Abstract
Natural killer (NK) cells can kill a wide range of cancer cells and are a promising tool for cell therapy of cancer. NK cells cytotoxicity is regulated by a balance between stimulatory and inhibitory signals. Interleukin-2 is known to increase NK cell cytotoxicity. Although many cytokines have been studied in efforts to induce durable NK cell expansions, most reports indicate a rather modest effect and the requirement for additional stimuli. We found that contact with the K562 myeloid leukemia cell line, genetically modified to express a membrane-bound form of interleukin-15 and the ligand for the costimulatory molecule 4-1BB, induced vigorous expansion of NK cells from peripheral blood. Based on these findings, we developed a method for large-scale clinical-grade expansion of NK cells. This method is currently used to expand allogeneic NK cells for infusion in patients with leukemia and solid tumors. We here summarize methods for expansion and activation of NK cells from human peripheral blood mononuclear cells as well as clinical-scale methods to produce NK cells for immunotherapy under Current Good Manufacturing Practices (cGMP) conditions.
Keywords: NK cells, cell therapy, acute myeloid leukemia, acute lymphoblastic leukemia, chimeric receptors
INTRODUCTION
Natural killer (NK) cells comprise 5% to 20% of human peripheral blood lymphocytes and are derived from CD34+ hematopoietic progenitor cells [1]. The precise physiologic sites where NK cells mature and the mechanisms that drive the development of their functional characteristics have not yet been fully clarified but recent studies indicate that these occur in the bone marrow and the lymphnodes [2-4].
NK cells have the morphology of large granular lymphocytes, and are phenotypically defined by the expression of CD56 and the lack of CD3 and T-cell receptor molecules [3, 4]. Approximately 10% of NK cells express very high levels of CD56 and also have dim expression of FcgRIII (CD16), a receptor that binds the Fc portion of IgG [5]. These cells mostly are believed to have primarily an immunoregulatory role exerted through the secretion of cytokines and chemokines [5]. Although less common in blood, bone marrow and spleen, this cell subset predominates in the secondary lymphoid tissue [6]. The remaining 90% of NK cells in blood express lower levels of CD56 and high levels of CD16 [5]. These cells appear to function predominantly in direct cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) [5, 7]. NK cells can directly induce apoptosis via the perforin-granzyme pathway, or by expressing death-receptor ligands on their cell surface. Such ligands include tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), or the Fas ligand, which directly trigger cell death via their respective receptors[8]
NK cells can kill target cells without the need for prior sensitization, an effect that is regulated by the balance of stimulatory and inhibitory signals [4, 9, 10]. Many of the signaling receptors on the surface of NK cells engage major histocompatibility complex (MHC) class I and MHC class I-like molecules. A well-described mechanism is the inhibition of NK activity by increasing expression of MHC or human leukocyte antigen (HLA) class I in target cells [11]. NK cells express killer immunoglobulin-like receptors (KIRs), most of which recognize specific corresponding HLA class I molecules on target cells, and deliver inhibitory signals [4, 9, 10],. These inhibitory signals from HLA can override activating signals and suppress the function of NK cells. A concept that has recently emerged is that interaction between NK cells and HLA molecules might also be important for their functional maturation and the generation of NK cells that are tolerant towards self molecules, a process which has been termed “licensing” [12].
This review summarizes methods for expansion and activation of NK cells from human peripheral blood mononuclear cells as well as clinical-scale methods to produce NK cells for immunotherapy under Current Good Manufacturing Practices (cGMP) conditions.
METHODS TO EXPAND NK CELLS
Cytokines and stimulants
Several protocols for NK-cell expansion have been reported (summarize in Table; see also www.nkcells.info/wiki/index.php/NK_cell_expansion). Many cytokines have been studied in efforts to induce durable NK cell expansion as well as increase their cytotoxicity [13]. Interleukin (IL)-2 can enhance the cytotoxicity of NK cells within 24 hours of incubation [14, 15]. IL-2 can also stimulate their proliferation but only a minority of NK cells can maintain proliferation after the initial response [14, 16, 17]. IL-4, IL-7 and IL-12 also induced some proliferative stimuli but are overall less potent than IL-2 [18]. Likewise, IL-15 alone or in combination with IL-2 typically results in minimal NK cell expansion [8]. We found that IL-2 (1000 IU/mL) or IL-15 (10 ng/mL) did not induce significant expansion of NK cells [19]. It appears that cytokines may be necessary but not sufficient for optimal proliferation of NK cells.
