Abstract
Characterizing connectivity in the spinal cord of zebrafish embryos is not only prerequisite to understanding the development of locomotion, but is also necessary for maximizing the potential of genetic studies of circuit formation in this model system. During their first day of development, zebrafish embryos show two simple motor behaviors. First, they coil their trunks spontaneously, and a few hours later they start responding to touch with contralateral coils. These behaviors are contemporaneous until spontaneous coils become infrequent by 30 h. Glutamatergic neurons are distributed throughout the embryonic spinal cord, but their contribution to these early motor behaviors in immature zebrafish is still unclear. We demonstrate that the kinetics of spontaneous coiling and touch-evoked responses show distinct developmental time courses and that the touch response is dependent on AMPA-type glutamate receptor activation. Transection experiments suggest that the circuits required for touch-evoked responses are confined to the spinal cord and that only the most rostral part of the spinal cord is sufficient for triggering the full response. This rostral sensory connection is presumably established via CoPA interneurons, as they project to the rostral spinal cord. Electrophysiological analysis demonstrates that these neurons receive short latency AMPA-type glutamatergic inputs in response to ipsilateral tactile stimuli. We conclude that touch responses in early embryonic zebrafish arise only after glutamatergic synapses connect sensory neurons and interneurons to the contralateral motor network via a rostral loop. This helps define an elementary circuit that is modified by the addition of sensory inputs, resulting in behavioral transformation.
Keywords: neurotransmitter, synapse, circuit, reflex, glutamate receptor
INTRODUCTION
Zebrafish embryos and larvae provide a unique system in which to study motor control because of the ability to combine genetics, imaging, and electrophysiology (Fetcho et al., 2008; McLean and Fetcho, 2008). However, these kinds of analyses are hampered by a lack of a precise understanding of the neurotransmitters and neuronal circuits that underlie the most basic motor behaviors. To address this deficit, we have focused on the glutamatergic system in the developing zebrafish embryo and its role in two simple motor behaviors.
Zebrafish show highly stereotyped escape responses and swimming that have been shown to be dependent on the hindbrain (Ritter et al., 2001; Drapeau et al., 2002). While the circuitry underlying these behaviors at larval stages has been probed extensively, it remains unclear how early behaviors including the touch response develop in zebrafish embryos (Saint-Amant and Drapeau, 1998; Downes and Granato, 2006). The first coordinated motor behavior in zebrafish appears at 17 h post-fertilization (hpf). It consists of spontaneous coiling of the trunk and is driven by activity in the spinal cord, independent of supraspinal input (Saint-Amant and Drapeau, 1998). As development proceeds, the spontaneous coiling frequency decreases ten-fold over the course of 12 h. Around 21 hpf, a new motor behavior appears in zebrafish embryos as they respond to mechanical stimuli with contralateral coiling of the trunk.
Spontaneous coils are dependent on electrical coupling of the neuronal network within the spinal cord. While wholesale blockade of chemical synaptic transmission does not affect spontaneous neural activity, uncouplers of gap junctions completely abolish the activity that underlies spontaneous coiling (Saint-Amant and Drapeau, 2001). The pacemaker mechanism for the spontaneous coils is unknown, but the likely cellular candidates for this mechanism are descending interneurons such as the ipsilateral projecting interneurons (IC) and circumferential descending interneurons (CiD; Hale et al., 2001; Saint-Amant and Drapeau, 2001). These interneurons are particularly interesting as their axons descend the length of the spinal cord; they have larger currents during spontaneous activity, more dynamic membrane potentials, and stronger electrical connections than other spinal interneurons (Saint-Amant and Drapeau, 2001). Analogous neurons in Xenopus laevis tadpole spinal cord and hindbrain (dlNs) also show electrotonic coupling, and this is thought to contribute to the synchronization of activity during swimming (Li et al., 2009). Primary ascending commissural interneurons (CoPA) and primary motoneurons are also spontaneously active, but may be passively driven by descending neurons as demonstrated by their smaller currents and more stable membrane potentials (Saint-Amant and Drapeau, 2000, 2001, 2003; Saint-Amant, 2006). These data point to spontaneous coils being generated and propagated by an extensive electrotonic network of neurons interconnected in the spinal cord by gap junctions.
The developmental decrease in frequency of spontaneous coils likely results from the inability of gap junctions to carry enough current to trigger behavior in the context of a generalized decrease in electrical resistance of the rapidly developing spinal neurons (Saint-Amant and Drapeau, 2000). Thus, the immature zebrafish spinal cord may need additional synaptic inputs during this same period to maintain excitability. Although glutamatergic neurotransmission within the spinal cord is unnecessary for motor output at very early stages (Saint-Amant and Drapeau, 2000, 2001; Saint-Amant, 2006), neurons expressing glutamatergic genes such as the vesicular glutamate transporters (vglut1, 2a, and 2b) are present and have extended axons by this stage, but whether they contribute to these early behaviors is still unclear (Higashijima et al., 2004a,b). These include the sensory Rohon-Beard (RB) neurons and CoPA interneurons (Higashijima et al., 2004a). Also, genes for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) glutamate receptor subunits are expressed in the spinal cord at this time (Cox et al., 2005; Hoppmann et al., 2008). These observations suggest that, during the early period of spontaneous coiling, glutamatergic synapses may be immature or that glutamatergic transmission may play only a minor role. However, this role may be gradually expanded as the gap junction network becomes less effective.
In contrast to the clear definition of the circuitry necessary for spontaneous coiling, there has been some controversy about the location of the neural structures that are required for the appearance of touch responses. An early study suggested a requirement for the hindbrain to obtain the full touch response (Saint-Amant and Drapeau, 1998), while others have recently suggested that the touch response circuitry is located completely within the spinal cord in zebrafish and Xenopus tadpoles (Li et al., 2003; Downes and Granato, 2006). In this study, we set out to examine this controversy by taking a closer look at the changes in circuitry that mediate the appearance of touch responses in zebrafish embryos.
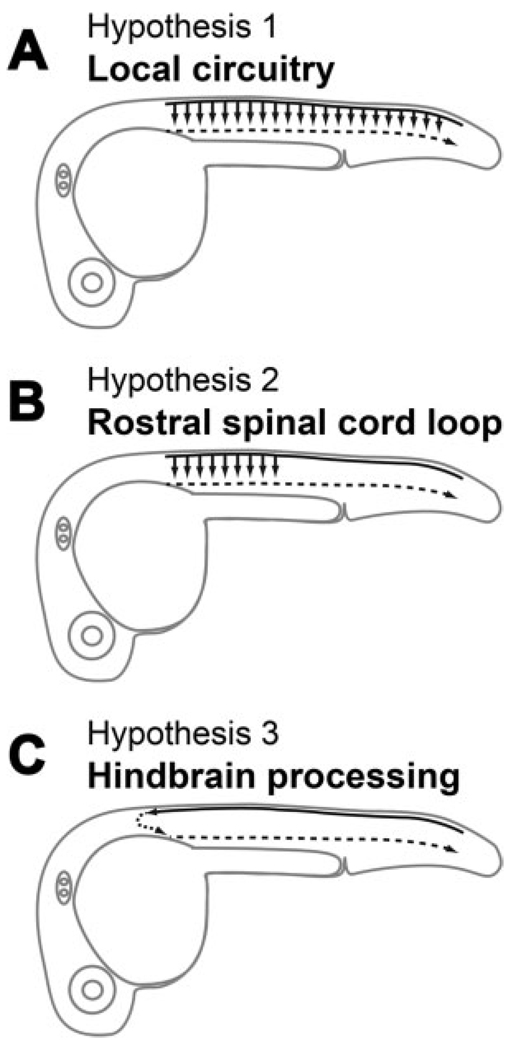
We present three hypotheses for the changes in circuitry that lead to the appearance of touch responses (see Fig. 1). Common to these three hypotheses is the role of the sensory RB cells. Previous research has shown that stimulation of a single RB cell in zebrafish and Xenopus larvae results in the triggering of a swimming episode (Clarke et al., 1984; Douglass et al., 2008), suggesting that these sensory neurons sit at the top of a very powerful cascade, which radically amplifies sensory input. Zebrafish embryos with mutations that abolish action potentials in RB cells (e.g. macho) are unresponsive to touch but retain spontaneous coiling (Ribera and Nusslein-Volhard, 1998). RB cells start to extend their axons rostrally and caudally in the dorsal longitudinal fascicles (dlf) around 17 hpf, and by 24 hpf they cover the entire length of the spinal cord and project into the hindbrain (Metcalfe et al., 1990). Therefore, to get the touch response to function, RB cells must be integrated into the motor network. We know that RB axons are in close proximity to the cell bodies of CoPA cells and have been hypothesized to connect en passant (Gleason et al., 2003; Downes and Granato, 2006). In Xenopus tadpole spinal cord, the Rohon-Beard cells form glutamatergic synapses onto dlc interneurons, the analogous neurons to CoPAs in this system (Li et al., 2003); however, it still remains to be determined whether these synapses are also formed in zebrafish. Thus, the second common element to our hypotheses is the role of CoPA interneurons. These neurons, through their commissural ascending axon, may provide the second site of sensory integration in addition to providing the contralateral switch needed for the proper execution of the contralateral coil in response to touch.
Figure 1.
