Abstract
Background
Rural populations as well as less educated people in the U.S. are less likely to receive colorectal cancer (CRC) screening than people living in urban areas and more educated people.
Methods
We tested a computer tablet, Patient/Provider Communication Assistant (PPCA), which collected data, educated patients, and printed personalized notes to patients and providers encouraging conversation about CRC screening. Mixed model analyses using a pre-post quasi-experimental design compared patient results during the comparison and intervention periods in 5 rural primary care practices on provider discussion about CRC screening, provider recommendation, and patient intention to be screened. Models including age, education and literacy measures as covariates were examined.
Results
Providers talked with patients about CRC screening in general, and colonoscopy specifically more frequently after the PPCA than with the comparison group (p values =.04 and .01, respectively). Providers recommended CRC screening more often to patients in the intervention group than to the comparison group (p=.02). Patients planned to be screened and specifically with colonoscopy more frequently following the intervention than in the comparison group (p=.003). There were no interactions between group and any of the covariates. Ninety-five percent of the patients regardless of age or education found the PPCA easy to use.
Conclusion
Results indicated increased provider discussion and recommendation, and patients' intentions to obtain CRC screening, and in particular colonoscopy, for patients exposed to the intervention, regardless of the patients' age or literacy levels. The PPCA is a promising intervention method that is acceptable to rural patients.
Keywords: colorectal cancer screening, rural, literacy, computer tablet
INTRODUCTION
Although colorectal cancer (CRC) screening can save lives through prevention and early detection many people do not participate in regular screening as recommended by national guidelines.1, 2 Rural as well as less educated people in the U.S. are less likely to be screened than urban and more educated people.3–6 A survey of rural patients suggested that the primary barrier to CRC screening was inadequate discussion of CRC between patient and physician.7
Similar to other cancer screening behaviors, multiple studies have shown that a provider recommendation is a strong predictor of CRC screening participation.3, 4, 8, 9 The odds of participating in any method of CRC screening for all age groups increased by at least a factor of 8 with a clinician's recommendation.9 The complexity of the CRC screening guidelines compared with other cancers may be a deterrent to more active recommendation of these tests by providers, however.10, 11 It also has been shown that individual providers' intentions, perceptions, and understanding vary greatly with regard to CRC screening.10–14
Discussion about CRC screening most frequently occurs at preventive care visits.3, 4, 15, 16 However, a study reported that only one third of all patients had undergone a routine physical examination within the past two years.14 In general, providers frequently do not have sufficient time to cover all preventive recommendations 6, 13 or adequate systems in place to identify patients in need of CRC screening.14
Patients' expectations and anxiety also may influence whether or not they are recommended for a screening test.17,18 Results of a large national study found that the majority of unscreened respondents were neither aware of the need nor counseled about CRC screening.5
Because both patient and provider characteristics and behavior influence CRC screening participation our challenge was to develop an intervention that educated patients, identified when they were not up to date with CRC screening, learned their plans (or intentions) to be screened, and stimulated a discussion and recommendation at a medical visit.19 Tailored computer-based interactive interventions have been used to inform, influence, and motivate individuals about health behaviors20–22 including help with making recommendations.23, 24 We developed and pilot tested a computer-based intervention, the Patient / Provider Communication Assistant (PPCA), to facilitate discussion and provider recommendations for CRC screening. We hypothesized that patients exposed to the PPCA would be more likely to have a discussion with and recommendation from their provider, which would lead to a plan to receive CRC screening.
METHODS
We used a pre-post quasi-experimental design applied to primary care practices. Five practices in rural communities were recruited into this pilot study. In each practice a comparison group of all patients ages 50–80 during 1 week were invited by their primary care provider to participate in a 5 minute exit interview concerning discussion and recommendations from providers, and patient intentions regarding colorectal screening. The comparison group served as the control for patients in the same practice who received the intervention. In the same 5 practices a similar group of patients were recruited in an equivalent way about 1 month later to participate in the PPCA intervention immediately before their visit (intervention group). Patients answered questions on the PPCA about their history of CRC screening, intentions to screen in the future and risk factor information. A written summary of the responses to these three categories of questions and a recommendation to speak with the health care provider about CRC screening was provided to the patient and the provider before the visit; the provider then sent these patients to the research assistant to be interviewed following their visit similar to the comparison group exit interview. This study was approved by the Institutional Review Board at the University of Vermont and all participants provided written informed consent.
Intervention development
We used the Transtheoretical Model (TTM) to characterize people's readiness to adopt or maintain CRC screening behavior. 25 Once we identify the stage of change of the individual patient we prepared an educational message specifically aimed at their readiness to adopt or maintain the CRC screening behavior (Table 1). The PRECEDE Model26 was the conceptual framework to conduct the formative and process evaluation to learn about the predisposing, enabling and reinforcing factors from the two participant groups in this study: Patients and PCP (providers and their office practices). We developed the intervention to address the barriers while using the facilitators we identified in the formative evaluation as the foundation of this intervention.
