Abstract
Introduced by Henri Kagan more than three decades ago, samarium diiodide (SmI2) has found increasing applications in chemical synthesis. This single-electron reducing agent has been particularly useful in C–C bond formations, including those found in total synthesis endeavors. This Review highlights selected applications of SmI2 in total synthesis, with special emphasis on novel transformations and mechanistic considerations. The examples discussed are both illustrative of the power of this reagent in complex molecule construction and inspirational for the design of synthetic strategies toward such targets, both natural and designed.
Keywords: Domino reactions, Natural products, Reduction, Samarium, Total synthesis
1. Introduction
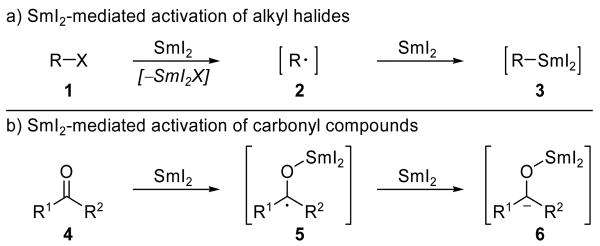
Complex target total synthesis provides a compelling proving ground for promising new reagents and novel applications of existing reagents, some of which eventually become recognized as invaluable tools in the synthetic chemist's arsenal. Those that achieve this level of importance do so because of their useful reactivities, versatility, predictable selectivities, functional group tolerance, and simplicity of handling, as demonstrated by a track record of successful applications in the synthesis of both simple and complex targets. First used in organic chemistry by Kagan and coworkers in 1977,[1] samarium diiodide (SmI2) has since been employed in the development of a wide variety of reactions and featured in hundreds of syntheses.[2] The large reduction potential of SmI2 (up to −2.05 V in the presence of HMPA)[3] allows access to a rich array of reactive intermediates. As shown in Scheme 1a, the reduction of an alkyl halide (1) can generate either radical species 2 (through a single-electron reduction), or organosamarium intermediate 3 (through two successive single-electron reductions). Likewise, a carbonyl moiety (4, Scheme 1b) can be reductively activated to form a reactive ketyl radical (5) which, under appropriate conditions, can be further reduced to provide access to carbanion 6. Access to this diverse group of high energy species allows for a broad range of reactivities. However, these possibilities do not render SmI2 an indiscriminant reducing agent, for its powerful reactivity is highly tunable through careful optimization of reaction conditions. Indeed, the ability to selectively access both radical[4] and ionic reaction manifolds, sometimes in the course of the same reaction,[5] makes SmI2 particularly versatile in organic synthesis. As a result of this potent combination of useful reactivity and tunable selectivity, SmI2 is widely employed and recognized as one of the premier single-electron reducing agents in the synthetic toolbox.
Scheme 1.
Common mechanisms of SmI2-mediated activation of a) alkyl halides and b) carbonyl compounds.
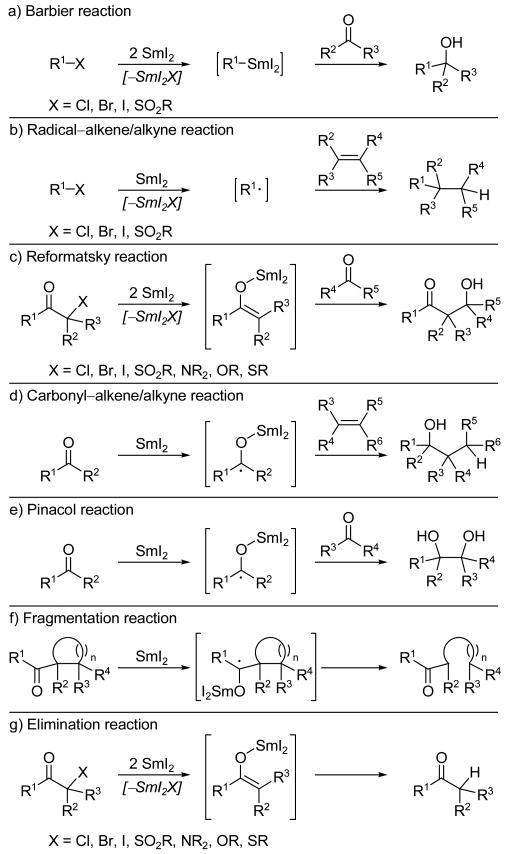
The present Review seeks to highlight the power and versatility of SmI2 by examining selected examples of its elegant application in total synthesis, a topic not fully explored in previous reviews.[2] The featured examples are grouped by the type of SmI2-mediated reaction into the following categories: Barbier, radical–alkene/alkyne, Reformatsky and aldol-type, carbonyl–alkene/alkyne, pinacol-type, fragmentation, and elimination reactions (see Scheme 2). Cascade sequences[6] will be discussed separately. It must be noted that the reaction classes depicted in Scheme 2 in no way cover all of the useful reactions of SmI2; indeed, the deoxygenation of epoxides, cleavage of heterocycles, β- and 1,2-eliminations, and deprotection of nitrogen-containing functionalities are but a few of the many reactions that are not represented in Scheme 2.