Diagram of the potential circuitry underlying the touch response. (A) Sensory input may be relayed locally all along the spinal cord for transmission to motoneurons. (B) Sensory input is routed through spinal interneurons in rostral spinal cord before reaching the motoneurons. (C) Sensory input is processed in the hindbrain that then drives the motoneurons.
The first hypothesis [Fig. 1(A)] for the onset of touch responsiveness is that CoPA interneurons connect directly with motoneurons on the contralateral side throughout the spinal cord, as has been shown in Xenopus tadpoles [Fig. 1(A); Li et al., 2003]. We know as stated above that extensive electrical coupling between primary interneurons and motoneurons persists at this stage of development and is required for the function of the touch response (Saint-Amant and Drapeau, 2001). In this scenario, synapses between CoPA interneurons and neurons in the contralateral electrotonic network in the spinal cord, but not the hindbrain, would trigger the same circuitry that is involved in spontaneous coils. This hypothesis implies that lesions at progressively more caudal levels of the spinal cord should not affect the touch response in an all or none manner, but rather in a progressive manner because all levels of the spinal cord would have a local circuit, which can individually trigger a contraction. The second hypothesis is that CoPA interneurons through their ascending axons may contact descending neurons located only in the rostral spinal cord, which are an integral part of the gap junction coupled network, thus triggering coils [Fig. 1(B)]. This hypothesis implies that lesions removing the more rostral levels of the spinal cord should markedly attenuate the touch response, because a large driving force would be lost. The third hypothesis [Fig. 1(C)] is that connections are made to hindbrain interneurons that were not previously involved in the spontaneous coiling and that these neurons can, in turn, recruit neurons at all levels of the gap junction coupled network via glutamatergic synapses, thus triggering coils [Fig. 1(C)]. This connectivity could exist by the time touch responses start as RB cell axons first reach premotor reticulospinal interneurons, such as the Mauthner cells in the developing hindbrain that mediate the startle response, around the time that the touch response becomes active (Kimmel et al., 1990; Metcalfe et al., 1990). Reticulospinal neurons project axons caudally in the spinal cord and synapse directly onto primary motoneurons (PMNs; Jontes et al., 2000; Mongeon et al., 2008). This hypothesis implies that lesions removing the hindbrain should remove the touch response and that the touch response may be much more flexible than spontaneous coils in terms of amplitude and pattern.
Here, we provide electrophysiological evidence for the involvement of glutamatergic synapses to CoPA interneurons in the initial step of the touch response and pharmacological evidence for the importance of AMPA-type glutamatergic transmission in the integration of sensory stimuli in the spinal cord. We also provide evidence for our second hypothesis from kinematic analysis of lesion experiments, which show that the hindbrain is unnecessary for touch responses while the caudal spinal cord is not sufficient. Our results thus settle the ongoing controversy about the location of the drive for touch responses by showing that the drive for both touch responses and spontaneous coiling may emanate from a section of the rostral spinal cord. Furthermore, we provide morphological evidence for contacts between CoPA cells and descending interneurons in the rostral spinal cord. We conclude that the appearance of the touch response in zebrafish embryos requires formation of glutamatergic synapses, which connect the sensory component of the spinal cord to the rostrally located motor circuitry of the spinal cord.
METHODS
Animals
Zebrafish of the AB strain and of the Tg(−3.1xngn1: GFP)sb2 line were maintained according to Westerfield (2000). Experiments were conducted in embryos and larvae raised at 28.5°C. All procedures were carried out in compliance with the guidelines of the University of Oregon and Université de Montréal Animal Care and Use Committees.
Video Recording and Kinematic Analysis
The movements of embryos were recorded under a stereo-microscope (Leica LZMFIII) using a monochrome digital camera (PixeLINK PL-A741). The rate of spontaneous coil was evaluated in free moving embryos at 30 frames/s over a period of 2 min in batches of five to ten embryos per video. To observe the movement of the trunk of the embryos, they were mounted in a drop of 2.5% low-melting point agarose on a coverslip dorsal side up. The preparations were submerged in embryo medium (Westerfield, 2000), and the trunks of the embryos were freed from the agarose. Embryos were allowed to recover for 20 min at room temperature before being subjected to mechanical stimulation and video recording at 100 frames/s. Mechanical stimulation was performed using an insect pin attached to a micromanipulator. Light touches were applied to the side of the embryo at somites 14–16, at the level of the caudal part of the yolk tube. Each trial consisted of a recording of the spontaneous activity for 2 min, followed by five to seven stimulations, each separated by at least 1 s. The lack of response to each tactile stimulus was counted and reported as a percentage of the total number of stimuli for each fish (failure rate). Analysis of the movement was performed using Image Pro Plus software. For each movement, the first time point (t = 0) was defined as the moment the probe touched the embryo for the sensory-evoked response or the frame preceding the onset of the coiling. The tip of the tail was then tracked from t = 0 to the time point at which the tip of the tail reached its apex during coiling [see Fig. 2(B)]. The duration from t = 0 to the onset of the coiling (latency) and the active contraction phase (coiling) were measured as two separate parameters. To distinguish spontaneous coiling from touch responses, coiling with a latency greater than 250 ms was considered failures to respond to touch and therefore evaluated as a spontaneous coil.
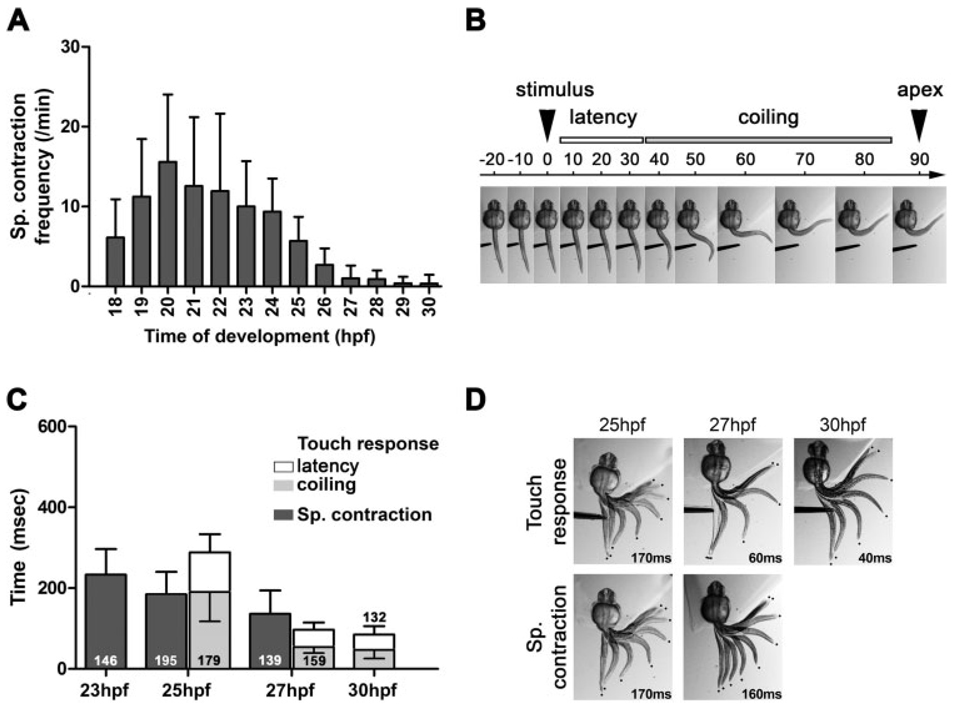
Figure 2.
Spontaneous and evoked coiling show kinetic differences. (A) Frequency of the spontaneous coils is represented between 17 and 30 hpf as SC/min. Over 70 freely moving embryos were recorded for each developmental time point. (B) Example of touch response analysis of a 27 hpf embryo. The latency phase is defined as the time when the sensory stimulus contacted the embryo (t = 0 ms) to the frame preceding active coil (t = 30). The coiling is defined by the frame of active coil (t = 40) to the frame at which the tip of the tail reached the apex of its movement (t = 80) (error bars = s.d.). (C) Kinetic analysis of the spontaneous coiling and touch response at 23, 25, 27, and 30 hpf, expressed as time taken in milliseconds (ms). Latency (open bars) and coiling (shaded bars) are indicated for the touch response. Stacked bars are cumulative, thus also representing the duration for the entire behavior. The number of individual movements analyzed is indicated in each column. A minimum of 21 embryos was analyzed for each behavior and time point (error bars = s.d.). (D) Examples of the coiling phase of spontaneous coil and touch response behaviors as depicted by overlaying successive frames from a high-speed video recording. The total duration of each coil is reported in milliseconds (ms). Note that while the duration of the coiling may vary between spontaneous and touch-evoked behavior, the shape of the movement is almost identical. Coiling appears to be the result of contraction of the muscles localized immediately caudal to the yolk sack, while the caudal aspect of the trunk remains completely relaxed. The interframe interval for each overlay is 20 ms (touch response and sp. coil at 25 hpf and sp. coil at 27 hpf); 10 ms (touch response at 27 hpf); and 5 ms (30 hpf).
Two-tailed unpaired t-tests were used to test the significance between data sets. Analysis of variance was also performed using an F-test. In cases where a significant difference in variance was detected, Welch’s correction was applied to the t-test. In Figure 3(B) and Figure 4(C), one sample set did not present variance; one-way ANOVA with Dunnett’s multiple comparison was then used instead of the t-test. In the figures, all data are represented as mean ± standard deviation and the numbers represent the number of fish (frequency of spontaneous coiling and failure rate of touch response) or the number of events analyzed (kinetic analysis). Significance of the t-test is represented in the graphs as *** for p < 0.001, ** for p < 0.01, and * for p < 0.05.
