Abstract
Aging frequently leads to a functional decline across multiple cognitive domains, often resulting in a severe reduction in life quality and also causing substantial care-related costs. Understanding age-associated structural and functional changes of neural circuitries within the brain will be required to improve successful aging. In this review, we focus on age-dependent alterations of the hippocampus and the decline of hippocampal function, which are critically involved in processes underlying certain forms of learning and memory. Despite the dramatic reductions in hippocampus-dependent function that accompany advancing age, there is also striking evidence that even the aged brain retains a high level of plasticity. Thus, one promising avenue to reach the goal of successful aging might be to boost and recruit this “plasticity,” which is the interplay between neural structure, function, and experience to prevent age-related cognitive decline and age-associated co-morbidities.
Keywords: adult neurogenesis, aging, plasticity, hippocampus, learning and memory
Introduction
The mammalian brain is challenged throughout life with novel experiences that shape behavior, personality, and cognitive abilities. Thus, the brain has to be able to integrate novelty into the neural circuitries that were formed based on earlier experiences. The dynamic but constant adaptation of the brain to external stimulation, which seems to be a pivotal component of the central nervous system (CNS) in order to fulfill its manifold tasks, is thought to be based on structural changes within neural networks that are induced by what we see, hear, feel, dream, taste, think or, in more general terms, “experience.” For a long time, it was thought that the complex architecture and the relative stability of certain memories strongly argued against a constant remodeling of neural circuitries. However, modern molecular and electrophysiological techniques, as well as in vivo imaging tools, have provided strong support for the concept of a constantly adapting central nervous system (Bliss & Collingridge, 1993; Cole, Saffen, Baraban, & Worley, 1989; Lendvai, Stern, Chen, & Svoboda, 2000; Toni, Buchs, Nikonenko, Bron, & Muller, 1999). The interaction between the brain and the environment has generated the concept of “plasticity.” There is no strict definition of the term, but plasticity is mostly used to describe the effects of experience onto the structure, connectivity, and functional behavior of either single cells or neural ensembles. The classic example of plasticity within the adult brain is long-term potentiation (LTP): high frequency stimulation leads to long-lasting changes in synaptic potentiation, resulting in structural and functional modifications of connected neurons (Bliss & Collingridge, 1993). Even though direct proof is still missing, a current hypothesis is that LTP might be the cellular correlate of learning and memory-associated events in the mammalian brain (Kandel, 2001). Importantly, plasticity can also occur at the molecular level (e.g., gene transcription, protein stabilization; Morgan, Cohen, Hempstead, & Curran, 1987) as well as at the systems level (e.g., alterations of other organs outside the central nervous system; e.g., Miller, 1969).
One brain area that is especially subject to plastic alterations in the mammalian CNS is the hippocampus, which is critical for certain forms of learning and memory (Squire, Stark, & Clark, 2004). The hippocampus serves as a filter station that basically “decides” which experiences or learning contents are transformed into long-term memory storage. The function of the hippocampus is conserved over species (Milner, Squire, & Kandel, 1998; Squire, 1992). How the hippocampus fulfills this task on a cellular level is not truly understood, but plastic events such as LTP and restructuring of neuronal connections seem to be involved. However, it is not only preexisting structures that are subject to plasticity-inducing events in the adult hippocampus. The finding that neural stem cells (NSCs) persist in the adult brain and give rise to new neurons throughout life has not only ended the long-standing dogma in the neurosciences that neurogenesis tapers off with the end of embryonic development and does not occur in the adult but has also added a completely new level of complexity to adult brain function (F. Gage, 2000; Gross, 2000).
A large body of data derived from human and rodent studies indicate that hippocampal function gradually declines with age, potentially leading to severe and disabling cognitive impairment (Plassman et al., 2008, for a review see Rapp & Heindel, 1994). The underlying causes of age-associated alterations in hippocampus-dependent behavior appear to be manifold. Without a doubt, findings from the hippocampus cannot be bluntly extrapolated to the rest of the brain. However, plasticity and age-dependent changes that appear in the hippocampus can serve as an example of how age effects brain function on a more general level. In this tutorial we will discuss age-related changes in hippocampal plasticity with a focus on NSCs, their potential contribution to cognitive impairment, and the “plastic capacity” that even the aged brain retains and that might be used for future therapeutic strategies to enhance successful aging.