Scheme 2.
Some representative SmI2-mediated transformations: a) Barbier, b) radical–alkene/alkyne, c) Reformatsky, d) carbonyl–alkene/alkyne, e) pinacol, f) fragmentation, and g) elimination reactions.
2. Barbier Reaction
The Barbier reaction (Scheme 2a) is a reductive addition of an alkyl halide or, more rarely, alkyl sulfone to a carbonyl group.[7] It is differentiated from a Grignard-type coupling by the presence of the reactive halide and the carbonyl coupling partner in the same reaction mixture. Kagan and coworkers discovered the intermolecular SmI2-mediated Barbier reaction in 1977.[1] In 1986, Molander and colleagues reported the intramolecular version.[8] Unlike other common Barbier variants, which employ metals such as magnesium, lithium, or zinc, the SmI2-mediated variation occurs in a homogeneous reaction mixture and is often associated with superior chemoselectivity. Originally proposed to involve coupling of an alkyl radical and a ketyl radical,[9] the SmI2-mediated Barbier reaction is now believed to proceed in both inter- and intramolecular cases through the intermediacy of an organosamarium species formed through two successive single-electron reductions, as depicted in Scheme 2a.[10] The Barbier reaction is one of the most commonly employed SmI2-mediated reactions, and the intramolecular variant in particular has proven to be popular for the formation of 5- to 8-membered carbocycles.
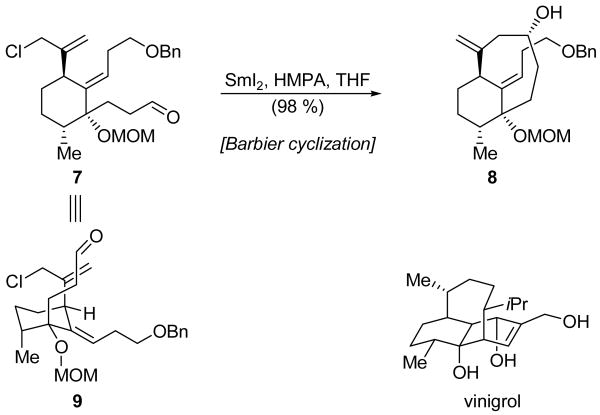
Matsuda and coworkers employed the intramolecular SmI2-mediated Barbier reaction in the construction of the synthetically daunting 8-membered ring within their vinigrol model 8 (Scheme 3).[11] Thus, advanced intermediate 7 smoothly reacted with SmI2 in the presence of HMPA at room temperature to deliver cyclization product 8 in a highly satisfying 98 % yield without any requirement for high dilution or slow addition. The use of HMPA as a means to enhance the reduction potential of SmI2 was critical to the success of this transformation. In its absence, desired product 8 was obtained in only 15 % yield, and the major product was the primary alcohol resulting from direct aldehyde reduction. Though the reaction proceeded best at ambient temperature, smooth ring closure was observed even at −78 °C, with only a modest drop off in yield. This surprisingly facile 8-membered ring closure was attributed to a preference for intermediate 7 to adopt conformation 9, a supposition that was supported by NMR spectroscopic analysis. This conformation places the reacting functionalities in axial positions of a chair cyclohexane system to avoid the significant A1,3 strain that would otherwise exist, and, as a result, preorganizes the reacting groups in close proximity.
Scheme 3.