Table 1.
Algorithms for stages of change (Transtheoretical model)
| Patient status | Educational message | Stage |
|---|---|---|
| - No history of CRC screening - No intentions to obtain in next 6 months |
- Acknowledge and provide tailored information about any risk factors - Encourage to reconsider decision showing self efficacy by role models (video clips) - Recommend screening |
Pre-contemplator |
| - No history of CRC screening - Intends to be screened in next 6 months |
- Acknowledge and provide tailored information about any risk factors - Provide social support - Recommend screening |
Contemplator |
| - Appropriately screened* now - Intends to be screened at appropriate intervals |
- Promote self efficacy by reinforcing current compliance - Emphasize benefits from screening - Acknowledge and provide tailored information about any risk factors - Recommend screening |
Action |
Adapted from Marcus et al. Promoting cancer screening among the first degree relatives of breast and colorectal cancer patients: The design of two randomized trials. Prev Med. 1999 Mar;28(3):229–42.
Appropriately screened will mean compliance with the ACS guidelines: FOBT annually, FOBT annually combined with Sigmoidoscopy every 5 years, Sigmoidoscopy or Double-contrast barium enema every 5 years, Colonoscopy every 10 years.
We worked with representatives from the intended patient audience to conduct 3 levels of formative research to develop the intervention.27 In 2005 we held 6 focus groups with 18 people aged 50–80 with low education to learn about their specific barriers and facilitators to participating in regular CRC screening. We then developed sample computer screens following guidelines for developing health education and computer programs for low literacy28 and elderly populations.29 An expert on teaching low literate persons reviewed the intervention to assure appropriate reading level and concept development. After the screens were developed we tested them with 2 gender-specific focus groups and with volunteers at a local senior center and Adult Basic Education Center to assess visual appeal, wording and comprehension. Finally, 10 volunteers of mixed educational levels and computer experience tested the computer program for ease of use.
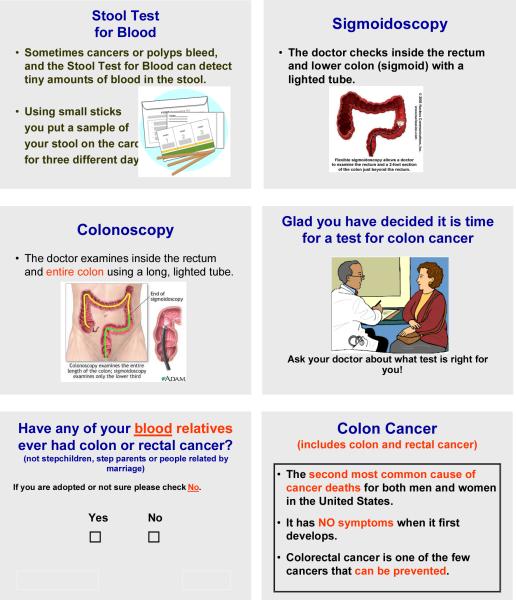
The program provided informed consent information followed by one question at a time in large print about CRC screening history, intentions, risk factors and demographics. As words appeared on a screen they were also read aloud via an audio component. Examples of the screens can be viewed in Figure 1. The program presented information about CRC, including risk factors, and a description of 3 screening tests (fecal occult blood test (FOBT), sigmoidoscopy and colonoscopy) including how the test is done and the advantages and disadvantages of each screening method.30 The educational screens included pictures of each test and pictures in the final screens appropriate to the recommendation. The computer selected 1 appropriate video clip, depending on age and gender of the patient, of a personal testimonial about participating in CRC screening. The final screens were personalized recommendations based on the responses to the prior questions. At the end of the PPCA the computer automatically printed a personalized recommendation for the patient and a provider recommendation for the patient's chart. A sample of the printout for a woman with no risk factors who has never been but intends to be screened included the following wording in a letter format:
It is great news that you are planning to get a test for colon cancer. As you get older your chance of getting colon cancer increases. A screening test can find small growths (polyps) before they develop into cancer. These can be removed to prevent cancer. There are three different tests for colon cancer. Talk with your doctor today about which test is right for you.
Intervention participants completed the PPCA in 10 –14 minutes in a private setting with a research assistant available to provide instruction if necessary.
Figure 1.
Examples from PPCA: Description of tests, final recommendation screen for women with intentions to be screened, question screen, and educational screen
We also met with each participating primary care practice's office and medical staff to introduce the program and identified the barriers and facilitators within the practice to participating in this study. We designed how, when and where we worked with patients based on what the practices were able and willing to do.