Hippocampus-dependent behavioral impairment associated with age
The hippocampus receives sensory input from cortical areas that are processed within the hippocampal circuitry and are then sent back to cortical association areas (Sheperd, 2003). The fundamental importance of the hippocampus in the transition from short-term (lasting minutes) to long-term (requiring de novo gene transcription and protein synthesis) memory first became evident with patients whose hippocampi were removed due to refractory epilepsy (Milner et al., 1998). These patients – the most prominent among them H.M. – suffered post-surgery from an anterograde amnesia. They were not able to transfer any new memory into long-term storage. One element of episodic memory that is also accessible in rodents is spatial memory (Bird & Burgess, 2008), and aging induces a decrease in performance in spatial navigation tasks across species (e.g., humans, non-human primates, rats, mice) (Barnes, 1979; F. H. Gage, Dunnett, & Bjorklund, 1984; Lai, Moss, Killiany, Rosene, & Herndon, 1995; Rapp, Kansky, & Roberts, 1997; Wilkniss, Jones, Korol, Gold, & Manning, 1997). Aging not only impacts retention of spatial memory; it also leads to a decline in the capacity to encode memory quickly, which might be crucial to cope with changing environments or experiences (Rosenzweig & Barnes, 2003; Wilson, Ikonen, Gallagher, Eichenbaum, & Tanila, 2005). Notably, in addition to spatial learning and memory, a variety of other hippocampus-dependent functions such as trace fear conditioning and trace eyeblink conditioning are affected by aging and decline over a life span (Finkbiner & Woodruff-Pak, 1991; Kishimoto, Suzuki, Kawahara, & Kirino, 2001; Moyer & Brown, 2006).
Structural changes and alterations in the connectivity of the hippocampal circuitry associated with aging
The underlying cause of the age-associated impairment of hippocampal function is not fully understood, but previous explanations were based on the finding that aging appeared to induce a dramatic loss of neurons and led to major alterations of neuronal morphology (for a review see: Burke & Barnes, 2006). However, more careful selection of healthy aged individuals (excluding patients with dementia) and rigorous quantitative analyses using stereological measures have revealed that these structural changes in the hippocampal formation are rather region-specific and probably less dramatic than previously thought (Keuker, Luiten, & Fuchs, 2003; West, Coleman, Flood, & Troncoso, 1994). Indeed, there does not seem to be any reduction in dendritic complexity in all hippocampal subfields in humans, a finding that was confirmed in rodents using stereological estimates (Flood, Guarnaccia, & Coleman, 1987; Hanks & Flood, 1991). Based on more recent experiments, there are also no striking reductions in the number of spines, at least in the dentate gyrus and in area CA1 of aged humans and rats (Curcio & Hinds, 1983; Markham, McKian, Stroup, & Juraska, 2005; Williams & Matthysse, 1986), in contrast to earlier studies. However, the number of axo-dendritic synapses between perforant path fibers and dentate granule cells appears to be reduced with age (Barnes & McNaughton, 1980; Geinisman, de Toledo-Morrell, Morrell, Persina, & Rossi, 1992). There is also an increased gap-junction-mediated coupling between granule cells in the dentate gyrus of aged rodents and a change in receptor and ion channel expression that might lead to changes in synaptic connectivity within the circuitry (Barnes, Rao, & McNaughton, 1987). In addition, reduced levels of numerous growth factors, such as brain derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), could alter the behavior of neural circuits in aged rodents (Hattiangady, Rao, Shetty, & Shetty, 2005; Shetty, Hattiangady, & Shetty, 2005). Thus, small but not excessive structural changes in neuronal morphology occur over a life span.