Formation of the 8-membered ring of vinigrol model 8 by a Barbier cyclization (Matsuda et al., 1997).[11]
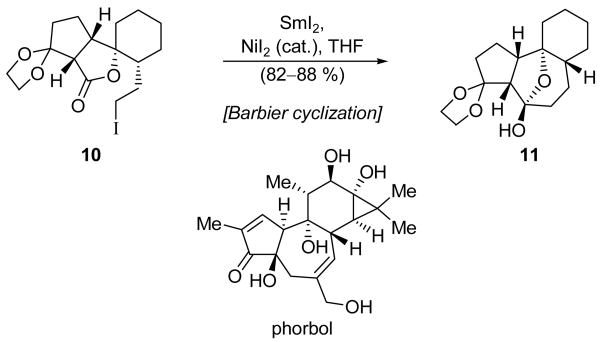
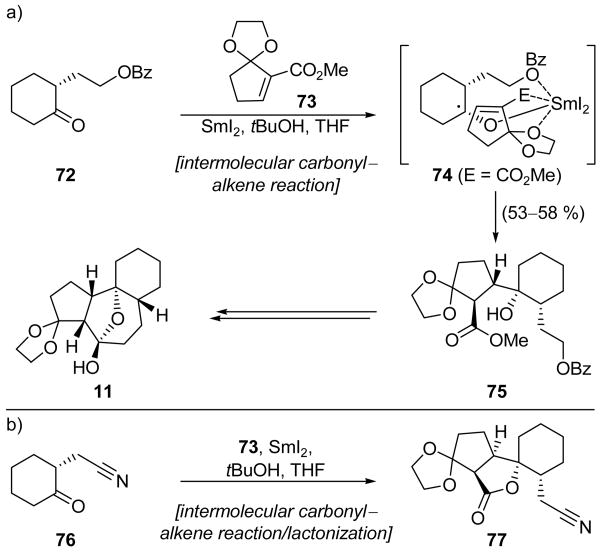
Carroll and Little employed two SmI2-mediated transformations in their rather concise synthesis of phorbol system 11 (Scheme 4).[12] We shall return to the first use of SmI2 later (Scheme 18), but the second application is shown in Scheme 4. Thus, iodide 10 underwent a SmI2-promoted Barbier cyclization to give hemiketal 11. The yield of 11 was initially only 43–68 %. However, the addition of catalytic NiI2, a modification to the SmI2-mediated Barbier reaction which was first reported by the Kagan group,[13] resulted in both an enhanced reaction rate and improved efficiency, providing phorbol model 11 in 82–88 % yield. While NiI2 and other transition metal salts have been extensively employed to catalyze SmI2-mediated reactions, the cause of the improvements to both reaction rate and efficiency remains to be determined.
Scheme 4.
Construction of phorbol system 11 by a Barbier cyclization (Carroll and Little, 2000).[12]
Scheme 18.
a) Carbonyl–alkene fragment coupling in the synthesis of phorbol system 11 and b) a stereochemically distinct coupling result (Carroll and Little, 2000).[12]
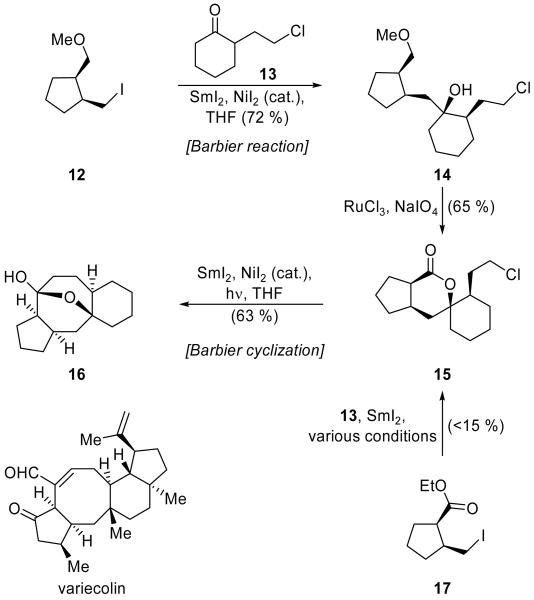
One of the more complex examples of the use of the SmI2-promoted Barbier reaction in total synthesis may be found in the Molander group's synthesis of variecolin model 16 (Scheme 5).[14] The sequence of events includes both inter- and intramolecular Barbier reactions, and leverages the different reactivity of SmI2 toward alkyl iodides and chlorides. The direct Barbier coupling of ester iodide 17 and chloro ketone 13 provided the desired coupling product 15 in <15 % yield under all reaction conditions screened. This is presumably due to intramolecular attack of the ester functionality of 17 by the intermediate organosamarium species generated from the primary iodide and formation of the resulting cyclobutanone intermediate, which can decompose through various pathways. Therefore, methoxy iodide 12 was employed instead, and, pleasantly, the resulting Barbier reaction, promoted by stoichiometric SmI2 and catalytic NiI2,[13] gave tertiary alcohol 14 in 72 % yield. (The product was isolated as a 1:1 mixture of diastereomers since ketone 13 was employed in racemic form.) The primary chloride of 14 was untouched in this reaction, and, therefore, could be carried along in unmasked form. The methyl ether of 14 was then oxidized through the Sharpless procedure (RuCl3, NaIO4) to afford, after spontaneous lactonization, spirocycle 15 in 65 % yield. A photoactivated[15] SmI2-mediated Barbier cyclization then cast the 8-membered ring of 16 in 63 % yield. This example highlights some of the many possible means of tuning both the substrate and the reaction conditions in order to achieve the desired outcome.