Recruitment of practices and patients
All 38 primary care physicians (7 OBGYNs), representing 20 practices (8 solo practices) in 2 rural counties were mailed a letter from the principal investigator (PI) and medical director of the Area Health Education Center explaining the study and inviting their practice to participate. A respected Vermont colon surgeon and the PI also conducted primary care grand rounds at the hospitals in these counties. Twelve practices initially were recruited but seven withdrew for various reasons (staff illness, physician sent to Iraq, PCP who performed colonoscopies as a major part of practice, starting electronic medical records). The three solo practices withdrew because there was insufficient space to conduct the study. All 5 participating practices were large family practices including 2 community health centers with a total of 12 physicians. In addition, there were 3 nurse practitioners and 3 physician's assistants who delivered primary care to these patients. In rural practices patients are often seen by mid level practitioners and regard them as their provider.
All patients between ages 50 and 80 were eligible to be in the study regardless of the type of medical visit scheduled. For both study conditions, eligible patients were initially recruited by a receptionist's invitation to talk to a research assistant. For the comparison group, after the provider's visit interested patients were directed to a research assistant who completed the informed consent process and exit interview. For intervention group participants, eligible patients were immediately sent to talk with the research assistant. Intervention participants engaged in the tablet-based educational program before their visit and signed the informed consent and answered a few visit related questions after the provider visit. We invited all patients between the ages of 50–80, regardless of their CRC screening status, to participate in the intervention. The TTM encourages reinforcement of appropriate behavior (action) and therefore we decided to congratulate those up to date on CRC screening. The questions asked on the PPCA determined whether the patient was currently screened.
Measurement
The exit interview for the comparison group included questions measuring demographics, risk factors, CRC screening history, interactions with the provider, and plans to be screened. Intervention participant exit interviews included questions about interactions with provider, plans to be screened and evaluation of the computer program. We simplified questions from the National Health Interview Survey (NHIS)31 and the Behavioral Risk Factor Surveillance System (BRFSS)32 and tested these questions using focus groups. Words and terms were defined in simple language before using in a question. For example, we never used the term “colorectal” but instead said “colon cancer” and defined it to include both colon and rectal cancer. A sample of the outcome measures assessing the provider's interaction with the patients asked, “Did your doctor talk with you about a stool test for blood today?” and “(If yes) Did your doctor today recommend the test or give you a kit?” A sample outcome measure of the patient's intention to obtain CRC screening was, “Do you plan to do the stool test for blood?”
Literacy was measured by three questions from two validated studies. The first question, “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” was used in another Vermont study33, while the last two questions, “How often do you read books?” and “When you are asked questions about your health what do you prefer?” (Like to read them yourself; like the questions read to you), combined with level of education, is a literacy measure developed by Lobach et al. 34,35
We developed and tested questions to measure the use and perceptions of the PPCA.
Statistical analyses
The comparison and intervention groups were compared with respect to demographics, risk factors and their CRC screening history, and then on the outcomes of interest: 1. whether or not the doctor talked about CRC screening, 2. whether or not the doctor recommended CRC screening and 3. patient intentions to receive CRC screening. Due to the nested nature of the data (participants nested within each practice) a mixed model analysis of variance approach was used running the GLIMMIX procedure in SAS for Windows version 9.1 (SAS Institute; Cary, NC) with the binary distribution for dichotomous outcome measures and the multinomial distribution for outcome measures with greater than two levels. The model included the two groups (comparison, intervention) as a fixed main effect, primary care practice as a random main effect and group by primary care practice as a random interaction effect. Subsequent models included the addition of participant level covariates (age, education and literacy) and their interactions with group (comparison, intervention) to determine if they modified the effect of group on the outcome measures. Subjects missing any of the variables included in a particular model were excluded from the analysis.
RESULTS
We recruited 177 patients into the comparison group and 142 into the intervention group. The participation rate for all but 2 practices is unknown because practices were required to invite the patients into the study and 3 were not able to share patient schedules due to HIPAA concerns. In the 2 practices that provided data, participation rates were 74% (63/85) and 69% (55/80) for the comparison and intervention groups respectively (p-value =.27). Of the intervention patients who were referred to the research assistant, 4 decided not to participate after they read the informed consent information, and 1 ran out of time. The comparison and intervention populations were similar in demographics, risk factors and their CRC screening history. The only significant difference between the 2 groups was that the comparison group was more likely to want health questions read to them (Table 2).
Table 2.