Basic electrophysiological properties of principal neurons in the hippocampus, such as resting membrane potential and input resistance, are not dramatically affected by advancing age (for a review see: Barnes, 1994). However, synaptic plasticity and LTP are altered in aged rodents. Both the induction and maintenance of LTP are impaired in aged rats, and this impairment holds true for LTP in the dentate gyrus, CA3, and area CA1 (Barnes, 1979; Barnes & McNaughton, 1980; Dieguez & Barea-Rodriguez, 2004). Why that happens is not fully understood, but it appears that Ca2+ signaling and altered dynamics of Ca2+ elevation at activated synapses are involved in reduced synaptic plasticity associated with aging (Thibault, Hadley, & Landfield, 2001). Furthermore, the “filter” function of the dentate gyrus, which is the first input of cortical connections into the hippocampal circuitry, is altered with age; in young rodents, stimulation of dentate granule cells results in suppression or no change of field potentials in target area CA3, a fact that is probably due to strong inhibition (for a review see: Patrylo & Williamson, 2007). In striking contrast, in a subset of aged rodents the same stimulation paradigm elicits trains of population spikes, suggesting an altered connectivity between the dentate gyrus and area CA3 that might contribute to age-associated alterations in hippocampal function by increasing basal activity in area CA3, that could eventually disrupt the signal to noise ratio of dentate gyrus CA3 connectivity (Patrylo, Tyagi, Willingham, Lee, & Williamson, 2007). In summary, it appears that the effects of aging on neuronal structure are rather subtle (at least at the level of current analyses) but that the functional connectivity within the hippocampal circuitry is strongly altered over the life span.
Regulation and functional significance of adult neurogenesis with age
Aging not only affects pre-existing structures but also has a strong effect on the activity and differentiation capacity of endogenous NSCs within the dentate gyrus area, because aging leads to strongly reduced levels of newborn neurons within the dentate area (Kuhn, Dickinson-Anson, & Gage, 1996). But what are the basic mechanisms of ongoing neurogenesis in the young adult brain and what changes over the life span?
The finding that, even in the adult brain, NSCs give rise to new granule cells has added a new level of complexity to adult brain function (Ming & Song, 2005). Newborn neurons become structurally and functionally integrated into the dentate circuitry after undergoing distinct steps of neuronal development (Jessberger & Kempermann, 2003; Kempermann, Jessberger, Steiner, & Kronenberg, 2004; Piatti, Esposito, & Schinder, 2006; van Praag et al., 2002). Importantly, not all immature neurons survive; only a fraction (approximately 30-40%) of new cells become stably integrated following an activity-dependent selection process, which happens about 1-3 weeks after the cells are born (Tashiro, Sandler, Toni, Zhao, & Gage, 2006). There is growing evidence that newborn neurons might be functionally significant at early stages of their neuronal development (approximately 3-5 weeks after they are born) (Ge, Yang, Hsu, Ming, & Song, 2007; Schmidt-Hieber, Jonas, & Bischofberger, 2004; Wang, Scott, & Wojtowicz, 2000). During this immature phase, newborn neurons are more excitable compared to pre-existing mature granule cells, making them “special” within the dentate circuitry (Schmidt-Hieber et al., 2004). Even though definitive proof is still missing (mostly due to the technical challenge of selectively ablating neurogenesis without substantial side effects), recent data suggest that, in young adult rodents, hippocampal neurogenesis is involved in spatial memory and contextual fear, and might also be required for the behavioral effects of antidepressant treatment (Santarelli et al., 2003; Saxe et al., 2006; Winocur, Wojtowicz, Sekeres, Snyder, & Wang, 2006; Zhang, Zou, He, Gage, & Evans, 2008). How new neurons might shape hippocampal connectivity and function and whether they serve any special function remain unclear but a large number of hypotheses have been formulated over the last years (Aimone, Wiles, & Gage, 2006; Becker, 2005; Doetsch & Hen, 2005; Kempermann, 2008; Schinder & Gage, 2004).
The number of newborn granule cells is not static but rather dynamically regulated by a variety of factors. Physical activity and environmental enrichment of young adult mice enhance the generation of functional neurons and are simultaneously associated with improved performance in hippocampus-dependent tasks, indicating a contribution of newborn neurons to hippocampal function (Kempermann, Kuhn, & Gage, 1997b; Van Praag, Christie, Sejnowski, & Gage, 1999). Interestingly, the cellular mechanisms how physiological regulators increase the number of new neurons are remarkably different: enrichment enhances the survival of new neurons whereas physical activity induces increased proliferation of neural progenitors. However, the molecular mechanisms underlying enrichement and physical activity-induced enhanced neurogenesis remain largely unknown. Other regulators include genetic background, dietary intake, head trauma, seizures, and learning (Chirumamilla, Sun, Bullock, & Colello, 2002; Elizabeth Gould, Beylin, Tanapat, Reeves, & Shors, 1999; Kempermann, Kuhn, & Gage, 1997a; Lee, Duan, Long, Ingram, & Mattson, 2000; Parent et al., 1997).