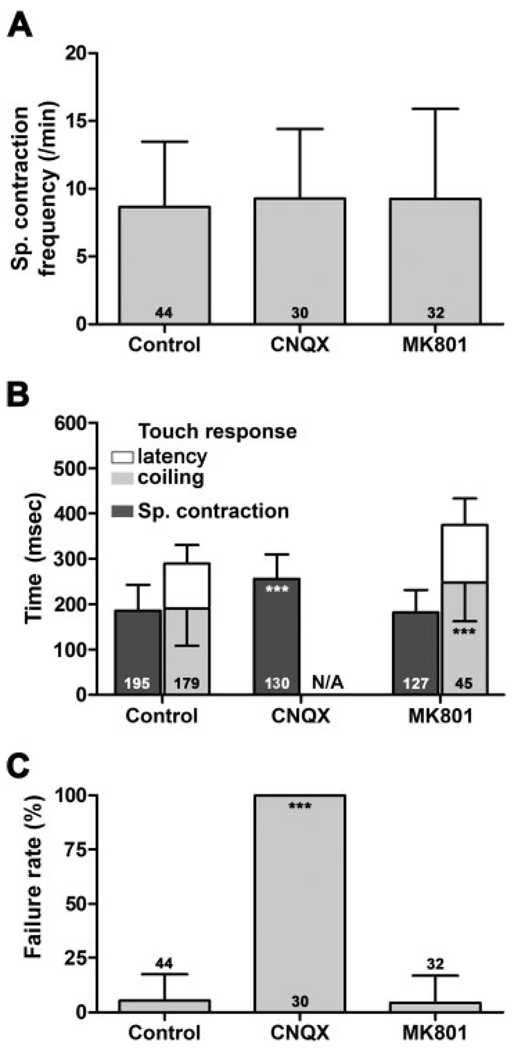
Figure 3.
AMPA-type glutamate receptors are necessary for evoked coiling. (A) Frequency of the spontaneous coils, (B) kinetic analysis of the touch response, and (C) failure rate after injection of embryo medium (control), AMPA receptor, or NMDA receptor antagonists (CNQX and MK801, respectively). Stacked bars in B are cumulative, thus also representing the duration for the entire behavior. The number of movements analyzed is indicated in each column in B, whereas the number of embryos analyzed is indicated in each column in A and C (error bars = s.d.).
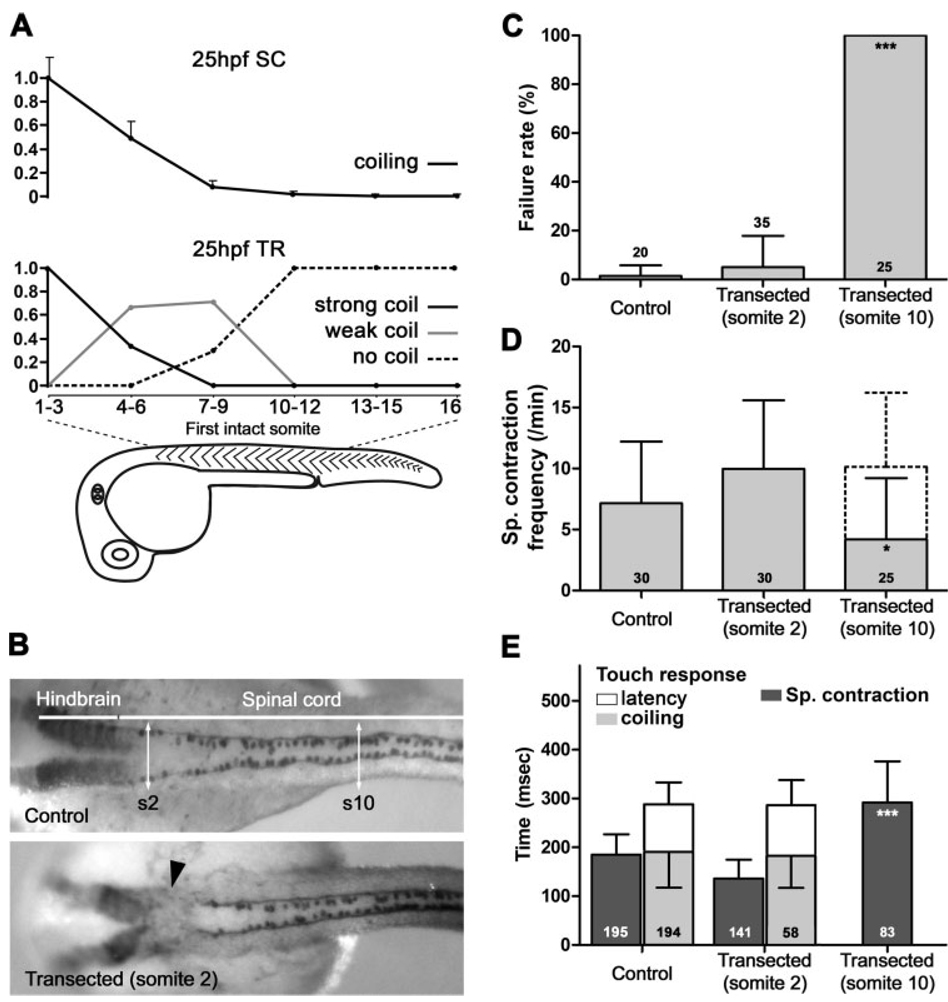
Figure 4.
Circuitry necessary for touch response resides in the anterior spinal cord. (A) The trunks of embryos at 25 hpf were imaged after complete transection at the level of somites indicated. Movies were quantified for spontaneous coiling frequency normalized to the most rostral transection (upper panel). Embryo trunks from complete transections at the levels indicated were analyzed for touch response. Responses were recorded as the percentage of embryos showing strong, weak, or no coils (lower panel; n = 6, 9, 7, 13, 5, and 3, respectively, for each lesion bin; error bars = s.e.). (B) Immunolabeling with GFP antibodies in ngn1: GFP transgenic zebrafish line [Tg(−3.1ngn1:GFP)sb2] reveals RB cell bodies and their axons (upper panel). The level of the hindbrain and spinal cord along the anteroposterior axis is indicated, as well as the levels of somite 2 (s2) and somite 10 (s10). Transection at the level of somite 2 (arrowhead, lower panel) abolishes immunolabeling at that level. (C) Failure rate of the touch response, (D) frequency of the spontaneous coils, and (E) kinetic analysis of the both behaviors for control embryos or embryos with spinal cord transections at somites 2 and 10 measured at 25 hpf. Anomalous spontaneous activity (pendulum-like motion) is included as a dashed box. Stacked bars in D and E are cumulative. The number of embryos analyzed is indicated in each column in C and D, while the number of movements analyzed is indicated in each column in E (error bars = s.d. in C–E).
Drug Applications and Spinal Cord Transections
To examine the behavioral response to different drugs, zebrafish embryos were embedded in 0.6% low melting temperature agarose, and 8–12 nL of drugs was injected through a microcapillary by pressure pulse into either the heart primordium or the third ventricle of the brain. No differences were noticed on the basis of sites of injection, suggesting that diffusion throughout the embryo is not a constraining factor. Drugs were prepared in embryo medium (Westerfield, 2000) at the following concentrations: MK801 at 100 µM or CNQX at 1 mM (Sigma-Aldrich, USA) and were estimated to be diluted 15-fold in the embryos. Injections with embryo medium were performed as controls. After injection, embryos were freed from agarose and allowed to recover at 28.5°C for 40 min. Embryos were then mounted in agarose as indicated above and recorded. Although CNQX is also an antagonist of kainate-type glutamate receptors, we conclude that AMPA receptors mediate the touch response because injection of the AMPA-selective noncompetitive antagonist GYKI 52466 also severely impaired the touch response similarly as CNQX (78% failure rate, not shown). CNQX did not adversely affect the health of the embryo, as by 30 hpf the majority of CNQX-injected embryos were responsive and none failed 100% of the time. However, the average rate of failure was still significantly above control-injected levels (20%, p = 0.035). Additional experiments suggested that complete elimination of the drug effects is achieved after 8 h (data not shown). Additional evidence that MK801 acts as an antagonist of zebrafish NMDA receptors comes from previous studies (Schmidt et al., 2000).
Complete transections of the trunk were performed on 23 hpf embryos in Evans buffer (134 mM NaCl, 2.9 mM KCl, 2.1 mM CaCl, 1.2 mM MgCl2, 10 mM glucose, 10 mM HEPES, adjusted to pH 7.8 with NaOH, 290 mOsm) with 100 µg/mL ampicillin and 0.02% tricaine (ethyl-3-aminobenzoate methanesulfonic acid, Sigma, MS-222), then washed with Evans buffer and ampicillin (without tricaine) multiple times before behavioral assays. Embryos were allowed to recover at 28.5°C to 25 hpf. The site of lesion was assessed individually for each embryo according to Kimmel et al. (1995), and embryos were binned per three somites for analysis. Spontaneous coils were video recorded over a 2-min period for each embryo. A touch stimulus was applied with forceps with the same force required to elicit touch responses in 100% of control embryos.