Scheme 5.
Synthesis of variecolin model 16 through halide-selective Barbier reactions (Molander et al., 2001).[14]
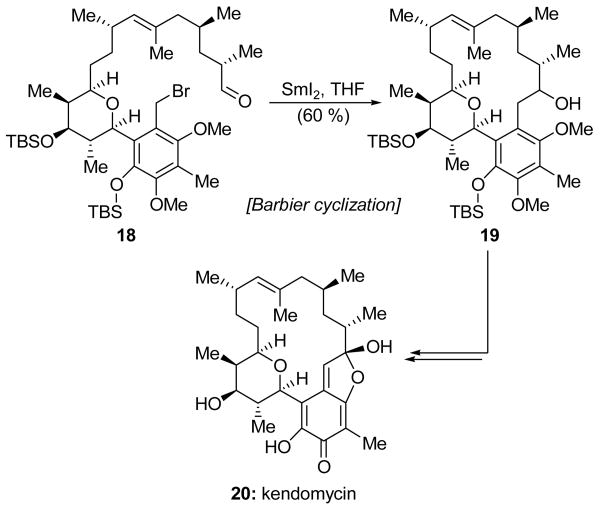
While the SmI2-mediated Barbier cyclization is most commonly used to construct small- and medium-sized rings, Lowe and Panek recently reported a total synthesis of kendomycin (20, Scheme 6) in which this reaction was employed to forge a macrocycle for the first time in natural product synthesis.[16] Treatment of advanced intermediate 18 with a dilute solution of freshly prepared SmI2 at room temperature led smoothly to a macrocyclization, delivering the desired product 19 in 60 % yield. While this intermediate was created as a single stereoisomer, the configuration of the alcohol was inconsequential (and left unassigned) since it was subsequently oxidized to a carbonyl moiety in the course of elaborating 19 into kendomycin (20).
Scheme 6.
Barbier macrocyclization in a total synthesis of kendomycin (20) (Lowe and Panek, 2008).[16]
3. Radical–Alkene/Alkyne Reaction
The SmI2-mediated radical–alkene/alkyne reaction (Scheme 2b) is initiated by a single-electron reduction of a halide or sulfone to generate a radical intermediate which undergoes subsequent addition to an alkene or alkyne. The first example of this transformation was reported in 1981, by Kagan and coworkers during a study on the mechanism of action of SmI2.[9] As with other radical carbon–carbon bond forming processes,[4] the SmI2-mediated variant is best for 5-membered ring construction and can employ activated[17] or unactivated alkenes and alkynes. This is a relatively uncommon use of SmI2, and we shall highlight but one example in this section; however, others may be found as parts of cascade sequences (vide infra). The use of SmI2 offers some advantages over more popular reagents such as nBu3SnH/AIBN, including reduced toxicity and improved ease of separation from reagent byproducts, which may be valuable in the synthesis of certain fine chemicals.
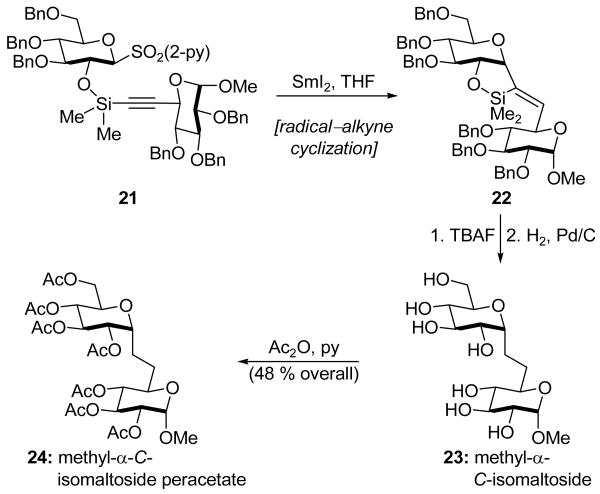
Beau, Skrydstrup, and coworkers reported the use of SmI2 as an alternative to the more commonly employed tin hydrides in the construction of C-glycosides.[18] Following a modification of a procedure developed by the Stork group,[19] a temporary silicon group was used to tether the two glycoside units, as shown in 21 (Scheme 7). Through the course of this project, it was discovered that the use of a 2-pyridinyl sulfone group, instead of the more commonly employed phenyl sulfone moiety, eliminated the need for HMPA. Previously, and in contrast to the above observation, only geminal bis-sulfones were susceptible to SmI2 in the absence of HMPA.[20] Therefore, silicon-tethered intermediate 21, containing a 2-pyridinyl sulfone functionality, was exposed to SmI2 in the absence of HMPA to effect a 5-exo-dig cyclization, giving vinylsilane 22. Cleavage of the temporary silicon tether (TBAF) and hydrogenation (H2, Pd/C) with concomitant hydrogenolysis of the benzyl ethers gave methyl-α-C-isomaltoside (23), which was then masked (Ac2O, py) as the more readily purified peracetylated disaccharide 24 in a pleasing 48 % overall yield from 21. This is the first example of a stereoselective synthesis of a disaccharide through a 5-exo-dig radical cyclization reaction.
