Summary
The induction of genetic competence is a strategy used by bacteria to increase their genetic repertoire under stressful environmental conditions. Recently, Streptococcus pneumoniae has been shown to co-ordinate the uptake of transforming DNA with fratricide via increased expression of the peptide pheromone responsible for competence induction. Here, we document that environmental stress-induced expression of the peptide pheromone competence-stimulating peptide (CSP) in the oral pathogen Streptococcus mutans. We showed that CSP is involved in the stress response and determined the CSP-induced regulon in S. mutans by microarray analysis. Contrary to pneumococcus, S. mutans responds to increased concentrations of CSP by cell lysis in only a fraction of the population. We have focused on the mechanism of cell lysis and have identified a novel bacteriocin as the ‘death effector’. Most importantly, we showed that this bacteriocin causes cell death via a novel mechanism of action: intracellular action against self. We have also identified the cognate bacteriocin immunity protein, which resides in a separate unlinked genetic locus to allow its differential regulation. The role of the lytic response in S. mutans competence is also discussed. Together, these findings reveal a novel autolytic pathway in S. mutans which may be involved in the dissemination of fitness-enhancing genes in the oral biofilm.
Introduction
Free-living bacteria are at the mercy of a variety of environmental stress conditions that impose constant selective pressure on the microorganism. To compete or simply survive in their ecological niche, bacteria must rely on the ability to sense and respond to stress. Often, the response to stress involves the induction of a transient state of hyper-mutability, which is argued to increase the probability of generating adaptive variants in the bacterial population (Bjedov et al., 2003). Although some debate exists as to whether mutagenesis is an inductive strategy or simply a by-product of the accumulation of DNA lesions, stress-induced mutations certainly participate in the adaptive evolution of bacteria (Bjedov et al., 2003).
Although an increased mutation rate may lead to the chance development of a fitness-enhancing phenotype, the probability of such an event occurring is limited to the available DNA sequence in an organism's own genome. However, naturally transformable bacteria are able to sample the DNA pool of an entire community during stress and acquire fitness-enhancing genes across species barriers. The major human pathogen Streptococcus pneumoniae and the soil-dweller Bacillus subtilis are the best-characterized naturally transformable Gram-positive bacteria. Although they employ different mechanisms to achieve the competent state, both organisms turn on their competence regulons in response to specific environmental stresses, which may improve fitness by generating genetic diversity through natural transformation (Claverys et al., 2006).
As the major aetiological agent of human dental caries (Mitchell, 2003), the naturally transformable oral bacterium Streptococcus mutans is well studied at the genetic and physiological level. The regulatory system that governs genetic competence in this species is homologous to the system in S. pneumoniae (Håvarstein et al., 1996) and is composed of a peptide pheromone (CSP, competence-stimulating peptide), the ComDE two-component signal transduction system and the alternate sigma factor ComX (Li et al., 2001a). Although competence is regulated by similar signalling systems in both streptococcal species, important differences separate the two species’ response to the pheromone. First, while competence develops uniformly across a population of S. pneumoniae (Håvarstein et al., 2006), it is well established that only a fraction of the S. mutans population (~1%) ever becomes CSP-responsive (Li et al., 2001a; Aspiras et al., 2004; Qi et al., 2005). Moreover, the competence cascade in S. mutans is known to incorporate inputs from additional two-component systems (Qi et al., 2004; Senadheera et al., 2005; Ahn et al., 2006; Perry et al., 2008). Finally, while S. pneumoniae controls expression of its bacteriocins through the dedicated BlpRH system (de Saizieu et al., 2000), S. mutans controls the expression of many of its bacteriocins through ComDE (Hale et al., 2005a; Kreth et al., 2005; 2006a; 2007; van der Ploeg, 2005). The co-ordination of bacteriocin production and competence suggests that S. mutans can generate DNA for uptake from lysis of neighbouring species (Kreth et al., 2005) in what may be an evolutionary adaptation to the multispecies oral biofilm environment.
Streptococcal CSP pheromone was originally thought to accumulate passively in proportion to population density and act as a classical quorum-sensing signal to activate the competence regulon at a specific cell density (Håvarstein et al., 1995; 1996; Li et al., 2001a). However, early work done in S. mutans (Li et al., 2001b; 2002) suggested an intimate link between the competence cascade and the organism's response to acid stress. A link between competence and oxidative stress has also been made in S. mutans (Wen et al., 2005; Senadheera et al., 2006), but a mechanistic explanation for these phenotypes has remained elusive. Evidence for stress-induced genetic plasticity has also accumulated in regard to S. pneumoniae (Claverys et al., 2000; Chastanet et al., 2001; Prudhomme et al., 2006), where it has been suggested that pneumococcal CSP may act as a secreted stress-induced pheromone (or ‘alarmone’) that triggers expression of stress-responsive genes (Claverys et al., 2006).
Here, we present evidence that S. mutans integrates its response to specific environmental stresses with its competence cascade via the CSP pheromone and describe for the first time the global transcriptome analyses of CSP-regulated genes in S. mutans. Our most important finding was that in the presence of high concentrations of CSP pheromone, the unprocessed form of mutacin V acted as an intracellular auto-active bacteriocin causing S. mutans autolysis. To our knowledge, this is a completely novel mechanism of action for a bacteriocin. Moreover, the impaired ability of S. mutans cells lacking mutacin V to become competent indicates that stress-induced lysis in a subpopulation may be required for the acquisition of diversity through genetic transformation in the surviving cells.
Results
Stress induces expression of the CSP pheromone
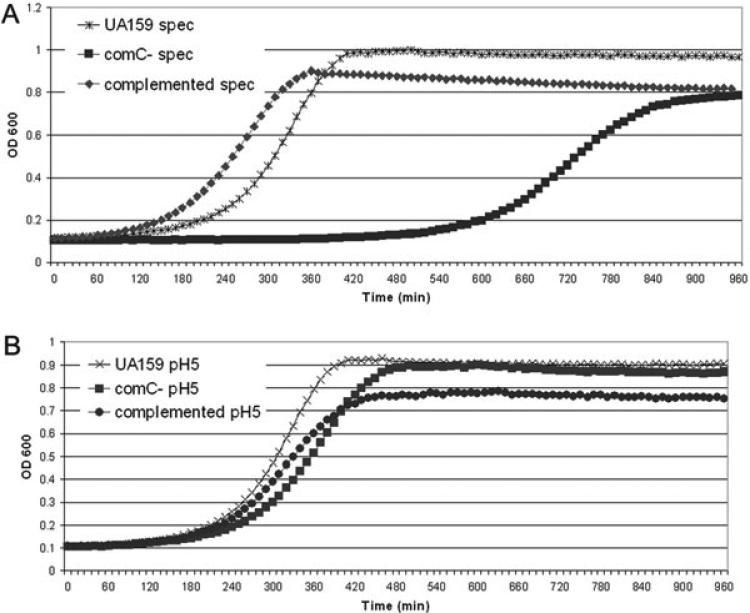
We asked whether environmental stress could activate the S. mutans competence regulon by monitoring the expression of the CSP pheromone-encoding gene (comC) under stress. Levels of comC transcript were significantly induced by acidic conditions at pH 5.0 (4.8 ± 0.8-fold) and in the presence of a subinhibitory concentration of the protein synthesis-inhibitor antibiotic spectinomycin (8.9 ± 3.8-fold). Levels of comC transcript were, however, unchanged in the presence of the DNA-damaging agent mitomycin C, the antimetabolite serine hydroxamate and the antibiotic erythromycin under the conditions assayed. These data suggest that upregulation of expression of the CSP pheromone may link the competence cascade and the stress response in S. mutans under some conditions. To test the impact of CSP on the stress response directly, cells of a mutant defective in the CSP pheromone-encoding gene (ΔcomC) were exposed to antibiotic and acid stress, harvested and resuspended in fresh medium for growth analysis. The same experiment was carried out with the ΔcomC mutant complemented with a functional comC gene in trans. When exposed to a subinhibitory concentration of spectinomycin, ΔcomC mutant showed a significant increase of lag phase before recovering from the antibiotic stress, while the complemented strain showed better recovery (Fig. 1A). In keeping with the less-pronounced induction of comC expression under low pH stress condition, the lag phase of ΔcomC mutant was significantly shorter in acid stress (Fig. 1B) than in antibiotic stress (Fig. 1A). As observed under spectinomycin-stressed condition, complementation of the ΔcomC mutant under acid stress resulted in an initial increase in growth rate followed by an eventual decline in cellular yield compared with the wild-type UA159 strain. These results suggested to us that the upregulation of CSP expression that occurs under stress actively contributes to the initial stages of recovery as indicated by resumed cell growth but is detrimental if it is allowed to continue accumulating in the culture and may even cause cell death if overproduced.
