Abstract
The effect of timing in providing dexamethasone treatment after intracerebral hematoma was evaluated in rats with hematoma induced by a subcortical collagenase injection. Male Sprague–Dawley rats (n = 30; body weight, 185 to 230 g) received dexamethasone (1 mg/kg) intraperitoneally at 2 h, 4 h, or 6 h (1 group per time point) after intracerebral collagenase injection, with another dose (1 mg/kg) administered at 24 h after collagenase injection. Neurologic examinations and rotarod treadmill tests were used to evaluate motor behavior before and at 24 and 48 h after intracerebral injection. Rats were euthanized after the last behavioral test. Brains were evaluated for hematoma size, number of penumbral necrotic neurons, neutrophils within the hematoma, and astrocytic response. Compared with the control and other treatment groups, rats treated with dexamethasone at 2 and 24 h after intracerebral collagenase injection scored significantly better on neurologic exams and rotarod tests. Hematoma volume was significantly smaller in all treated groups than in the control group but did not differ between treatment groups. Fewer neutrophils were seen in the perihematoma region of all treated rats compared with controls, but the number of necrotic neurons was decreased significantly only in the group treated with dexamethasone at 2 and 24 h. These results indicate that a 1-mg/kg dose of dexamethasone is beneficial for treatment of intracerebral hemorrhage, particularly if administered early after the hemorrhagic insult.
Traumatic cerebral hemorrhages, which in veterinary patients are caused mainly by automobile accidents and falls, are diagnostic and therapeutic challenges.15 Animal models have played an important role in elucidating the cascade of cellular and biochemical events occurring after traumatic brain injury.7 These models have helped to elucidate various aspects of the pathogenesis and treatment of intracranial hemorrhaging. Among these, the collagenase-induced intracerebral hematoma model14 is highly reproducible and shows many characteristics of the intracerebral hemorrhagic process in mammals. In addition, the short- and long-term histologic and behavioral changes associated with this model have been evaluated.5,6 Compared with the blood infusion model, the collagenase model causes greater primary injury that occurs distal to the hematoma, and neurologic deficits resolve less rapidly over time, making the collagenase model more appealing for long-term studies.12
The efficacy of corticosteroids for the treatment of brain hemorrhages has been evaluated in different experimental studies with conflicting results.3,8-11 Administration of corticosteroids at 1 h after hematoma induction is beneficial for the treatment of this condition.10,11,16 Although these studies have promising results, the interval between trauma and treatment might greatly influence the response to corticosteroid treatment. The aim of this study was to assess the motor performance and histopathology associated with dexamethasone treatment at 2, 4, and 6 h after hematoma induction in the intracranial collagenase rat model. A second dexamethasone dose was administered 24 h after collagenase injection.
Materials and Methods
Animals.
Male Sprague–Dawley rats (n = 30; weight, 185 to 230 g; Charles River, Montréal, Canada) were used in this study. These animals were free of common rodent viruses, bacteria, and parasites (Charles River standard microbiologic profile). After their arrival at our facility, they were housed conventionally (room temperature, 21 ± 2 °C; 15 air changes per hour; 12:12-h light:dark cycle) in polycarbonate cages (Ancare, Bellmore, NY) on hardwood bedding (Betachips, Northeastern Products, Warrensburg, NY) and acclimated for 5 d before initiation of the study. Rats received tap water and rodent chow (Rodent Chow 5075, Charles River) ad libitum. The University of Montreal Faculty of Veterinary Medicine Institutional Animal Care and Use Committee approved the experimental protocol before animal use, in accordance with the guidelines of the Canadian Council on Animal Care.4
Experimental design.
Dexamethasone (sodium monophosphate dexamethasone; Professional Veterinary Laboratories, Winnipeg, Canada) was administered intraperitoneally at a dose of 1 mg/kg (2 mg/mL) at 2, 4, or 6 h (1 group per time point; n = 6 per group) after injection of intracerebral collagenase; all treated rats received a second dexamethasone dose at 24 h after collagenase injection. One control group (n = 6) received intracerebral injection of collagenase but no steroid treatment (surgical controls). The other control group (n = 6) treated with the vehicle only received dexamethasone only (steroid-treatment controls) without intracranial collagenase to evaluate the effects of dexamethasone on the neurologic exam and rotarod treadmill test. Rats in which hematoma was induced were assigned randomly to groups to achieve a similar mean body weight among groups.