Patient characteristics for the comparison and intervention groups
| Patient characteristics | Comparison (N=177) | Intervention (N=142) | ||
|---|---|---|---|---|
| (n) % | % | (n) | % | |
| Age | ||||
| 50–60 | (85) | 48.0 | (59) | 42.8 |
| 61–70 | (59) | 33.3 | (48) | 34.8 |
| 71–80 | (33) | 18.6 | (31) | 22.5 |
| Gender | ||||
| Male | (75) | 42.4 | (56) | 41.2 |
| Educational attainment | ||||
| < High School (HS) | (26) | 14.7 | (12) | 9.0 |
| HS or General Education Development (GED) | (77) | 43.5 | (57) | 42.9 |
| Some college or trade school | (34) | 19.2 | (31) | 23.3 |
| College | (23) | 13.0 | (19) | 14.3 |
| > college | (17) | 9.6 | (14) | 10.5 |
| How often need help reading? | ||||
| Never/rarely | (140) | 79.1 | (108) | 78.8 |
| Sometimes/often/always | (37) | 20.9 | (29) | 21.2 |
| How often do you read books? | ||||
| Never, < once a week | (65) | 36.9 | (39) | 28.5 |
| ≥Once a week | (111) | 63.1 | (98) | 71.5 |
| When you are asked questions about your health what do you prefer?** | ||||
| Read yourself/no pref. | (109) | 62.3 | (110) | 81.5 |
| Have read to you. | (66) | 37.7 | (25) | 18.5 |
| Currently smokes cigarettes | (33) | 18.6 | (17) | 12.5 |
| Has a first degree relative with CRC | (43) | 24.3 | (27) | 19.4 |
| Has personal history of: | ||||
| Colorectal cancer | (0) | 0.0 | (5) | 3.8 |
| Colon or rectal polyp removed | (55) | 31.1 | (42) | 31.8 |
| Ulcerative colitis | (8) | 4.5 | (6) | 4.7 |
| Crohn's disease | (1) | 0.6 | (2) | 1.5 |
| Currently up to date* on CRC screening✪ | ||||
| FOBT | (14) | 7.9 | (8) | 5.6 |
| Sigmoidoscopy | (3) | 1.7 | (8) | 5.6 |
| Colonoscopy | (106) | 59.9 | (75) | 52.8 |
| Not up to date on CRC screening | (54) | 30.5 | (51) | 35.9 |
| Patients at | ||||
| Practice 1 | (35) | 19.8 | (27) | 18.9 |
| Practice 2 | (45) | 25.4 | (42) | 29.4 |
| Practice 3 | (28) | 15.8 | (19) | 13.3 |
| Practice 4 | (35) | 19.8 | (26) | 18.2 |
| Practice 5 | (34) | 19.2 | (29) | 20.3 |
At time of doctor visit
Significant at .02 between comparison and intervention groups
Self-report of CRC screening includes all the tests that patients reports and are not mutually exclusive
The proportions of patients reporting that their doctor talked with them about CRC screening and specifically about colonoscopy were significantly greater in the intervention than in the comparison group (p=.04, .04, respectively). The same effect was seen in the doctor's recommendation to the patient to be screened (p=.02) and a recommendation for colonoscopy (p=.01). For patients who reported not being up to date on CRC screening, those exposed to the intervention were significantly more likely to plan on getting screened than those in the comparison group (p=.01). When including all patients whether current on screening or not, we found that significantly more patients exposed to the intervention were planning to participate in CRC screening (p=.003), particularly with colonoscopy (p=.003) (Table 3).
Table 3.
Comparison and intervention groups results for main outcome variables
| Comparison (N=177) | Intervention (N=142) | P value* | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Dr talked about: | |||||
| CRC screening | 50 | 29.6 | 71 | 54.2 | .04 |
| FOBT | 21 | 11.9 | 25 | 19.2 | .21 |
| Sigmoidoscopy | 8 | 4.6 | 10 | 7.6 | .29 |
| Colonoscopy | 43 | 25.3 | 67 | 51.2 | .04 |
| Dr. Recommended: | |||||
| CRC screening | 38 | 23.0 | 64 | 49.2 | .02 |
| FOBT |
11 | 6.3 | 15 | 11.6 | .25 |
| Sigmoidoscopy | 5 | 2.9 | 3 | 2.3 | .77 |
| colonoscopy | 30 | 18.1 | 56 | 43.4 | .01 |
| Participants not currently up to date on CRC screening plan to: | |||||
| Get screened | 23 | 43.4 | 45 | 91.8 | .01 |
| To do FOBT | 12 | 22.2 | 17 | 36.2 | .18 |
| Get a sigmoidoscopy | 0 | 0.0 | 1 | 2.1 | .37 |
| Get a colonoscopy | 16 | 30.8 | 31 | 66.0 | .07 |
| All participants plan to: | |||||
| Get screened | 88 | 50.6 | 121 | 91.7 | .003 |
| To do FOBT | 49 | 27.7 | 48 | 36.9 | .35 |
| Get a sigmoidoscopy | 5 | 2.9 | 6 | 4.6 | .81 |
| Get a colonoscopy | 58 | 33.1 | 94 | 72.3 | .003 |
p-values from the GLIMMIX mixed model
footnote: The totals for Dr. talked about and recommended “CRC screening”, and participants plan to “Get screened” include a participant only once. The participant had the opportunity to check more than one screening test for all four questions so that these do not necessarily add up to the total.