Fig. 1.
Recovery of S. mutans from stress. To test the contribution of endogenous CSP to stress recovery, early-log phase cells of the UA159 wild-type, a ΔcomC mutant (no CSP production) and an ΔcomC complemented strain (overproduction of CSP) were grown in THYE (control), in THYE containing spectinomycin (A) or in THYE at pH 5.0 (B) for 2.5 h. Cells were harvested and resuspended in fresh THYE at 1/100 dilution. Absorbance of the growing cultures was then automatically recorded for 16 h. Cells that recovered from stress in the absence of CSP showed a growth defect, while the CSP overproducing cells recovered from stress more quickly but attained lower growth yields than the wild type. These results imply that the S. mutans CSP pheromone is important in the stress response to acid and the antibiotic spectinomycin.
Competence-stimulating peptide pheromone triggers autolysis in a fraction of the population
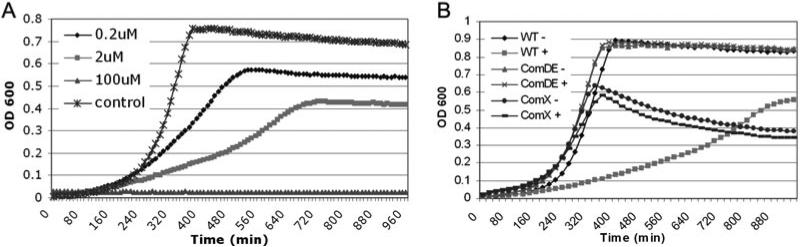
These results suggest a mechanistic link for competence induction under stress in S. mutans and broaden the implications of previous work done by Claverys’ group (Dagkessamanskaia et al., 2004; Prudhomme et al., 2006) in S. pneumoniae, by demonstrating a new role for the CSP pheromone in the stress response of more than one streptococcal species. However, as important differences exist between the CSP-induced competence cascade in S. mutans and S. pneumoniae (Martin et al., 2006), we examined the phenotypic effect of increasing the concentration of CSP in cultures of S. mutans. We used exogenously added synthetic CSP (sCSP) to study the effect of the CSP pheromone reproducibly, without secondary complications from the stress (e.g. protein synthesis inhibition from spectinomycin). We routinely use 0.2 μM sCSP to induce competence in S. mutans (Li et al., 2001a) and based our choice of sCSP concentrations on our stress experiments (which showed up to a 15-fold increase in comC transcript levels). It is possible that even higher localized endogenous CSP concentrations exist in the biofilm environment, but we attempted to avoid potential artefacts associated with overloading a signalling system by restricting the amount of exogenously added sCSP to concentrations close to those which induce competence in vitro. By monitoring the growth kinetics of planktonic cultures of S. mutans in the presence of increasing concentrations of sCSP, we found a ComX-dependent decrease in the growth rate and a ComDE-dependent decrease in the final growth yield of S. mutans proportional to the concentration of sCSP (Fig. 2). This phenotype differs from autolysis in S. pneumoniae, in which the whole population lyses in the presence of CSP (Ronda et al., 1987).
Fig. 2. S. mutans growth kinetics.
A. Growth of S. mutans in the presence of sCSP. To artificially mimic the high CSP concentrations induced by stress, cells were diluted into media supplemented with increasing concentrations of sCSP or without (control) and grown for 16 h. Cultures responded to sCSP with a slower growth rate and lower growth yield proportional to the increase in sCSP concentration. To ensure the specificity of the phenotype, a peptide was synthesized with the same amino acid composition as S. mutans CSP but with the order randomized. The randomized peptide did not have any effect on S. mutans growth (not shown).
B. Growth of S. mutans wild-type (WT), ΔcomDE mutant and ΔcomX mutant in the absence (–) and presence (+) of 2 μM CSP pheromone. While eliminating ComDE restores both the growth rate and yield of the culture in the presence of sCSP, the ΔcomX mutant shows a defect in growth yield both in the absence and presence of sCSP. We infer from these data that the altered growth yield of the culture in the presence of sCSP is due to ComE-controlled genes.
Our cultures responded to high sCSP concentrations with a decreased growth rate but stable plateau, which we attributed either to the average of two distinct populations of CSP-induced (lysing) and uninduced (resistant) cells (known as all-or-none induction; reviewed in Davidson and Surette, 2008) or to a uniform state of bacteriostasis in the whole population. To distinguish between these two phenomena, we monitored the supernatants of S. mutans cultures constitutively expressing a β-glucuronidase (GUS) reporter gene for release to the culture medium of intracellular GUS by cell lysis. GUS activity was significantly increased in the supernatant of cultures grown with sCSP, indicating cell lysis occurred at high sCSP concentrations (Fig. S1). We next quantified the extent of cell death in mid-log phase cultures grown in the presence of 2 μM sCSP using fluorescent staining for viability and found that < 10% of the S. mutans UA159 population stains with propidium iodide because of cell death (Fig. S2). This result is in agreement with previous work done on S. mutans strain UA140 using fluorescence viability staining, in which only a subpopulation of cells lysed no matter what the concentration of exogenous sCSP (Qi et al., 2005). Finally, we restored the growth of the culture to wild-type levels by subculturing sCSP-exposed cells into fresh medium (Fig. S2), confirming the existence of a CSP-resistant subpopulation even at concentrations of sCSP up to 200 μM. We conclude from these results that the CSP pheromone induces a state of population-level stasis in S. mutans and invokes lysis in a fraction of the population while sparing the remainder.
Genome-wide expression response to CSP: identification of mutacin V
Microarray-based expression profiling showed that 2 μM of sCSP altered the expression (± ≥ twofold) of 277 genes in the S. mutans UA159 genome (Table S1). As CSP induces gene expression both directly through ComE signalling and secondarily through ComX, we also determined the expression response to sCSP in the absence of ComX by microarray (Table S2). We found that ComE controls the expression of 37 genes, among which are the S. mutans bacteriocins and comX (Table 1). Like S. pneumoniae, S. mutans ComX is a competence-specific sigma factor responsible for expression of the entire competence regulon, including the CSP-encoding gene (Peterson et al., 2004).
Table 1.