Scheme 7.
Application of a radical–alkyne cyclization in the synthesis of methyl-α-C-isomaltoside (23) and its peracetate (24) (Beau, Skrydstrup et al., 1994).[18]
4. Reformatsky and Aldol-Type Reactions
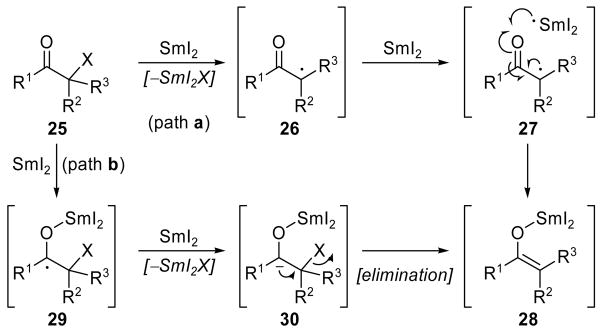
First demonstrated by Kagan and coworkers in 1977,[1] the SmI2-mediated Reformatsky reaction[21] proceeds through initial reductive cleavage of a heteroatom-containing substituent vicinal to a carbonyl to form a SmIII enolate, which then attacks a carbonyl functionality in an aldol fashion (Scheme 2c). Depending on the identity of the initial functionality, two possible mechanisms for the formation of the SmIII enolate intermediate may be envisioned.[22] If the starting material (25, Scheme 8) possesses a moiety in the α position to the carbonyl or carboxyl functionality that is amenable to a direct reductive cleavage, such as a halide or sulfone, SmI2 may induce a single-electron reduction to form stabilized radical 26 (path a, Scheme 8). A subsequent reduction (see 27) then provides SmIII enolate 28, containing an oxygen–samarium bond. Alternatively, if the vicinal group is not reductively labile, the reaction will proceed through initial formation of ketyl radical 29 (path b, Scheme 8), which then undergoes a second reduction to form carbanion 30. Spontaneous elimination of the vicinal group then generates SmIII enolate 28. The intermolecular reaction is rarely employed because of the presence of many possible side reactions. In contrast, the intramolecular variant generally enjoys high yields and stereoselectivities, presumably due to chelation of the SmIII ion with the two reacting functionalities. Though not as popular as traditional aldol reactions, the SmI2-mediated Reformatsky reaction has been featured prominently in several total syntheses, including instances where the zinc-promoted variant failed to provide the desired product.
Scheme 8.
Two possible mechanisms of SmIII enolate formation.