Table.
Selected protocols for expansion and activation of NK cells
| Protocol | Median fold expansion after culture (% of CD56+CD3- cells) |
Reference | ||
|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | ||
| PBMC from donor, OKT3 (10ng/mL), IL2 (500 IU/mL) |
- | - | 193 (55) |
[21] |
| PBMC from myeloma, OKT3 (10ng/mL), IL2 (500 IU/mL) |
- | - | 1625a (65) |
[20] |
| Non-adherent PBMC from ALL, RPMI8866, IL2 + IL15 |
- | 40b (62–95) |
- | [49] |
| PBMC from donor, HFWT, IL2 (200IU/mL) | - | 58–401c (77.4–85.6) |
- | [28] |
| NK cell enriched from donor, IL2 (100IU/mL) + IL15 (10IU/mL) + PHA (100ug/mL) + ionomycin (1μM/mL) |
- | 80–200 | - | [29] |
| PBMC from donor, K562-mIL15–41BBL, IL2 (10–100 IU/mL) |
21.6 (62.9) |
152 (90) |
277 (96.8) |
[43] |
Measured on day 20
day 10
day 10–21
Abbreviations: PBMC, peripheral blood mononuclear cells; ALL, acute lymphoblastic leukemia
Recently, Alici E et al [20] used culture conditions using IL2 (500 IU/mL) with an anti-CD3 antibody (Orthoclone OKT-3; 10 ng/mL) and reported that the number of NK cells from the peripheral blood of 7 newly diagnosed, untreated patients with multiple myeloma had expanded on average 1,600-fold after 20 days of culture. This is in striking contrast with the 190-fold expansion obtained using NK cells from healthy individuals [21], implying that NK cells from myeloma patients may somehow be more susceptible to stimulation than NK cells from healthy individuals. It is also unclear how CD3 stimulation contributed to the expansion of CD3-NK cells in these studies.
It should be noted that the cell populations that results from the stimulation of peripheral blood mononuclear cells with IL-2 termed lymphokine-activated killer (LAK) cells are comprised of mostly of polyclonal T-cells and only a small fraction is CD56+ NK cells [22].
Expansion of NK cell with accessory cells
Most investigators believe that sustained proliferations of NK cells require additional signals[23, 24], such as the presence of monocytes [23] or B-lymphoblastoid cells [16, 25, 26]. Miller et al. [23] reported an approximate 30-fold expansion of NK cells after 18 days of culture with 1000 IU/mL IL-2 and monocytes. Perussia et al. [27] found that contact with irradiated B-lymphoblastoid cells induced as high as a 25-fold expansion of NK cells after 2 weeks of stimulation. Harada et al [28] reported that HFWT, a Wilm’s tumor-derived cell line, stimulates up to 400-fold NK expansion after 2 weeks. Other investigators have used allogeneic mononuclear cells[29], autologous lymphocytes[30], mitogen activated lymphocytes[25], and umbilical cord mesenchymal cells [31]
Contact with K562 cells, a cell line derived from a patient with myeloid blast crisis of chronoc myelogenous leukemia and bearing the BCR-ABL1 translocation, is known to induce modest proliferations of NK cells [28, 32], and augment NK cell proliferation in response to IL-15 [24]. We genetically-modified K562 cells to express two NK stimulatory molecules. One, the ligand for 4-1BB (4-1BBL), induces activation signals through 4-1BB (CD137), a costimulatory molecule expressed on the surface of NK cells [33]. The other, IL-15, is known to support NK-cell maturation and survival [34-37]. IL-15 has greater activity when bound to the cell membrane of stimulatory cells, rather than in its soluble form [38-42]; hence, we made a construct containing the human IL-15 gene fused to the gene encoding the human CD8α