Transections, in which only the spinal cord was severed, were performed using a microcapillary on 24-hpf-old embryos individually restrained in agarose. Part of the agarose was then removed to allow the trunk free range of movement, and video recording was performed as indicated above 30 min after transection. We transected the spinal cord at the level of somite 2 to disconnect the hindbrain from the spinal cord and at the level of somite 10 to separate the rostral spinal cord from its more caudal aspect.
Backfilling
Backfilling of neurons was performed as previously documented (Mendelson, 1986) with small modifications. Briefly, 24 hpf embryos were anesthetized with 0.02% tricaine, and the spinal cord was transected with a tungsten micropin at the level of somite 10. Subsequently, a micropin loaded with biotin-labeled tetramethylrhodamine-dextran 3000 (Invitrogen D-7162) was inserted into the lesion. Embryos were allowed to recover for 1 h and were then anesthetized and fixed with 4% paraformaldehyde in PBS for 3 h.
Immunolabeling
After video recording, spinal cord-transected or intact Tg(−3.1ngn1:GFP)sb2 embryos were fixed at 4°C for 3 h in 2% trichloroacetic acid, washed several times in PBS, 0.1% Triton X100 (PBST), and blocked in PBST/10% fetal calf serum (blocking solution) for 1 h. After blocking, embryos were incubated overnight at 4°C in 1:1000 dilution of anti-GFP antibody (Invitrogen, A11122) in blocking solution, and then washed six times for 10 min in PBST. Embryos were incubated overnight at 4°C in 1:500 dilution of HRP-coupled antirabbit antibody (Jackson ImmunoResearch, 711-035-152), then washed six times for 10 min in PBST. Two additional washes were performed in PBS. Horseradish peroxidase was revealed with the DAB peroxidase substrate kit from Vector Laboratories (SK-4100) according to the manufacturer’s protocol. Images of dorsal views of the embryo were captured with a digital camera on a Leica stereomicroscope.
After backfilling, embryos were incubated in 5% BSA, 2% goat serum in PBST (blocking solution) overnight, and then incubated with anti-CON1 (1:150; gift from J.Y. Kuwada, University of Michigan) and streptavidin-Cy3 (1:20; Invitrogen S-A1010) in blocking solution for 3 h. After washing extensively in PBST, embryos were incubated with AlexaFluor 488-coupled antimouse IgG (1:250; Invitrogen A-11001) in blocking solution overnight. Embryos were washed extensively in PBST and then mounted in 80% glycerol. Imaging was performed on a Nikon TE2000U microscope using a 60× water immersion objective (1.2NA) and an EZ-C1 confocal system (Nikon). Optical slices were taken sequentially for each chromophore with 0.2-µm steps. Stacks were then projected as a single image for presentation purposes using EZ-C1 viewer software (Nikon).
Electrophysiology
Methods for recording from spinal neurons in zebrafish embryos were published previously (Saint-Amant and Drapeau, 2003). Briefly, embryos obtained from wild-type adults, aged 25–28 hpf during recordings, were anesthetized with 0.02% tricaine during pinning and dissection. The spinal cord was exposed over somites 10–12 with a mild collagenase treatment to loosen and remove muscle cells. The bath solution consisted of Evans buffer with 15 µM d-tubocurarine (Sigma T2379). In some experiments, 20–40 µM CNQX was added to the bath solution. We used standard whole-cell recording techniques in vivo (Drapeau et al., 1999) at room temperature (22°C). Preparations were discarded after 45 min. Patch-clamp electrodes (5–8 MΩ resistance) were filled with an intracellular solution consisting of 105 mM potassium gluconate, 16 mM KCl, 2 mM MgCl2, 10 mM HEPES, 10 mM EGTA, and 4 mM Na3ATP, at pH 7.2, 273 mOsm. The pipette solution has an estimated junction potential of −5 mV that was not corrected for. The theoretical reversal potential for chloride with these solutions is calculated to be −46 mV in our experiments. All embryonic neurons were labeled during whole-cell experiments by including 0.1% sulforhodamine B (Sigma, S1402) in the patch pipette. PMNs were identified by their large ventrally located cell bodies and their axons exiting the local ventral root and extending to the muscle; CoPAs were identified by their large dorsally located cell bodies and their ascending commissural axons that project in the contralateral dlf. Touch responses were evoked by squirting bath solution on the tail of the embryos using a glass micropipette, located at somite 20, connected to a Picospritzer 2 (20 psi, 5–20 ms; Parker Instrumentation). We have measured that the Picospritzer takes 6.5 ms from trigger to release of liquid. This delay has been deducted from measured latencies to estimate the latency from skin stimulus to recorded potentials [Fig. 5(C)]. In some experiments, transections were performed with a glass micropipette at somite 10. Whole-cell current was recorded with an Axopatch-1D amplifier (Axon Instruments), filtered at 5 kHz (−3 dB) and digitized at 20–50 kHz. Data were acquired with pClamp 8 software and were analyzed off-line with Clampfit software (Axon Instruments). The recordings were not analyzed if the resting potential was more positive than −30 mV or if the input resistance was below 500 MOhm. Student t-tests were performed to assess the significance of the data. All figures were arranged for presentation purposes using Adobe Photoshop CS2.
Figure 5.
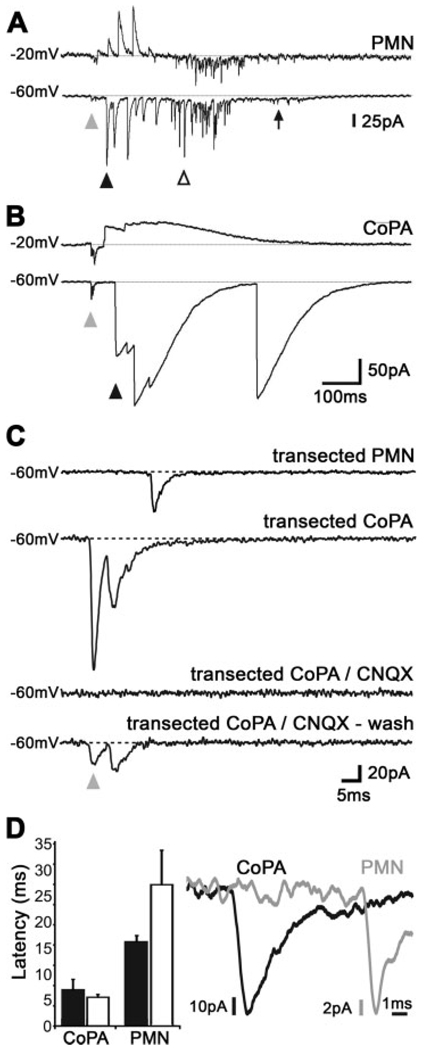
Short latency AMPA currents in CoPA interneurons. (A) Voltage clamp recording of currents elicited in a motoneuron following tactile stimulation of the tail. The recording shows a baseline of 100 ms before the stimulus, which was followed by a small amplitude sensory-evoked synaptic current (SESC) of very short latency (gray arrowhead), then by later arriving large amplitude events that were reversed at −20 mV (black arrowhead). The next events were rapid and of large amplitude and were not reversed at −20 mV (empty arrowhead), and the final event is a slow inward current (arrow). (B) Voltage clamp recording of a CoPA interneuron, which only showed two types of events, a small amplitude and short latency SESC (gray arrowhead), and a slow, large amplitude event that were reversed at −20 mV (black arrowhead). (C) The short latency SESCs in PMNs and CoPA interneurons were insensitive to transections of the spinal cord at somite 6, but they were sensitive to AMPA receptor blockade with CNQX (20 µM). In contrast, the large amplitude depolarizations were abolished by transection. (D) SESCs in CoPA interneurons occurred earlier than in motoneurons, suggesting that CoPAs are upstream to motoneurons with regards to sensory input. Black bars represent intact embryos, open bars transected embryos. Note the 20-fold change in the x axis scale bar as compared to A and B (error bars = s.e.).
RESULTS
Spontaneous and Touch-Evoked Coiling Show Kinetic Differences
To investigate the circuitry underlying early behaviors of zebrafish embryos, we first characterized these behaviors in detail by kinematic analysis of high-speed video recordings. We quantified the kinetics of spontaneous coiling and touch-evoked coiling between 17 and 30 hpf, a developmental time period during which these behaviors appear and overlap. We found that free-moving embryos start to coil after 17 hpf [Fig. 2(A)]. The frequency of coiling increased dramatically to peak at 20 hpf, before gradually decreasing to a very low frequency at 30 hpf. At that time, most of the embryos (95/101) did not display spontaneous coiling during our recording period of 2 min. The timing of the increase and decrease in spontaneous coiling frequency is consistent with previous reports (Saint-Amant and Drapeau, 1998; Cui et al., 2005; Saint-Amant, 2006); however, the frequency we measured differed by a factor of four [15.6 ± 8.5 spontaneous coils/min as compared to around 60 per min (Saint-Amant and Drapeau, 1998)]. This difference can be explained by the different genetic background of the fish used in our study (AB strain) and, as reported previously (Saint-Amant and Drapeau, 1998), the temperature at which we conduct our experiments (room temperature, 22–23°C). Indeed, when the fish were observed immediately after removal from the incubator (28.5°C), the rate of coiling was greater (data not shown). Because maintaining the temperature constant at 28.5°C during video analysis for all further experiments proved difficult, we ensured that embryos were always acclimated to room temperature for about 20 min prior to image acquisition.
