50% reduction in platelet counts, 5-10% of them show a more severe thrombocytopenia associated with increased reticulated platelets, suggesting the presence of clearance-inducing antiplatelet antibodies (CIAA). In support of that inference, 90% of the latter mice show detectable serum antiplatelet antibodies. The antibodies are primarily IgG, and are also detected in over 30% of CD47(−/−) males. WASP(−)μMT(−/−) males, which demonstrate no serum or platelet associated antibodies, show a degree of thrombocytopenia similar to that of WASP(−) males. Their platelet clearance rates remain accelerated – more so in WASP(−)μMT(−/−) than WASP(+)μMT(−/−) recipients.
Conclusions:
These findings suggest that platelet WASP deficiency results in an increase in platelet clearance rates by two mechanisms: an antibody independent mechanism which largely requires WASP deficiency in trans, and an antibody dependent mechanism which does not. Both an increased incidence of antiplatelet antibodies and an increased susceptibility to their effects contribute to antibody dependent clearance of WASP(−) platelets.
Introduction
The Wiskott-Aldrich Syndrome is an X-linked condition manifesting in most affected children as a clinical triad of immunodeficiency, thrombocytopenia with unusually small platelets, and severe eczema. Platelet kinetic studies in WAS patients[1-3] demonstrate rapid platelet clearance. Although this can be seen in severe thrombocytopenia (platelet counts less than 50×109/L) even when the latter is due to impaired production[4], at least some of the WAS patients studied had platelet counts above that threshold. Rapid clearance of WASP-deficient platelets appears to involve an intrinsic platelet defect, as it was reproduced on infusion of WASP-deficient platelets into normal volunteers. Allogeneic platelet consumption is normal in most, but not all[3], WAS patients. A concurrent impairment of platelet production was inferred from platelet counts and consumption rates in some cases, and has received further support from reports of impaired thrombopoiesis and abnormal megakaryopoiesis in clinical WAS[5, 6], and abnormal thrombopoiesis in murine WAS [7]. WAS patients have a high rate of autoimmune complications (72% in one study[8]), including autoimmune hemolytic anemia and glomerulonephritis. Both anti-nuclear antibodies and glomerulonephritis have been documented in WASP(−) mice[9].
These findings raise the question of whether antiplatelet antibodies could contribute to the thrombocytopenia of WAS. Support for this possibility comes from the fact that splenectomized WAS patients have a high incidence (23% in one study[10]) of episodic thrombocytopenias meeting the diagnostic criteria for ITP. The incidence of immune-mediated platelet destruction might be expected to be substantially higher in non-splenectomized WAS patients. In some cases, WAS patients present with a fluctuating course of thrombocytopenias resembling ITP[11]. And there are several published reports of increased amounts of platelet associated antibodies in these patients[12-15].
We previously showed[16] that WASP(−) mice have a more significant thrombocytopenia on the B6 background than was evident on the original SvEv background; that the consumption rate of WASP(−) platelets is increased, more so in WASP(−) recipients than in WT; that opsonization with a hamster anti-mouse CD61 antibody accelerates the in vivo consumption of WASP(−) platelets more than that of WT platelets; and that antibody opsonization induces greater uptake of WASP(−) platelets by bone marrow derived macrophages than is seen with opsonized WT platelets. We also found that a fraction of the WASP(−) males in our colony show an unusually low platelet count and an increased fraction of reticulated platelets (RP). This suggests the presence of clearance-inducing antiplatelet antibodies in this subset. We reported direct evidence for such antibodies in one such WASP(−) mouse. Here we use a more sensitive detection method to measure the incidence of these antibodies, and correlate their presence with evidence of rapid platelet clearance.
Materials and Methods
Reagents
Hamster anti-mouse CD61, Hamster anti-CD42d, FITC goat anti-mouse IgG/M, FITC mouse anti-mouse IgM (clone AF6-78), APC-B220, and PE-anti-mouse CD41 were obtained from BD Biosciences. FITC goat anti-mouse IgG was from Abd-Serotec. 6A6 antibody, originally derived by Dr. R.A. Good[17], was prepared by standard methods from hybridoma cells provided by Dr. Jeffrey Ravetch (The Rockefeller University). Rabbit polyclonal anti-WASP antibody was a gift of Dr. Hans Ochs (University of Washington). AF-448 donkey anti-rabbit IgG was obtained from Invitrogen. PGE-1 was obtained from Sigma. CMFDA (“celltracker green”) and BMQC (“celltracker violet”) were obtained from Invitrogen.
Mouse strains
WASP(−) mice originally derived by Snapper et al.[18] were crossed onto the C57Bl/6J background for at least 8 generations. Platelet and reticulated platelet counts were routinely performed on all WASP(−) males prior to their use as blood product donors or recipients. μMT(−/−) mice[19] (strain B6.129S2-Igh-6tm1Cgn/J) were obtained from Jackson Laboratories. CD47(−/−) mice (C57Bl/6J) were obtained from the Jackson Laboratory. WASP(−)CD47(−/−) males were derived as previously described[16]. To generate WASP(−), μMT(−/−) mice, WASP(−/−) females were crossed with μMT(−/−) males. The resultant double heterozygote females were bred with μMT(−/−) males, and progeny were screened to identify WASP(−)μMT(−/−) genotypes. Flow cytometric screening for WASP deficiency was performed by permeabilizing peripheral blood leukocytes (Cytofix/cytoperm, BD Biosciences), exposure to polyclonal rabbit-anti-WASP antibody, and detection with AF-488-donkey-anti-rabbit IgG. μMT deficiency was identified by screening for B220+ peripheral blood lymphocytes. All mice were bred and maintained in specific pathogen free environments at the Memphis VA Medical Center and at Seattle Children's Hospital.