transmembrane domain, and used it to transduce K562 cells. The resulting cell line (K562-mb15-41BBL) induced 21.6-fold expansion of CD56+ CD3− NK cells from peripheral blood (range, 5.1–86.6-fold; n = 50) after 7 days, which was considerably superior to that produced by stimulation with interleukin (IL)-2, IL-12, IL-15 and/or IL-21 [19, 43]. These cultures were performed using irradiated K562-mb15-41BBL cells and 10 IU/mL IL-2. We observed minimal or no proliferation of CD3+ lymphocytes. NK cells could be further expanded with K562-mb15-41BBL cell stimulation by prolonging the cultures and adding 100 IU/mL IL-2 after day 7. Thus, median NK cell recovery increased to 152-fold after 14 days of culture, and 277-fold after 21 days [19, 43]
NK cells expanded by K562-mb15-41BBL cells stimulation were significantly more potent than purified unstimulated or IL-2-stimulated NK cells against acute myeloid leukemia (AML) cells in vitro and could eradicate AML in murine models [43]. Preliminary studies indicate that these NK cells are also cytotoxic against cell lines derived from patients with Ewing sarcoma, rhabdomyosarcoma and neuroblastoma (D. Cho, D. Shook, D. Campana, unpublished results).
Although expanded NK cells acquired powerful cytotoxicity against a variety of cancer cell types, their capacity to kill acute lymphoblastic leukemia cells (ALL) remained overall limited. To overcome this resistance we transduced expanded NK cells with artificial receptors directed against CD19, a molecule expressed by ALL cells and cells of other B-cell malignancies. Anti-CD19 receptors linked to CD3ζ markedly enhanced NK-cell mediated killing of ALL cells, a result that was further improved by adding the 4-1BB costimulatory molecule to the chimeric anti-CD19-CD3ζ receptor [19]. Addition of 4-1BB was also associated with increased production of IFN-γ and GM-CSF [19].
CLINICAL LARGE-SCALE NK CELL ACTIVATION AND EXPANSION
Methodologic considerations
It is now feasible to obtain clinical-grade purified functional NK cells for infusion [44]. NK cells can be obtained from different sources: from the patient (autologous), the patient’s human leukocyte antigen (HLA)-matched siblings, or haploidentical family members or unrelated donors. It might be convenient if NK cells could be collected in advance, cryopreserved and thawed before infusion. Unstimulated cryopreserved and thawed NK cells have phenotype and cytotoxicity that resembles that of fresh cells[45]. Whether this is also the case for activated and expanded NK cells remains to be established.
For clinical-grade NK cell activation and expansion, cells need to be cultured for a period of time that varies between less than 24 hours to several weeks [20, 46]. Several tissue culture media have proven effective including stem cell growth medium (SCGM) (CellGenix, Freiburg, Germany) [20, 43] and X-VIVO serum-free media (BioWhittaker, Verviers, Belgium) [47]. The media can be supplemented with fetal bovine serum (from certified sources) or human serum from AB blood donor [47] is used for clinical applications. NK cells can be cultured in flasks or in bags such as Teflon (FEP) bags[29, 48] and Baxter Lifecell bags [8]. If stimulatory cells are used, it is important to prevent their overgrowth and to ensure than no viable cells are infused with the cultured NK cells. Irradiation, at doses of 30 Gy [49], 50 Gy [28], 70 Gy [29] or 100 Gy [19] is a safe and effective method.