Surgery.
The surgical procedures have been previously described.10 Rats were anesthetized with isoflurane (Aerane, Baxter, Mississauga, Ontario, Canada) in medical oxygen (100% O2, Praxair, St Hubert, Canada). A collagenase solution composed of 0.5 U collagenase type VII (Sigma–Aldrich, Oakville, Ontario, Canada) in 2 µL saline (Abbott Laboratories, Montreal, Canada) then was injected over a period of 10 min through a burr hole in the right caudoputamen nucleus, 5 mm below the dura (stereotaxic coordinates: 0.0 mm anteroposterior and 3.0 mm lateral), by using a 5-µL Hamilton syringe. Collagenase was prepared fresh (1500 U in 10 mL saline in a sterile 50-mL beaker and aliquotted into sterile 1-mL microfuge tubes) on the day of surgery and administered within 5 h of its preparation.
Rectal temperature was monitored (Thermalert TH8, Physitemp, Clifton, NJ) and maintained within normal limits (36.6 to 37.5 °C) throughout surgery by keeping rats on an electrical heating pad.
Behavioral evaluations.
Behavioral evaluations (rotarod treadmill test and neurologic exam) were performed on all rats before and at 24 and 48 h after intracerebral collagenase injection. Postoperative values for these evaluations are expressed as a percentage of preoperative values for each rat. The neurologic exam10 comprised the following tests scored on a scale of 0 (poor or absent) to 4 (normal): activity (exploration of the immediate environment when the animal was placed on a novel hard surface); locomotion (graded from no movement and unilateral rotation to incomplete movement and linear locomotion); positional passivity (flexion response was evaluated when the posterior limbs were pulled away from the rat during hand restraint); visual positioning (evaluated when rats held by the tail above a contact surface were lowered toward the surface); climbing (skill and symmetry were observed while the rat climbed on a wire grid); tail rigidity (the tail was elevated at midlength; rigidity was scored from no flexibility to complete rigidity); and tremor (rats were held by the tail and the hind paws were observed for tremor or stability while the front paws remained in contact with a hard surface). More extensive batteries of behavioral tests have been used in rodents,17 but we used the tests that we previously used to evaluate corticosteroid treatments.10,11
Rats also were tested on a rotarod treadmill (ENV576, Med Associates, St Albans, VT) set to accelerate from 5 to 35 revolutions per minute over 5 min. The maximal time the rat stayed on the rotarod (maximum, 5 min) was recorded for each performance. Rats were trained daily for 5 consecutive days, and results of the next 3 d were used as a baseline. A mean of 3 trials was used to represent the animal's daily performance for the baseline and postsurgical evaluations. Behavioral results are reported as a percentage of each individual baseline evaluations.
Histopathology.
After all behavioral tests, rats were placed under deep anesthesia (pentobarbital, 100 mg/kg IP; MTC Pharmaceuticals, Toronto, Canada), perfused through the heart with a physiologic dextrose–sucrose solution followed by a 10% buffered formalin solution, and then euthanized. For microscopic evaluation, brains were fixed in formalin for 48 h and embedded in paraffin. Transverse sections (5 μm) were taken every 100 μm from the beginning to end of the hematoma and were stained with hematoxylin and saffron. Evaluation of hematoma volume (central zone of necrotic tissue, not including the peripheral zone with necrotic glial cell and neurons) and neutrophil and necrotic neuron counts at a magnification of ×200 (dorsal, ventral, medial, and lateral fields in the perihematoma region) were obtained by using an image analysis system (Compix Imaging Systems, Sewickley, PA). Nuclear shape and relative size (number of pixels) were used first to select neutrophils and macrophages, which then were differentiated individually by cell size and nuclear shape and confirmed by a board-certified pathologist (P Hélie). To determine astrocytic reactivity (an indicator of neuronal hyperactivity and necrosis), immunohistochemistry for glial fibrillary acidic protein was performed by using polyclonal antibodies obtained from BioGenex Laboratories (San Ramon, CA). The immunoglobulin fraction was composed of rabbit antisera diluted in PBS (pH 7.6) in 1% bovine serum albumin. Antibodies were stained with a commercially available immunoperoxidase procedure (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). In addition, positive and negative controls for all brain slices were provided for proper identification of reactive astrocytes.