There were no significant interactions between group and any of the covariates. The inclusion of just the main effect of the covariates did not detect any association between the covariate and the outcomes, and did not modify the association between group and the outcomes.
Patients who used the PPCA were asked 4 questions to evaluate using the intervention. Responses are shown in Table 4 stratified by age and educational attainment. Virtually everyone found the program easy to use. Older participants and less educated participants found the sound (reading the information and questions on each screen) helpful compared with the younger and the more educated participants. A slightly higher percentage of the older and the less educated participants found the length of the program too long compared to the younger and the more educated participants. Few reported that the video testimonials was their favorite part of the program while most found the information about colon cancer their favorite part followed by either the questions they answered or the final recommendation screens at the end of the program.
Table 4.
Evaluation of the Patient – Physician Communication Assistant (PPCA) by age and education
| Age 50–60 | Age 61–70 | Age 71–80 | ≤ H.S. | > H.S. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n) | % | (n) | % | (n) | % | (n) | % | (n) | % | |
| How easy was it to use the computer today? | ||||||||||
| Very easy | (54) | 96.4 | (42) | 91.3 | (26) | 96.3 | (59) | 92.2 | (58) | 96.7 |
| Somewhat easy | ( 2) | 3.6 | ( 4) | 8.7 | ( 0) | 0.0 | ( 4) | 6.2 | ( 1) | 1.7 |
| A little difficult | ( 0) | 0.0 | ( 0) | 0.0 | ( 1) | 3.7 | ( 1) | 1.6 | ( 1) | 1.7 |
| Very difficult | ( 0) | 0.0 | ( 0) | 0.0 | ( 0) | 0.0 | ( 0) | 0.0 | ( 0) | 0.0 |
| How did you find the sound? | ||||||||||
| Very helpful | (19) | 33.9 | (20) | 43.5 | (16) | 59.3 | (36) | 56.3 | (16) | 26.7 |
| Somewhat helpful | (18) | 32.1 | (14) | 30.4 | ( 3) | 11.1 | (15) | 23.4 | (18) | 30.0 |
| Not helpful | ( 0) | 0.0 | ( 3) | 6.5 | ( 1) | 3.7 | ( 3) | 4.7 | ( 1) | 1.7 |
| Annoying | ( 4) | 7.1 | ( 0) | 0.0 | ( 3) | 11.1 | ( 3) | 4.7 | ( 4) | 6.7 |
| Did not use | (15) | 26.8 | ( 9) | 19.6 | ( 4) | 14.8 | ( 7) | 10.9 | (21) | 35.0 |
| Was the length of the computer program…? | ||||||||||
| Too short | ( 0) | 0.0 | ( 1) | 2.2 | ( 0) | 0.0 | ( 1) | 1.6 | ( 0) | 0.0 |
| Just right | (55) | 98.2 | (42) | 91.3 | (24) | 88.9 | (58) | 90.6 | (57) | 95.0 |
| Too long | ( 1) | 1.8 | ( 3) | 6.5 | ( 3) | 11.1 | ( 5) | 7.8 | ( 3) | 5.0 |
| What was you favorite part of the computer program? | ||||||||||
| Was it the: | ||||||||||
| information about colon cancer | (30) | 58.8 | (29) | 67.4 | ( 9) | 36.0 | (37) | 61.7 | (27) | 50.0 |
| questions that you answered | ( 5) | 9.8 | (5) | 11.6 | ( 9) | 36.0 | (10) | 16.7 | ( 8) | 14.8 |
| videos of others talking about their experiences | ( 2) | 3.9 | ( 0) | 0.0 | ( 2) | 8.0 | (3) | 5.0 | ( 1) | 1.9 |
| screens at the end that recommend screening | (14) | 27.5 | ( 9) | 20.9 | ( 5) | 20.0 | (10) | 16.7 | (18) | 33.3 |
DISCUSSION
This study provided preliminary evidence that the PPCA designed to educate patients about their personal need for colorectal cancer screening and to alert their providers about CRC screening status promoted patient-provider discussion, provider recommendations, and positive patient intentions to obtain screening. The intervention was designed to provide simple personalized information about need for screening in a form that was readily accessible to patients. Rather than tailoring the intervention to literacy levels we used language, concepts and visuals suitable to people with varying degrees of education and health literacy. Process data showed that the PPCA was highly acceptable to all patients, including those who were older and had less education. Similar to several other studies, the intervention appears to have facilitated a clinical situation where prepared patients and providers were more likely to engage in productive discussions of a challenging topic.36 – 38
Strengths of this pilot study included recruitment of intervention and comparison groups from five representative rural group practices. The outcome variables for the study provided evidence that the intervention stimulated higher levels of effective interaction between patient and provider, as intended, with significant effects on provider recommendations and patient intentions, two strong predictors of screening behavior. Consistent results favoring the intervention group across 5 practices provided confidence in the generalizability of results to the rural northern communities in which the intervention was tested.