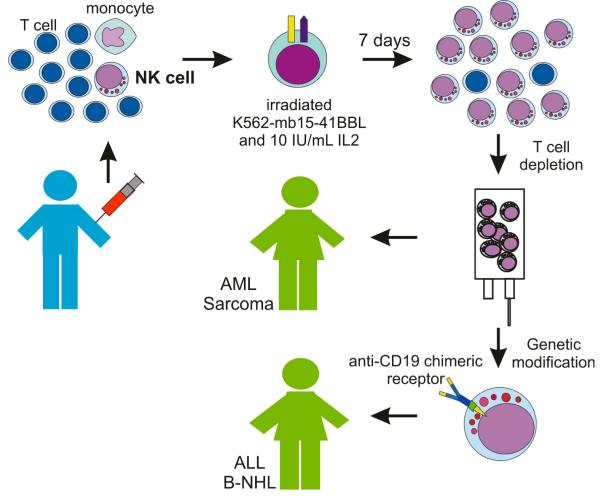
Our clinical protocol used cGMP guidelines to process apheresis products and obtain expanded activated NK cells [43] (Figure). Mononuclear cells are Ficoll-separated and placed in culture in SCGM medium supplemented with 10% FBS, gentamicin sulfate (50 mg/L), and 10 IU/mL human IL-2 at a concentration of 0.5 × 105 CD56+ CD3− cells/mL. Irradiated (100 Gy) K562-mb15-41BBL cells (from a Master Cell Bank) are added at a ratio of 1 CD56+ CD3− cell: 10 K562-mb15-41BBL cells. Cultures are performed in a closed VueLife bags system (American Fluoroseal, Gaithersburg, MD). Cells are fed after 2 and 5 days, and harvested after 7 days of culture. The cell product is then depleted of residual T cells using the CliniMACS System (Miltenyi). Finally, cells are washed and resuspended in PlasmaLyte-148 (Baxter, Deerfield, IL) with 0.5% human serum albumin. Under these conditions, we obtained a median 90.5-fold expansion of CD56+ CD3− NK cells (n = 12) after 7 days of culture [43]. The expansion in these large-scale cultures was higher than that of observed in small-scale experiments, most likely because of to the use of SCGM tissue culture medium instead of RPMI-1640 (SCGM appears to be well suited to support NK cell growth [20]. We therefore estimate that it should be feasible to obtain the number of NK cells planned for infusion in our protocol (maximum dose, 5 × 107 NK cells/kg) and more from a leukapheresis product. For example, considering that an average apheresis from a normal adult donor gives us about 100 × 108 nucleated cells and the average percentage of NK cells is 7.0%, a 90-fold expansion would result in 6.3 × 1010 NK cells. If necessary, larger numbers could be obtained by prolonging the cultures beyond 7 days.
Figure. Schematic representation of protocols using expanded NK cells at St Jude Children’s Research Hospital.
The leukapheresis product obtained from a haploidentical donor is mixed with irradiated K562-mb15-41BBL cells. After 7 days of culture, most cells recovered are activated NK cells. After T-cell depletion using the CliniMACS system, NK cells are infused in patients with NK-sensitive malignancies such as acute myeloid leukemia (AML), Ewing sarcoma or rhabdomyosarcoma. For patients whose neoplasia is less sensitive to NK cytotoxicity, such as B-lineage acute lymphoblastic leukemia (ALL) or B-cell non-Hodgkin lymphoma (B-NHL), expanded NK cells are transduced with an anti-CD19 chimeric receptor before infusion.
An alternative to expand NK cells is represented by the continuously growing NK cell lines NK-92, derived from a patient with non-Hodgkin lymphoma that is cytotoxic against a wide range of malignant cancer cells [50]. These cells have practical appeal, but irradiation is mandatory before infusion in patients, which may limit their efficacy in vivo.
Therapeutic applications of NK cells
In the setting of hematopoietic stem-cell transplantation, donor NK cells may exert an anti-leukemia effect if they do not express KIRs reacting with the HLA class I epitope expressed by the patient’s leukemia cells. In animal models, donor NK cells killed host leukemic cells and lympho-hematopoietic cells without affecting non-hematopoietic tissues [51], suggesting the possibility of an NK-mediated graft-versus-leukemia (GVL) effect without systemic disease. Therefore, it is now a common practice at some clinical centers to select donors with an HLA and KIR type that facilitates NK-cell activation [52-54].