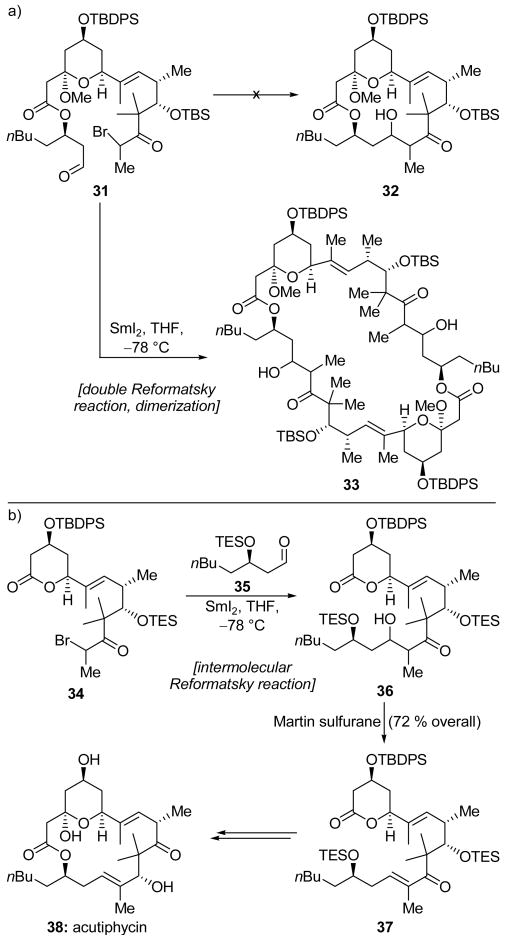
In Moslin and Jamison's original strategy towards acutiphycin (38, Scheme 9b),[23] a late-stage intramolecular Reformatsky reaction was envisioned in order to close the macrocyclic ring of the molecule. However, and as depicted in Scheme 9a, they discovered that slow addition of α-bromoketone 31 to a dilute solution of SmI2 yielded not the expected monomeric macrocycle 32, but the dimeric product 33. Though intramolecular Reformatsky reactions to form medium and large carbocyclic rings were reported to be favored over dimerizations,[24] in this case, dimerization evidently prevailed because of various steric interactions, notably at the geminal dimethyl group, conspiring to reduce the rate of the competing cyclization. In their revised strategy, shown in Scheme 9b, enone 37 was targeted. However, common methods of coupling fragments 34 and 35 (or their derivatives) were unsuccessful. For example, a base-promoted aldol reaction favored reaction at the lactone ring system of 34, and attempts at a Mukaiyama aldol, Horner–Wadsworth–Emmons olefination, or zinc-promoted Reformatsky reaction yielded only unreacted starting materials. Inspired by their earlier undesired dimerization (Scheme 9a), Moslin and Jamison proposed a bold intermolecular SmI2-mediated Reformatsky reaction in order to couple their fragments. Gratifyingly, α-bromoketone 34 and aldehyde 35 were smoothly joined through the action of SmI2 at −78 °C to give the expected product 36, dehydration (Martin sulfurane)[25] of which afforded the target enone 37 in 72 % overall yield for the two steps. The latter compound was successfully elaborated to synthetic acutiphycin (38).
Scheme 9.
a) An unexpected intermolecular Reformatsky reaction/dimerization and b) an intermolecular Reformatsky reaction used in the total synthesis of acutiphycin (38) (Moslin and Jamison, 2006).[23]
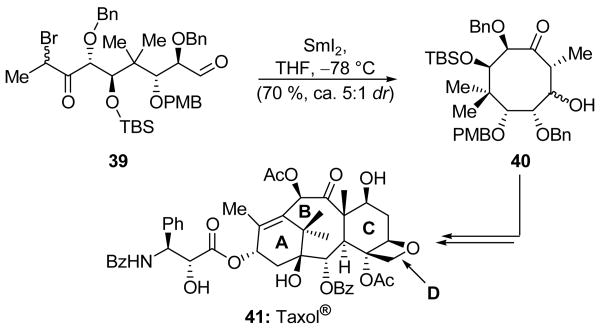
Another interesting application of the Reformatsky reaction may be found in the Mukaiyama group's total synthesis of Taxol® (41, Scheme 10).[26] The team first targeted the formation of the highly congested B ring precursor 39 as an inconsequential mixture of bromide epimers. An intramolecular SmI2-promoted Reformatsky reaction then delivered the highly substituted cyclooctane system 40 in 70 % yield, and as a ca. 5:1 ratio of inconsequential epimers at the newly-formed secondary hydroxyl group. The high efficiency of this process is impressive in light of the heavily functionalized nature of the product and the general difficulty of synthesizing 8-membered carbocyclic rings. Compound 40 was a critical building block for the Mukaiyama total synthesis of Taxol® (41).
Scheme 10.