However, there are also conditions in which neurogenesis is greatly reduced. For example, stress (which is strongly associated with major depression) and inflammation dramatically reduce the number of newborn cells (E. Gould & Tanapat, 1999; Monje, Toda, & Palmer, 2003). As mentioned above, there is an exponential decrease of neurogenesis throughout the life span in rodents, non-human primates, and potentially in humans (Eriksson et al., 1998; E. Gould et al., 1999; Kempermann, Kuhn, & Gage, 1998; Kronenberg et al., 2006; Kuhn et al., 1996; Rao, Hattiangady, & Shetty, 2006). With advancing age, the proliferative activity of hippocampal NSCs and neuronal differentiation capacity decline, leading to a dramatic, approximately 10-fold reduction in net neurogenesis between the age of 2 months and 2 years in a rodent's life (Kempermann, Gast, & Gage, 2002; Kuhn et al., 1996; van Praag, Shubert, Zhao, & Gage, 2005). Analyzing levels of neurogenesis over a life span provided similar observations in non-human primates (E. Gould et al., 1999). Interestingly, at least in some studies, the reduction of neurogenesis appears to be correlated with age-associated cognitive deficits (Bizon & Gallagher, 2003; Bizon, Lee, & Gallagher, 2004; Drapeau et al., 2003, but see also Merrill, Karim, Darraq, Chiba, & Tuszynski, 2003), suggesting that newborn neurons are either causally involved in age-induced cognitive impairment or that the levels of neurogenesis correlate with or predict the overall plasticity of the hippocampal circuitry. At the moment it is not clear whether neurogenesis is actively downregulated with age or if the reduction of newborn cells is rather caused by i) an exhaustion of the stem cell pool, ii) alterations in the neurogenic niche, such as reduced neurotrophic factor availability, iii) age-associated reduced levels of excitability within the dentate circuitry that could translate into less net neurogenesis, or iv) alterations in humoral factors with age, such as corticosteroid levels. In fact, it has been shown that reduction of corticosteroid levels in aged rats to the levels of young adults was sufficient to restore neurogenesis almost completely, suggesting that general aging-associated changes in the organism might indeed play a pivotal role in reduction of neurogenesis with age (Cameron & McKay, 1999). Supporting this assumption, levels of corticosteroids in the plasma correlate with the number of new neurons in aged rats (Montaron et al., 2006).
Without a doubt, adult neurogenesis is not a one-step process, as new neurons have to progress through several distinct developmental steps before they fully integrate and eventually become indistinguishable from granule cells born during embryonic or early postnatal development (Laplagne et al., 2006). The effect of age on the speed of neuronal maturation of individual newborn cells remains unclear. There is some evidence suggesting a delayed maturation in aged rats compared to cells that were born in younger adults (Rao, Hattiangady, Abdel-Rahman, Stanley, & Shetty, 2005). However, other reports suggest that once a cell is born and starts to mature into a neuron, the rate and steps of neuronal maturation are comparable between young and aged rodents (Couillard-Despres et al., 2006; van Praag et al., 2005; Jessberger & Gage, unpublished observation). Experiments are under way aiming to characterize cell-autonomous versus niche-dependent alterations of the NSC activity and potential neuronal maturation processes in young versus old mice. Clearly, a decline in neurogenesis is associated with advancing age, indicating that one feature of neural plasticity –the addition of new neurons into the dentate circuitry – is dramatically reduced over the life span.
NSC-based therapeutic strategies to ameliorate age-related cognitive decline
Due to alterations in the demographic composition of societies with increasing numbers of old people, aging will potentially become an issue that affects more than the aged individuals themselves (Wilmoth, 1998). Even though many domains of life might be affected with age, the prevention or amelioration of cognitive decline is one of the key aspects for successful aging.