Because the high rate of spontaneous coiling might lead to misidentification of touch-evoked responses, we decided to measure the kinetics of touch-evoked coiling during the decreasing phase of spontaneous coiling frequency [Fig. 2(A)]. After 25 hpf, it was possible to distinguish spontaneous from touch-evoked responses and to separate the touch response behavior into two distinct phases. We defined the time from start of the stimulus (touch from an insect pin) to the initiation of self-propelled movement as “latency,” and the time from the initiation of self-propelled movement to the apex of the coil as touch-evoked “coiling” [Fig. 2(B)].
In a side-by-side comparison of the kinetics of spontaneous coiling and touch response, it was apparent that the duration of both coiling motions decreased with development of the embryo [Fig. 2(C,D)]. Zebrafish embryos completed spontaneous coiling in 233 ± 64 ms at 23 hpf and in 136 ± 58 ms at 27 hpf. Because of the very low frequency of spontaneous coiling at 30 hpf, it was not possible to obtain significant data for this time point. Touch-evoked coiling took 190 ± 73 ms at 25 hpf and 47 ± 21 ms at 30 hpf and was very similar in duration to spontaneous coiling at 25 hpf [190 ± 73 ms compared to 185 ± 54, p = 0.48; Fig. 2(C)]. During the period of time analyzed, the decrease in duration of the spontaneous coiling appears roughly linear, whereas touch-evoked coiling duration showed a marked difference (d) between 25 and 27 hpf (d = 136 ± 6 ms; p < 0.0001), but only a small difference between 27 and 30 hpf (d = 7 ± 2 ms; p = 0.001). In addition, the latency was reduced from 99 ± 45 ms at 25 hpf to 39 ± 21 ms at 30 hpf, mirroring the changes in coiling duration. Interestingly, although the duration for spontaneous coiling and the touch-evoked coiling was comparable at 25 hpf, the time to perform a spontaneous coil is more than double the time for touch-evoked coiling at 27 hpf [136 ± 58 and 54 ± 14 ms, respectively; Fig. 2(C,D)], showing that with development touch-evoked coils quickly become more vigorous than the spontaneously occurring ones.
The embryos are growing and developing at a rapid rate during the period analyzed [23–30 hpf; Fig. 2(D)], and so it is possible that measuring the duration of these behaviors does not take into account the change in shape and length of the trunk and tail. To normalize for growth of the embryo, we also analyzed the speed of movement of the tip of the tail. These analyses demonstrated that speeds of contraction increased for both spontaneous coiling and touch response during this period and showed a similar trend as seen in the duration measurements (data not shown). This suggests that the contraction increases proportionately with growth of the embryos. We conclude from our kinematic analysis that the speed of spontaneous coiling and touch response is significantly different by 27 hpf [Fig. 2(C)], even though the shape of the movement (a stereotypical c-bend) underlies both behaviors [Fig. 2(D)]. This is in contrast to older embryos and larvae, which display swimming activity, consisting of contractions, which propagate along the trunk and produce an s-bend (McLean and Fetcho, 2008).
AMPA-Type Glutamate Receptors Are Necessary for Evoked Coiling
Previous studies demonstrated that although spontaneous coiling does not rely on glutamatergic transmission, the touch response is dependent on glutamate (Saint-Amant and Drapeau, 2000; Saint-Amant, 2006). Both AMPA receptors and NMDA receptors are expressed in the developing spinal cord at this time (Cox et al., 2005; Hoppmann et al., 2008). To investigate the potential contribution of these two important glutamatergic receptor types to the early motor behaviors, we injected the competitive AMPA receptor antagonist CNQX or the noncompetitive NMDA receptor antagonist MK801 into embryos at 24 hpf and analyzed the kinetics of spontaneous coiling and touch response 1 h later.
Injection of embryos with either CNQX and MK801 or embryo medium (control) did not result in a difference in spontaneous coiling frequencies [p = 0.61 and 0.65, respectively; Fig. 3(A)], confirming that glutamatergic transmission is not necessary to elicit spontaneous coils (Saint-Amant and Drapeau, 2000). However, analysis of the duration of spontaneous coiling revealed an interesting effect of the AMPA receptor antagonists on this behavior [Fig. 3(B)]. The duration of spontaneous coiling was increased after CNQX injection (257.0 ± 55 ms in comparison to 185 ± 55 ms for control-injected fish, p < 0.0001), suggesting that in embryos old enough to show touch responses, AMPA receptors might provide some synaptic drive in addition to the gap junctions to increase the speed of coiling during the spontaneous behavior. Blocking NMDA receptors with MK801, on the other hand, had no effect on the duration of the spontaneous coil [183 ± 49 ms compared to 190 ± 73 ms, p = 0.35; Fig. 3(B)]. This lack of effect for MK801 may indicate that the activity level during spontaneous coils is too low to remove the magnesium block from NMDA receptors (Jahr and Stevens, 1987). These results suggest that although not necessary for the execution of spontaneous coils, glutamatergic transmission is still used by the coiling network and can strengthen spontaneous coils.
We found that CNQX had a profound effect on triggering the touch response. Indeed, blocking AMPA receptors resulted in a 100% failure to respond to touch stimuli [Fig. 3(C)]. Because of this failure rate, it is impossible to study if AMPA receptors can modulate the duration of the coils as the response never gets triggered. NMDA receptors, on the other hand, were not necessary as all MK801 injected embryos responded to stimuli [Fig. 3(B)]. However, MK801 had a significant effect on the duration of touch-evoked coiling [242 ± 76 ms vs. control: 189 ± 73 ms; p < 0.0001, Fig. 3(B)]. These results are consistent with the critical requirement of AMPA-mediated synaptic transmission for the sensory trigger, as well as a minor role for NMDA-mediated transmission during the touch-evoked coils.
Circuitry Necessary for Touch Response Passes Through Rostral Spinal Cord
We considered three possible sites for sensory-motor integration: (1) throughout the spinal cord, (2) in rostral spinal cord, and (3) in the hindbrain (see Fig. 1). We distinguished among these three hypotheses by performing transections of the spinal cord in various locations and analyzing behavior at 25 hpf. We first transected the entire trunk of the embryo as performed in other studies (Saint-Amant and Drapeau, 1998; Downes and Granato, 2006). We then analyzed the remaining trunk/tail portions of the embryo for spontaneous coiling and touch response.
Complete transections of the trunk resulted in spontaneous coiling becoming progressively less frequent [Fig. 4(A), upper panel]. Transections removing the first seven somites eliminated over 92% of spontaneous coils, while transections caudal to somite 10 virtually eliminated all spontaneous activity [99%; Fig. 4(A), black trace]. The same trunks revealed similar results when analyzed for touch responsiveness, with removal of the first seven somites abolishing touch responses in 30% of the embryos, while removing segments caudal to somite 10 resulted in complete elimination of the touch response [Fig. 4(A), lower panel]. Additionally, transections at intermediate locations (somites 1–9) resulted in progressively weaker responses in progressively caudal locations, i.e. the fraction of embryos responding strongly decreased and the fraction of weakly responding embryos increased until being eliminated by transections past somite 10. These data suggest that the neural substrate necessary for triggering spontaneous coiling and providing drive for touch-evoked behaviors is localized rostral to the tenth somite of the spinal cord.
To characterize the kinetics of responses, we performed experiments in which we transected only the spinal cord (not the entire trunk), leaving the rest of the embryo essentially intact [Fig. 4(B)]. This approach minimized trauma and allowed us to embed the head and yolk of the embryo for video analysis. In this way, control and transected embryos would have exactly the same leverage during the coiling motion, allowing a comparison of kinetics. Spinal cord transections at somite 2 did not perturb either the spontaneous coiling or response to touch when stimulated at somite 16 [Fig. 4(C,D)], as was expected from our complete transections [Fig. 4(A)]. Frequency of spontaneous coiling, touch response failure rate, and the kinetics of spontaneous coiling and touch response were identical to control embryos [p > 0.05; Fig. 4(C–E)]. Given the lack of effect on both spontaneous coiling and touch response behaviors, we performed transections on transgenic embryos expressing GFP in RB cells [Tg(−3.1ngn1: GFP)sb2]. Immunolabeling of GFP after transection and video recording demonstrated the absence of RB cells and their axons at the lesion site [Fig. 4(B), bottom panel]. This confirmed that our transection procedure was effective in efficiently cutting the spinal cord. Furthermore, it demonstrates that the high regenerative capability of the axons in the zebrafish embryo and larva (Becker et al., 1997; Bhatt et al., 2004) could not explain the lack of effect of the lesion at somite 2.
