Abstract
Background and Objective
Inflammation is pivotal in atherosclerosis. C-reactive protein (CRP), in addition to being a cardiovascular risk marker may also be pro-atherogenic. We have previously shown that in addition to the liver, human aortic endothelial cells (HAEC) synthesize and secrete CRP. While CRP levels are increased in obesity, metabolic syndrome and diabetes, levels of adiponectin are reduced in these conditions. We tested the hypothesis that adiponectin reduces CRP synthesis and secretion in HAECs under normoglycemic (5.5mM glucose) and hyperglycemic conditions (15mM glucose).
Methods and results
Adiponectin dose-dependently reduced CRP mRNA and protein from HAEC. Adiponectin treatment of HAEC significantly decreased Iκb phosphorylation and NFκb binding activity. There was no effect of adiponectin on STAT or C/EBP transcriptional activity. Adiponectin also activated AMP kinase resulting in decreased NFκb activity and decreased CRP mRNA and protein. These effects of adiponectin were mimicked by AICAR, an activator of AMPK and reversed by inhibition of AMPK. Thus, adiponectin reduces CRP synthesis and secretion from HAEC under hyperglycemia via up regulation of AMP kinase and down regulation of NFκb. Similar findings were observed in rat primary hepatocytes.
Conclusions
Thus, in obesity and diabetes, the hypoadiponectinemia could exacerbate the pro-inflammatory state by inducing CRP production.
Introduction
Inflammation is pivotal in all phases of atherogenesis (1, 2). CRP, the prototypic marker of inflammation in man, has been shown in several studies to be a cardiovascular risk marker with high levels of CRP predicting cardiovascular events (1-3). Much recent data challenges the dogma that CRP is exclusively produced by the liver (3). Indeed, cogent data suggest that it is produced in atherosclerotic lesions, the kidney, neurons and alveolar macrophages (4, 5). mRNA and protein for CRP is expressed in arterial plaque tissue and both CRP mRNA and protein levels are 10-fold higher in plaque when compared to the normal artery, suggesting that CRP is produced in atherosclerotic lesions (6). Furthermore, we showed that human aortic endothelial cells synthesize and secrete CRP (7). The most potent agonist for CRP production from HAEC is the combination of IL-1 and IL-6 (8). In addition, the secretion of CRP is augmented 100-fold in presence of macrophage conditioned media (MCM) (7). Thus, stimulated synthesis and secretion of CRP by cells in the atherosclerotic lesion by paracrine/autocrine loops could result in local concentrations of CRP far in excess of plasma concentrations and could contribute to proinflammatory, proatherogenic effects. This could contribute to the poorer prognosis in patients with elevated CRP levels and acute coronary syndromes (9, 10). Furthermore, the adipose tissue, previously thought to be an inert triglyceride depot has been shown to produce numerous adipokines including adiponectin.
Adiponectin is a potent adipocytokine (11-16). Decreased levels of adiponectin are found in obesity, type 2 diabetes and coronary artery disease. Low levels of adiponectin are inversely associated with high levels of CRP. Furthermore, adiponectin has been shown to up regulate eNOS, decrease cytokine and chemokine synthesis from HAEC. Recent studies have shown that recombinant globular adiponectin, a proteolytic cleavage product of total adiponectin is pharmacologically active, up regulates eNOS in vascular endothelial cells, and decreases atherosclerosis in apo E-/- mice (13, 16, 17). Also, there is emerging data indicating that CRP impairs insulin signaling and adiponectin is able to improve insulin sensitivity (18,19). A strong and inverse correlation has also been documented between CRP and adiponectin mRNA in human adipose tissue (20). Thus, adiponectin could be regulating CRP. However, there is a paucity of data examining the effect of adiponectin on CRP synthesis and secretion from HAEC. In this study, we provide novel data that CRP synthesis and secretion from HAEC is augmented under hyperglycemia and that pretreatment with adiponectin significantly down regulates synthesis and secretion of CRP from HAEC.