Relative expression levels of highly expressed CSP-induced S. mutans genes encoding putative and known bacteriocins and their accessory genes.
| Locusa | Common name, putative function | Foldb |
|---|---|---|
| SMU.150 | NlmA mutacin IV | +11.19 |
| SMU.151 | NlmB mutacin IV | +12.40 |
| SMU.423 | Putative bacteriocin | +14.55 |
| SMU.925 | Putative immunity factor | +18.20 |
| SMU.1897 | ABC transporter, ATP-binding; ComA | +9.19 |
| SMU.1898 | Putative ABC transporter, ATP-binding and permease | +4.18 |
| SMU.1899 | Putative ABC transporter fragment | +5.22 |
| SMU.1900 | ABC transporter; ComB | +5.92 |
| SMU.1902 | GG-motif-containing peptide | +9.76 |
| SMU.1903 | Hypothetical protein | +15.97 |
| SMU.1904 | Hypothetical protein | +12.25 |
| SMU.1905 | GG-motif-containing peptide | +10.11 |
| SMU.1906 | Putative bacteriocin | +11.36 |
| SMU.1907 | Hypothetical protein | +8.93 |
| SMU.1908 | Hypothetical protein | +18.27 |
| SMU.1909 | Hypothetical protein | +19.61 |
| SMU.1910 | Hypothetical protein | +18.26 |
| SMU.1912 | Hypothetical protein | +22.23 |
| SMU.1913 | Putative immunity protein | +15.23 |
| SMU.1914 | Bacteriocin, mutacin V | +20.41 |
| SMU.1915 | Precursor of CSP pheromone | +3.76 |
| SMU.1916 | Histidine kinase ComD | +10.50 |
| SMU.1917 | Response regulator ComE | +11.32 |
| SMU.1997 | Sigma factor ComX | +14.27 |
Results for selected genes were ordered based on their position in the UA159 chromosome. The grey highlighted area indicates the genes located in a 13.5 kb bacteriocin-related genomic island. Putative bacteriocin-encoding genes are in bold.
Transcripts levels were measured by cDNA hybridized to a fourfold redundant S. mutans microarray and averaged for three replicated hybridizations. Quantitative real-time RT-PCR was performed on selected genes to confirm the results obtained using microarray.
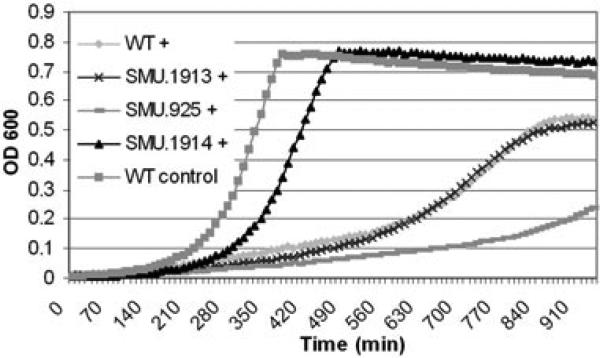
The products of six CSP-responsive genes (cbpD, lytA, comM, cibA, cibB and cibC) have been directly implicated in S. pneumoniae autolysis (Guiral et al., 2005; Håvarstein et al., 2006). Our search for homologous effectors among the CSP-induced genes identified by microarray yielded no significant sequence homologies but suggested the involvement of a bacteriocin in S. mutans CSP-induced lysis. Our search was also guided by experiments performed in Streptococcus thermophilus and Streptococcus salivarius, showing similar dose-dependent growth inhibition in the presence of the species-specific CSP paralogue BIP (Fig. S3), a signalling peptide known to induce bacteriocin expression (Fontaine et al., 2007). S. mutans isogenic mutants were generated to be defective in mutacin IV (SMU.150, SMU.151), mutacin V (SMU.1914) and GG-motif-containing peptides (SMU.423, SMU.1906). Importantly, inactivation of SMU. 1914, encoding the non-lantibiotic peptide bacteriocin mutacin V (Hale et al., 2005a), drastically attenuated the response to sCSP in S. mutans (Fig. 3). To ensure that the observed phenotype was not due to comC repression, we performed transcriptional analysis by quantitative real-time PCR and found that the expression of the CSP-encoding gene was not affected in the Δ1914 mutant (data not shown).
Fig. 3.
Effect of 2 μM sCSP pheromone (+) on S. mutans wild-type (WT) and mutants defective in the bacteriocin CipB (SMU.1914) and its putative immunity factors SMU.1913 and CipI (SMU.925). Growth of the WT strain in THYE alone provided a baseline for comparison (control).
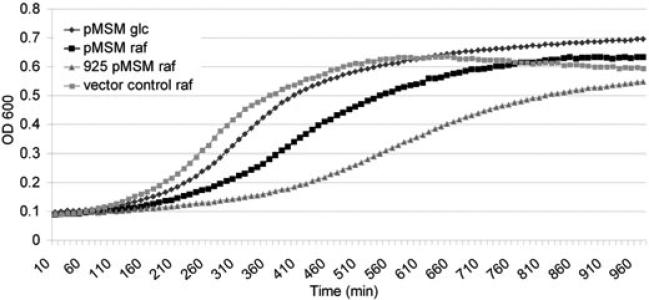
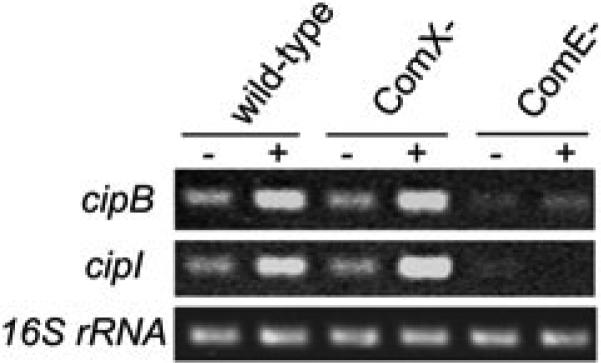
To confirm the bacteriocin-like activity of SMU.1914, we performed a series of agar overlay assays using the indicator strain Lactococcus lactis I6. As observed by Hale et al. (2005a), the Δ1914 mutant had a reduced zone of inhibition that was not observed for the mutacin IV mutant against L. lactis I6 (Fig. S4). As some bacteriocins produced by Gram-positive bacteria require two peptides for activity, we also investigated whether mutacin V (SMU.1914) caused cell lysis alone or in combination with CSP (SMU.1915), given that the two peptide-encoding genes share an overlapping (but divergent) promoter region (Kreth et al., 2007). We constructed a CSP-independent raffinose-inducible expression system to express mutacin V alone in S. mutans from a multicopy plasmid, bypassing the need for exogenous sCSP addition. This system allowed us to rule out the contribution of sCSP to our experimental system, given that excesses of signalling peptide have been documented to function as bacteriocin-like peptides at extremely high concentrations (Anderssen et al., 1998). Using this CSP-independent inducible expression system, we found that mutacin V gene expression was induced 5.0 ± 1.6-fold in raffinose (inducer) vs. in glucose (repressor). Although this level falls short of the 20-fold induction of mutacin V we observed with sCSP (Table 1), we were still able to induce a growth defect similar to sCSP using the raffinose-inducible system (Fig. 4). This result confirms that mutacin V is necessary for the observed autolytic phenotype in sCSP. We confirmed that SMU.1914 was regulated directly by ComE (Fig. 5) and was transcribed in direct proportion to the amount of sCSP (data not shown) or the concentration of naturally accumulating CSP in a growing culture of S. mutans (Fig. S5). Although Hale et al. (2005a) proposed that SMU.1914 be designated nlmC (gene product mutacin V), we instead suggest the three-letter prefix cip for CSP-induced peptide and propose the name CipB for this self-acting bacteriocin.
Fig. 4.