In sharp contrast, transections at somite 10 completely abolished the touch response when stimulated caudal to the transection, at around somite 16 [Fig. 4(C)]. Importantly, however, these spinal cord-transected embryos were spontaneously active [Fig. 4(D)], complementing our data for complete transections [Fig. 4(A)], in which we observed spontaneous movement in the trunk rostral to the lesion site (data not shown). Embryos transected at somite 10 performed normal full coiling, as well as unusual movements. These movements did not lead to a coiling of the trunk, but rather only a side-to-side swing of the trunk like a moving pendulum. We refer to this activity as “pendulum-like” movement. Among the 25 embryos transected at somite 10, two only exhibited normal coiling movements (a full stereotypical c-bend), fourteen a mix of normal and pendulum like-movements, and nine only the latter. On average, normal coiling represented one-third of all movements analyzed (36 ± 33%). If only the normal coiling movement is considered [Fig. 4(D), plain box], the average frequency of the spontaneous coiling was significantly reduced (4.2 ± 5 per min) in comparison to control embryos [7.3 ± 4.8 per min; p = 0.046; Fig. 4(D)]. However, if all spontaneous movements are considered together, then the frequency of total spontaneous activity was not different from control embryos [p = 0.07; Fig. 4(D), dashed box]. The duration of spontaneous coiling was significantly increased by transection at somite 10 [p < 0.0001; Fig. 4(E)]. This increase of the duration and the reduction in range of motion of the spontaneous coiling may be caused by the lack of firing of PMNs caudal to somite 10 or could be attributed to lesion of overlying musculature during the transection procedure. These data suggest that embryos transected at somite 10 exhibit spontaneous activity, but do not respond to a sensory stimulus caudal to the site of transection. When considered together with the complete transection data [Fig. 4(A)], we conclude that the neural circuitry sufficient for triggering spontaneous coiling as well as driving sensory-evoked coiling at 25 hpf lies between somites 2 and 10. These results are in agreement with our second hypothesis for a rostrally located relay of sensory input.
CoPA Interneurons Receive Glutamatergic Input From RBs
CoPA cells are prime candidates to relay the sensory stimulus to the rostral spinal cord, because their axons project rostrally in the contralateral dlf (Hale et al., 2001) and receive direct sensory input from glutamatergic RB cells through glutamatergic synapses located on their dendrites at 3 days postfertilization (Gleason et al., 2003). However, it is unclear whether contacts between RB axons and the CoPA cell bodies constitute chemical synapses at 24 hpf (Downes and Granato, 2006), a time at which CoPAs have not yet extended dendrites. To test the possibility that these contact sites might be the site of glutamatergic sensory input into the motor network, we performed electrophysiological recordings from CoPA interneurons and motoneurons (somites 10–12) while stimulating the skin on the ipsilateral side of the embryo (somites 18–20). The responses obtained from both types of neurons were compared and contrasted to assess their respective roles.
Responses to stimulation in the intact embryo revealed sensory-evoked synaptic currents (SESCs) with a short latency [Fig. 5(A,B), gray arrowheads], followed by much larger currents that appeared with a longer delay in both PMNs and CoPAs [Fig. 5(A,B), black arrowheads]. We measured an average latency of 6.9 ± 1.9 ms for the time from stimulation to an SESC in CoPA interneurons and 16.2 ± 1.2 ms for PMNs [p = 0.01, n = 3; Fig. 5(D)], suggesting that CoPAs are located more upstream in the circuit than PMNs with regards to the sensory input. Our results are thus in line with recordings from dlc interneurons in Xenopus laevis tadpole spinal cord that receive direct input from RB cells with a 4–10 ms latency (Sillar and Roberts, 1988).
To examine the nature of the inputs, we recorded currents while depolarizing the membrane to a holding potential of −20 mV. This would reverse glycine-mediated chloride currents, which are inward at this time in development (Reynolds et al., 2008), but would not reverse excitatory sodium currents. SESCs were not reversed by depolarizing the membrane suggesting that these are excitatory currents [Fig. 5(A,B)]. In contrast to the SESCs, the next currents to appear were reversed by depolarizing the membrane to −20 mV suggesting they are mediated by glycine [Fig. 5(A,B), black arrowheads]. These inward currents may not result in excitation however, as they presumably inhibit coincident excitatory inputs due to a shunting of the membrane resistance (Cui et al., 2005). The CoPAs spend the rest of their activity in this presumed shunting mode [Fig. 5(B)]. In addition to these common types of activity, PMNs also showed a third type of activity consisting of fast events that were not reversed by holding the membrane at −20mV [Fig. 5(A); open arrowhead], which suggests that these inward currents are mediated by glutamatergic and/or gap junction transmission (Saint-Amant and Drapeau, 2001). A low amplitude slow inward current was often seen at the end of the PMN responses [Fig. 5(A), arrow]. This current is similar to the periodic inward currents that were shown previously to underlie spontaneous coils (Saint-Amant and Drapeau, 2000). This sequence of synaptic inputs to motoneurons perfectly fits the behavior. As should be the case for an output neuron ipsilateral to the stimulus, they first receive a barrage of inhibitory inputs during the contralateral coil and then excitatory transmission during a secondary ipsilateral coil.
To confirm our behavioral results that the circuits for the touch response form an amplifying loop through rostral spinal cord, we recorded from PMNs and CoPAs in embryos in which the rostral spinal cord had been removed by transection at somites 8–10. While three of the six PMNs were not active in response to tactile stimuli, three PMNs showed single short latency SESCs [Fig. 5(C)]. Importantly, the SESCs were not followed by barrages of large inhibitory or excitatory currents, as seen in intact embryos [Fig. 5(A)]. It is thus highly unlikely that these very brief and small amplitude SESCs (21.6 ± 8.6 pA) are sufficient to produce anything more than a single spike in the motoneurons and thus could not drive the full coiling behavior, which can last for hundreds of milliseconds in the intact embryo. We consistently recorded short latency SESCs in CoPAs of transected embryos [3/3; Fig. 5(C,D)]. As with motoneurons, the large secondary currents normally seen in the CoPAs of intact embryos were completely absent in transected embryos [Fig. 5(C)]. SESCs in both PMNs and CoPAs were completely blocked by bath application of AMPA receptor antagonist CNQX [Fig. 5(C), n = 3 and 2, respectively], mirroring the previously described elimination of touch responses by AMPA blockade (see Fig. 3), and the inhibition of touch-evoked currents in intact embryos (Saint-Amant, 2006). The latency of responses was similar to those in the intact embryo: 5.5 ± 0.6 ms for CoPAs and 27.2 ± 6.6 ms for PMNs [p = 0.03, n = 3; Fig. 5(D)]. When these electrophysiological results are taken together, they support the second hypothesis in which RBs synapse directly onto CoPA interneurons in zebrafish, which in turn relay the sensory signal rostrally and contralaterally where the amplification and patterning of the complete motor response occurs.
CoPAs Contact Descending Interneurons in Rostral Spinal Cord
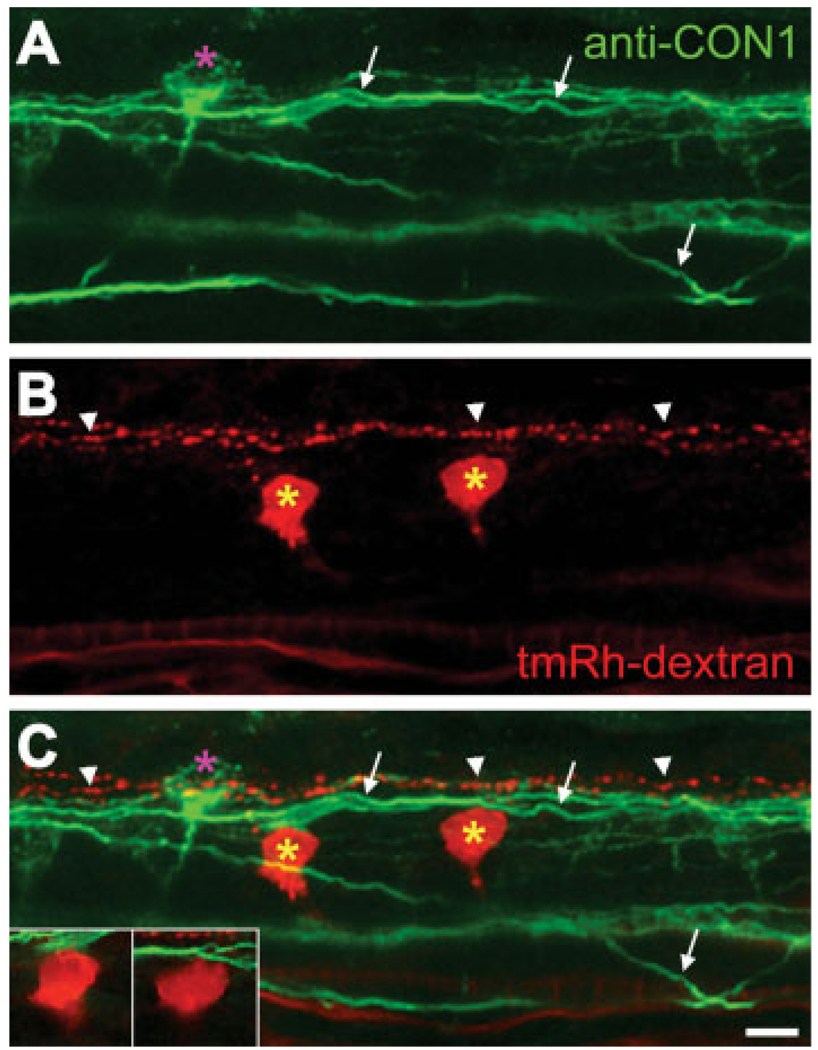
Our experiments suggest that CoPAs receive direct sensory input from RB cells in response to touch and that significant drive of the motor response is generated in the rostral spinal cord. To characterize where the sensory input propagated by the CoPA cells might be transmitted through rostral spinal cord, we examined the morphology of CoPA projections in that region. To visualize descending interneurons, likely candidates for intermediates in a rostral spinal cord loop, we combined retrograde labeling from the level of somites 10, with subsequent immunolabeling of CoPA cells and their axons (Bernhardt et al., 1990). Imaging of the spinal cord rostral to the labeling site allowed us to visualize contacts between axons and retrogradely labeled neurons (see Fig. 6). CoPA cells were visible (Fig. 6, pink asterisk), identified by their axons that pass to the contralateral side of the spinal cord close to the floor plate and project dorsally to the dlf (Fig. 6, arrows). Descending interneurons (yellow asterisks) had been successfully backfilled and were recognizable by virtue of ventral and caudal projections of their axons. The majority of retrogradely labeled neurons examined were seen to be in close contact with CoPA axons either in the dlf or as they passed rostrally and dorsally toward the dlf (19/23 in 6 embryos). Most of the descending interneuron cell bodies lay in a dorsal position (14/19; Fig. 6), suggesting they were CiD interneurons (Bernhardt et al., 1990; Higashijima et al., 2004b). The other backfilled neuron cell bodies lay in a more ventral position and were contacted by the commissurally projecting axons of CoPA cells (5/19; not shown). Their ventral and rostral cell body location suggests they may be IC cells (Mendelson, 1986). Interestingly, RB axons that lie in the dlf were also backfilled and appear to lie more dorsal to the CoPA axons (Fig. 6, arrowheads). These did not contact backfilled interneuron cell bodies (see Fig. 6). From these experiments, we conclude that CoPA cell axons possess the correct anatomical projections to form a loop through descending interneurons such as CiD or IC cells in the rostral spinal cord.