Platelet and reticulated platelet counts
Platelet counts were performed via quantitative flow cytometry by reference to an internal fluorescent particle control, as previously described[16]. For measurement of reticulated platelets, platelets were resuspended in a thiazole orange solution (“Retic-Count”, BD Biosciences) for 30 minutes at room temperature, then analyzed by flow cytometry with gating of as described by Ault et al.[20]. All flow cytometric data was analyzed using Flowjo software (Tree Star Inc, Ashland, OR).
Antiplatelet antibody assays
Target platelets were obtained by bleeding donors via tail clipping directly into microfuge tubes containing 800 uL buffer A, which consisted of modified Tyrode's buffer (20 mM HEPES, 137 mM NaCl, 13.8 mM NaHCO3, 2.5 mM KCl, 0.36 mM NaH2PO4-H2O, 5.5 mM glucose, 0.25% bovine serum albumin, 1 mM MgCl2) supplemented with 0.38% sodium citrate, and 1 ug/mL PGE-1 (Sigma, St Louis, MO, USA). Platelets were separated from other blood components via Ficoll density gradient (Fico/Lite platelets; Atlanta Biologicals (Atlanta, GA, USA); 350 RCF, 15 minutes, room temperature), diluted in modified Tyrode's buffer supplemented with 1 ug/ml PGE-1, and centrifuged at 6000 RCF for 4.5 minutes. Platelets were resuspended at approximately 1 × 109 per ml in modified Tyrode's buffer supplemented with 1 ug/ml PGE-1 (Sigma). Paraformaldehyde (Electron Microscopy Sciences) was added to 1% and the platelets were left at room temperature for 5 min. After addition of 10 volumes of modified Tyrode's buffer supplemented with 1 ug/ml PGE-1, platelets were centrifuged again (6000 RCF, 5 minutes), resuspended in the same buffer, and counted using a Beckman-Coulter (Fullerton, CA, USA) Model Z2 particle count and size analyzer.
Sera were prepared (simultaneous with or less than three weeks after platelet and RP counts were obtained) by centrifuging blood (obtained by tail clipping or retro-orbital bleed) in serum separator tubes (Becton Dickinson) at 6.8 thousand RCF for 2 minutes at room temperature. Sera were stored in aliquots at −20 C and not re-frozen. Binding to target platelets took place in 40 ul net volumes of modified tyrode's buffer containing 5 × 105 platelets, appropriate dilutions of serum, 0.1 ul per assay of “carrier” mouse serum (Sigma cat. #M5905), and in some cases appropriate dilutions of antibodies as described in the text. The mixtures were incubated at 4 degrees for 75 minutes with gentle rocking. After addition of 500 ul of modified tyrode's buffer, the tubes were centrifuged at 6000 RCF for 4.5 minutes at room temperature. Pellets were resuspended in 50 ul of modified tyrode's buffer plus secondary antibody (FITC anti-IgG/M) and incubated at 4 degrees C for 30 minutes with gentle rocking. 350 ul of PBS supplemented with 2% inactivated fetal bovine serum was added, and the samples were immediately analyzed on either a FacsCalibur or LSR (Becton Dickinson) flow cytometer. Assays were performed in duplicate for each serum dilution tested. Averages of the FITC geometric mean fluorescence intensity (gMFI) values were normalized to those of concurrently run controls receiving no serum. For a subset of the assays, the amount of “carrier” serum was reduced to 0.03 ul per assay. For this subset, a separate set of WT control sera (set 2) was also evaluated (for redefinition of the normal range) by the same method.
For assays using non-fixed platelets, serum dilutions and assays were performed in the presence of 0.2% Tween-20 (Fisher), and 0.02 ul rabbit serum (Sigma, catalogue number R9133) per assay. Platelet preparation was otherwise as described above.
In vivo platelet clearance assays
Platelet preparation, labeling of platelets with CMFDA, injection of labeled platelets, and quantification of the fraction of peripheral blood platelets labeled was performed as previously described[16]. In some cases, platelets labeled with BMQC (“celltracker violet”, Invitrogen) were co-injected with CMFDA-labeled platelets. Briefly, platelets were prepared as for the “target platelets” described above, but without PFA treatment. Platelets at 2.9 × 105 per ul were incubated fluorescently labeled in modified tyrode's buffer, 1 ug/ml PGE-1, and 1.25 uM CMFDA or 1.25 uM BMQC at 37 degrees C for 20 minutes. After addition of five volumes modified tyrode's buffer + 1 ug/ml PGE-1, platelets were centrifuged at 2000 RCF for 10 minutes at room temperature and resuspended at approximately 1 × 105 per ul in modified tyrode's buffer. Opsonization was performed at 5 × 105 platelets per ul in complete tyrode's buffer at 15 ng 6A6 per million platelets, for 20 minutes at room temperature. After addition of 3 volumes of modified tyrode's buffer plus 1 ug/ml PGE-1, platelets were centrifuged at 6000 RCF for five minutes, resuspended in modified tyrode's buffer, and counted. Labeled platelets or mixtures of labeled platelets were brought to 2.5 × 105 per ul and injected via tail vein into the recipients described in the text. Typical injection volumes of 400 to 600 ul yielded platelet dosages of 1 to 1.5 × 108 platelets, resulting in most cases in 2% to 4% labeling of circulating platelets at T0 (5 minutes). Recipients were bled retro-orbitally at T0 and at daily intervals thereafter. In some cases, as noted in the figure legends, the fraction of platelets labeled was determined after gating platelets via forward vs. side scatter. In others, gating also employed the addition of a PE-CD41 marker.
Statistics
Logistic regression and correlation were performed with SAS software. All other statistical analyses were performed with Microsoft Excel.