Growth kinetics of S. mutans UA159 strain containing the shuttle plasmid pDL277 (Leblanc et al., 1992) harbouring the CipB-encoding gene under the control of the raffinose-inducible promoter (notation ‘Pmsm’) of the S. mutans multiple-sugar metabolism operon (McLaughlin and Ferretti, 1996). Growth was monitored by following OD600 for 16 h in TYE containing either 0.5% raffinose (inducer, notation ‘raf’) or 0.5% glucose (repressor, notation ‘glc’). The vector alone (no insert) was used as control. Also plotted is the growth of a mutant defective in the putative immunity gene SMU.925 carrying the pMSM construct following induction with 0.5% raffinose.
Fig. 5.
RT-PCR gene expression profiles of cipB and cipI, encoding the bacteriocin and immunity protein respectively, in S. mutans wild-type strain and mutants defective in the alternate sigma factor ComX and the response regulator ComE. In addition to the lack of detectable transcript in the ΔcomE mutant, both cipB and cipI have putative ComE binding sites in their promoter regions (van der Ploeg, 2005), suggesting that ComE activates transcription of these genes directly. The constitutively expressed 16SrRNA gene served as a loading control.
CipB bacteriocin likely acts intracellularly
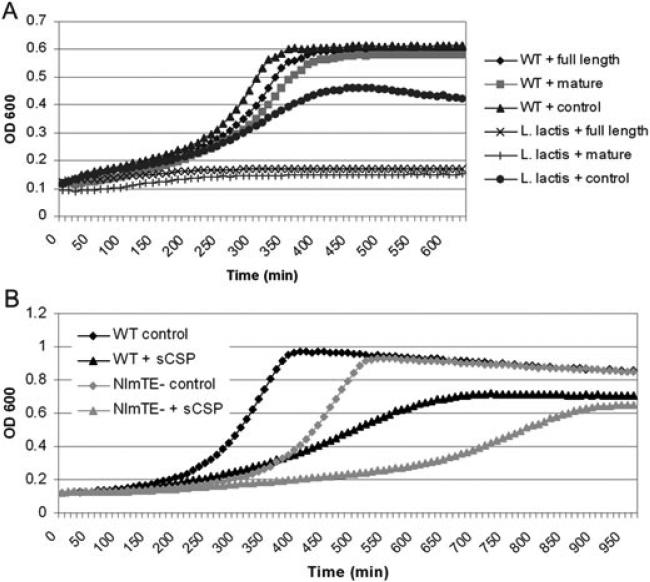
We reasoned that CipB's action against self would occur from: (i) a position tethered to the cell wall via cell-to-cell contact, (ii) the extracellular environment or (iii) intracellularly via membrane insertion from the cytoplasmic side. The Cib bacteriocins of S. pneumoniae are tethered to the cell wall after export and able to kill neighbouring cells via cell-to-cell contact (Guiral et al., 2005). We cocultured sCSP-induced wild-type cells with ΔcipB mutant cells to provide cell contact but did not observe any functional complementation of the mutant (results not shown), again establishing that these organisms differ in their autolytic pathways. We investigated activity from the extracellular environment by growing the ΔcipB mutant in cell-free supernatant from a sCSP-induced wild-type culture and in medium supplemented with purified recombinant CipB peptides (precursor and mature form). We found no effect on the growth of the S. mutans ΔcipB mutant in conditioned medium or at concentrations of recombinant CipB peptides that completely inhibited the indicator strain L. lactis I6 (Fig. 6A), indicating that extracellular concentrations of the recombinant bacteriocins had no effect on the producing strain. Importantly, we found that the recombinant bacteriocin precursor peptide was equally effective at killing L. lactis, implying that export-dependent processing was not required for activation of the CipB bacteriocin. This result suggests that an intracellular accumulation of the unprocessed bacteriocin may be lethal to the producing cell. To further test if intracellular accumulation of CipB could cause lysis, we inactivated the dedicated ABC transporter NlmTE, required for the export of S. mutans non-lantibiotic bacteriocins including CipB (Hale et al., 2005b). We confirmed that the ΔnlmTE mutant was unable to secrete CipB using an agar overlay assay with L. lactis indicator cells (Fig. S4). We found a significant growth defect in the ΔnlmTE mutant compared with the wild-type strain at all sCSP concentrations tested (Fig. 6B), which we attribute to an increased intracellular accumulation of unprocessed CipB. Interestingly, we also observed a longer lag phase in the ΔnlmTE mutant in the absence of sCSP following dilution from an overnight culture. As the CipB bacteriocin was highly expressed in overnight cultures (Fig. S5), this initial growth defect supports the hypothesis that intracellular CipB accumulation was lethal to the producing cell.
Fig. 6. CipB may act intracellularly.
A. Growth kinetics of S. mutans (WT) and L. lactis I6 in the presence of recombinant CipB (precursor and mature form). The full-length precursor peptide represents the intracellular form of the bacteriocin, including its leader sequence. The mature peptide represents the extracellular form of the bacteriocin, having been cleaved at the GG-motif. Importantly, the precursor peptide is equally effective against L. lactis, implying that export-dependent processing is not necessary for activity.
B. Effect of 2 μM sCSP (+) on the wild-type strain and a mutant defective in NlmTE, the ABC transporter responsible for export of CipB. The ΔnlmTE mutant was assayed over a range of sCSP concentrations and showed growth defects compared with the wild-type strain at all concentrations assayed (not shown), likely because of the intracellular accumulation of CipB. The ΔnlmTE mutant has a growth defect even in the absence of sCSP, possibly because of the accumulation of intracellular CipB induced by the high endogenous CSP concentration found in the overnight culture from which it was diluted.
Of final note, the expression of nlmTE decreased approximately twofold in the presence of 2 μM sCSP (Table 1), which was reflected in decreased zones of inhibition by agar overlay (Fig. S4). Given that the expression of cipB was increased 20-fold in these cells (Table 1), it appears that CipB accumulated to high intracellular levels in the presence of high concentrations of sCSP. This result was important in ruling out the notion that excess sCSP could have simply overwhelmed the normal CipB export pathway. Instead, an active decrease in expression of the transporter coupled with increased expression of the bacteriocin suggested that intracellular accumulation of CipB may be an active process. Together, these data strongly suggested a completely novel mechanism of action for a bacteriocin: intracellular action against self.
The small protein CipI (SMU.925) confers immunity
Bacteriocin-encoding genes are usually cotranscribed with their cognate immunity genes, which are protective against the action of the bacteriocin (Abee, 1995; Diep et al., 2007). The sCSP exposure induced two candidate immunity genes, SMU.925 and SMU.1913 (Table 1), which share 82% amino acid identity and have putative ComE binding sites in their promoter regions (van der Ploeg, 2005). Unexpectedly, the growth of a Δ1913 mutant was identical to the wild-type strain in the presence of sCSP (Fig. 3), indicating that, although SMU.1913 was cotranscribed with cipB (data not shown), it did not prevent CSP-induced lysis. In contrast, inactivation of SMU.925 resulted in almost complete growth arrest at high sCSP concentrations (Fig. 3). To confirm the role of SMU.925 in immunity to cell death, wild-type cells expressing SMU.925 from its own native promoter on a multicopy plasmid were exposed to sCSP. The cells overexpressing SMU.925 were significantly more resistant to sCSP than the wild-type ones (Fig. S6). We also transformed the Δ925 mutant with the same CSP-independent raffinose-inducible cipB expression construct used above. Cells deficient in the immunity gene were more susceptible than the wild-type ones to high levels of cipB expressed in presence of raffinose (Fig. 4), consistent with the proposed role for CipI as the immunity gene. We thus propose the designation cipI (CSP-induced peptide immunity) for SMU.925.