Obviously, aging in humans occurs with a tremendous degree of individual variability; where some centenarians are highly active, physical and cognitive decline occurs much earlier in others without any evidence of neurodegenerative disease. The same holds true for genetically related individuals. Thus, it seems plausible to speculate that the speed and degree of aging are not determined only by genetic disposition but are rather regulated by the dynamic interplay between genes, behavior, and experience. Indeed, previous studies showed that physical activity as well as cognitive challenge leads to improved cognitive performance in aged humans, supporting the concept of “use it or lose it” (Churchill et al., 2002; Kramer et al., 2002; Kramer et al., 1999). Interestingly, there is increasing evidence that experience alters not only brain function but also neural structure in humans; for example, hippocampal volume increased in medical students after extensive studying for an exam (Draganski et al., 2006).
As outlined above, aging is associated with a dramatic decrease in the number of newborn granule cells. However, this decline might be avoidable and changeable even at advanced stages: outdoing the effects of environmental enrichment (EE) in young adult mice, EE leads to an approximately 5-fold increase of newborn granule cells in aged mice that were kept for 10 months under enrichment conditions (Kempermann et al., 2002). In this experiment enrichment started at the age of 10 months and the observed pro-neurogenic effect was largely due to increased neuronal differentiation (Kempermann et al., 2002). Strikingly, mice that had lived in the EE performed significantly better in spatial memory tasks compared to sedentary controls, supporting the hypothesized link between neurogenesis and hippocampus-dependent behavior (Kempermann et al., 2002). Similarly, voluntary physical exercise in aged mice (19 months old) for approximately 7 weeks enhanced the number of newly generated cells approximately 5-fold (van Praag et al., 2005). Again, this increase in neurogenesis was associated with improved performance in the Morris water maze (van Praag et al., 2005). However, an important difference between the two studies was that, in the latter study, running started very late in life (at 19 months, with an average life expectancy of about 26 months in the C57Bl/6 mice). Thus, even in advanced stages of aging, the brain retains the ability to react to exogenous stimuli that are associated with improved cognitive performance. Even more surprising, also very short periods of EE exposure (1 week) are sufficient to enhance both neurogenesis and behavioral performance in aged mice (Jessberger & Gage, unpublished observation), similar to the effects in young adults (Tashiro, Makino, & Gage, 2007). This finding is important because previously used manipulations of the environment (either EE or physical activity) were presented for extended times (> 7 weeks for running, 10 months for EE), making it difficult to directly test these effects in humans. But there is already evidence that physical activity in the elderly is beneficial for cognition (for a review see: Kramer et al., 1999). As non-invasive imaging techniques for NSC activity and neurogenesis in humans become increasingly available (Manganas et al., 2007; Pereira et al., 2007), future studies will determine whether physical activity also affects hippocampal neurogenesis in humans.
Preventing or ameliorating age-associated cognitive decline might not be the only application for the activation of NSC-related plasticity. Age is also the major risk factor for neurodegenerative diseases such as Alzheimer's disease (AD) (Lindsay et al., 2002). Interestingly, recent evidence suggests that, in patients and animal models of AD, there might be alterations in hippocampal neurogenesis that contribute to cognitive impairment (for a review see: Steiner, Wolf, & Kempermann, 2006). However, it is not clear at this time whether alterations of NSCs are causally involved or are a mere byproduct of the disease process. In any case, the therapeutic targeting of endogenous NSCs (either by drugs or with environmental challenges) might be a promising line of treatment in the future.
The same is true for age-associated affective disorders such as depression. One clinical feature of depression is the inability to successfully integrate new experiences, and it has been previously speculated that adult hippocampal neurogenesis might be critically involved in major depression (Sahay & Hen, 2007). There are two lines of evidence supporting a role for newborn neurons in emotional behavior: i) many antidepressant drugs increase the number of newborn neurons in the dentate area (Schmidt & Duman, 2007), and ii) the effectiveness of certain antidepressant such as fluoxetine seems to require ongoing neurogenesis, at least in young mice (Santarelli et al., 2003). Thus, restoring hippocampal neurogenesis in the aging process, either by environmental stimulation or drug treatment, might not only be beneficial for age-related cognitive decline but might also ameliorate neurological diseases that are associated with age.