Results
Assay development
Our objective in designing a flow cytometric assay for serum antiplatelet antibodies was to maximize its sensitivity by using intact platelets as targets. Bound antibody is then detected with a fluorescent secondary antibody specific for both IgG and IgM. The resultant geometric mean fluorescence intensity (gMFI), normalized to that of control target platelets receiving no serum, can be compared to a normal range derived from measurements of multiple control sera. While this method lacks the enzymatic signal amplification of an ELISA, it effectively amplifies signals via the copy numbers of the targeted antigens.
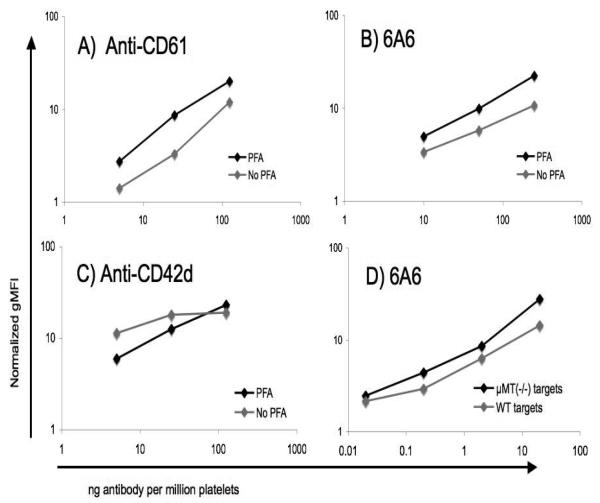
Because paraformaldehyde (PFA) fixation of target platelets has been shown to result in sensitive detection of antiplatelet antibodies in other contexts[17], we assessed whether its use enhanced the sensitivity of flow cytometric detection. For two of three antibodies tested, we found a consistently greater signal strength with PFA-fixed targets (figure 1).
Figure 1. Effect of formalin fixation and μMT(−/−) target platelets on antiplatelet antibody assay sensitivity.
A, B, C) WT platelets were briefly fixed in 1% paraformaldehyde (PFA), or not, and exposed to the indicated amounts of the antibodies shown. Antibody binding was then detected with PE anti-hamster IgG (A, C) or FITC goat-anti-mouse IgG/M (B). D) Paraformaldehyde fixed WT platelets were exposed to the indicated amounts of 6A6 antibody, and antibody binding was detected with FITC goat-anti-mouse IgG/M. All geometric mean fluorescence intensities in the figure were normalized to controls receiving no primary antibody.
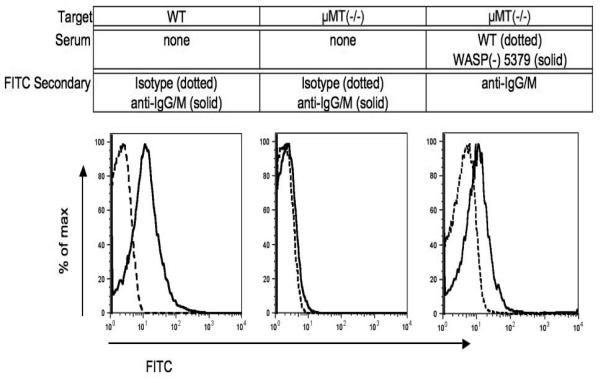
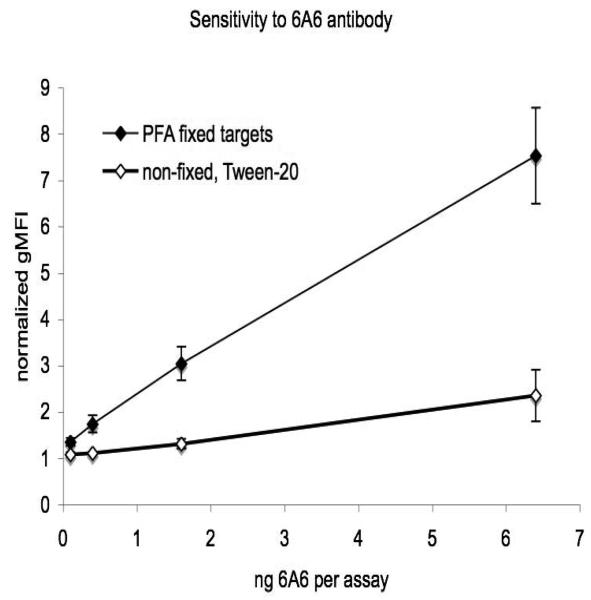
Human platelets show significant “background” levels of immunoglobulins[21], as do murine platelets (figure 2). Because this high background could reduce the sensitivity of the assay, we assessed the use of PFA-fixed target platelets from mice lacking circulating antibodies (μMT(−/−)). μMT(−/−) mice fail to express the heavy chain of IgM (μ chain), and as a consequence their B-cells are absent[19]. This also increased the sensitivity of detection of a test antibody (6A6), although the effect was slight (figure 1). With the use of both PFA fixation and μMT(−/−) targets, the method consistently detects less than 1 ng of 6A6 antibody (Figure 3).
Figure 2. Background and serum-derived antibody detection on target platelets.
Paraformaldehyde-fixed platelets, untreated or exposed to 0.03 ul per 0.5 million platelets of the indicated sera shown, were exposed to the secondary antibodies shown as described in materials and methods.
Figure 3. Antiplatelet antibody assay sensitivity.
Antiplatelet antibody assays were performed as described in figures 1 and 2. The indicated quantities of 6A6 antibody were added per assay (0.5 × 106 μMT(−/−) target platelets per assay). Data for PFA fixed targets is from 10 consecutive experiments in which 6A6 was used as a positive control. Non-fixed target data are means of four assays from two separate experiments. Error bars are standard errors.