As for cipB, cipI was found to be dependent on ComE for its transcription (Fig. 5) and was transcribed in proportion to the amount of sCSP added (data not shown). However, unlike cipB, the expression of cipI showed an additional level of density-dependent control, as dilution from an overnight culture (high to low cell density) caused an increase in its expression (Fig. S5). We considered that a second mechanism controlling cipI expression might exist to prevent cell lysis at low cell density and tested whether autoinducer-2 (AI-2) might be the signalling molecule regulating this second system. No growth defect was observed when a ΔluxS mutant deficient in the LuxI-type AI synthetase (enzyme responsible for the synthesis of AI-2; Bassler and Losick, 2006) was grown in the presence of increasing sCSP concentrations (results not shown), indicating that AI-2 is likely not the second signal molecule.
Role of CSP-induced lysis in genetic competence
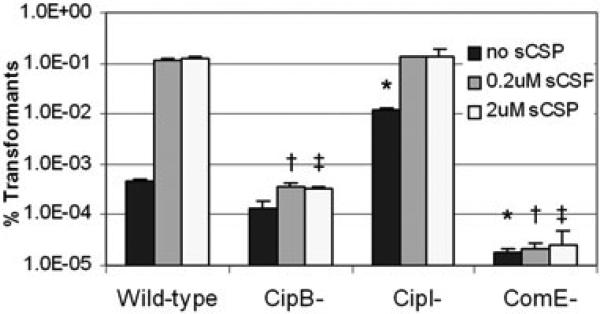
In addition to its known role in competence (Li et al., 2001a), we have shown that S. mutans CSP is involved in the stress response and can trigger autolysis. Bacteria are unicellular organisms and would seem incapable of altruistic behaviours like cellular suicide. For an altruistic trait like autolysis to be preserved by evolution, it must be linked to a behaviour that benefits genetically identical sibling cells (Keller and Surette, 2006). We reasoned that the linkage of the stress response, competence induction and autolysis through the CSP pheromone could serve to facilitate the exchange of fitness-enhancing DNA under stress and maintain the autolysis pathway through evolution. If our hypothesis is correct, S. mutans cells must be equally transformable at the high sCSP concentrations in which we observe cell lysis. Indeed, we found cells exposed to 2 μM sCSP to be transformed at frequencies similar to those obtained using 0.2 μM sCSP (Fig. 7). We next examined whether lysis and competence development could occur simultaneously in a culture using fluorescent staining for viability and a GFP reporter fused to the promoter of the gene encoding the sigma factor responsible for induction of the CSP-dependent competence regulon, comX. We observed sporadic expression of PcomX–gfp throughout the culture, consistent with the hypothesis that only a fraction of the S. mutans population is sCSP-induced (and competent) at any one time (Fig. 8A). However, when sCSP-treated cultures were counter-stained with propidium iodide, we observed overlap between PcomX–gfp expression and cell death in the majority of cases (Fig. 8B and C). This result was not surprising, as both cipB and comX are regulated directly via CSP-ComDE signalling, and suggests that sCSP-induced competent cells continue to accumulate CipB under continuous sCSP stimulation, resulting in cell death. We therefore tested the transformation efficiency of the ΔcipB mutant in the presence of sCSP to determine the transformation efficiency at high sCSP concentrations in the absence of cell death. When cell death in the culture was prevented by inactivation of cipB, the transformation efficiency in the presence of sCSP could not be induced beyond sCSP-independent levels (no sCSP added) (Fig. 7). Conversely, when cell death in the culture was promoted by removal of the immunity protein, ΔcipI cultures reached levels of transformation comparable to wild-type sCSP-induced levels in the absence of exogenously added sCSP (Fig. 7). These results indicated that the sCSP-induced competence cascade is connected to CipB-mediated autolysis in S. mutans. We are currently investigating whether factors released via lysis of a sub-population of cells contribute to the development of competence in the surviving population.
Fig. 7.
Transformation efficiency of S. mutans wild-type strain and its mutants deficient in the CipB bacteriocin and CipI immunity protein. Sheared genomic DNA carrying an antibiotic resistance gene was added alone or with 0.2 μM or 2 μM sCSP to growing cultures. Cells were grown for a further 2.5 h before differential plating. Results obtained for the wild-type strain showed that transformation is possible and equally efficient at the concentrations of sCSP that induce cell lysis. Mutants unable to undergo cell lysis (CipB–) showed no increase in transformation frequency in the presence of sCSP, while mutants with increased lysis potential (CipI–) showed increased transformation in the absence of sCSP. Transformation efficiencies are expressed as the number of antibiotic-resistant CFUs divided by the total number of CFUs. A ComE-deficient strain served as a transformation-deficient control.
Fig. 8.
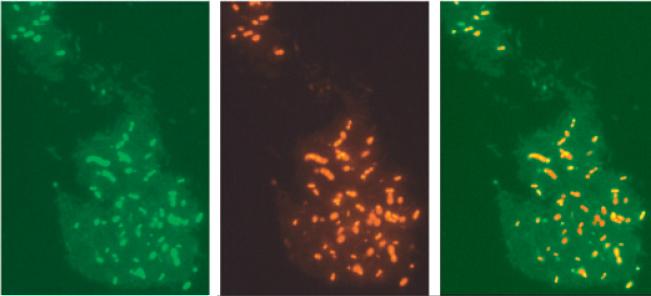
Competence and lysis in cultures of S. mutans. The alternate sigma factor ComX is induced by ComE and is responsible for induction of the CSP-dependent competence regulon. A PcomX–gfp reporter fusion was used to monitor the development of competence in the presence of 2 μM sCSP
(A). Cultures were then counter-stained with propidium iodide (B) to determine cell death. When images were merged (C), it was apparent that cells expressing comX were also undergoing cell lysis.
The results obtained with PcomX–gfp suggest that the sCSP-dependent competence pathway does not contribute to the uptake of fitness-enhancing genes in a stressed population, as sCSP-induced cells are killed because of CipB accumulation. However, we examined whether stress alone (in the absence of sCSP) could induce transformation in S. mutans using subinhibitory concentrations of spectinomycin known to increase comC expression. We found a 4.0 ± 1.3-fold increase in transformation efficiency in stressed cultures vs. control cultures. Importantly, the transformation efficiency of a ΔcomC mutant strain was identical to the wild type in presence of subinhibitory concentration of spectinomycin, indicating that the increase in transformation we observed was through the CSP-independent competence pathway. Together, these data suggest that the increase in CSP pheromone production under stress causes S. mutans autolysis through the ComDE signal transduction system, which releases DNA for uptake via the CSP-independent competence pathway (Fig. 9).
Fig. 9.
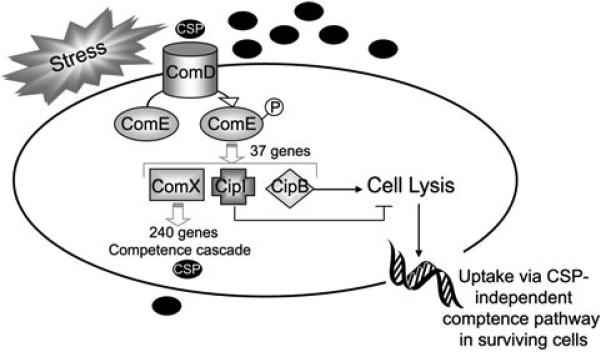
Summary of data. The sensor histidine kinase ComD is activated either directly by stress or by an accumulation of the CSP pheromone and activates its cognate response regulator ComE by phosphorylation. Activated ComE then directly regulates the expression of 37 genes, including the alternate sigma factor ComX, the CipB bacteriocin and its immunity protein CipI. CipB causes cell lysis in a fraction of the population, which potentially contributes DNA for uptake and other secondary signals to trigger genetic competence in the surviving population. Expression of 240 genes, including the competence cascade and the CSP molecule itself, are directly controlled by ComX. Open grey arrows: direct genetic regulation. Solid black arrow: phenotype caused.