Conclusion
Aging affects the structural and functional connectivity of the mammalian hippocampus. One outstanding alteration that occurs with advancing age is the dramatic decline in the number of newborn neurons generated by endogenous NSCs. However, even the aged brain retains the capacity to react to environmental stimuli with enhanced plasticity, opening the possibility of targeting NSCs and other aspects of ongoing neural plasticity to prevent or ameliorate age-related neurological deficits. Future studies will have to determine the exact degree of plasticity that persists into old age and the causal relationship between enhanced neurogenesis and behavioral function. Importantly, first attempts using non-invasive imaging methods to analyze the extent of neurogenesis in humans are promising (Manganas et al., 2007; Pereira et al., 2007) and will together with improved histological and molecular techniques of human post-mortem samples hopefully close the apparent gap between animal and human research in the context of adult neurogenesis.
Figure 1.
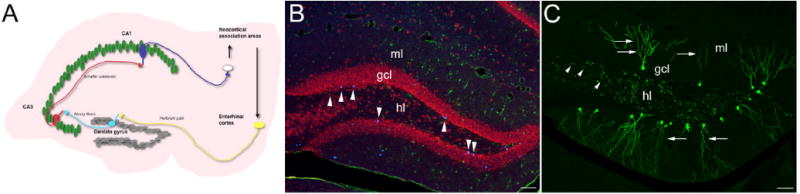
Neurogenesis persists in the adult hippocampus. (A) Schematic representation of hippocampal connectivity. The hippocampus receives its main input via the entorhinal cortex from necortical association areas. The axons of entorhinal cortex neurons (perforant path fibers) synapse onto dentate gyrus granule cells that send their axons (mossy fibers) to area CA3. CA3 pyramidal neurons send axons to area CA1 (Schaffer collaterals). CA1 pyramidal cells then signal back to neocortical association areas. (B) Newborn cells in young adult mice were labeled by the injection of the thymidine analogue BrdU 4 weeks before the animals were killed. Note the BrdU-labeled (blue) newborn cells expressing the pan-neuronal marker NeuN (red) within the inner parts of the dentate gyrus granule cell layer. Only very few astrocytes (S100β in green) are newly generated in the adult hippocampus. (C) Labeling of dividing NSCs and their progeny with a retrovirus reveals the distinct, highly polarized morphology of 4-week-old, newborn granule cells with an apical dendrite extending toward the molecular layer (arrow) and axonal processes growing to area CA3 (arrowheads). gcl, granule cell layer; ml, molecular layer; hl, hilus. Scale bars in B, C: 150 μm.
Figure 2.
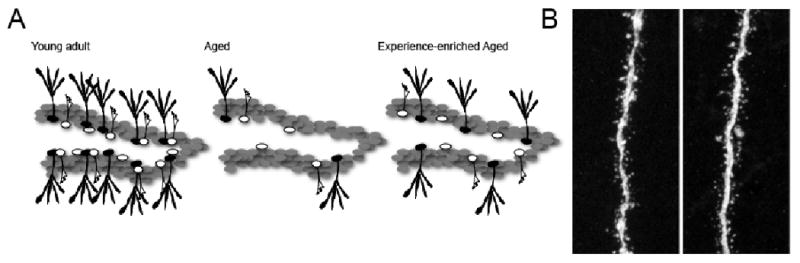
Age-dependent regulation of hippocampal neurogenesis. (A) Young adult mice have a relatively high number of NSCs (which consist presumably of different subtypes, see (Kempermann et al., 2004), depicted in black, that produce robust numbers of newborn granule cells (white, left panel). With advancing age, there is a dramatic drop in the amount of hippocampal neurogenesis (middle panel). At the moment, it is not fully understood if this decrease is due to a drop in NSC proliferative activity, number of NSCs, or alterations in their differentiation capacity. Importantly, even the aged brain responds to external stimuli such as environmental enrichment and physical activity, indicating the persistent potential for plasticity even in the aged brain (right panel). (B) Retroviral labeling allows detailed morphological analyses of newborn cells. The number and shape of dendritic spines extending from 4-week-old granule cells in young mice (left panel) and aged, 19-month-old mice (right panel) are not different, suggesting that the speed of neuronal maturation is not substantially affected in aged rodents.