The PPCA achieved its goals of engaging rural patients from a wide range of ages and educational backgrounds and providing them with personalized educational messages about CRC screening. The program was carefully developed to meet the needs of patients with lower as well as higher levels of knowledge and skills. The amount of information presented on any screen, the formats for presenting the information, and the branching pathway for question and information personalization were all carefully designed to be usable and appealing to most adults. It is possible also that success in appealing to patients with lower levels of education or low literacy was achieved by the delivery of messages through sound as well as text; process evaluation suggested that this was an appealing feature for older patients and those with less education. Nearly all participants, regardless of age or education, found the tablet program to be “very easy” to use. The efficient flexibility of the PPCA in collecting personal information and delivering these message qualities was a significant advantage, and builds upon other research with tablet computers that successfully collected patient history and risk factor information to stimulate screening behavior.21, 39
The provider component of the intervention also appeared to work well. This component consisted of a simple notice placed in the patient's chart prior to their medical visit indicating self-reported CRC screening status, intentions to be screened, and risk factors. Discussion of colonoscopy screening was reported for approximately twice as many intervention as comparison patients. Brief interviews with staff and providers at the end of the study found that this intervention was not intrusive and most providers found it useful.
The increase in likelihood of provider recommendation to obtain CRC screening and particularly a colonoscopy as a result of the intervention is notable since this factor has been found to be one of the major influences on patients' decision to be tested.3, 4, 40 Although this assessment is based solely on posttest reports, the results are consistent with the conceptual framework that focused on facilitating discussion between patient and provider about the advantages and disadvantages of this sometimes difficult decision as a step toward formation of positive intentions.25, 26
Some barriers to wider use of this type of intervention in rural primary care practices were encountered. Solo practices were not able to complete the study because there was insufficient space for a research assistant; lack of flexibility in solo practices could be an obstacle to introduction of even simple technology innovations. A related issue was encountered when practices required patients to use the PPCA in a private area rather than in the waiting room. The practices in general did not want to manage patient questions about how to operate the tablet program. These types of issues represent challenges to intervention developers to design simple, self-explanatory programs that also provide convincing patient privacy protection.
Limitations
The design for this pilot study had some features that limit confidence in the results. Although the study included a comparison group, patients were not randomly allocated to conditions, and the comparison group was systematically recruited at a slightly earlier time. The outcomes were important predictors of obtaining screening, but did not assess actual screening behaviors. The project did not have sufficient resources to conduct a complete chart review of all participants to see if screening actually occurred. Intentions to screen and actually being screened may be different. The practices were targeted for recruitment because they were representative of primary care services in a rural area but were not randomly chosen. The practices that volunteered may be different from other rural practices and may be better at influencing patient screening intentions. It should also be noted that the levels of patient reported CRC screening in these practices was relatively high. Previous studies of the accuracy of self-reported CRC screening are mixed but increase with age.41, 42 The study provided useful information about intervention effects within this demographic segment, but no information was obtained for impact on other racial/ethnic groups or non-rural patients.
Implications
Results of this study provide insights on methods for increasing participation in CRC screening among difficult to reach populations. The design of this intervention was intended to facilitate discussions about screening between patients with low levels of education or health literacy and their providers; the outcomes suggest that this goal was achieved with a positive effect on patient screening intentions. Higher levels of patient readiness to receive and act on a provider screening recommendation may increase the chances that a provider will invest the time needed to present a rationale and make a recommendation, and increase the chances that the recommendation will be followed. The general strategy of focused preparation of patients immediately prior to a medical visit could be extended to other cancer prevention and control problems. A further finding of interest is that the intensive efforts to shape the messages built into the PPCA for the needs of lower education and health literacy patients appeared to have a favorable effect for other patients; simple well-presented messages about important health issues may generally be beneficial for all patients.43 The trend of using computer tablets in health care settings as a data-gathering tool is likely to continue because of the expected efficiencies when this approach is linked with electronic health records.44
This pilot study had limited resources to take advantage of all the potential tailoring that was possible from the data collected. One of the notable benefits of using a computer tablet is that it is able to tailor messages to the individual by characteristics such as race and ethnicity, educational skill level, age, risk of a disease and by interest in obtaining and using health information.20, 45 This flexibility of data collection and presentation of personalized health information also will allow for selection of multiple languages and cultural customization for patients from diverse populations in the same setting. The next step is to test this intervention in a randomized controlled trial with a more diverse population. The next trial would continue the formative research with new populations of intended audiences and take advantage of the PPCA's ability to tailor the messages and the presentation to the individual.