Antibody incidence
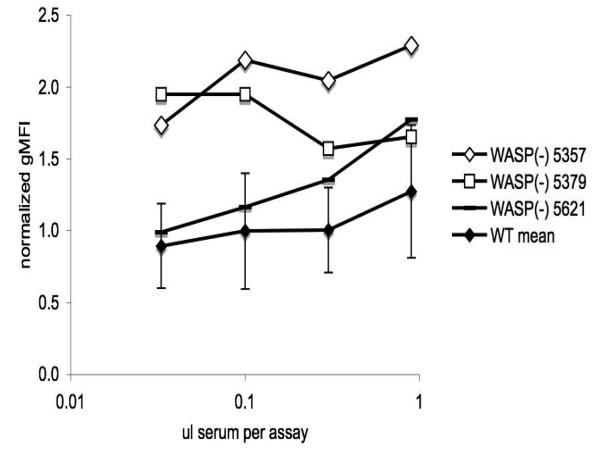
We determined a normal range of results for the assay, at several serum dilutions, by testing sera from age-matched WT B6 males. We then tested sera from multiple WASP(−) males and found several which induced normalized gMFI values well above the normal range at multiple serum dilutions. Examples are shown in figure 4. To compile and analyze these results for multiple serum samples, we categorized them at each dilution as “antibody positive” if they fell more than two standard deviations (SD) above the WT mean (as shown in figure 4). A finding of three consecutive serum dilutions inducing normalized gMFI values more than one SD above the mean was also considered positive. We defined the “detection threshold” as the volume of serum (per assay) at or above which antibody is detected.
Figure 4. Detection of serum antiplatelet antibodies.
Paraformaldehyde fixed μMT(−/−) platelets were exposed to the indicated amounts of test sera from three wasp− males (tag numbers 5357, 5379, and 5621). Antibody binding was detected with FITC goat-anti-mouse IgG/M. Geometric mean fluorescence intensity (gMFI) values were normalized to concurrent controls receiving no test sera. WT mean values are for sera from 12 males (set 1, table 2). Error bars are two standard deviations. Inferred detection thresholds are 0.03 ul (5357, 5379) and 0.3 ul (5621).
A weak positive correlation was seen between WT donor age and nGMFI at each serum dilution. Linear adjustment of the normal range to be used as a function of the age of the “test” serum donor had no effect on detection threshold results for any of the sera tested (data not shown).
We evaluated detection thresholds for sera from 37 WASP(−) males, and correlated them with platelet and reticulated platelet counts. 9 of the mice were selected because they showed evidence of clearance-inducing antiplatelet antibodies (the “CIAA” subset). The rest were selected randomly from mice not showing such evidence. Criteria for CIAA were A) unusually low platelet counts (>1SD below the WASP(−) mean, i.e. < 435×109/L), AND B) unusually high percent RP (> 1SD above the WT mean, i.e. > 6.56%). The mean and SD values used to generate these criteria are shown in table 1. The WT mean and SD were chosen for the second criterion because they derive from a unimodal distribution lacking the “tail” of high RP values seen with the WASP(−) values (data not shown). By these two criteria, 7% (5 of 70) of the WASP(−) mice we previously described[16] are in the CIAA subset.
Table 1.
Platelet and reticulated platelet count reference data
| age at assay | retic plts (%) | platelets (×10E9/L) |
||
|---|---|---|---|---|
| WASP(−) | mean | 9.2 | 5.98 | 578 |
| median | 8.60 | 5.28 | 588 | |
| n | 70 | 70 | 70 | |
| sd | 3.3 | 2.88 | 153 | |
| WT | mean | 12.1 | 5.53 | |
| median | 11.2 | 5.27 | ||
| n | 34 | 34 | ||
| sd | 2.9 | 1.03 | ||
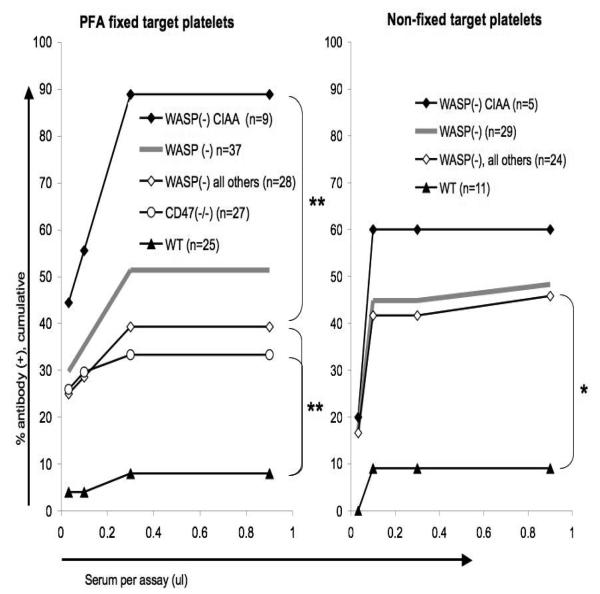
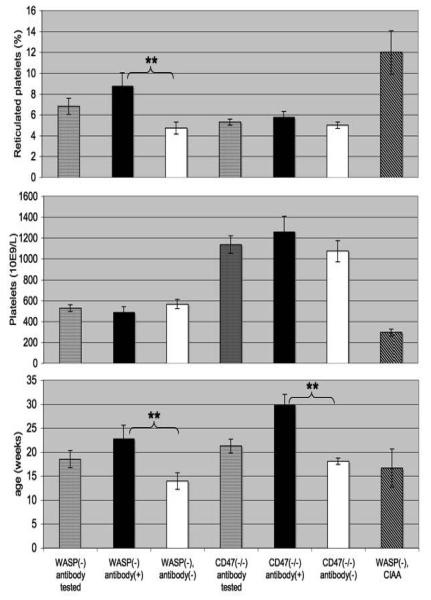
We found an overall antibody incidence exceeding 50% in the WASP(−) males tested (figure 5, left). The CIAA subset showed a significantly higher incidence than did those not in that subset. Antibody(+) mice showed a higher mean %RP than antibody(−) mice, were older, and did not show a significantly lower platelet count (figure 6). In multivariate analysis, antibody incidence is associated both with % RP (p=.0153) and age (p=.0264). %RP is not correlated with age (Pearson correlation=.25, p=.1311).