Methods
Cell Culture
HAEC were used between passages 3-5 and cultured in normoglycemic media (NG, 5.5mM glucose) in presence or absence of globular adiponectin ( 0-10μg/mL) in 12-well culture plates. Also, total adiponectin was used in some studies. Both total and globular adiponectin were obtained from Peprotech Inc, Rocky Hill, NJ. HAEC were incubated in serum-and growth factor-free media for the expression of CRP under normoglycemic conditions (5.5mM glucose) or hyperglycemic conditions (15mM) for 24 or 48 hours and RNA or protein/media were collected for CRP measurements, respectively. Mannitol was used as hyperosmolar control. All media were endotoxin-free and adiponectin was passed through Detoxigel.
For experiments with adiponectin, endothelial cells were pre-treated with adiponectin, (0,2.5,5 and 10μg/mL) for 2 hours before incubating in hyperglycemic conditions. Concentrations of adiponectin that have been used are physiological as reported previously(12-14, 18).
CRP Protein Expression
The cells were collected in M-PER (Pierce Biotechnologies, CA). Western blotting for CRP was performed as described previously (7) using rabbit anti-human CRP (Calbiochem, San Diego, CA) antibody. Western blotting for phospho p65, phospho p38MAPK and phospho AMPK was performed with monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA and Abcam Inc, Cambridge, MA respectively) and ß-actin (mouse monoclonal antibody; Sigma Aldrich, St. Louis, MO) or the respective unphosphorylated total antibody (anti- p65, anti-p38 MAPK from Santa Cruz Biotechnology, Santa Cruz, CA and anti-AMPK antibody from Abcam Inc, Cambridge, MA) was used as an internal control (7).
Secreted CRP by ELISA
CRP levels in the supernatants of HAEC (pooled from 3 different wells and concentrated) were measured using an enzyme-linked immunosorbent assay (ELISA) specific for human CRP (Alpco Laboratories, Windham, NH) as described previously and reported as ng/mg cell protein (7).
CRP mRNA
CRP mRNA was assessed by semi quantitative RT-PCR and real time RTPCR. RNA was isolated from the cells using TRIzol(Invitrogen, Carlsbad, CA). For the RT reaction, 1μg of total RNA was used based on the standard curve, to synthesize the cDNA. For semi-quantitative PCR, the primers used for human CRP were: forward: 5'TCG TAT GCC ACC AAG AGA CAA GAC A 3' and reverse: AAC ACT TCG CCT TGC ACT TCA TAC T 3' and 18s RNA primer pair was obtained from R&D (Minneapolis, MN). PCR reaction was performed using Invitrogen master mixes as previously described (7, 21) using 50ng of cDNA. CRP and 18s RNA yield a band between 400-600bp, were resolved on a 2% agarose gel. Band intensities were determined using Image quant software (GE Healthcare and Biosciences, Piscataway, NJ) (7). For real time RT-PCR, we used 25ng of cDNA for PCR reaction. Human CRP (amplicon size 80bp, assay id# Hs00357041_m1) and 18s (amplicon size 187bp; assay id#:Hs99999901-s1) gene expression assays were purchased from Applied Biosystems (Foster City, CA) and followed the recommended protocol. No RT, no template, and positive controls were run in parallel. The optimal amount of RNA was determined by generating a standard curve using serial dilutions of control RNA (concentration of RNA vs Ct values) with fixed number of cycles, and subjecting the PCR products to gel electrophoresis for further confirmation using 18s RNA as the reference.Data were calculated using the 2-ΔΔCT method and presented as CRP/18s mRNA ratio normalized to mannitol/low glucose.
