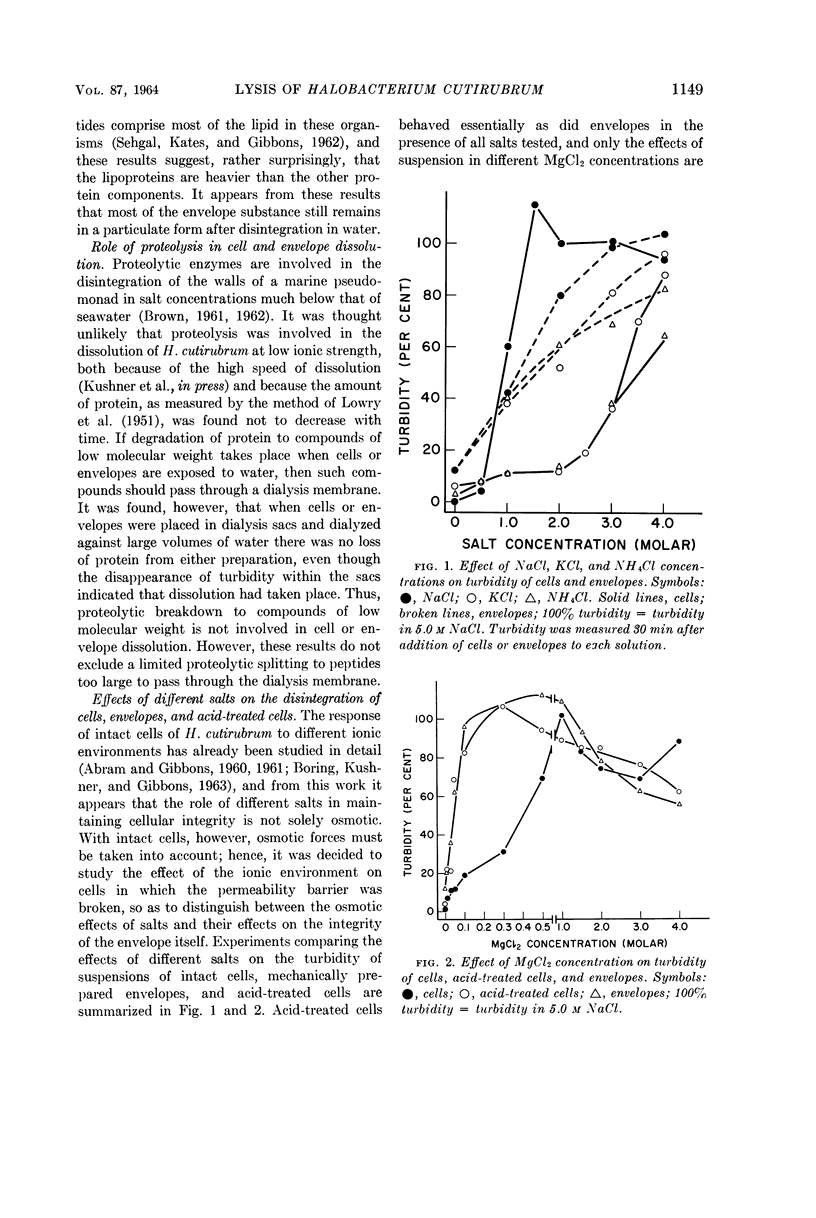
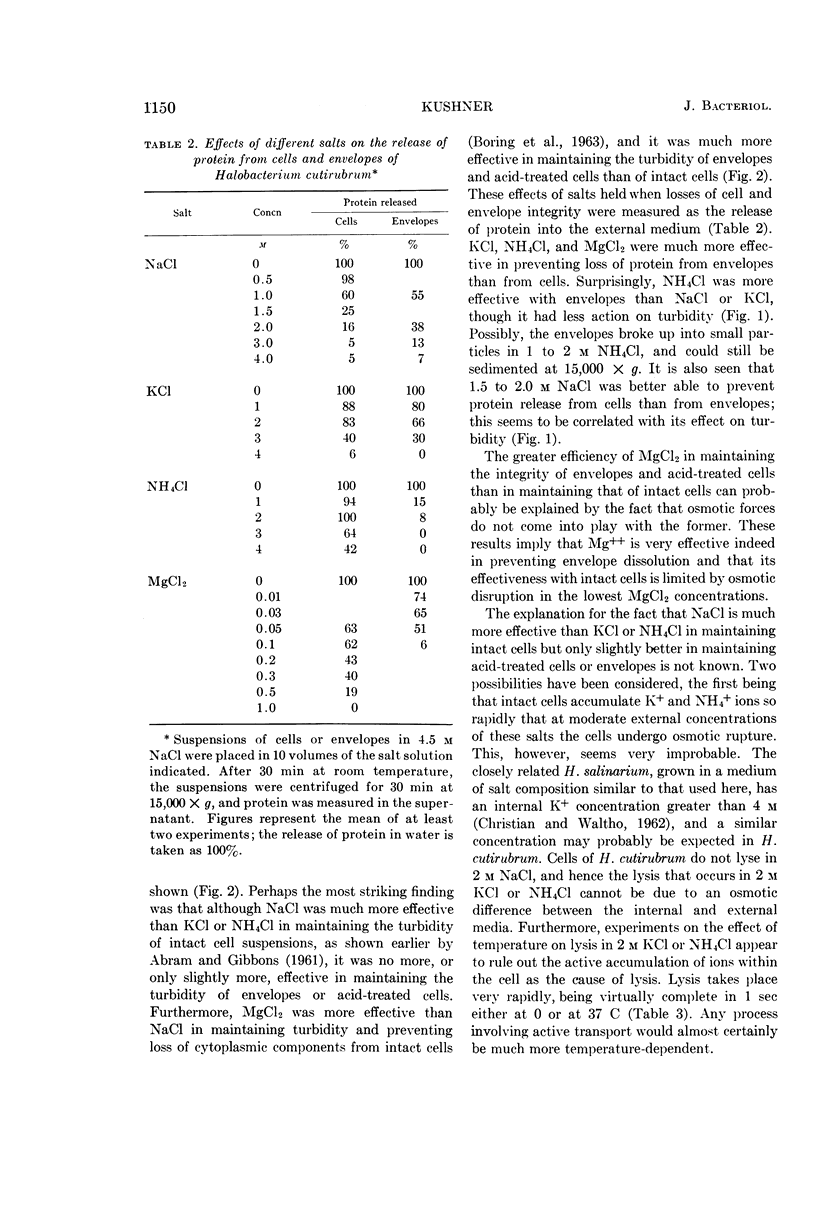
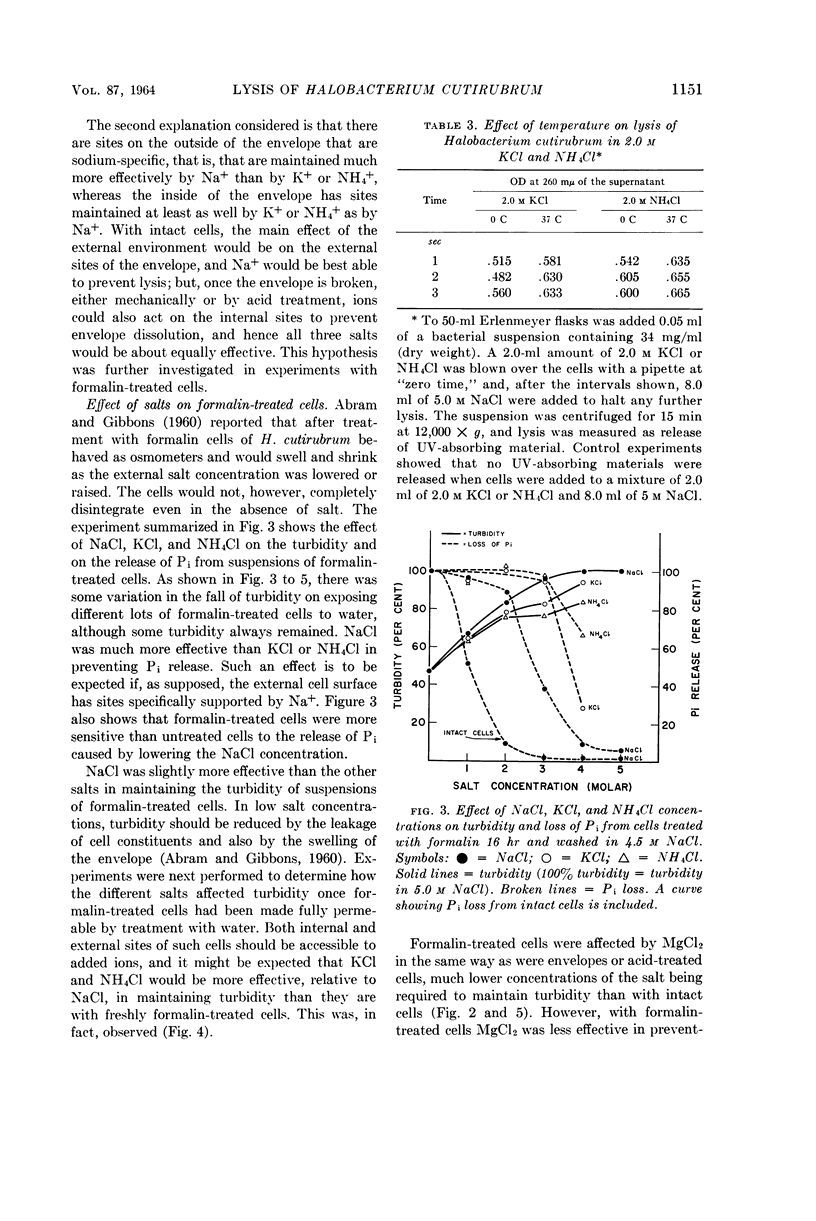
Abstract
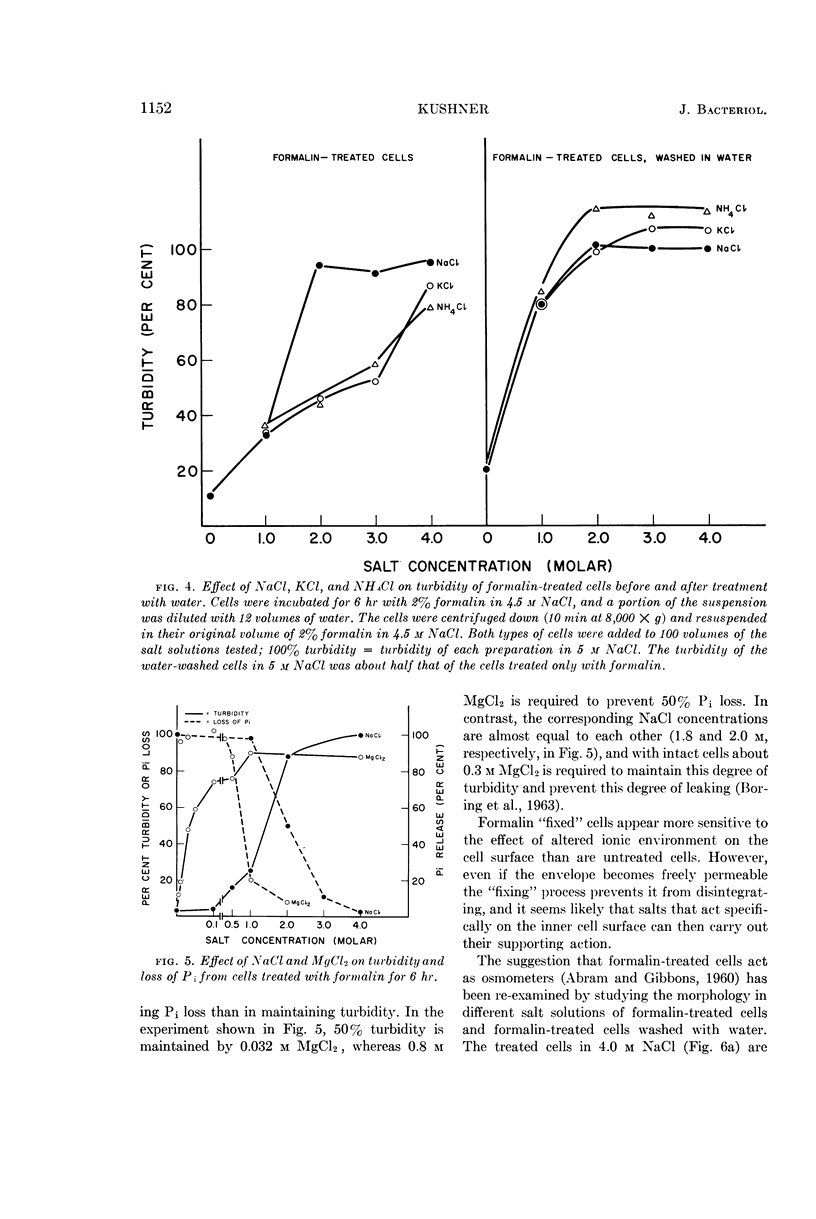
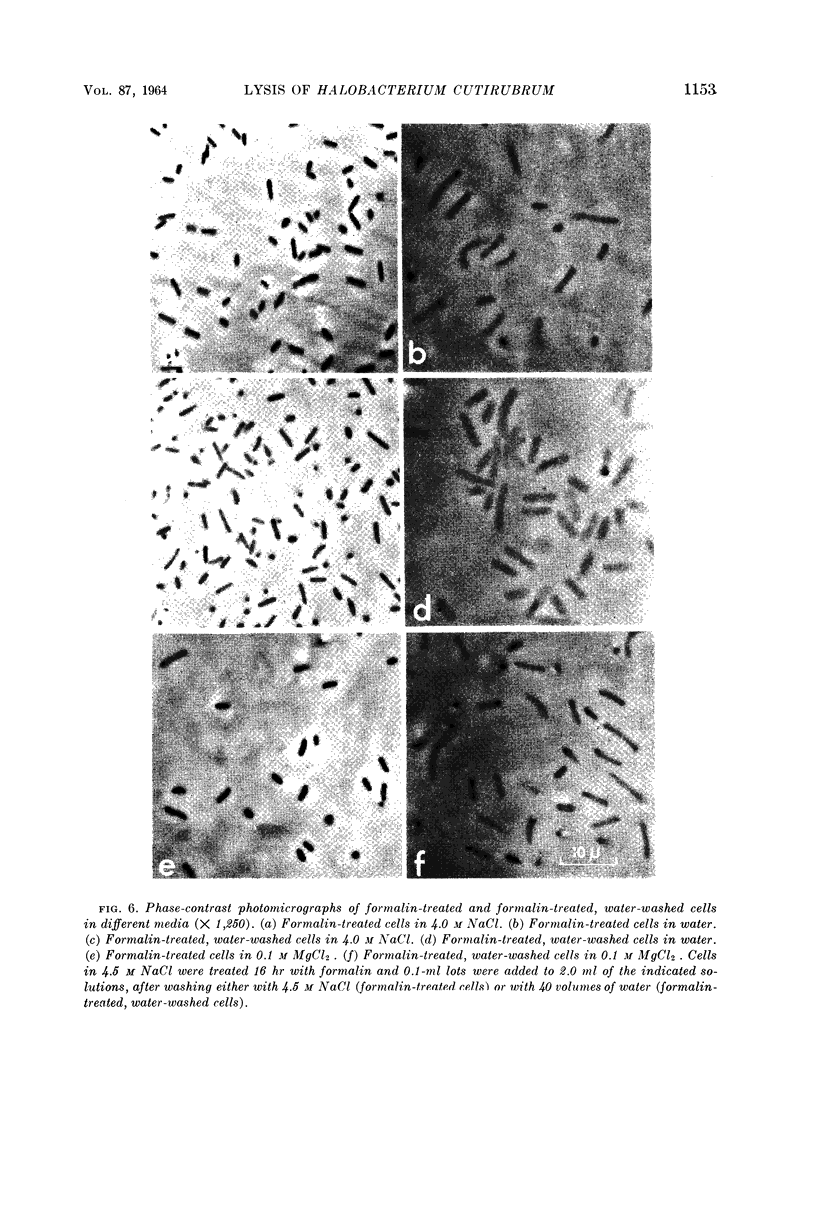
Kushner, D. J. (National Research Council, Ottawa, Ontario, Canada). Lysis and dissolution of cells and envelopes of an extremely halophilic bacterium. J. Bacteriol. 87:1147–1156. 1964.—Envelopes of the extremely halophilic bacterium, Halobacterium cutirubrum, disintegrate in the absence of salt to form much smaller particles. Extensive proteolytic breakdown to compounds of low molecular weight