Acknowledgments
We would like to thank Mary Lynn Gage for editing this manuscript. We apologize to all the authors whose work we could not cite due to space limitations.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/pag.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17(1):13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. J Comp Neurol. 1987;259(4):549–558. doi: 10.1002/cne.902590405. [DOI] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15(6):722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9(3):182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18(1):215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3(4):227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2(10):894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19(6):693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23(5):941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340(6233):474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, et al. Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci. 2006;24(6):1535–1545. doi: 10.1111/j.1460-9568.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Hinds JW. Stability of synaptic density and spine volume in dentate gyrus of aged rats. Neurobiol Aging. 1983;4(1):77–87. doi: 10.1016/0197-4580(83)90058-1. [DOI] [PubMed] [Google Scholar]
- Dieguez D, Jr, Barea-Rodriguez EJ. Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse. 2004;52(1):53–61. doi: 10.1002/syn.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15(1):121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100(24):14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging. 1991;6(1):109–117. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- Flood DG, Guarnaccia M, Coleman PD. Dendritic extent in human CA2-3 hippocampal pyramidal neurons in normal aging and senile dementia. Brain Res. 1987;409(1):88–96. doi: 10.1016/0006-8993(87)90744-x. [DOI] [PubMed] [Google Scholar]
- Gage F. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5(1):43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A Critical Period for Enhanced Synaptic Plasticity in Newly Generated Neurons of the Adult Brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippoampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult old world primates. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46(11):1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1(1):67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer's disease. I. CA1 of hippocampus. Brain Res. 1991;540(1-2):63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18(10):2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21(5):565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for. Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52(2):135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997a;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997b;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001;12(15):3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Colcombe S, Erickson K, Belopolsky A, McAuley E, Cohen NJ, et al. Effects of aerobic fitness training on human cortical function: a proposal. J Mol Neurosci. 2002;19(1-2):227–231. doi: 10.1007/s12031-002-0038-y. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16(6):947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4(12):e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15(2):99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404(6780):876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15(1):97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol. 2003;459(2):201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Miller NE. Psychosomatic effects of specific types of training. Ann N Y Acad Sci. 1969;159(3):1025–1040. doi: 10.1111/j.1749-6632.1969.tb12995.x. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20(3):445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27(4):645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Impaired trace and contextual fear conditioning in aged rats. Behav Neurosci. 2006;120(3):612–624. doi: 10.1037/0735-7044.120.3.612. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Tyagi I, Willingham AL, Lee S, Williamson A. Dentate filter function is altered in a proepileptic fashion during aging. Epilepsia. 2007;48(10):1964–1978. doi: 10.1111/j.1528-1167.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Williamson A. The effects of aging on dentate circuitry and function. Prog Brain Res. 2007;163:679–696. doi: 10.1016/S0079-6123(07)63037-4. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti VC, Esposito MS, Schinder AF. The timing of neuronal development in adult hippocampal neurogenesis. Neuroscientist. 2006;12(6):463–468. doi: 10.1177/1073858406293538. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21(2):464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5(6):545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Heindel WC. Memory systems in normal and pathological aging. Curr Opin Neurol. 1994;7(4):294–298. doi: 10.1097/00019052-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8(8):1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology (Bethesda) 2004;19:253–261. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(5-6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Sheperd GME. The Synaptic Organization of the Brain. 5th Oxford University Press; USA: 2003. [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51(3):173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]; Psychol Rev. 1992 Jul;99(3):582. published erratum appears in. [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Steiner B, Wolf S, Kempermann G. Adult neurogenesis and neurodegenerative disease. Regen Med. 2006;1(1):15–28. doi: 10.2217/17460751.1.1.15. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21(24):9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402(6760):421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42(2):248–257. [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344(8925):769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wilkniss SM, Jones MG, Korol DL, Gold PE, Manning CA. Age-related differences in an ecologically based study of route learning. Psychol Aging. 1997;12(2):372–375. doi: 10.1037//0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- Williams RS, Matthysse S. Age-related changes in Down syndrome brain and the cellular pathology of Alzheimer disease. Prog Brain Res. 1986;70:49–67. doi: 10.1016/s0079-6123(08)64297-1. [DOI] [PubMed] [Google Scholar]
- Wilmoth JR. The future of human longevity: a demographer's perspective. Science. 1998;280(5362):395–397. doi: 10.1126/science.280.5362.395. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25(29):6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]