Figure 5. Serum antiplatelet antibody detection thresholds.
Cumulative incidences of antibody detection are shown for sera with detection thresholds at or below four serum volumes. Asterisks denote significant differences (** <0.01; * <0.05, fisher's exact test, two tailed, comparing the pooled positive rates for all dilutions in each group).
Figure 6. Platelet counts, reticulated platelet counts, and ages of the mice tested.
Results are for the mice used in figure 5 (left). Error bars are standard errors. Asterisks denote significant differences (** <0.01, student's t-test).
Because CD47(−/−) mice are prone to autoimmunity, and to increased platelet clearance after infusion of an antiplatelet antibody[22], we evaluated sera from these mice as well. Our results show a significant incidence of such antibodies (figure 5, left). The antibody(+) mice were older than the antibody negative mice (figure 6). In multivariate analysis, however, antibody incidence for these mice is not significantly associated with age (p= .17) due to a weak concurrent correlation between antibody incidence and %RP (p = 0.20). % RP is again not correlated with age (p=0.13, Pearson correlation = 0.30). We also tested several sera from WASP(−)CD47(−/−) males we had derived previously[16], and found an incidence of antibody similar to that seen in WASP(−) males (data not shown).
Antibody characteristics
We were initially unable to detect antibodies when we exposed several antibody(+) sera to target μMT(−/−) platelets which had not been treated with PFA (data not shown). This raised the question of whether some of the antibodies we detected are directed at antigens internal to the PFA-treated target platelets. After modifying the assay conditions via the use of a non-ionic detergent (Tween-20) and a different source of ‘carrier’ serum, however, we were able to detect antibodies in sera from WASP(−) males at a similar rate using non-PFA fixed target platelets (figure 5, right). The normal range was again established with a set of WT sera. The two methods did not agree in all cases (Table 3). As an example, use of non-fixed targets in the presence of Tween-20 results in much less sensitive detection of 6A6 antibody(figure 3).
Table 3.
Comparison of results obtained with fixed vs. non-fixed target platelets
| PFA fixed | ||||
|---|---|---|---|---|
| + | − | total | ||
| Non-fixed Tween-20 |
+ | 6 | 1 | 7 |
| − | 4 | 7 | 11 | |
| total | 10 | 8 | 18 | |
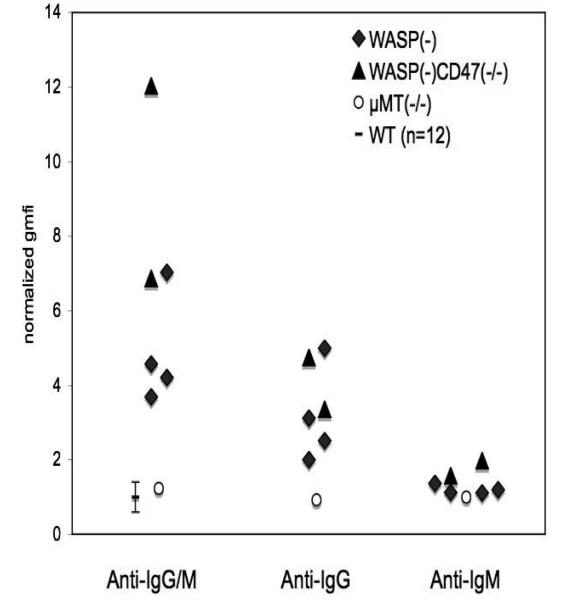
We used secondary antibodies specific for mouse IgG or IgM to evaluate several of the antibody positive sera. All of those tested demonstrate IgG antibodies, although we cannot rule out low levels of concurrent IgM in some cases (figure 7).
Figure 7. Classes of antibodies detected.
Sera which were positive for antibody using FITC anti-IgG/M secondary antibody (as described in figure 4) were evaluated via the same method (0.2 ul per assay) with two additional secondary antibodies, FITC-anti-IgG (polyclonal) and FITC anti-IgM (monoclonal). Sera from μMT(−/−) males were used as internal negative controls. WT data is from figure 4 (0.1 ul per assay) +/− 2SD.
In vivo effects of specific and non-specific antibodies on platelet clearance
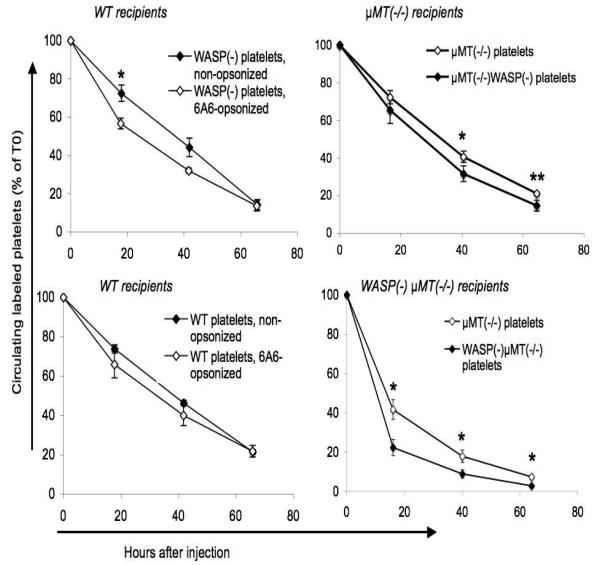
We previously showed that the in vivo consumption rate of WASP(−) platelets is more accelerated by opsonization with an anti-CD61 antibody than is that of WT platelets[16]. We have confirmed this finding with a second antibody, 6A6 (figure 8, left). 6A6 targets two presumably related, uncharacterized murine platelet surface proteins of approximately 100 kDa and 43 kDa[17], and is known to induce rapid in vivo platelet clearance[23]. It was originally described as an IgG1[17], but was subsequently shown to be in the IgG2a class [23].