Statistical analyses.
Kruskall–Wallis 1-way analysis by ranks was used to evaluate changes in the results of the neurologic exams and rotarod treadmill tests. A linear model with repeated measures was used to evaluate statistical significance to assess whether changes in the number of neutrophils, necrotic neurons, and hematoma volume at 24 or 48 h after treatment were significantly different from results obtained from the surgical control group. Statistical analyses were performed by using SAS software (version 9.1, SAS Institute, Cary, NC). The threshold for significance was set at 0.05.
Results
Behavioral evaluations.
Semiquantitative neurologic exams and rotarod tests demonstrated a beneficial effect of dexamethasone treatment after intracerebral hematoma induction in rats (Figures 1 and 2). Baseline (presurgical) scores showed no difference between groups for both the neurologic exam (maximal score of 22 for all animals) and rotarod treadmill test (2-, 4-, and 6-h dexamethasone-treatment groups: 117 ± 18, 122 ± 13, and 131 ± 15 s, respectively; surgical control group, 125 ± 10 s; and dexamethasone control group: 121 ± 17 s). At 24 and 48 h after surgery, the surgical control group showed a significant (P < 0.05) drop in neurologic exam scores and rotarod performances when compared with presurgical evaluations.
Figure 1.
Neurologic exam scores (mean ± SD; expressed as a percentage of baseline performance) at 24 and 48 h after surgery of male Sprague Dawley rats that received intracerebral collagenase without (surgical control group; black) and with intraperitoneal dexamethasone treatment (1 mg/kg) at 2 and 24 h (white), 4 and 24 h (light gray), and 6 and 24 h (dark gray) after injection of collagenase. At 24 and 48 h after intracerebral injection of collagenase, neurologic exam scores differed significantly (*; P < 0.01) between animals treated at 2 and 24 h and the surgical control group.
Figure 2.
Rotarod scores (mean ± SD; expressed as a percentage of baseline performances) at 24 and 48 h after surgery of male Sprague Dawley rats that received intracerebral collagenase without (surgical control group, black) and with intraperitoneal dexamethasone treatment (1 mg/kg) at 2 and 24 h (white), 4 and 24 h (light gray), and 6 and 24 h (dark gray) after the collagenase injection. At 24 and 48 h after the intracerebral collagenase injection, rotarod scores differed significantly (*; P < 0.01) between animals treated at 2 and 24 h and the surgical control group.
At 24 h after collagenase injection, rats had significantly (P < 0.02) higher scores on their neurologic and rotarod evaluations when dexamethasone treatments began at 2 h after hematoma induction but not when treatments were initiated at 4 and 6 h (Figures 1 and 2). Average neurologic scores were reduced to 73% ± 19% of baseline values and rotarod results to 48% ± 18% for the group treated at 2 and 24 h after surgery. In contrast, groups treated at 4 and 24 h showed an average reduction to 53 ± 13% % in the neurologic scores and 29% ± 21% in rotarod performance; neurologic and rotarod scores for rats treated with dexamethasone at 6 and 24 h were 61% ± 16% and 20% ± 18% of baseline values, respectively.
The most striking effects were noted at 48 h after surgery. Rats that received their first dexamethasone injection at 2 h after surgery had significantly (P < 0.02) higher neurologic scores (84% ± 21% of baseline) than controls, whereas the scores of animals first treated at 4 or 6 h after surgery did not differ from those of untreated surgical controls. For the rotarod treadmill test, rats treated at 2 and 24 h after collagenase injection had significantly higher scores (P < 0.02) whereas no significant difference was noted for rats treated at 4 and 24 and 6 and 24 h. Rats treated at 4 and 24 h and 6 and 24 h showed an average reduction of neurologic scores (56% and 55%, respectively) and rotarod performances (33% and 27%, respectively) after collagenase injection.