Figure 6.
CoPA axons contact descending interneurons. Confocal image of rostral spinal cord (somites 3 and 4) at 26 hpf. This is a projection of 62 optical sections taken at 0.2-µm intervals. (A) CoPA axons (arrows) were labeled with anti-CON1 (green). (B, C) Descending interneurons (yellow asterisks) were backfilled with tetramethylrhodamine–dextran (tmRh–dextran) at somite 10. Sites of contact between CoPA axons and filled interneurons are visible also in single optical sections (insets in C). Backfilled RB axons (arrowheads) do not contact the backfilled interneurons.
DISCUSSION
We embarked upon characterizing the neuronal pathways underlying the touch response in zebrafish embryos by proposing three hypothetical circuits based upon existing studies (see Fig. 1). Central to each hypothesis is the role of the touch-sensitive RB cells and CoPA interneurons. One possibility was that CoPA cells receive glutamatergic input from RB cells and pass this activity to the contralateral side of the spinal cord in a local circuit [Fig. 1(A)]. This configuration was previously described in Xenopus laevis tadpoles (Li et al., 2003) and was suggested to be the case in zebrafish embryos, too (Downes and Granato, 2006). A second possibility was that CoPA cells could pass sensory activity to a locomotor network located in rostral spinal cord [Fig. 1(B)]. A third possibility suggested that sensory input passes through the hindbrain [Fig. 1(C)], as has been established for sensorimotor behavior in older zebrafish larva (Liu and Fetcho, 1999). However, this third hypothesis seemed unlikely in embryos, because hindbrain transections did not eliminate the touch response (Downes and Granato, 2006). Here, we provide behavioral, pharmacological, electrophysiological, and morphological evidence supporting our second hypothesis that glutamate released from RB cells activates CoPA cells, which then convey this information via a commissural rostral loop, where they presumably which synapse with second-order descending interneurons, generating the drive necessary to pattern the response [Fig. 1(B)].
From our experiments [Fig. 3(B,C)] and previous studies (Saint-Amant, 2006), it is apparent that glutamatergic transmission, specifically AMPA-mediated activity, is essential for the touch response, but is dispensable for spontaneous coils. Primary requirement of AMPA receptors for the transmission of the sensory input is in line with previous results obtained in Xenopus as electrophysiological recording from dlc interneurons (homologous to CoPA interneurons in zebrafish) show that new synapses mainly express AMPA receptors (Rohrbough and Spitzer, 1999) and AMPA receptor currents are predominant at synapses with sensory neurons (Li et al., 2003). Conversely, recordings in reticulospinal neurons and motoneurons in zebrafish (Ali et al., 2000) and motoneurons in Xenopus (Li et al., 2003) have shown that newly formed synapses have either mixed AMPA/NMDA or purely “silent” NMDA receptor currents. Silent (NMDA receptor-only) synapses might therefore be involved in different subsets of interneurons in the course of development of zebrafish. Our data demonstrating an effect of the NMDA receptor antagonist on touch response coiling, but not on spontaneous coiling [Fig. 3(B)], is consistent with the idea of silent synapses existing at this time point in zebrafish spinal cord. However, it is hard to imagine how neurons spiking during spontaneous coiling would not activate NMDA receptors, as they presumably would do during evoked coiling. Recent studies suggest that neurons connected via electrical synapses, especially if these are localized on the axon, experience low-pass filtering (Li et al., 2009), resulting in minimal activation of cell bodies and dendrites that would not permit activation of NMDA receptors. Thus, the effect of blocking NMDA receptors on the speed of touch-evoked coils, but not on spontaneous coils, may reflect a greater activation of neurons during touch responses, which then enables the recruitment of NMDA receptors.
Our recordings from CoPA cells suggest that synapses between CoPA cells and RB cells are the first site of glutamatergic transmission in the sensorymotor response. It was important to establish this, because only axonal proximity had been demonstrated previously (Downes and Granato, 2006). Because all CoPA cells showed synaptic depolarizations when the skin was stimulated and our recordings were distant from the site of stimulation (at least five somites), it is highly likely that a single RB cell synapses onto multiple—or even all—CoPA cells on one side of the spinal cord. Furthermore, due to the axon projection of the RB cells, it is probable that a single CoPA cell receives input from many if not all RB cells on one side of the cord. This divergence and convergence of inputs suggests that stimulation of any single RB cell will lead to multiple CoPA cells transmitting a signal to the next level of the circuit, thereby amplifying the signal.
Because the kinetics of both spontaneous and touch-evoked behaviors were very similar, at least at 25 hpf [Fig. 2(C)], it is possible to conceive a motor circuit common to both spontaneous and touch-evoked behaviors being triggered by the glutamatergic input onto CoPA cells. This common circuit was further suggested when we measured a slight decrease in spontaneous coil strength caused by the glutamate receptor antagonist CNQX [Fig. 3(A)]. However, we must note that AMPA receptors may directly modulate the neuromuscular junction, as application of glutamate receptor agonists, albeit at a later stage in development, results in an increase in spontaneous acetylcholine release at the neuromuscular junction (Todd et al., 2004). Alternatively, a new network, independent of the premotor circuitry underlying the spontaneous coiling, might be at the origin of the sensorymotor response. However, this is unlikely at the onset of the touch response (21 hpf), because no new interneurons have extended axons that could form an alternate pathway. At 24 hpf, a second set of interneurons (CiD, CiA, CoB, and CoSA) start to develop their axons and may also therefore participate in this new motor behavior (Bernhardt et al., 1990). Since secondary interneurons are rapidly integrated into the spontaneous coil circuitry by becoming coupled to the pre-existing electrotonic network at 24 hpf (Saint-Amant and Drapeau, 2001), the same basic network of interneurons can support spontaneous and evoked responses. Thus, based on the pharmacological and transection data, we propose that both behaviors use the same circuitry and may differ kinetically only because of differences in the trigger and strength of recruitment through the appearance of glutamatergic neurotransmission.
This common circuit hypothesis was further verified by our lesion experiments. Indeed, our behavioral observations and electrophysiological recordings from transection experiments clearly demonstrate that sensory input from the tip of the tail needs to proceed to the rostral spinal cord to produce a response (Fig. 4 and Fig 5). This result is unexpected given previous work in zebrafish embryos and Xenopus tadpoles (Sillar and Roberts, 1988; Downes and Granato, 2006) and contradicts earlier findings demonstrating the requirement of the hindbrain (Saint-Amant and Drapeau, 1998). It is important to note that our spinal cord transections were performed slightly differently to previous spinalizations (Saint-Amant and Drapeau, 1998; Downes and Granato, 2006). In addition to complete transections which separate the rostral portion of the embryo from the trunk and tail, we performed spinal cord transections that left the rest of the body intact. This kind of transection afforded us the possibility to determine that spontaneous rhythmic coiling activity occurring in the rostral part of the tail is not dependent on long-range rostral–caudal connections. In contrast, the touch responses in the same animals were abolished by the severing of these connections. Thus, when put together, our drug and lesion experiments strongly point to a common rostral circuitry for spontaneous coils and touch responses. It is entirely possible that neurons rostral to somite 2 are activated during a touch response, because the necessary axonal projections are in place at 24 hpf: RB and CoPA axons project to the hindbrain and eventually contact reticulospinal neurons such as the descending and contralaterally projecting Mauthner cells (Metcalfe et al., 1990). However, our results clearly demonstrate that circuitry within the spinal cord is sufficient for performing the touch response, because removal of the hindbrain did not produce any alteration of the motor response [Fig. 4(C–E)].