An intramolecular Reformatsky reaction to form B ring system 40 during the total synthesis of Taxol® (41) (Mukaiyama et al., 1997).[26]
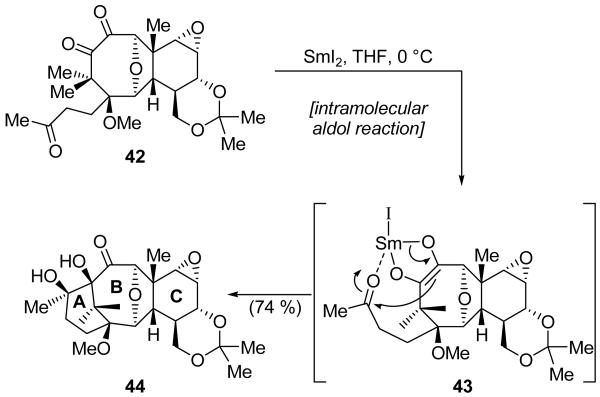
The Arseniyadis group also employed a SmI2-mediated aldol-type reaction in their synthesis of Taxol® ABC ring model system 44 (Scheme 11).[27] Thus, exposure of α-diketone 42 to SmI2 led to rapid formation of SmIII enediolate 43,[28] which underwent an intramolecular aldol reaction to give diol 44 in 74 % yield. The observed stereochemistry is proposed to be the result of chelation of the methyl ketone of 43 to the samarium counterion.
Scheme 11.
Formation of the A ring of Taxol® ABC model system 44 through an aldol-type reaction (Arseniyadis et al., 2005).[27]
5. Carbonyl–Alkene/Alkyne and Related Reactions
The carbonyl–alkene/alkyne reaction arguably is the most important SmI2-mediated reaction in total synthesis. As shown in Scheme 2d, the carbonyl moiety is initially reduced to generate a ketyl radical, which then attacks an unsaturated system. The ketyl–olefin coupling was first described by Molander and coworkers,[29] and variations of this reaction have been explored in many laboratories. As with the radical–alkene/alkyne reaction, the carbonyl–alkene/alkyne reaction may be performed with both activated[30] and unactivated alkenes and alkynes. When the alkene/alkyne partner is part of an α,β-unsaturated carbonyl moiety, alternative reaction pathways, such as reductive enolate formation from the α,β-unsaturated system and subsequent aldol coupling, may be operative.[31] Intramolecular cyclizations to cast 4- to 8-membered rings are the most common, but both inter- and intramolecular variants are routinely employed. We shall highlight some particularly elegant and innovative reactions of this general class as applied to targets of varying degrees of complexity, but with so many examples from which to choose, our survey is necessarily of rather limited scope.
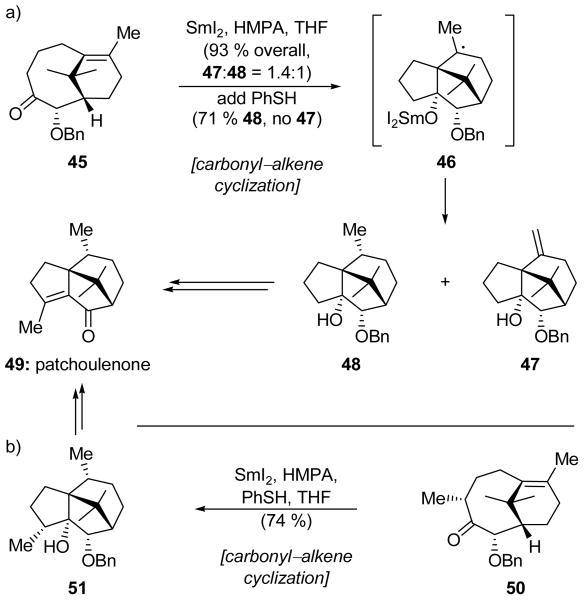
Banwell and coworkers commenced the construction of patchoulenone (49, Scheme 12a)[32] with an anion-accelerated oxy-Cope rearrangement to give bridging bicycle 45. The latter compound was a substrate for an acid-catalyzed Prins reaction which delivered the tricyclic skeleton of patchoulenone in high yield. Though this sequence was rather efficient, a challenging hydrogenation required for the completion of the synthesis prompted an evaluation of alternative strategies. In particular, they explored a SmI2-mediated carbonyl–alkene reaction (a reductive process) as a potential replacement for the Prins reaction (a redox neutral process) in hope that it might circumvent the problematic hydrogenation. Initially, this route was rather disappointing; although the desired tricyclic core, found in both products 47 and 48, was formed in 93 % overall yield upon exposure to SmI2 and HMPA, the reaction gave a 1.4:1 ratio of alkene product 47 (which requires the problematic hydrogenation step) and the desired reduction product 48. Apparently, ketyl radical formation and subsequent ring closure generated tertiary radical 46, which, under the reaction conditions, proceeded to disproportionate and give both alkene 47 and the desired product 48 in comparable amounts. With this mechanistic rationale in mind, PhSH was added as a hydrogen radical donor, and, much to their delight, the Banwell team found that this operationally simple modification resulted in exclusive formation of the reduced product 48 in 71 % yield.