Figure 8. In vivo platelet clearance.
Platelets labeled with fluorescent markers were injected via tail vein. The fraction of labeled platelets was determined at 5 minutes after injection (T0) and at the intervals shown. All error bars are standard errors. Left: Effect of opsonization with 6A6 antibody on platelet clearance. CMFDA-labeled platelets were opsonized with 6A6 antibody and injected into WT recipients. Platelets were identified via log forward vs. log side scatter. N=3 for each type of injected platelet. Right: Effect of μMT(−/−) genotype on platelet clearance. Platelets were labeled with either CMFDA or BMQC. Mixtures of the two genotypes shown in the top or bottom figures were injected into the recipients shown; their clearance was followed simultaneously. Platelets were gated using both log forward vs. log side scatter and a PE-anti-CD41 marker. N=5 recipients in each group. Asterisks indicate p values less than 0.05 (*) or 0.01 (**), (student's two-tailed t-test).
Non-opsonized WASP(−) platelets are also consumed more rapidly in vivo than WT platelets, although the effect is of significantly greater magnitude in WASP(−) recipients[16]. These findings raised the question of whether the rapid clearance of non-opsonized WASP(−) platelets is dependent on the normal “background” level of platelet surface antibodies.
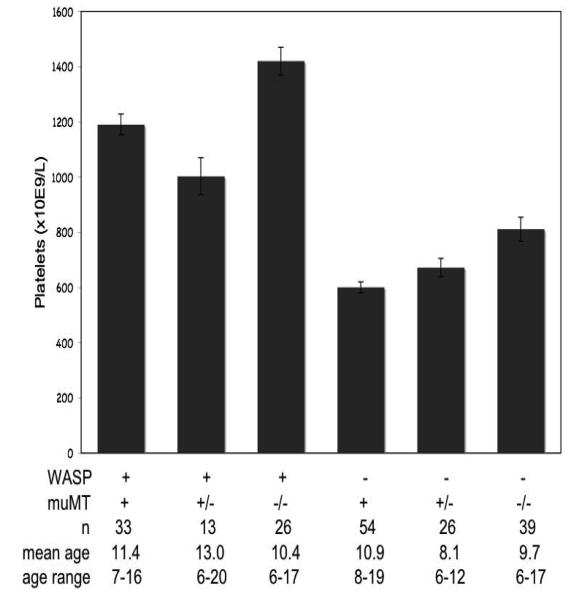
To address this question, we eliminated platelet surface antibodies from the system by crossing WASP(−) mice onto the μMT(−/−) background. Removal of antibodies from the WASP(−) mice by this method resulted in only a slight increase in platelet counts, comparable to that seen when we remove them from WT mice (figure 9). In vivo clearance rates of WASP(−) platelets in the absence of platelet surface antibodies remained significantly greater than those of WT platelets, although as on the B6 background this effect is minimal in WASP(+) recipients (figure 8, right). WT platelet clearance rates were also increased in WASP(−) recipients, as we previously reported[16]. Clearance in this experiment was measured using both CMFDA and BMQC-labeled platelets; concurrent control studies showed no effect of either marker on clearance rates relative to the other (data not shown). As is true on the B6 background, WASP(−)μMT(−/−) males demonstrate splenomegaly in association with markedly enhanced extramedullary hematopoiesis (data not shown).
Figure 9. Effect of μMT− genotypes on platelet counts in WASP− and WASP(+) males.
Mean platelet count values are shown. Error bars are standard errors.
Discussion
The thrombocytopenia of WAS is thought to be due to both reduced platelet production and increased platelet destruction, the latter due to an intrinsic platelet defect. A murine model of WAS demonstrates a milder thrombocytopenia, rapid platelet consumption, and a significant increase in bone marrow and splenic megakaryocytes. It is not clear whether platelet production is reduced in the murine model[16], although platelet yield per megakaryocyte is likely to be reduced.
To determine whether anti-platelet antibodies contribute to the thrombocytopenia of murine WAS, we have quantified the binding of serum antibodies to target platelets lacking surface antibodies. We find such antibodies in approximately 40% of WASP− males. 5-10% of WASP− males show a more severe thrombocytopenia and an increased fraction of reticulated platelets. We detected serum antibodies in 90% of the latter mice. This supports the biological significance of a subset of the antibodies we detected. Also, the in vivo clearance of WASP− platelets is more accelerated by antibody opsonization with either of two antibodies (anti-CD61[16] or 6A6) than is that of WT platelets. The increased in vivo clearance rate correlates with increased ex vivo phagocytosis of antibody opsonized WASP− vs. WT platelets[16]. Thus WASP deficiency results both in an increased incidence of antiplatelet antibodies and an increased sensitivity to antibody induced platelet clearance.
Like WAS, CD47 deficiency predisposes toward autoimmunity[24]. In this case the mechanism is better characterized: CD47, on red cells or platelets, binds a macrophage surface protein (SIRP-alpha) which subsequently inhibits signal transduction through macrophage Fc receptors. The absence of CD47 therefore promotes phagocytosis of antibody opsonized red cells or platelets. This presumably promotes autoimmunity by augmenting host antigen presentation, although this mechanism has not been clearly established. It is tempting to speculate that WASP deficiency could promote autoimmunity by a similar mechanism.