NFκb, STAT, C/EBP Binding Activity Assay
Activation of NFκb, STAT, C/EBP in nuclear extracts was determined using TransAM assay (Active Motif, Carlsbad, CA). Nuclear extracts were suspended in TransAM lysis buffer and nuclear proteins (5 μg total protein) were incubated with immobilized oligonucleotides containing the NFκb consensus DNA-binding site (5'-GGGACTTTCC-3') for 1 hour at room temperature. After washing, 100 μL of p65 subunits monoclonal antibody (1:1000 dilutions) were added for 1 hour at room temperature. After 3 washes, 100 μL of horseradish-peroxidase-conjugated secondary antibody (1:1000 dilutions) were added to each well for 1 hour at room temperature. The absorbance at 450 nm was determined using a standard for NFκb. STAT and C/EBP activities were examined similarly using the respective Trans-AM kits (21,22).
Luciferase transactivation assay- Deletion promoter constructs and mutants were obtained from the Samols laboratory. HAEC (50-80% confluent) were transfected with 2 μg of DNA (1 μg luciferase reporter-CRP promoter construct and 1 μg transcription factor expression vector). Luciferase transactivation assays were performed utilizing CRP promoter constructs with mutated NFκb sites to evaluate their role in mediating the effect of HG and/or adiponectin. After 24 hours, luciferase assays were performed (Promega, Madison, WI). Luciferase activity was normalized to the protein concentration of the extract measured by using a BioRad DC protein assay kit.
Isolation of rat primary hepatocytes-Primary cultures of rat hepatocytes were prepared by the method of Gores et al. (23). Briefly, rats were anesthetized, and isolated livers were perfused via the portal vein with Ca2+- and Mg2+-free HEPES buffer. Subsequent perfusion included 1.0 mM Ca2+ and 0.02% collagenase. The livers were then gently raked, the cell suspension was centrifuged, and the resulting cell pellet was resuspended in DMEM containing 0.1% BSA, 200U/ml penicillin, and 200mg/ml streptomycin. The cells were plated at a density of 5 × 105 cells on rat tail collagen-coated plates in absence and presence of adiponectin and challenged with IL-1+IL-6 as described previously (7).
Statistical analyses was performed using GraphPad Prizm software. Analysis of variance followed by paired t-tests were used to determine significant differences between treatments, and significance was set at P < 0.05.
Results
Adiponectin decreases CRP synthesis and secretion induced by HG in HAEC
We demonstrate that CRP mRNA and protein are significantly increased under hyperglycemic (HG) conditions and that CRP protein and mRNA are inhibited by pretreatment with globular adiponectin (Fig 1a and Fig 1b). Furthermore, secreted CRP is significantly increased in HG conditions and treatment with both globular (dose-dependently) and total adiponectin inhibited CRP secreted protein induced by hyperglycemia (Fig 1c).
Figure 1.

Effect of Adiponectin on HG-induced CRP synthesis and secretion (n=5). HAEC were incubated with mannitol, NG/HG (5.5/15mMglucose) in absence and presence of globular or total adiponectin.
a. Representative RT-PCR with CRP/18S densitometric ratios.
b. Representative Western blot with CRP/β-actin ratios.
c. Secreted CRP.
; *p<0.01 vs NG; #p<0.05 vs HG
Effect of adiponectin on transcriptional factors involved in CRP synthesis
Since the promoter for CRP in hepatocytes contains binding elements for NFκb, STAT and C/EBP, we examined the effect of HG on these 3 transcriptional factor activities, in absence and presence of adiponectin. While HG up regulated STAT-1 (Fig 2a), C/EBP-β (Fig 2b) and NFκb (Fig 2c) activities in nuclear extracts of HAEC, adiponectin failed to affect STAT and C/EBP-β activities. However there was a significant down regulation of NFκb transcriptional activity following pretreatment with adiponectin (Fig 2c).
Figure 2.

Effect of Adiponectin on HG- induced nuclear transcription factor activity: HAEC were incubated with NG/HG ± adiponectin.
2a). STAT1 and STAT3; 2b). C/EBPβ; 2c). NFκb. *p<0.01 vs control.
*p<0.001 vs NG and #p<0.01 vs HG.