Rats in the steroid-treatment control group showed no significant differences between the pre- and posttreatment neurologic and rotarod treadmill tests (results not shown), suggesting that dexamethasone alone had no effect on motor behaviors.
Histopathologic findings.
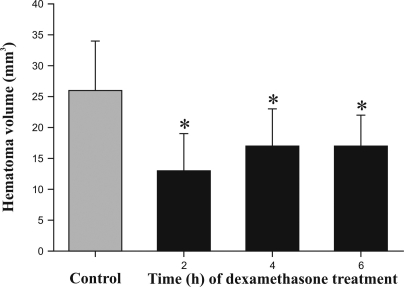
The hematoma area consisted of clusters of erythrocytes, cellular debris, neutrophils, and necrotic parenchyma. No macrophages were detected. Clear spaces within the neuropil, interpreted as edema, surrounded the perihematoma region and extended along the corpus callosum in the ipsilateral and contralateral hemispheres. Hematoma volume was significantly (P < 0.02) smaller in all dexamethasone-treated animals (12.5 ± 2.17 mm3 in rats treated at 2 and 24 h, 16.6 ± 2.46 mm3 in those treated at 4 and 24 h, and 16.7 ± 2.46 mm3 in those treated at 6 and 24 h after collagenase injection) when compared with nontreated animals (26.4 ± 2.66 mm3); however, there was no difference between treated groups (Figure 3). In addition, dexamethasone treatment led to significant differences in the number of selected cell types. Neutrophils were mainly at the periphery of lesions and were more abundant in controls (1581 ± 123) than in dexamethasone-treated animals (2 and 24 h, 4 and 24 h, and 6 and 24 h: 710 ± 100, 883 ± 114, and 1097 ± 114, respectively; P < 0.01). Rats treated at 6 and 24 h after intracerebral collagenase injection had significantly (P < 0.02) higher neutrophil counts than did those treated at 2 and 24 h after surgery (Figure 4). The number of necrotic neurons decreased significantly (P < 0.05) only when dexamethasone treatment started at 2 h after intracranial collagenase injection (148 ± 33) when compared with that of surgical controls (223 ± 36). The number of necrotic neurons were the same as that for controls when dexamethasone treatment at 4 h (219 ± 33) or 6 h (298 ± 33) after surgery (Figure 5).
Figure 3.
Intracerebral hematoma volume (mm3; mean ± SD) at 48 h after intracerebral collagenase injection in male Sprague Dawley rats without (surgical control group) and with intraperitoneal dexamethasone treatments at 2 and 24 h, 4 and 24 h, and 6 and 24 h after surgery. *, P < 0.02 for all treated groups when compared with the surgical controls.
Figure 4.
Neutrophil counts (mean ± SD) at 48 h after intracerebral collagenase injection in male Sprague Dawley rats without (surgical control group) and with intraperitoneal dexamethasone treatments at 2 and 24 h, 4 and 24 h, and 6 and 24 h after surgery. *, P < 0.01 for all treated groups when compared with surgical controls.
Figure 5.
Number of necrotic neurons (mean ± SD) at 48 h after intracerebral collagenase injection in male Sprague Dawley rats without (surgical control group) and with intraperitoneal dexamethasone treatment at 2 and 24 h, 4 and 24 h, and 6 and 24 h after surgery. The number of necrotic neurons differed (*, P < 0.05) between the control and treated groups only when treatment started at 2 h after collagenase injection.
As in a previous study,10 induction of hematoma by intracerebral injection of collagenase was accompanied by vasculitis, erythrocytes, and reactive astrocytes in our rats. Vasculitis was characterized by infiltration of inflammatory cells, fibrin deposits, and loss of integrity of the vascular wall. Astrocytic cells were reactive because they were swollen and had numerous cytoplasmic projections, as shown by immunohistochemistry. No differences in the incidence of these features were noted between rats first treated with dexamethasone at 4 or 6 h and nontreated animals. However, the group treated at 2 and 24 h after the insult had qualitatively fewer erythrocytes, fewer marked vascular changes, and less neuronal degeneration that did the other groups.