Discussion
Experimental evidence has long supported a link between the competence cascade and stress response in S. mutans (Li et al., 2001b; 2002; Qi et al., 2004; Ahn et al., 2006), but a mechanistic explanation for these phenotypes has remained elusive. In this study, we report the characterization of a novel self-acting intracellular bacteriocin which functions as a mediator of autolysis in S. mutans. We have characterized this autolytic response in the physiological context of an elevated concentration of the CSP pheromone under stress, which provides the proverbial ‘missing link’ between stress and competence in S. mutans. Moreover, our results broaden the implications of previous work in pneumococci (Claverys et al., 2006) by demonstrating the presence of a peptide ‘alarmone’ in phylogenetic groups of streptococci beyond the mitis group. However, like in pneumococci (Prudhomme et al., 2006), not all stressful conditions assayed with S. mutans triggered CSP upregulation. While we have focused on elucidating the downstream pathways triggered by CSP upregulation in S. mutans, future work directed towards understanding why some stresses trigger CSP upregulation while others do not will complete our understanding of the stress response in this organism.
We focused on the effect of the stress-induced upregulation of the CSP pheromone in the absence of the stress using exogenously added sCSP. We demonstrated a slower growth rate and a reduction in growth yield at high concentrations of sCSP because of a balance between cell growth and autolysis in S. mutans. This response differs from the uniform autolytic response mounted by S. pneumoniae in the presence of high CSP. S. pneumoniae achieves both a death-susceptible and -resistant population by expressing two different CSP pherotypes (Claverys et al., 2006). However, S. mutans has been shown to encode a single CSP pherotype (Allan et al., 2007). The nature of cell lysis (and competence) responses in S. mutans is therefore different from the response in S. pneumoniae and may be closer to the other well-characterized naturally transformable Gram-positive organism, B. subtilis, whose competence response occurs in only a fraction of the population because of bistability (Smits et al., 2005). The bistable response occurs when positive feedback into a system occurs on a fast timescale and negative feedback occurs on a slower timescale (Davidson and Surette, 2008). The result is an amplification of transcriptional ‘noise’ in a system, which manifests as two transient but distinct populations of induced and uninduced cells (Dubnau and Losick, 2006; Davidson and Surette, 2008). We envision a situation in S. mutans in which stress-induced CSP upregulation serves to ‘prime the pump’ of bistability in the CSP-ComDE circuit, causing some cells to respond with a rapid and extreme upregulation of CSP-controlled genes (including comC), because of positive feedback. What is unique about the genetic organization of comC in S. mutans is its divergent regulation from mutacin V (Kreth et al., 2006b; 2007). Kreth and colleagues showed that this genetic organization results in repression of comC transcription when SMU.1914 expression is activated by ComE binding to their common intergenic region (Kreth et al., 2007). When SMU.1914 expression was high, a built-in ‘safety mechanism’ prevented the further accumulation of CSP in the culture by repressing comC transcription, which eventually feeds back into the loop to prevent autolysis of the whole population.
We identified the type II bacteriocin mutacin V (CipB) as a major factor in CSP-induced lysis using genome-wide gene expression analysis and mutagenesis. Importantly, we have demonstrated that its activity against self is due to intracellular accumulation in the producing strain. We have presented evidence that CipB acts alone to cause cell death, that the unprocessed form of the bacteriocin is active against an indicator strain and that inactivation of the NlmTE transporter causes cell death. This intracellular mechanism makes sense when examined from an environmental context – a secreted or surface-located ‘death peptide’ would have the potential to wreak havoc in the tightly packed oral biofilm environment. Bacteriocins generally act by creating channels or pores in the cell membrane that destroy the membrane potential and cause cell death by cellular energy depletion (Nes et al., 2007). CipB likely causes cell death initially via a similar mechanism and cell lysis secondarily by activating murein hydrolases and/or lytic enzymes (Galvez et al., 1990). Alternatively, intracellular accumulation of CipB may cause cell lysis in a manner analogous to the holin/antiholin system characterized in Staphylococcus aureus, in which a pore-forming peptide (the holin) inserts into the cytoplasmic membrane to allow degradative enzymes to access the cell wall (Rice et al., 2003). The antimicrobial activity of CipB is directed mainly against non-streptococcal species but not against traditional mutacin-target organisms like Streptococcus gordonii and S. salivarius (Hale et al., 2005a). Given our data showing that CipB acts intracellularly, we suggest that the main function of this peptide is CSP-mediated autolysis but not as an exported bacteriocin. It is possible that the export of CipB outside S. mutans by NlmTE transporter is part of a detoxification mechanism and that the few bacterial species that are susceptible to it are bystanders rather than primary targets. Data showing a significant growth defect in S. mutans NlmTE-deficient cells compared with the parental strain support this argument. We also show the potential for a similar auto-active bacteriocin in members of the salivarius group of streptococci.
Unexpectedly, inactivation of SMU.1913 excluded its involvement in CSP-mediated autolysis. Instead, the product of the cipI gene encodes the immunity factor involved in S. mutans autolysis. Although it is unexpected to find genes paired in function unlinked on the genome, the need for redundant controls over this autolytic pathway may have necessitated this duplication. Our view is supported by evidence showing that, in addition to ComE regulation at high CSP concentrations, an additional (and currently unknown) regulatory pathway triggered activation of cipI expression at low cell density. This safety mechanism would be impossible if the immunity protein was cotranscribed with the bacteriocin.
We hypothesized that competence and lysis would allow the exchange of fitness-enhancing DNA under stress. However, when we monitored the induction of cell death and competence on a cell-by-cell basis using a transcriptional GFP reporter gene fusion, we found that the same population became competent and lysed. This finding was not entirely surprising for both competence and lysis are triggered by the CSP-ComDE circuit. Instead, we showed that transformation can occur in a CSP-independent manner in spectinomycin stress, providing an alternative pathway for the acquisition of fitness-enhancing genes. The lack of transformation in the ΔcipB mutant is somewhat paradoxical, given that these cells are the survivors in a CSP-induced population and would be the expected recipients of transforming DNA. However, the contrary result with ΔcipI implies that cell death in the CSP-responsive members of a population may trigger genetic competence in the non-CSP-responsive survivors, and it also suggests that cellular factors released via lysis could provide secondary signals to induce competence via a CSP-ComDE-independent pathway. The ability to sense cell lysis would permit naturally competent bacteria to turn on their uptake machinery when DNA is available in their environment. We are currently exploring this fascinating possibility.
Experimental procedures
Culture conditions
The S. mutans strains used in this study are shown in Table S3. Mutants were constructed in S. mutans UA159 wild-type as previously described (Lau et al., 2002). Strains were grown in Todd-Hewitt–Yeast Extract (THYE) broth at 37°C with 5% CO2. Growth was monitored using a microbiology workstation (Bioscreen C Labsystems). Coculture experiments were conducted by adding equal volumes of each strain, and colony-forming units (CFUs) were enumerated by plating. Viability staining was performed using the LIVE/DEAD BacLight kit (Invitrogen) according to the manufacturer's directions. Lysis was assessed by harvesting the supernatant of cultures expressing a GUS reporter gene cloned into a theta-replicating plasmid (Biswas et al., 2008) in the absence and presence of 2 μM sCSP. Supernatants were combined in equal parts with 2× GUS buffer [100 mM Na2HPO4, 20 mM β-mercaptoethanol, 2 mM EDTA, 0.2% Triton X -100, 1 mM PNPG substrate (Sigma)]. Absorbance at 420 nm was measured after 15 min of colour development. GUS activity was expressed as (1000 × A420)/[time (min) × OD600] in Miller units.