2d). A representative blot of phospho p65 expression with densitometric ratios of pp65/p65 (n=3).
Furthermore, in nuclear extracts, adiponectin pre- treatment of HAEC (0-10μg/mL for 24 hrs) significantly decreased phosphorylation of p65 induced by HG (Fig 2d).
Loss of NFκb abrogates HG-induced CRP synthesis in HAEC
To confirm the role of NFκb, we transfected HAEC with dominant negative NFκb or control vector. When NFκb was inhibited, there was significant decrease in CRP synthesis and secretion with HG (Figs 3 a and 3b). Furthermore, luciferase transactivation assays performed utilizing CRP promoter constructs with mutated NFκb sites (PC3) resulted in loss of CRP promoter activity in presence of HG indicating the critical involvement of NFκb in CRP transcription in HAEC (please see http://atvb.ahajournals.org online supplement).
Figure 3.
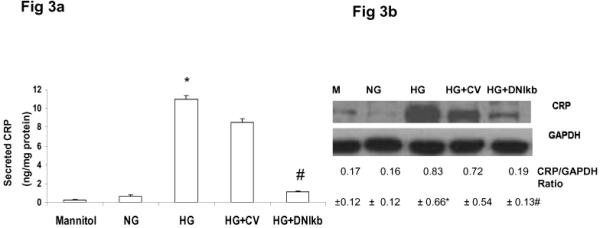
Loss of NFκb abrogates HG-induced CRP: HAEC were transiently transfected with control or dominant negative Iκb vector for 24 hours in presence of NG or HG.
3a). Secreted CRP
3b). Representative RT-PCR gel of CRP with ratios.
*p<0.001 compared to mannitol/NG; #p<0.01 vs. HG, n=3.
Effect of Adiponectin on MAPK in HAEC
Since HG activated p38MAPK but not ERK/JNK, we tested the effect of adiponectin on HG-induced phosphorylation of p38MAPK and adiponectin failed to have any significant effect (data not shown).
Adiponectin Upregulates AMPK activity in HAEC
Several reports indicate that adiponectin also activates AMP kinase, we tested the effect of adiponectin on AMPK activity and subsequent CRP release under HG conditions. Adiponectin pretreatment resulted in significant up regulation in AMPK activity and this was mimicked by AICAR, 250 μM (AICAR is a cell-permeable adenosine analogue that can be phosphorylated to ZMP, an AMP analogue and known AMPK activator) (Fig 4a). In presence of Compound C, 100 nM, (AMPK inhibitor, Calbiochem) there was a significant reversal of adiponectin's effects on AMP kinase phosphorylation , NFκb activity and CRP mRNA and protein expression (Fig 4a-4d). Also pretreatment with either adiponectin or AICAR resulted in decreased NFκb binding (Fig 4d), and decreased CRP mRNA and protein (Fig 4b and Fig 4c respectively) and this was reversed with Compound C (Figs 4b-4d).
Figure 4.
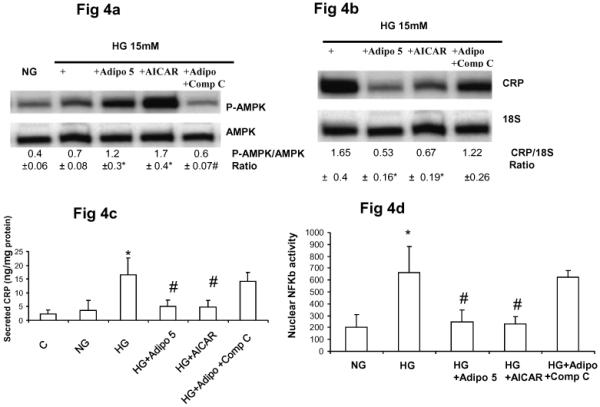
Effect of Adiponectin on HG-induced AMPK and NFκb activity in HAEC.
4a. Representative Western blot for pAMPK/AMPK with densitometric ratios.