Paraformaldehyde fixation of the platelets used as targets in the assay could in principle allow detection of (presumably biologically irrelevant) antibodies specific for internal antigens. However, we detected antibodies at a similar rate using a modified method targeting non-fixed platelets. Nonspecific binding of antigen-antibody complexes to the target platelets could generate similar findings, but the lack of an Fc receptor on murine platelets makes this unlikely.
Most WASP− mice do not express detectable antiplatelet antibodies. To ask whether the normal background level of antibodies on their platelets is required for the rapid clearance of these platelets, we generated WASP− males deficient in antibody production. Platelet counts were only mildly increased in these animals, and rapid platelet consumption was again seen. As we observed previously[16], rapid consumption was largely dependent on WASP deficiency in trans (it is difficult to detect in WASP(+) recipients), whether on a WT or μMT(−/−) background. Normal levels of platelet surface antibodies therefore appear to play no role in the thrombocytopenia.
These results suggest that platelet WASP deficiency induces rapid platelet consumption by two physiologic mechanisms: an antibody independent mechanism, which is largely dependent on WASP deficiency in trans, and an antibody-dependent mechanism, which does not require WASP deficiency in trans.
The antibody independent mechanism appears to predominate in murine WAS. No molecular mechanism for it has been identified; deficiencies in the platelet actin cytoskeleton have not been consistently seen in WASP− platelets[25]}[26, 27]. It is also not clear what mediates the trans effect. The splenomegaly seen in WASP− mice, in association with a marked increase in splenic hematopoiesis[28], is not likely to do so, as the clearance rate of WASP− platelets in splenectomized WASP− mice is significantly greater than that of WT platelets in splenectomized WT mice[16]. That result might have been expected, since splenomegaly, if secondary only to increased EMH, does not by itself induce rapid platelet clearance[29, 30]. The trans effect cannot be due to thrombocytopenia itself, since splenectomy nearly normalizes platelet counts in WASP− mice but, again, does not normalize platelet clearance rate[16]. An understanding of the trans effect is likely to require studies of which cells, in which organs, take up these platelets rapidly.
We have not detected, in those WASP− mice with evidence of CIAA, increased levels of platelet associated antibodies (data not shown). Possible reasons for this include the rapid turnover of antibody-opsonized platelets in these animals, and the ability of platelets to clear surface antibodies[31, 32]. If binding of the antibodies we have detected to platelets in vivo results in a race toward those two outcomes, the fraction of platelets demonstrating increased levels of surface antibodies could be small.
Given the strikingly high incidence of other forms of autoimmunity in WAS, it would be surprising if WAS patients did not develop antiplatelet antibodies – particularly those patients with fluctuating or severe thrombocytopenia. Previous reports of increase platelet associated antibodies in WAS patients[12-15], however, are problematic due to the low specificity of that method (see, for example, Hezard et al (2008)[33]. Our results provide more reasons to try to detect such antibodies and evaluate their contribution to the thrombocytopenia of clinical WAS.
Table 2.
Ages of mice tested
| Antibody testing | ||||||
|---|---|---|---|---|---|---|
| WT, set 1 | WT, set 2 | WASP(−) | WASP(−), CIAA |
WASP(−), non-CIAA |
CD47(−/−) | |
| n | 12 | 13 | 37 | 9 | 28 | 27 |
| mean age | 19.3 | 22 | 18.4 | 16.7 | 19 | 21.3 |
| age range | 8.4 - 29.0 | 8.9 - 38.0 | 5.3 - 40.3 | 5.3 - 37.1 | 6.0 - 40.3 | 14.6 - 35.7 |
| Platelet counts: | ||||||
| WASP genotype | + | + | + | − | − | − |
| muMT genotype | + | +/− | −/− | + | +/− | −/− |
| n | 33 | 13 | 26 | 70 | 26 | 39 |
| mean age | 11.4 | 13 | 10.4 | 9.6 | 8.1 | 9.7 |
| age range | 7-16 | 6-20 | 6-17 | 6-19 | 6-12 | 6-17 |
Acknowledgements
This work was supported by NIH NHBLI awards 1 K08 HL72865 (TS) and 1R56AI71163, and R01AI071163 (DJR, TS); The Department of Veterans Affairs (Merit Review, TS); Research Inc., a nonprofit corporation associated with the Memphis VA Medical Center (Memphis, TN). Alexander Astrakhan is supported by the University of Washington's Cell & Molecular Biology training grant (T32GM007270) and the Molecular Medicine fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
No financial interest/relationships with financial interest relating to the topic of this article have been declared
References
- 1.Baldini MG. Nature of the platelet defect in the Wiskott-Aldrich syndrome. Ann N Y Acad Sci. 1972;201:437–444. doi: 10.1111/j.1749-6632.1972.tb16316.x. [DOI] [PubMed] [Google Scholar]
- 2.Grottum KA, Hovig T, Holmsen H, Abrahamsen AF, Jeremic M, Seip M. Wiskott-Aldrich syndrome: qualitative platelet defects and short platelet survival. Br J Haematol. 1969;17:373–388. doi: 10.1111/j.1365-2141.1969.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 3.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–252. [PubMed] [Google Scholar]
- 4.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66:1105–1109. [PubMed] [Google Scholar]