In addition to their use in the context of allogeneic stem cell transplantation, allogeneic NK cells can be directly infused in non-myeloablated patients. Miller et al. [46] first demonstrated the potential utility of this approach. These investigators treated 19 adult patients with high risk AML with cyclophosphamide (60 mg/kg for 1 or 2 doses), fludarabine (25 mg/m2 daily for 5 doses), IL-2 (10 million units per dose for 6 to 9 doses), and an infusion of 2 × 107/kg CD3-depleted NK-cell product (NK cells enriched to approximately 40%) that was activated for 18 hours with 1000 IU/mL IL-2. Eight of 15 AML patients showed at least 1% engraftment at day 7 or later after the infusion, and 5 patients achieved a complete remission. Interestingly, lymphodepletion induced higher levels of IL-15 which in turn might have been important in prolonging the survival of the infused NK cells [46]. Our current protocol using NK cells expanded by stimulation with the K562-mb15-41BBL cell line uses a framework identical to that described by Miller et al. [46]. Thus, patients are treated for 7 days with cyclophosphamide and fludarabine and receive subcutaneous IL-2 after infusion of the expanded NK cells.
If cancer cells present a tumor-specific antigen in the HLA context they can be recognized and lysed by cytotoxic T lymphocytes (CTL) specific for the antigen. For example, expanded T lymphocytes specific for Epstein-Barr virus (EBV)-associated molecules have been applied for the treatment and prophylaxis of EBV-associated lymphoproliferative disease and lymphoma [55]. Other EBV-associated tumors may also be amenable to this form of therapy [56, 57]. However, most cancers lack identifiable virus-associated antigens [58], although other molecules, such as WT1 and Pr3, are overexpressed in some cancer cells and are possible targets for adoptive T-cell therapy [59, 60] NK cells offer some potential advantage over CTL therapy. First, a wide range of cancer cells appear to be sensitive to NK cell cytoxicity. In addition to AML, NK-sensitive malignancies include soft-tissue sarcomas (D. Cho, D.Shook, D. Campana, unpublished), neuroblastoma [59, 60] and malignant glioma [59]. Second, they can be used in an allogeneic setting without the risk of graft versus-host-disease [44]. Thirdly, with the method described in this review, large numbers of cytotoxic NK cells can be reliably obtained in a relatively short period of time.
For malignancies that are relatively resistant to NK cells, strategies that can be explored include genetic modification with artificial receptors, such as the one that we reported using anti-CD19 receptors for the treatment of B-cell neoplasias [19]. To this end, the overall strategy that we have described is not limited to CD19+ leukemia and lymphoma cells but is also applicable to many other molecules express by cancer cells and can be implemented by replacing the anti-CDc19 scFv with the scFv of another antibody [61, 62] .
Acknowledgments
This work was supported by grants CA113482 and CA21765 from the National Cancer Institute, grants from the Assisi Foundation and from the Fondation de Gouverneurs de l’Espoir, and by the American Lebanese Syrian Associated Charities (ALSAC)
REFERENCES
- 1.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–73. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 2.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–37. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56 bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 7.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–25. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava S, Lundqvist A, Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy. 2008;10:775–83. doi: 10.1080/14653240802648181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 13.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–94. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160:1147–69. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips JH, Lanier LL. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986;164:814–25. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London L, Perussia B, Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J Immunol. 1986;137:3845–54. [PubMed] [Google Scholar]
- 17.Lanier LL, Buck DW, Rhodes L, Ding A, Evans E, Barney C, et al. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988;167:1572–85. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson MJ, Manley TJ, Donahue C, Levine H, Ritz J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J Immunol. 1993;150:1705–14. [PubMed] [Google Scholar]
- 19.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alici E, Sutlu T, Bjorkstrand B, Gilljam M, Stellan B, Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–62. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 21.Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001;62:1092–8. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 23.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–9. [PubMed] [Google Scholar]
- 24.Robertson MJ, Cameron C, Lazo S, Cochran KJ, Voss SD, Ritz J. Costimulation of human natural killer cell proliferation: role of accessory cytokines and cell contact-dependent signals. Nat Immun. 1996;15:213–26. [PubMed] [Google Scholar]