4b. Representative CRP RT-PCR gel and densitometric ratios.
4c. Secreted CRP
4d. Nuclear NFκb DNA binding activity.
*p<0.01 compared to C, NG and #p<0.01 compared to HG, n=4 experiments.
Adiponectin decreases CRP synthesis in Primary Rat Hepatocytes
Lastly, since a large part of transcriptional regulation of CRP in hepatocytes has been studied in hepatoma cell lines (HepG2 or Hep3B cells), we isolated primary rat hepatocytes and examined the effect of adiponectin on IL-1 and IL-6 mediated CRP synthesis and secretion in these primary cells. As shown in Fig 5a, the combination of IL-1 and IL-6 resulted in significant up regulation in secreted CRP. This was abrogated in presence of adiponectin. Similar results were observed for CRP mRNA (data not shown). Furthermore, the combination of IL-1+IL-6 resulted in significant increase in C/EBP-β, STAT 3 and NFκb activities and adiponectin pre-incubation decreased NFκb activity (Fig 5b).
Figure 5.
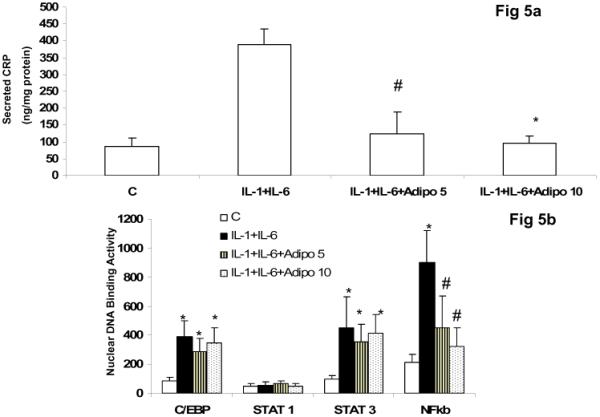
Effect of Adiponectin on CRP secretion in Primary Rat hepatocytes incubated with IL-1b and IL-6 in absence and presence of adiponectin.
5a. Secreted CRP levels *p<0.001 vs. IL-1+IL-6 and #p<0.05 vs. IL-1+IL-6.
5b. Nuclear C/EBP-beta, STAT1, STAT3 and NFκb activity.
*p<0.001 vs.Control and #p<0.05 vs. IL-1+IL-6; n=3.
Discussion
We report two novel findings in this manuscript. First, high glucose results in increased synthesis and secretion of CRP from human aortic endothelial cells and this appears to be via up regulation of NFκb. Secondly, we demonstrate that adiponectin abrogates the HG-induced CRP synthesis and secretion via up regulation of AMP kinase and down regulation of NFκb activity. Furthermore, we confirm these inhibitory effects of adiponectin on CRP synthesis in primary rat hepatocytes.
Conditions associated with diabetes and obesity exhibit increased cardiovascular morbidity and mortality. Thus, it is important to clarify the molecular pathways between fat accumulation and vascular disease. While adiponectin is expressed largely in adipose tissue, circulating levels of adiponectin are significantly decreased in obesity, diabetes, metabolic syndrome and CAD (11-17) . Conversely, hsCRP (high sensitivity CRP) levels are higher in these conditions of hypoadiponectinemia. High levels of CRP are associated with increased cardiovascular events (2,3). Also, CRP is produced locally in vascular tissue and this leads to concentrations 10-fold excess of that in plasma (1,4, 20). CRP has been shown to be produced in macrophages, in neurons and in the kidney and adipocytes. We have previously shown that CRP is synthesized and secreted by human aortic endothelial cells and that this is augmented several fold in presence of macrophage conditioned media, indicating that there is cross-talk between vascular cells, i.e. macrophages and endothelial cells resulting in increased inflammation (7). Ouchi has previously shown a strong inverse correlation between CRP and adiponectin mRNA (20). However, this does not imply cause and effect. In a recent report, it has been suggested that CRP inhibits adiponectin secretion in 3T3 cells (24). Since there are many contaminants associated with CRP and the authors do not appear to purify their CRP, this finding needs to be interpreted with caution. If confirmed, it will suggest that both adiponectin and CRP can modulate each others secretion and thus account for the pro-inflammatory burden of obesity and diabetes. We were unable to confirm the inhibitory effect of CRP on adiponectin using carefully purified CRP (25) ( data not shown).