Discussion
Together with our previous findings,10 the current behavioral results indicate that intraperitoneal administration of a 1-mg/kg dose of dexamethasone within 2 h after the induction of an intracerebral hematoma is beneficial and minimizes the size of the hematoma and its repercussions on motor behavior in rats. Our histologic findings suggest that beneficial effects are achieved even when treatment is initiated 6 h after the cerebral trauma in this model. This result suggests that a contributing factor other than the hematoma itself (for example, edema—which is known to decrease after dexamethasone treatment)16 may be important. Rats treated with dexamethasone at 2 and 24 h after intracerebral collagenase injection had better behavioral and rotarod performances than did those treated at 4 and 24 h and 6 and 24 h after trauma. Although intraperitoneal administration is a common route of drug dosage in rodents, dexamethasone could be administered intravenously, thereby avoiding the effect of decreased absorption due to shock after trauma. Like the intravenous route, intraperitoneal dosage provides rapid drug exposure compared with other known routes (for example, intramuscular).
Hematoma size plays a role in the neurologic deficits that occur after trauma.6 Cerebral hematomas cause compression of the neuroparenchyma, resulting in neuronal degeneration and neurologic deficits, mainly because hematomas are space-occupying lesions and because of cerebral edema due to hemorrhage.2 Animals having minimal cerebral hemorrhage therefore experience few neurologic deficits. At 48 h, the volume of the hematoma was significantly smaller in treatment groups compared with controls, and although not significant the hematoma volume was the smallest when the treatment was initiated 2 h after the collagenase injection. Changes in motor behavior with treatment might also reflect a treatment-associated decrease in edema,16 but edema was not evaluated in the current study.
The administration of dexamethasone decreased the number neutrophils in the hemorrhagic zone of all 3 treatment groups. Neutrophils have been associated with ischemic damage in the brain.13 Corticosteroids, including dexamethasone, reduce neutrophil margination, perhaps explaining at least in part the smaller hemorrhagic volume seen with treatment.
The number of necrotic neurons in the perihematoma region differed between controls and the group for which dexamethasone treatment started at 2 h after intracranial collagenase injection. Because hematoma volume was the smallest in these animals, the absolute number of necrotic neurons surrounding the damaged parenchyma likely was smaller than for other treatment groups and may help to explain the behavioral results. Previous experiments10 have shown that dexamethasone treatment 1 h after collagenase injection decreased the number of necrotic neurons, most likely due to the rapid progression of the lesion within the first 2 h after the insult.
Astrocytes were present in the perihematoma region in every group (treated and surgical control) and showed characteristic hypertrophy (increase in body cell size, increase of the length and number of the cytoplasmic projections), which was easily visible by using immunochemistry for glial fibrillary acidic protein. No significant differences between treated and nontreated animals were observed in the number of reactive astrocytes in the penumbral zone. Glial fibrillary acidic protein therefore was not a useful criterion to evaluate dexamethasone treatment.
Previous studies10,11 have shown that corticosteroids administered shortly after the induction of an intracerebral hemorrhage are beneficial for the treatment of cerebral hematoma. Consistent with these results, administration of 1 mg/kg dexamethasone had beneficial effect on attenuating behavioral and histopathologic changes after intracerebral hemorrhage in rats. The treatment appeared to be more effective if administered within the first 2 h after the insult. Delayed administration of dexamethasone after hemorrhage therefore could be associated with poor recovery of motor performance. The beneficial effects of corticosteroids on reducing swelling and edema generally are accepted, but these drugs should be used with caution if pronounced hematologic changes (for example, decreased platelet function) are noted in traumatized patients. To validate our findings, behavior, histopathology, and edema should be evaluated for potential long-term abnormalities.
Intracerebral hemorrhage is the most serious type of stroke, and hematoma volume is the major determinant of outcome.3 Hematoma expansion occurring mainly in the first 24 h of the event leads to neurologic deterioration secondary to compression of neuronal tissue, edema, and inflammation. Treatments that target the hematoma therefore may have the greatest potential to affect outcome. Corticosteroids have been used extensively to treat vasogenic edema and inflammation after spinal cord injury.1 Associated neuroprotective mechanisms include inhibition of lipid peroxidation and antioxidative properties of steroids.9 However, current treatment recommendations based on recent clinical trials2 still reflect uncertainties about the efficacy of medical approaches to reduce hematoma expansion.