- 25.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell Immunol. 1991;135:454–70. doi: 10.1016/0008-8749(91)90290-r. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi T, Wynberg J, Srinivasan R, Becknell B, McCoy JP, Jr., Takahashi Y, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170–7. doi: 10.1182/blood-2003-12-4438. [DOI] [PubMed] [Google Scholar]
- 27.Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171–88. [PubMed] [Google Scholar]
- 28.Harada H, Saijo K, Watanabe S, Tsuboi K, Nose T, Ishiwata I, et al. Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line, HFWT. Jpn J Cancer Res. 2002;93:313–9. doi: 10.1111/j.1349-7006.2002.tb02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luhm J, Brand JM, Koritke P, Hoppner M, Kirchner H, Frohn C. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res. 2002;11:651–7. doi: 10.1089/15258160260194794. [DOI] [PubMed] [Google Scholar]
- 30.Condiotti R, Zakai YB, Barak V, Nagler A. Ex vivo expansion of CD56+ cytotoxic cells from human umbilical cord blood. Exp Hematol. 2001;29:104–13. doi: 10.1016/s0301-472x(00)00617-2. [DOI] [PubMed] [Google Scholar]
- 31.Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14:1031–8. doi: 10.1016/j.bbmt.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Phillips JH, Lanier LL. A model for the differentiation of human natural killer cells. Studies on the in vitro activation of Leu-11+ granular lymphocytes with a natural killer-sensitive tumor cell, K562. J Exp Med. 1985;161:1464–82. doi: 10.1084/jem.161.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 34.Carson WE, Fehniger TA, Haldar S, Eckhert K, Lindemann MJ, Lai CF, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–43. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 36.Fehniger TA, Caligiuri MA. Ontogeny and expansion of human natural killer cells: clinical implications. Int Rev Immunol. 2001;20:503–34. doi: 10.3109/08830180109054417. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–56. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 38.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–9. [PubMed] [Google Scholar]
- 39.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 40.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–8. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 41.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–7. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 43.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-08-3712. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung W, Iyengar R, Leimig T, Holladay MS, Houston J, Handgretinger R. Phenotype and function of human natural killer cells purified by using a clinical-scale immunomagnetic method. Cancer Immunol Immunother. 2005;54:389–94. doi: 10.1007/s00262-004-0609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meehan KR, Wu J, Webber SM, Barber A, Szczepiorkowski ZM, Sentman C. Development of a clinical model for ex vivo expansion of multiple populations of effector cells for adoptive cellular therapy. Cytotherapy. 2008;10:30–7. doi: 10.1080/14653240701762398. [DOI] [PubMed] [Google Scholar]
- 46.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 47.Klingemann HG, Martinson J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy. 2004;6:15–22. doi: 10.1080/14653240310004548. [DOI] [PubMed] [Google Scholar]
- 48.McKenna DH, Jr., Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 49.Torelli GF, Guarini A, Maggio R, Alfieri C, Vitale A, Foa R. Expansion of natural killer cells with lytic activity against autologous blasts from adult and pediatric acute lymphoid leukemia patients in complete hematologic remission. Haematologica. 2005;90:785–92. [PubMed] [Google Scholar]
- 50.Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003;5:259–72. doi: 10.1080/14653240310001523. [DOI] [PubMed] [Google Scholar]
- 51.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 52.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–9. [PubMed] [Google Scholar]
- 53.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–9. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 54.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–50. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 55.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 56.Wagner HJ, Bollard CM, Vigouroux S, Huls MH, Anderson R, Prentice HG, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11:81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 57.Comoli P, De Palma R, Siena S, Nocera A, Basso S, Del Galdo F, et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol. 2004;15:113–7. doi: 10.1093/annonc/mdh027. [DOI] [PubMed] [Google Scholar]
- 58.Klein G, Klein E. Surveillance against tumors--is it mainly immunological? Immunol Lett. 2005;100:29–33. doi: 10.1016/j.imlet.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 59.Main EK, Lampson LA, Hart MK, Kornbluth J, Wilson DB. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985;135:242–6. [PubMed] [Google Scholar]
- 60.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Morandi F, Bocca P, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Letters. 2005;228:155–61. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 61.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 62.Imai C, Campana D. Genetic modification of T cells for cancer therapy. J Biol Regul Homeost Agents. 2004;18:62–71. [PubMed] [Google Scholar]