Diabetes and metabolic syndrome are associated with increased inflammation (26-28). Both IL-1 and IL-6 are potent inducers of CRP synthesis and secretion (8). In this study, we show that under HG conditions, HAEC synthesize and secrete increased levels of CRP compared to normoglycemia. In addition, we demonstrate that adiponectin significantly abrogates HG-induced synthesis and secretion of CRP.
Adiponectin modulates endothelial function, improves endothelial NO, and has an inhibitory effect on proliferation of vascular smooth muscle cells induced by growth factors, inhibits collagen-induced platelet aggregation, suppresses foam cell formation via inhibiting scavenger receptor A and has direct effects on improving liver and muscle insulin sensitivity (10-15, 29). Overexpression of human adiponectin attenuated plaque formation in Apo E -/- mice. Thus, adiponectin could have a protective effect on atherosclerosis and it may be the link between obesity, type 2 diabetes and atherosclerosis. Yamauchi et al (13,30) showed that globular adiponectin, a proteolytic cleavage product of adiponectin, has a greater potency in reversing insulin resistance than native uncleaved adiponectin. In endothelial cells, globular adiponectin inhibits expression of cytokines, chemokines, ICAM, VCAM, up regulates eNOS and results in decreased monocyte-endothelial cell adhesion and NFκb (13,16,17,30). While there appears to be a reciprocal relationship between levels of adiponectin and CRP (20), especially in patients with the metabolic syndrome or diabetes, there exists no data on the regulation of CRP synthesis and secretion in EC with adiponectin. Also, previously another adipokine, leptin has been shown to up regulate CRP synthesis and secretion in cultured HCAEC (31). Here, we present novel data, that adiponectin down regulates CRP synthesis and secretion in HAEC.
In liver derived cell lines, the main regulators of cytokine induced CRP synthesis are C/EBP-β, STAT 3 and NFκb, transcriptional regulation in HAEC has not been delineated previously(8, 32,33). Here, we show that among the transcription factors affecting the CRP promoter, in presence of HG, there is increased STAT1, C/EBP-β and NFκb activity. In addition, using promoter deletion constructs for CRP, we show that CRP transcription under HG conditions in HAEC is NFκb centric. Furthermore, transfection of HAEC with dominant negative NFκb abrogated the HG-induced CRP synthesis and secretion, confirming the crucial role of NFκb in HG-induced transcription of CRP. Previously, adiponectin has been shown to down regulate NFκb activity in endothelial cells resulting in decreased expression of the cell adhesion molecules and IL-8 (34,35). In this study, we provide evidence that adiponectin down regulates CRP synthesis and secretion in HAEC under HG conditions via inhibition of NFκb.
AMPK is a highly conserved heterotrimeric signaling kinase responsive to hypoxia, exercise and cellular stress (36,37). Adiponectin metabolic signaling in liver, skeletal muscle and adipose has been shown to be mediated via AMPK activation (38-40). Also, adiponectin has been shown to increase nitric oxide synthesis via activation of AMPK (41). Under HG conditions, apoptosis in EC as well as diminished ability of insulin to activate Akt was prevented by activation of AMPK by AICAR, suggesting that AMPK may play a key role in protecting EC from the adverse effects of HG (39).
In support of these studies, we also show that adiponectin up regulates AMPK phosphorylation in HAEC under HG conditions, these effects are mimicked by AICAR, a known activator of AMPK activity and inhibited by addition of compound C, a specific inhibitor of AMPK phosphorylation. Adiponectin decreases macrophage phagocytic capacity through cross-talk between AMPK and NFκb signaling pathways (42, 43). We show in HAEC, that adiponectin upregulates AMPK, resulting in decreased NFκb activity, and subsequent synthesis and secretion of CRP under HG conditions.