Gene expression analysis
Transcriptional analysis of comC in environmentally stress-induced UA159 cells was conducted by real-time RT-PCR. Cells were grown in THYE until an OD600 of ~0.4 (mid-log phase) was reached, and an aliquot was reserved (prestress control). Cells were then resuspended in fresh THYE and exposed for 30 min at 37°C to the following stresses: acid shock (THYE acidified to pH 5.0 by the addition of HCl), amino acid starvation (100 μg ml−1 serine hydroxamate), DNA damage (50 ng ml−1 mitomycin C) and inhibition of RNA synthesis (erythromycin and spectinomycin at sub-MIC of 0.5 μg ml−1 and 50 μg ml−1 respectively). UA159 cells were processed with the Bio101 Fast Prep System (Qbiogen), and total RNA was extracted using Trizol reagent (Invitrogen). DNA-free RNA samples were subjected to reverse transcription using the First-Strand cDNA Synthesis Kit (MBI Fermentas). Real-time RT-PCRs were carried out using the QuantiTech SYBR Green PCR master mix (Qiagen) in an MX3000P System (Stratagene). A standard curve was plotted with cycle threshold (Ct) values obtained from amplification of known quantities of cDNAs. The standard curve was used to determine the efficiency (E) of comC primer set binding and amplification: E = 10−1/slope. Comparison of the expression of comC gene between its control and stress was determined using the formula: Ratio = (EcomC)ΔCt(control – stress)/(E16SrRNA)ΔCt(control – stress). The 16SrRNA gene was used as internal reference as we found the expression of this gene to be stable under the test conditions. All assays were performed in triplicate with RNA isolated from three independent experiments and using a P < 0.01.
DNA microarrays
Streptococcus mutans UA159 and ΔcomX cells were grown with 2 μM sCSP or without (uninduced control) to mid-log phase. Total RNA was extracted as described above. The cDNAs were prepared for hybridization using the PFGRC protocol (http://pfgrc.jcvi.org/index.php/microarray/protocols.html). Microarray chips were scanned using a Gene Pix 4000B (Axon) and analysed using the TM4 Microarray Software Suite (http://www.tm4.org/). Transcript levels were measured by cDNA hybridized to a fourfold redundant S. mutans microarray and averaged for three replicated hybridizations. Differential gene expression was based on a post-normalization cut-off of ±> twofold. Significance was determined using a one-class t-test.
Recombinant peptides
Recombinant CipB fusion peptides (precursor and mature form) were generated using the T7 expression vector pET28a(+) (Novagen). The full-length coding region of CipB (His6-fullCipB) and its mature form (His6-GGCipB) were PCR amplified using forward and reverse primers containing a NheI and XhoI restriction site respectively. The amplicons were cloned in frame downstream from the hexa-His sequence in the T7 expression vector pET28a(+) precut by the same enzymes and transformed into Escherichia coli BL21 (non-expression host) competent cells. The nucleotide sequences of the inserts were confirmed by DNA sequencing. Recombinant plasmids were then transformed into E. coli BL21(DE3) competent cells. E. coli transformant cells were incubated aerobically at 37°C in 100 ml LB-kanamycin 30 μg ml−1 supplemented with 1% glucose until the culture reached an OD600 of 0.6. IPTG was then added at a final concentration of 1 mM to induce expression of recombinant fusion proteins, and the incubation was continued for a further 3 h at 37°C. The cells were collected by centrifugation and resuspended in 1× binding buffer (Novagen) and disrupted on ice by sonication. The soluble fractions of the disrupted cells were recovered by centrifugation, and the hexa-His-tagged recombinant CipB proteins, His6-fullCipB and His6-GGCipB, were then purified by affinity chromatography on Ni2+-nitrilotriacetic acid resin (Novagen) as previously described (Levesque et al., 2004). A total cell protein extract was prepared from IPTG-induced vectorless E. coli BL21(DE3) and used as a negative control.
Bacteriocin overlay assays
Twenty microlitres of S. mutans cultures were spotted onto THYE agar plates directly from overnight cultures (control) or after growth to mid-log phase in the presence of 2 μM sCSP. Spots were allowed to dry and were then overlaid with a 1/100 dilution of the indicator å strain L. lactis I6 suspended in 3 ml of THYE top agar. Plates were allowed to set and were incubated overnight before analysis.
Transformation assays
S. mutans cells were exposed to stress at mid-log phase as described for gene expression analysis. To test the role of cell lysis in competence, cells were grown to OD600 of ~0.1 and divided into three aliquots: (i) no sCSP, (ii) 0.2 μM sCSP and (iii) 2 μM sCSP. Ten micrograms of streptococcal genomic DNA containing a spectinomycin resistance marker was added to the cultures, which were grown for a further 2.5 h before differential plating.
Supplementary Material
Acknowledgements
We thank John Tagg and Nicholas Heng for providing mutants related to mutacin V transport, Indranil Biswas for providing shuttle expression plasmids for S. mutans and Elena Voronejskaia for assisting with the mutant constructions. This work was supported by CIHR-Priority Announcement IMHA Grant FRN-90114 (to C. M. L) and by NIDCR Grant R01 DE013230-08 (to D. G. C.). DNA microarrays were supported through NIDCR via NIAID contract number N01-AI15447 to JCVI. J. A. P. is the recipient of a CIHR Strategic Training Fellowship in Cell Signaling in Mucosal Inflammation and Pain.