It is interesting to consider peculiarities of the rostral spinal cord that may underlie this circuitry. One anatomical difference with respect to the rest of the spinal cord is the presence of IC cells. They are located in caudal hindbrain and the rostral portion of the spinal cord, extending caudally until about somite 8 (Mendelson, 1986; Metcalfe et al., 1990). Neurons homologous to IC cells in Xenopus tadpole hindbrain and rostral spinal cord are connected by gap junctions and help to synchronize motor output during swimming (Li et al., 2009). Perhaps, IC cells constitute a conserved mechanism for coordinating multiple types of movement. However, our morphological analyses and backfill experiments suggested that relatively few IC cells, as compared to CiD interneurons, were in contact with CoPA axons (see Fig. 6). CiD interneurons, in contrast, are located throughout the spinal cord (Bernhardt et al., 1990). Additional studies will be required to determine which morphological contact results in a synapse. Furthermore, we cannot exclude the possibility that there may be multiple interneurons in series or in parallel that form part of this circuit. Another explanation for the importance of rostral spinal cord may lie in the rostro-caudal gradient of development (Myers et al., 1986; Raible et al., 1992). While this has not yet been studied in terms of neuronal connections, it is plausible that synaptic drive is much stronger in rostral spinal cord during this period of development, as seen in Xenopus tadpoles (Tunstall and Roberts, 1994).
We propose the following model for minimal circuitry underlying the touch response in the zebrafish embryo: RB cells form glutamatergic synapses en passant on the cell bodies of CoPA cells. The CoPA cells project their axons in the contralateral dlf toward the rostral portion of the spinal cord. There, CoPA axons potentially connect through both gap junctions and glutamatergic synapses to CiD interneurons and IC cells. These descending interneurons then project to ipsilateral motoneurons, where they also form connections via gap junctions and chemical synapses (Saint-Amant and Drapeau, 2001). Although some paired recordings have shown connectivity between CiD or IC cells and PMNs (Saint-Amant and Drapeau, 2001), further morphological and electrophysiological investigations using paired recordings will be necessary to completely characterize the network required for touch responsiveness.
In conclusion, our data strongly suggest that the same basic motor circuit may underlie both spontaneous and touch-evoked behavior in the developing zebrafish embryo. Circuitry contained in the rostral spinal cord is sufficient for both behaviors, whereas glutamate is only necessary for touch-evoked coiling. During a few hours of maturation, the spinal cord may switch from a coiling network heavily dependent on gap junctions and intrinsic pacemaking activity to a coiling network composed of the same neurons now driven by glutamatergic transmission and dependent on sensory stimulation.
Acknowledgments
Contract grant sponsor: National Institute of Neurological Disorders; contract grant number: R01NS065795 (to P.W.).
Contract grant sponsors: Autism Speaks (to T.P. and P.W.); GRSNC (to J.R.); CIHR (to L.S.-A.); FRSQ (to L.S.-A.), Whitehall Foundation (to P.W.).
We would like to thank Monte Westerfield, Pierre Drapeau, Alexandra Tallafuss, Sean E. Low and other members of our labs for comments on previous versions of this manuscript. We would also like to thank Keith Beadle and the University of Oregon Zebrafish Facility for zebrafish husbandry.
REFERENCES
- Ali DW, Buss RR, Drapeau P. Properties of miniature glutamatergic EPSCs in neurons of the locomotor regions of the developing zebrafish. J Neurophysiol. 2000;83:181–191. doi: 10.1152/jn.2000.83.1.181. [DOI] [PubMed] [Google Scholar]
- Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, Chitnis AB, Lindamer L, Kuwada JY. Identification of spinal neurons in the embryonic and larval zebrafish. J Comp Neurol. 1990;302:603–616. doi: 10.1002/cne.903020315. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Otto SJ, Depoister B, Fetcho JR. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. doi: 10.1126/science.1098439. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JA, Kucenas S, Voigt MM. Molecular characterization and embryonic expression of the family of N-methyl-d-aspartate receptor subunit genes in the zebrafish. Dev Dyn. 2005;234:756–766. doi: 10.1002/dvdy.20532. [DOI] [PubMed] [Google Scholar]
- Cui WW, Low SE, Hirata H, Saint-Amant L, Geisler R, Hume RI, Kuwada JY. The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. J Neurosci. 2005;25:6610–6620. doi: 10.1523/JNEUROSCI.5009-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol. 2008;18:1133–1137. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes GB, Granato M. Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J Neurobiol. 2006;66:437–451. doi: 10.1002/neu.20226. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Ali DW, Buss RR, Saint-Amant L. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J Neurosci Methods. 1999;88:1–13. doi: 10.1016/s0165-0270(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Brustein E. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MR, Higashijima S, Dallman J, Liu K, Mandel G, Fetcho JR. Translocation of CaM kinase II to synaptic sites in vivo. Nat Neurosci. 2003;6:217–218. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- Hale ME, Ritter DA, Fetcho JR. A confocal study of spinal interneurons in living larval zebrafish. J Comp Neurol. 2001;437:1–16. doi: 10.1002/cne.1266. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Mandel G, Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol. 2004a;480:1–18. doi: 10.1002/cne.20278. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Schaefer M, Fetcho JR. Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. J Comp Neurol. 2004b;480:19–37. doi: 10.1002/cne.20279. [DOI] [PubMed] [Google Scholar]
- Hoppmann V, Wu JJ, Soviknes AM, Helvik JV, Becker TS. Expression of the eight AMPA receptor subunit genes in the developing central nervous system and sensory organs of zebrafish. Dev Dyn. 2008;237:788–799. doi: 10.1002/dvdy.21447. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987;325:522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jontes JD, Buchanan J, Smith SJ. Growth cone and dendrite dynamics in zebrafish embryos: Early events in synaptogenesis imaged in vivo. Nat Neurosci. 2000;3:231–237. doi: 10.1038/72936. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Hatta K, Metcalfe WK. Early axonal contacts during development of an identified dendrite in the brain of the zebrafish. Neuron. 1990;4:535–545. doi: 10.1016/0896-6273(90)90111-r. [DOI] [PubMed] [Google Scholar]
- Li WC, Roberts A, Soffe SR. Locomotor rhythm maintenance: Electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J Physiol. 2009;587(Pt 8):1677–1693. doi: 10.1113/jphysiol.2008.166942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. The spinal interneurons and properties of glutamatergic synapses in a primitive vertebrate cutaneous flexion reflex. J Neurosci. 2003;23:9068–9077. doi: 10.1523/JNEUROSCI.23-27-09068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol. 2008;68:817–834. doi: 10.1002/dneu.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B. Development of reticulospinal neurons of the zebrafish. II. Early axonal outgrowth and cell body position. J Comp Neurol. 1986;251:172–184. doi: 10.1002/cne.902510204. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Myers PZ, Trevarrow B, Bass MB, Kimmel CB. Primary neurons that express the L2/HNK-1 carbohydrate during early development in the zebrafish. Development. 1990;110:491–504. doi: 10.1242/dev.110.2.491. [DOI] [PubMed] [Google Scholar]
- Mongeon R, Gleason MR, Masino MA, Fetcho JR, Mandel G, Brehm P, Dallman JE. Synaptic homeostasis in a zebrafish glial glycine transporter mutant. J Neurophysiol. 2008;100:1716–1723. doi: 10.1152/jn.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci. 1986;6:2278–2289. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Brustein E, Liao M, Mercado A, Babilonia E, Mount DB, Drapeau P. Neurogenic role of the depolarizing chloride gradient revealed by global overexpression of KCC2 from the onset of development. J Neurosci. 2008;28:1588–1597. doi: 10.1523/JNEUROSCI.3791-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera AB, Nusslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. J Neurosci. 1998;18:9181–9191. doi: 10.1523/JNEUROSCI.18-22-09181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DA, Bhatt DH, Fetcho JR. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Spitzer NC. Ca(2+)-permeable AMPA receptors and spontaneous presynaptic transmitter release at developing excitatory spinal synapses. J Neurosci. 1999;19:8528–8541. doi: 10.1523/JNEUROSCI.19-19-08528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L. Development of motor networks in zebrafish embryos. Zebrafish. 2006;3:173–190. doi: 10.1089/zeb.2006.3.173. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J Neurosci. 2000;20:3964–3972. doi: 10.1523/JNEUROSCI.20-11-03964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–1046. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Whole-cell patch-clamp recordings from identified spinal neurons in the zebrafish embryo. Methods Cell Sci. 2003;25:59–64. doi: 10.1023/B:MICS.0000006896.02938.49. [DOI] [PubMed] [Google Scholar]
- Schmidt JT, Buzzard M, Borress R, Dhillon S. MK801 increases retinotectal arbor size in developing zebrafish without affecting kinetics of branch elimination and addition. J Neurobiol. 2000;42:303–314. [PubMed] [Google Scholar]
- Sillar KT, Roberts A. Unmyelinated cutaneous afferent neurons activate two types of excitatory amino acid receptor in the spinal cord of Xenopus laevis embryos. J Neurosci. 1988;8:1350–1360. doi: 10.1523/JNEUROSCI.08-04-01350.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd KJ, Slatter CA, Ali DW. Activation of ionotropic glutamate receptors on peripheral axons of primary motoneurons mediates transmitter release at the zebrafish NMJ. J Neurophysiol. 2004;91:828–840. doi: 10.1152/jn.00599.2003. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Roberts A. A longitudinal gradient of synaptic drive in the spinal cord of Xenopus embryos and its role in co-ordination of swimming. J Physiol. 1994;474:393–405. doi: 10.1113/jphysiol.1994.sp020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. 4th ed. Eugene, OR: Institute for Neuroscience, University of Oregon; 2000. [Google Scholar]