- 5.Kajiwara M, Nonoyama S, Eguchi M, et al. WASP is involved in proliferation and differentiation of human haemopoietic progenitors in vitro. Br J Haematol. 1999;107:254–262. doi: 10.1046/j.1365-2141.1999.01694.x. [DOI] [PubMed] [Google Scholar]
- 6.Luthi JN, Gandhi MJ, Drachman JG. X-linked thrombocytopenia caused by a mutation in the Wiskott-Aldrich syndrome (WAS) gene that disrupts interaction with the WAS protein (WASP)-interacting protein (WIP) Exp Hematol. 2003;31:150–158. doi: 10.1016/s0301-472x(02)01023-8. [DOI] [PubMed] [Google Scholar]
- 7.Sabri S, Foudi A, Boukour S, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–140. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- 8.Dupuis-Girod S, Medioni J, Haddad E, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–627. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 9.Humblet-Baron S, Sather B, Anover S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117:407–418. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullen C, Anderson KD, Blaese, RM. Splenectomy and/or bone marrow transplantation in the management of the wiskott-aldrich syndrome: long-term followup of 52 cases. Blood. 1993;82:2961–2966. [PubMed] [Google Scholar]
- 11.Notarangelo LD, Mazza C, Giliani S, et al. Missense mutations of the WASP gene cause intermittent X-linked thrombocytopenia. Blood. 2002;99:2268–2269. doi: 10.1182/blood.v99.6.2268. [DOI] [PubMed] [Google Scholar]
- 12.Corash L, Shafer B, Blaese RM. Platelet-associated immunoglobulin, platelet size, and the effect of splenectomy in the Wiskott-Aldrich syndrome. Blood. 1985;65:1439–1443. [PubMed] [Google Scholar]
- 13.Kanegane H, Nomura K, Miyawaki T, et al. X-linked thrombocytopenia identified by flow cytometric demonstration of defective Wiskott-Aldrich syndrome protein in lymphocytes. Blood. 2000;95:1110–1111. [PubMed] [Google Scholar]
- 14.Litzman J, Jones A, Hann I, Chapel H, Strobel S, Morgan G. Intravenous immunoglobulin, splenectomy, and antibiotic prophylaxis in Wiskott-Aldrich syndrome. Arch Dis Child. 1996;75:436–439. doi: 10.1136/adc.75.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semple JW, Siminovitch KA, Mody M, et al. Flow cytometric analysis of platelets from children with the Wiskott-Aldrich syndrome reveals defects in platelet development, activation and structure. Br J Haematol. 1997;97:747–754. doi: 10.1046/j.1365-2141.1997.1132938.x. [DOI] [PubMed] [Google Scholar]
- 16.Prislovsky A, Marathe B, Hosni A, et al. Rapid platelet turnover in WASP(−) mice correlates with increased ex vivo phagocytosis of opsonized WASP(−) platelets. Exp Hematol. 2008;36:906–906. doi: 10.1016/j.exphem.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizutani H, Engelman RW, Kurata Y, Ikehara S, Good RA. Development and characterization of monoclonal antiplatelet autoantibodies from autoimmune thrombocytopenic purpura-prone (NZW × BXSB)F1 mice. Blood. 1993;82:837–844. [PubMed] [Google Scholar]
- 18.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 20.Ault KA, Rinder HM, Mitchell J, Carmody MB, Vary CP, Hillman RS. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am J Clin Pathol. 1992;98:637–646. doi: 10.1093/ajcp/98.6.637. [DOI] [PubMed] [Google Scholar]
- 21.George JN. Platelet immunoglobulin G: its significance for the evaluation of thrombocytopenia and for understanding the origin of alpha-granule proteins. Blood. 1990;76:859–870. [PubMed] [Google Scholar]
- 22.Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 24.Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leuk Lymphoma. 2004;45:1319–1327. doi: 10.1080/1042819042000201989. [DOI] [PubMed] [Google Scholar]
- 25.Gross BS, Wilde JI, Quek L, Chapel H, Nelson DL, Watson SP. Regulation and function of WASp in platelets by the collagen receptor, glycoprotein VI. Blood. 1999;94:4166–4176. [PubMed] [Google Scholar]
- 26.Rengan R, Ochs HD, Sweet LI, et al. Actin cytoskeletal function is spared, but apoptosis is increased, in WAS patient hematopoietic cells. Blood. 2000;95:1283–1292. [PubMed] [Google Scholar]
- 27.Falet H, Hoffmeister KM, Neujahr R, Hartwig JH. Normal Arp2/3 complex activation in platelets lacking WASp. Blood. 2002;100:2113–2122. [PubMed] [Google Scholar]
- 28.Andreansky S, Liu CH, Turner SJ, et al. WASP- mice Exhibit Defective Immune Responses to Influenza A Virus, Streptococcus pneumoniae, and Mycobacterium bovis BCG. Experimental Hematology. 2005;33:443–451. doi: 10.1016/j.exphem.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Birks JW, Klassen LW, Gurney CW. Hypoxia-induced thrombocytopenia in mice. J Lab Clin Med. 1975;86:230–238. [PubMed] [Google Scholar]
- 30.McDonald TP, Clift RE, Cottrell MB. Large, chronic doses of erythropoietin cause thrombocytopenia in mice. Blood. 1992;80:352–358. [PubMed] [Google Scholar]
- 31.Santoso S, Zimmermann U, Neppert J, Mueller-Eckhardt C. Receptor patching and capping of platelet membranes induced by monoclonal antibodies. Blood. 1986;67:343–349. [PubMed] [Google Scholar]
- 32.White JG, Escolar G. Induction of receptor clustering, patching, and capping on surface-activated platelets. Lab Invest. 1990;63:332–340. [PubMed] [Google Scholar]
- 33.Hezard N, Simon G, Mace C, Jallu V, Kaplan C, Nguyen P. Is flow cytometry accurate enough to screen platelet autoantibodies? Transfusion. 2008;48:513–518. doi: 10.1111/j.1537-2995.2007.01556.x. [DOI] [PubMed] [Google Scholar]