Footnotes
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abee T. Pore-forming bacteriocins of Gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol Lett. 1995;129:1–9. doi: 10.1016/0378-1097(95)00137-T. [DOI] [PubMed] [Google Scholar]
- Ahn SJ, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan E, Hussain HA, Crawford KR, Miah S, Ascott ZK, Khwaja MH, Hosie AHF. Genetic variation in comC, the gene encoding competence-stimulating peptide (CSP) in Streptococcus mutans. FEMS Microbiol Lett. 2007;268:47–51. doi: 10.1111/j.1574-6968.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- Anderssen EL, Diep DB, Nes IF, Eijsink VG, Nissen-Meyer J. Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin A. Appl Environ Microbiol. 1998;64:2269–2272. doi: 10.1128/aem.64.6.2269-2272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspiras MB, Ellen RP, Cvitkovitch DG. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol Lett. 2004;238:167–174. doi: 10.1016/j.femsle.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Biswas I, Jha JK, Fromm N. Shuttle expression plasmids for genetic studies in Streptococcus mutants. Microbiology. 2008;154:2275–2282. doi: 10.1099/mic.0.2008/019265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Tenaillon O, Gerard B, Souza V, Denamur E, Radman M, et al. Stress-induced mutagenesis in bacteria. Science. 2003;300:1404–1409. doi: 10.1126/science.1082240. [DOI] [PubMed] [Google Scholar]
- Chastanet A, Prudhomme M, Claverys JP, Msadek T. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J Bacteriol. 2001;183:7295–7307. doi: 10.1128/JB.183.24.7295-7307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J-P, Prudhomme M, Mortier-Barriere I, Martin B. Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol Microbiol. 2000;35:251–259. doi: 10.1046/j.1365-2958.2000.01718.x. [DOI] [PubMed] [Google Scholar]
- Claverys J-P, Prudhomme M, Martin B. Induction of competence regulons as a general response to stress in Gram-positive bacteria. Annu Rev Microbiol. 2006;60:451–475. doi: 10.1146/annurev.micro.60.080805.142139. [DOI] [PubMed] [Google Scholar]
- Dagkessamanskaia A, Moscoso M, Henard V, Guiral S, Overweg K, Reuter M, et al. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol Microbiol. 2004;51:1071–1086. doi: 10.1111/j.1365-2958.2003.03892.x. [DOI] [PubMed] [Google Scholar]
- Davidson CJ, Surette MG. Individuality in Bacteria. Annu Rev Genet. 2008;42:253–268. doi: 10.1146/annurev.genet.42.110807.091601. [DOI] [PubMed] [Google Scholar]
- Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. Common mechanisms of target cell recognition and immunity for class II bacteriocins. PNAS. 2007;104:2384–2389. doi: 10.1073/pnas.0608775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- Fontaine L, Boutry C, Guedon E, Guillot A, Ibrahim M, Grossiord B, Hols P. Quorum-sensing regulation of the production of Blp bacteriocins in Streptococcus thermophilus. J Bacteriol. 2007;189:7195–7205. doi: 10.1128/JB.00966-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A, Valdivia E, Martinez-Bueno M, Maqueda M. Induction of autolysis in Enterococcus faecalis S-47 by peptide AS-48. J Appl Bacteriol. 1990;69:406–413. doi: 10.1111/j.1365-2672.1990.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Guiral S, Mitchell TJ, Martin B, Claverys JP. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc Natl Acad Sci U S A. 2005;102:8710–8715. doi: 10.1073/pnas.0500879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JD, Ting YT, Jack RW, Tagg JR, Heng NC. Bacteriocin (mutacin) production by Streptococcus mutans genome sequence reference strain UA159: elucidation of the antimicrobial repertoire by genetic dissection. Appl Environ Microbiol. 2005a;71:7613–7617. doi: 10.1128/AEM.71.11.7613-7617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JDF, Heng NCK, Jack RW, Tagg JR. Identification of nlmTE, the locus encoding the ABC transport system required for export of Nonlantibiotic Mutacins in Streptococcus mutans. J Bacteriol. 2005b;187:5036–5039. doi: 10.1128/JB.187.14.5036-5039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håvarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håvarstein LS, Gaustad P, Nes IF, Morrison DA. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- Håvarstein LS, Martin B, Johnsborg O, Granadel C, Claverys JP. New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol Microbiol. 2006;59:1297–1307. doi: 10.1111/j.1365-2958.2005.05021.x. [DOI] [PubMed] [Google Scholar]
- Keller L, Surette MG. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol Microbiol. 2005;57:392–404. doi: 10.1111/j.1365-2958.2005.04695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Zhu L, Shi W, Qi F. Cell density- and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol Lett. 2006a;265:11–17. doi: 10.1111/j.1574-6968.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Zhu L, Shi W, Qi F. Cell density- and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol Lett. 2006b;265:11–17. doi: 10.1111/j.1574-6968.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Kreth J, Hung DCI, Merritt J, Perry J, Zhu L, Goodman SD, et al. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology. 2007;153:1799–1807. doi: 10.1099/mic.0.2007/005975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods. 2002;49:193–205. doi: 10.1016/s0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- Leblanc DJ, Lee LN, Abu-Al-Jaibat A. Molecular, genetic, and functional analysis of the basics replicon of PVA380-1, a plasmid of oral Streptococcal origin. Plasmid. 1992;28:130–145. doi: 10.1016/0147-619x(92)90044-b. [DOI] [PubMed] [Google Scholar]
- Levesque C, Vadeboncoeur C, Frenette M. The csp operon of Streptococcus salivarius encodes two predicted cell-surface proteins, one of which, CspB, is associated with the fimbriae. Microbiology. 2004;150:189–198. doi: 10.1099/mic.0.26592-0. [DOI] [PubMed] [Google Scholar]
- Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001a;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Hanna MN, Svensater G, Ellen RP, Cvitkovitch DG. Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J Bacteriol. 2001b;183:6875–6884. doi: 10.1128/JB.183.23.6875-6884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Lau PC, Tang N, Svensater G, Ellen RP, Cvitkovitch DG. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J Bacteriol. 2002;184:6333–6342. doi: 10.1128/JB.184.22.6333-6342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RE, Ferretti JJ. The multiple-sugar metabolism (msm) gene cluster of Streptococcus mutans is transcribed as a single operon. FEMS Microbiol Lett. 1996;140:261–264. doi: 10.1016/0378-1097(96)00191-7. [DOI] [PubMed] [Google Scholar]
- Martin B, Quentin Y, Fichant G, Claverys J-P. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 2006;14:339–345. doi: 10.1016/j.tim.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Mitchell TJ. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol. 2003;1:219–230. doi: 10.1038/nrmicro771. [DOI] [PubMed] [Google Scholar]
- Nes IF, Diep DB, Holo H. Bacteriocin diversity in Streptococcus and Enterococcus. J Bacteriol. 2007;189:1189–1198. doi: 10.1128/JB.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JA, Levesque CM, Suntharaligam P, Mair RW, Bu M, Cline RT, et al. Involvement of Streptococcus mutans regulator RR11 in oxidative stress response during biofilm growth and in the development of genetic competence. Lett Appl Microbiol. 2008;47:439–444. doi: 10.1111/j.1472-765X.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SN, Sung CK, Cline R, Desai BV, Snesrud EC, Luo P, et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol. 2004;51:1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- van der Ploeg JR. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J Bacteriol. 2005;187:3980–3989. doi: 10.1128/JB.187.12.3980-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys J-P. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science. 2006;313:89–92. doi: 10.1126/science.1127912. [DOI] [PubMed] [Google Scholar]
- Qi F, Merritt J, Lux R, Shi W. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect Immun. 2004;72:4895–4899. doi: 10.1128/IAI.72.8.4895-4899.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Kreth J, Levesque CM, Kay O, Mair RW, Shi W, et al. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol Lett. 2005;251:321–326. doi: 10.1016/j.femsle.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Rice KC, Firek BA, Nelson JB, Yang SJ, Patton TG, Bayles KW. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J Bacteriol. 2003;185:2635–2643. doi: 10.1128/JB.185.8.2635-2643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda C, Garcia JL, Garcia E, Sanchez-Puelles JM, Lopez R. Biological role of the pneumococcal amidase. Cloning of the lytA gene in Streptococcus pneumoniae. Eur J Biochem. 1987;164:621–624. doi: 10.1111/j.1432-1033.1987.tb11172.x. [DOI] [PubMed] [Google Scholar]
- de Saizieu A, Gardes C, Flint N, Wagner C, Kamber M, Mitchell TJ, et al. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J Bacteriol. 2000;182:4696–4703. doi: 10.1128/jb.182.17.4696-4703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera MD, Guggenheim B, Spatafora GA, Huang YC, Choi J, Hung DC, et al. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J Bacteriol. 2005;187:4064–4076. doi: 10.1128/JB.187.12.4064-4076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera MD, Lee AW, Hung DC, Spatafora GA, Goodman SD, Cvitkovitch DG. The Streptococcus mutans vicX gene product modulates gtfB/C expression, biofilm formation, genetic competence and oxidative stress tolerance. J Bacteriol. 2006;189:1451–1458. doi: 10.1128/JB.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits WK, Eschevins CC, Susanna KA, Bron S, Kuipers OP, Hamoen LW. Stripping Bacillus: ComK auto-stimulation is responsible for the bistable response in competence development. Mol Microbiol. 2005;56:604–614. doi: 10.1111/j.1365-2958.2005.04488.x. [DOI] [PubMed] [Google Scholar]
- Wen ZT, Suntharaligham P, Cvitkovitch DG, Burne RA. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect Immun. 2005;73:219–225. doi: 10.1128/IAI.73.1.219-225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.