Conclusions
A brief patient intervention prior to medical visits appeared to increase discussion of CRC screening needs at the visit, increase provider recommendations to obtain screening, and increase patients' intentions to obtain a screening test. Although the study design precluded clear cut conclusions, the consistency of the outcome and process results across multiple test sites provided encouragement for further exploration of this approach to increasing cancer screening participation.
ACKNOWLEDGMENTS
This study was funded by a grant from the National Cancer Institute CA107215. The manuscript's contents is solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
We want to thank the staff and physicians of the five primary care practices for their assistance and cooperation, and their patients who participated in the study. We also want to acknowledge the efforts of and thank David Little, M.D. and Neil Hyman, M.D. for their help in recruiting practices into the study.
REFERENCES
- 1.Klabunde CN, Frame PS, Meadow A, et al. A national survey of primary care providers' colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352–62. doi: 10.1016/s0091-7435(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 2.Ko C, Kreuter W, Baldwin L. Persistent demographic differences in colorectal cancer screening utilization despite Medicare reimbursement. BMC Gastroenterology. 2005;5(10):1–8. doi: 10.1186/1471-230X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy B, Dawson j, Hartz A, James P. Colorectal cancer testing among patients cared for by Iowa family physicians. Am J Prev Med. 2006;31(3):193–201. doi: 10.1016/j.amepre.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Klabunde C, Schenck A, Davis W. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313–19. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Wee C, McCarthy E, Phillips R. Factors associated with colon cancer screening: The role of patient factors and physician counseling. Prev Med. 2005;41(1):23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20(4):368–73. doi: 10.1111/j.1748-0361.2004.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 7.Greiner K, Engelman K, Hall M, Ellerbeck E. Barriers to colorectal cancer screening in rural primary care. Prev Med. 2004;38:269–75. doi: 10.1016/j.ypmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Brawarsky P, Brooks D, Mucci L, Wood P. Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detect Prev. 2004;28:260–68. doi: 10.1016/j.cdp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert A, Kanarek N. Colorectal cancer screening: Physician recommendation is influential advice to Marylanders. Prev Med. 2005;41:367–79. doi: 10.1016/j.ypmed.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med. 2003;36:525–35. doi: 10.1016/s0091-7435(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 11.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale-update based on new evidence. Gastroenterology. 2003;124(2):544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 12.Myers RE, Hyslop T, Gerrity M, et al. Physician intention to recommend complete diagnostic evaluation in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 1999;8(7):587–93. [PubMed] [Google Scholar]
- 13.Yarnell K, Pollak K, Ostbye T, Krause K, Michener J. Primary prevention: Is there enough time for prevention? Am J Public Health. 2003;93:635–41. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dulai G, Farmer M, Ganz P, et al. Primary care provider perceptions of barriers to and facilitators of colorectal cancer screening in a managed care setting. Cancer. 2004;100:1843–52. doi: 10.1002/cncr.20209. [DOI] [PubMed] [Google Scholar]
- 15.Zapka J, E P, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23(1):28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 16.Patel P, Forjuoh S, Avots-Avotins A, et al. Identifying opportunities for improved colorectal cancer screening in primary care. Prev Med. 2004;39(2):239–46. doi: 10.1016/j.ypmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Haggerty J, Tudiver F, Brown J, et al. Patients' anxiety and expectations: How they influence family physicians' decisions to order cancer screening tests. Can Fam Physician. 2005;51:1658–59. [PMC free article] [PubMed] [Google Scholar]
- 18.Vernon SW. Participation in colorectal cancer screening: A review. J Natl Cancer Inst. 1997;89(19):1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein M, Hennessy M, Kamb M, et al. Using intervention theory to model factors influencing behavior change. Project Respect. Eval Health Prof. 2001;24(4):363–84. doi: 10.1177/01632780122034966. [DOI] [PubMed] [Google Scholar]
- 20.Williams RB, Boles M, Johnson RE. Patient use of a computer for prevention in primary care practice. Pat Educ Counsel. 1995;25(3):283–92. doi: 10.1016/0738-3991(95)00800-f. [DOI] [PubMed] [Google Scholar]
- 21.Williams RB, Boles M, Johnson RE. A patient-initiated system for preventive health care. A randomized trial in community-based primary care practices. Arch Fam Med. 1998;7(4):338–45. doi: 10.1001/archfami.7.4.338. [DOI] [PubMed] [Google Scholar]
- 22.Stevens VJ, Glasgow RE, Toobert DJ, et al. One-year results from a brief, computer-assisted intervention to decrease consumption of fat and increase consumption of fruits and vegetables. Prev Med. 2003;36(5):594–600. doi: 10.1016/s0091-7435(03)00019-7. [DOI] [PubMed] [Google Scholar]
- 23.Shiffman R, Liaw Y, Brandt C, et al. Computer-based guideline implementation systems: A systematic review of functionality and effectiveness. J Am Med Inform Assoc. 1999;6:104–14. doi: 10.1136/jamia.1999.0060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strecher V, Kreuter M, Den Boer D, et al. The effects of computer-tailored smoking cessation messages in family practice settings. J Family Prac. 1994;39:262–70. [PubMed] [Google Scholar]
- 25.Prochaska J, DiClemente C. Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992;(28):183–218. [PubMed] [Google Scholar]
- 26.Green L, Kreuter M. Health promotion planning. An educational and environmental approach. Mayfield Publishing Co; Mountain View, CA: 1991. [Google Scholar]
- 27.Rudd RE, Comings JP. Learner developed materials: An empowering product. Health Educ Quarterly. 1994;21(3):313–27. doi: 10.1177/109019819402100304. [DOI] [PubMed] [Google Scholar]
- 28.Institute NC, editor. Clear & simple: Developing effective print materials for low-literate readers. National Institute of Health; Bethesda, MD: 1994. [Google Scholar]
- 29. Making you web site senior friendly: A checklist, http://www.nlm.nih.gov/pubs/checklist.pdf, Accessed June 10, 2003.