The liver is the primary source of CRP synthesis, thus we also examined the effect of adiponectin in primary rat hepatocytes that were induced to produce CRP with the combination of IL-1 and IL-6, since such treatment has previously been shown to induce CRP synthesis in Hep3B and HepG2 cells (8,33). We provide novel data that adiponectin decreases IL-1 and IL-6 induced CRP synthesis and secretion in primary rat hepatocytes and that this may be due to down regulation of C/EBP and NFκb activities. Further studies in this model will elucidate the molecular pathways by which adiponectin exerts these effects.
In addition to the growing anti-atherogenic and antidiabetic properties of adiponectin, we provide novel evidence that adiponectin abrogates HG -induced CRP synthesis and secretion via up regulation of AMPK and down regulation of NFκb. Our observations provide a fundamental mechanism for the link between adiposity and endothelial dysfunction and suggests that adiponectin up regulation could be beneficial in modulating the pro- inflammatory / prothrombotic effects of CRP (3).
Acknowledgements
a) Sources of Funding: NIH K24AT00596 and HL74360. b) We appreciate the gift from Dr. Samols of the CRP promoter deletion constructs. c ) Authors do not report any conflicts of interest.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113(17):2135–50. [PubMed] [Google Scholar]
- 4.Jabs WJ, Busse M, Krüger S, Jocham D, Steinhoff J, Doehn C. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005;68(5):2103–10. doi: 10.1111/j.1523-1755.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 5.Yasojima K, Schwab C, McGeer EG, McGeer PL. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer's disease. Brain Res. 2000;887(1):80–9. doi: 10.1016/s0006-8993(00)02970-x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Inoue N, Ohashi Y, Terashima M, Matsui K, Mori T, Fujita H, Awano K, Kobayashi K, Azumi H, Ejiri J, Hirata K, Kawashima S, Hayashi Y, Yokozaki H, Itoh H, Yokoyama M. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23(8):1398–404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 7.Venugopal SK, Devaraj S, Jialal I. Macrophage conditioned medium induces the expression of C-reactive protein in human aortic endothelial cells: potential for paracrine/autocrine effects. Am J Pathol. 2005;166(4):1265–71. doi: 10.1016/S0002-9440(10)62345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganapathi MK, Rzewnicki D, Samols D, Jiang SL, Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991;147(4):1261–5. [PubMed] [Google Scholar]
- 9.Yip HK, Hang CL, Fang CY, Hsieh YK, Yang CH, Hung WC, Wu CJ. Level of high-sensitivity C-reactive protein is predictive of 30-day outcomes in patients with acute myocardial infarction undergoing primary coronary intervention. Chest. 2005;127(3):803–8. doi: 10.1378/chest.127.3.803. [DOI] [PubMed] [Google Scholar]
- 10.Abbate A, Biondi-Zoccai GG, Brugaletta S, Liuzzo G, Biasucci LM. C-reactive protein and other inflammatory biomarkers as predictors of outcome following acute coronary syndromes. Semin Vasc Med. 2003;3(4):375–84. doi: 10.1055/s-2004-815695. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa Y. Adiponectin: Identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler. 2005;6(2):7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. Suppl. [DOI] [PubMed] [Google Scholar]
- 12.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257(2):167–75. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J. Globular adiponectin protects ob/ob mice from diabetes and Apo E-/- mice from atherosclerosis. J Biol Chem. 2003;278:2461–68. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 14.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278(45):45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 16.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–49. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 17.Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated Hsp90 binding to eNOS and activation of PI3-Akt pathway contribute to globular adiponectin induced NO production. Biochem Biophys Res Commun. 2005;332:200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 18.Yuan G, Zhou L, Tang J, Yang Y, Gu W, Li F, Hong J, Gu Y, Li X, Ning G, Chen M. Serum CRP levels are equally elevated in newly diagnosed type 2 diabetes and impaired glucose tolerance and related to adiponectin levels and insulin sensitivity. Diabetes Res Clin Pract. 