is not involved in this process or in the lysis of cells in the absence of salt. NaCl is much more effective than KCl or NH4Cl in preserving the integrity of intact cells, but is only slightly more effective in preserving the integrity of mechanically prepared envelopes, of cells made permeable by treatment with acid, and of cells made permeable by formalin fixation followed by exposure to water. MgCl2 is much more effective in preserving the integrity of these preparations than of intact cells. The results suggest that the exterior cell surface has sites specifically requiring Na+ to maintain their integrity, whereas the interior surface has sites whose integrity is maintained at least as well by K+ or NH4+ as by Na+.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., GIBBONS N. E. The effect of chlorides of monovalent cations, urea, detergents, and heat on morphology and the turbidity of suspensions of red halophilic bacteria. Can J Microbiol. 1961 Oct;7:741–750. doi: 10.1139/m61-088. [DOI] [PubMed] [Google Scholar]
- ABRAM D., GIBBONS N. E. Turbidity of suspensions and morphology of red halophilic bacteria as influenced by sodium chloride concentration. Can J Microbiol. 1960 Oct;6:535–543. doi: 10.1139/m60-062. [DOI] [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BROWN A. D., SHOREY C. D. Preliminary observations on the cell envelopes of two species of Halobacterium. Biochim Biophys Acta. 1962 May 7;59:258–260. doi: 10.1016/0006-3002(62)90731-x. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. The peripheral structures of gram-negative bacteria. III. Effects of cations of proteolytic degradation of the cell envelope of a marine pseudomonad. Biochim Biophys Acta. 1962 Jul 30;62:132–144. doi: 10.1016/0006-3002(62)90498-5. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- GROSSOWICZ N., ARIEL M. Mechanism of protection of cells by spermine against lysozyme-induced lysis. J Bacteriol. 1963 Feb;85:293–300. doi: 10.1128/jb.85.2.293-300.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- SEHGAL S. N., KATES M., GIBBONS N. E. Lipids of Halobacterium cutirubrum. Can J Biochem Physiol. 1962 Jan;40:69–81. [PubMed] [Google Scholar]
- SMITHIES W. R., GIBBONS N. E., BAYLEY S. T. The chemical composition of the cell and cell wall of some halophilic bacteria. Can J Microbiol. 1955 Oct;1(8):605–613. doi: 10.1139/m55-073. [DOI] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]