- 30.Burke W, Beeker C, Kraft J, Pinsky L. Engaging women's interest in colorectal cancer screening: A public health strategy. J Women Health Gender-Based Med. 2000;9(4):363–71. doi: 10.1089/15246090050020673. [DOI] [PubMed] [Google Scholar]
- 31.CDC Health statistics cancer 2000 module. ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/survey_Questionnaires/NHIS /2000/qcancerx.pdf, Accessed May 9, 2003.
- 32.CDC Behavioral risk factor surveillance questionnaires, English versions. http://www.cdc.gov/brfss/questionnaires/english.htm, Accessed May 9, 2003.
- 33.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: Evaluation of a brief instrument to identify limited reading ability. BMC Fam Prac. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobach DF, Hasswlblad V, Wildemuth BM. Evaluation of a tool to categorize patients by reading literacy and computer skill to facilitate the Computer-Administered Patient Interview. AMIA Ann Symp Proc. 2003:391–395. [PMC free article] [PubMed] [Google Scholar]
- 35.Lobach DF, Arbana JM, Mishra MS, Campbell M, Wildemuth BM. Adapting the human-computer inte for reading literacy and computer skill to facilitate collection of information directly from patients. MedInfo. 2004;11:1142–46. [PubMed] [Google Scholar]
- 36.Khankari K, Eder M, Osborn C, et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Jul 26; doi: 10.1007/s11606-007-0295-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker DM, Gomez EB, Kaiser DL, Yoshihasi A, Hodge RH., Jr. Improving preventive care at a medical clinic: How can the patient help? Am J Prev Med. 1989;5(6):353–9. [PubMed] [Google Scholar]
- 38.Turner RC, Waivers LE, OBrien K. The effect of patient-carried reminder cards on the performance of health maintenance measures. Arch Intern Med. 1990;150(3):645–7. [PubMed] [Google Scholar]
- 39.Baratiny GY, Campbell EM, Sanson-Fisher RW, Graham J, Cockburn J. Collecting cancer risk factor data from hospital outpatients: Use of touch-screen computers. Cancer Detect Prev. 2000;24(6):501–7. [PubMed] [Google Scholar]
- 40.Brawarsky P, Brooks D, Mucci L, Wood P. Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detect Prev. 2004;28:260–68. doi: 10.1016/j.cdp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Baier M, Calonge N, Cutter G, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9(2):229–32. [PubMed] [Google Scholar]
- 42.Mandelson M, LaCroix A, Anderson L, et al. Comparison of self-reported fecal occult blood testing with automated laboratory records among older women in a health maintenance organization. Am. J. Epidemiol. 1999;150(6):617–21. doi: 10.1093/oxfordjournals.aje.a010060. [DOI] [PubMed] [Google Scholar]
- 43.Davis T, Williams M, Marin E, et al. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134–49. doi: 10.3322/canjclin.52.3.134. [DOI] [PubMed] [Google Scholar]
- 44.Gamm L, Kash B, Bolin J. Organizational technologies for transforming care: Measures and strategies for pursuit of IOM quality aims. J Ambul Care Manage. 2007;30(4):291–301. doi: 10.1097/01.JAC.0000290397.58790.b9. [DOI] [PubMed] [Google Scholar]
- 45.Kreuter M, Lukwago S, Bucholtz R, et al. Achieving cultural appropriateness in health promotion programs: Targeted and tailored approaches. Health Educ Behav. 2003;30(2):133–46. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]