Acknowledgments
This work was supported by the Fond du Centenaire de la Faculté de Médecine Vétérinaire de l'Université de Montréal. We thank Guy Beauchamp for statistical analyses and Marie-Thérèse Parent for the preparation of the figures.
References
- 1.Alderson P, Roberts I. 2005. Corticosteroids for acute traumatic brain injury (Cochrane review). : Cochrane library, issue 2. Chichester (UK): John Wiley and Sons; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CS. 2009. Medical management of acute intracerebral hemorrhage. Curr Opin Crit Care 15:93–98 [DOI] [PubMed] [Google Scholar]
- 3.Braakman R, Schouten HJ, Blaauw-van Dishoeck M, Minderhoud JM. 1983. Megadose steroids in severe head injury. Results of a prospective double-blind clinical trial. J Neurosurg 58:326–330 [DOI] [PubMed] [Google Scholar]
- 4.Canadian Council on Animal Care 1993. Guide to the care and use of experimental animals. Ottawa (Canada): Canadian Council on Animal Care [Google Scholar]
- 5.Chesney JA, Kondoh T, Conrad JA, Low WC. 1995. Collagenase-induced intrastriatal hemorrhage in rats results in longterm locomotor deficits. Stroke 26:312–316 [DOI] [PubMed] [Google Scholar]
- 6.Del Bigio MR, Yan HJ, Buist R, Peeling J. 1996. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke 27:2312–2319 [DOI] [PubMed] [Google Scholar]
- 7.Finnie JW, Blumberg PC. 2002. Traumatic brain injury. Vet Pathol 39:679–689 [DOI] [PubMed] [Google Scholar]
- 8.Hall ED. 1985. High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J Neurosurg 62:882–887 [DOI] [PubMed] [Google Scholar]
- 9.Ildan F, Polat S, Oner A, Isbir T, Cetinalp E, Kaya M, Karadayi A. 1995. The effect of treatment of high doses of methylprednisolone on Na+/Mg+2 ATPase activity and lipid peroxidation and ultrastructural findings following cerebral contusion in rat. Surg Neurol 44:573–580 [DOI] [PubMed] [Google Scholar]
- 10.Lema PP, Girard C, Vachon P. 2004. Evaluation of dexamethasone for the treatment of intracerebral hemorrhage using a collagenase-induced intracerebral hematoma model in rats. J Vet Pharmacol Ther 27:321–328 [DOI] [PubMed] [Google Scholar]
- 11.Lema PP, Girard C, Vachon P. 2005. High doses of methylprednisolone are required for the treatment of collagenase-induced intracerebral hemorrhage in rats. Can J Vet Res 69:253–259 [PMC free article] [PubMed] [Google Scholar]
- 12.MacLellan CL, Sialsi G, Poon CC, Edmundson CL, Buist R, Pelling J, Colbourne F. 2008. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab 28:516–525 [DOI] [PubMed] [Google Scholar]
- 13.Matsuo Y, Onodera H, Shiga Y, Nakamura M, Ninomiya M, Kihara T, Kogure K. 1994. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke 25:1469–1475 [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. 1990. Collagenase-induced cerebral hemorrhage in rats. Stroke 21:801–807 [DOI] [PubMed] [Google Scholar]
- 15.Thomas WB. 1996. Cerebrovascular disease. Vet Clin North Am Small Anim Pract 26:925–943 [PubMed] [Google Scholar]
- 16.Vachon P, Moreau JP. 2003. Low doses of dexamethasone decrease brain water content of collagenase-induced cerebral hematoma. Can J Vet Res 67:157–159 [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Chen G, Zhang J. 2009. Edavarone reduces brain edema and attenuates cell death after intracerebral haemorrhage in mice. Brain Inj 23:353–357 [DOI] [PubMed] [Google Scholar]