2006;72(3):244–50. doi: 10.1016/j.diabres.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 19.D'Alessandris C, Lauro R, Presta I, Sesti G. C-reactive protein induces phosphorylation of insulin receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby impairing the insulin signalling pathway that promotes glucose transport. Diabetologia. 2007;50(4):840–9. doi: 10.1007/s00125-006-0522-y. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107(5):671–4. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 21.Singh U, Devaraj S, Dasu MR, Ciobanu D, Reusch J, Jialal I. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol. 2006;26(11):2469–75. doi: 10.1161/01.ATV.0000241572.05292.fb. [DOI] [PubMed] [Google Scholar]
- 22.Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab. 2007;293(1):E337–46. doi: 10.1152/ajpendo.00718.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gores GA, Nieminen A, Wray B, Herman B, Lemasters J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989;83:386–389. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan G, Chen X, Ma Q, Qiao J, Li R, Li X, Li S, Tang J, Zhou L, Song H, Chen M. C-reactive protein inhibits adiponectin gene expression and secretion in 3T3-L1 adipocytes. J Endocrinol. 2007;194(2):275–81. doi: 10.1677/JOE-07-0133. [DOI] [PubMed] [Google Scholar]
- 25.Dasu MR, Devaraj S, Du Clos TW, Jialal I. The biological effects of CRP are not attributable to endotoxin contamination: evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48(3):509–12. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma agonists. Am J Cardiol. 2007;99(4A):27B–40B. doi: 10.1016/j.amjcard.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;36(12):1331–6. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S, Swarbrick M, Singh U, Adams-Huet B, Havel P, Jialal I. CRP, Adiponectin and its Oligomers in the Metabolic Syndrome: Evaluation of New Laboratory-Based Biomarkers. Am J Clin Path. 2007 doi: 10.1309/RN84K51B2JJY1Y0B. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furler SM, Gan SK, Poynten AM, Chisholm DJ, Campbell LV, Kriketos AD. Relationship of adiponectin with insulin sensitivity in humans, independent of lipid availability. Obesity (Silver Spring) 2006;14(2):228–34. doi: 10.1038/oby.2006.29. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;2:941–46. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 31.Singh P, Hoffmann M, Wolk R, Shamsuzzaman AS, Somers VK. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(9):e302–7. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- 32.Cha-Molstad H, Young DP, Kushner I, Samols D. The interaction of C-Rel with C/EBPbeta enhances C/EBPbeta binding to the C-reactive protein gene promoter. Mol Immunol. 2007;44(11):2933–42. doi: 10.1016/j.molimm.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Ganter U, Arcone R, Toniatti C, Morrone G, Ciliberto G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J. 1989;8(12):3773–9. doi: 10.1002/j.1460-2075.1989.tb08554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314(2):415–9. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 35.Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97(12):1245–52. doi: 10.1161/01.RES.0000194328.57164.36. [DOI] [PubMed] [Google Scholar]
- 36.Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36(1):28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- 37.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3(4):340–51. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 38.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U.S.A. 2002;99(25):16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fattyacid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52(6):1355–63. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 41.Cheng KKY, Lam KSL, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adipoectin induced eNOS activation and NO production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–94. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, Froguel P, Nagai R, Kadowaki T. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr. Drug Targets Immune Endocr Metabol Disord. 2003;3(4):243–54. doi: 10.2174/1568008033340090. [DOI] [PubMed] [Google Scholar]
- 43.Saijo S, Nagata K, Nakano Y, Tobe T, Kobayashi Y. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Commun. 2005;334(4):1180–3. doi: 10.1016/j.bbrc.2005.07.016. [DOI] [PubMed] [Google Scholar]