Scheme 12.
Carbonyl–alkene cyclizations in the a) first- and b) second-generation total syntheses of patchoulenone (49) (Banwell et al., 1998).[32]
Though the desired product 48 was successfully converted into patchoulenone (49), a further refinement allowed a simpler end game. Thus, as shown in Scheme 12b, bicyclic intermediate 50, differing from 45 in the presence of an additional methyl group, underwent the same carbonyl–alkene reaction, promoted by SmI2 and PhSH, to give the desired product 51 in 74 % yield. Only benzyl ether cleavage, oxidation, and dehydration were required for the completion of this second generation synthesis of patchoulenone (49).
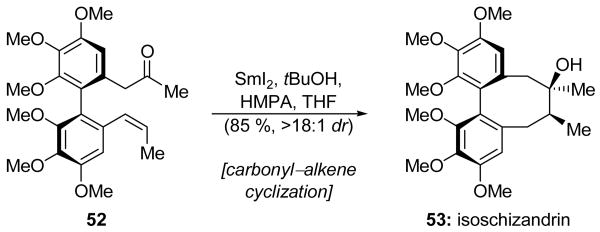
The Molander group employed an 8-endo-trig carbonyl–alkene cyclization for the final step of their isoschizandrin (53, Scheme 13) total synthesis.[33] The final intermediate, namely optically active biaryl compound 52, was exposed to SmI2 in the presence of tBuOH and HMPA to provide isoschizandrin (53) in 85 % yield and as a >18:1 mixure of diastereomers. The presence of the biaryl moiety is proposed to facilitate this ring closure by both lowering the SOMO/LUMO energy gap and reducing the entropic cost of ring closure by preorganizing at least four of the carbon atoms in the required conformation. The remarkable stereocontrol observed in this reaction is attributable to three factors. Dibenzocyclooctadiene systems normally exist in either the twist-boat-chair, or twist-boat conformations, and the presence of the Z-olefin prevents the adoption of a twist-boat-like conformation in the precursor, thus effectively prescribing the relative stereochemistry of the methine stereocenter. The steric demand of the bound HMPA ligands forces the SmIII ion to adopt a pseudoequatorial position and, therefore, enforces the relative stereochemistry at the newly-formed quaternary center. Finally, the preset absolute configuration of the biaryl ring system forces the ketone to approach from above the alkene, thus establishing the absolute sense of stereochemistry observed in isoschizandrin (53).
Scheme 13.
A carbonyl–alkene cyclization to complete the total synthesis of isoschizandrin (53) (Molander et al., 2003).[33]
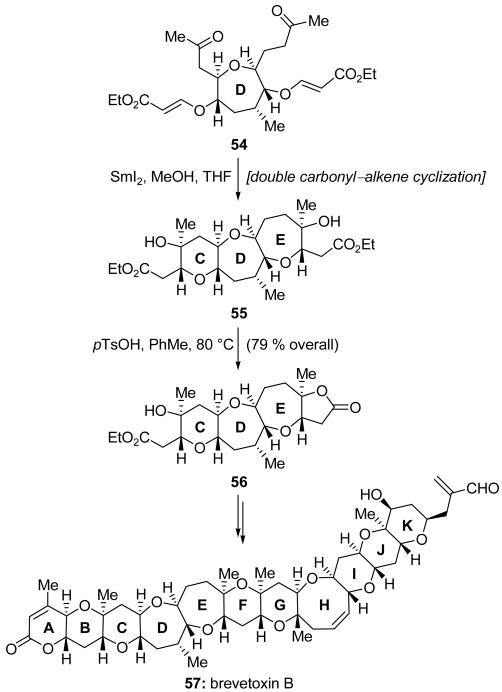
Nakata and coworkers reported in 1999[34] the use of a SmI2-mediated carbonyl–α,β-unsaturated ester cyclization reaction for a high-yielding and stereoselective formation of trans-fused polytetrahydropyran ring systems, a structural motif that is common in polyether marine natural products. This useful methodology was soon expanded to the synthesis of trans-fused cyclic ethers of other sizes[35] and has been applied by both the Nakata group and others toward the total synthesis of many natural products, including the marine polyether compounds gambierol,[36] brevetoxin B (57, Scheme 14),[37] and brevenal.[38] This SmI2-mediated cyclic ether formation was employed for the formation of four rings in the Nakata synthesis of brevetoxin B (57).[37] One example of the application of this cyclization reaction is shown in Scheme 14. Thus, D ring fragment 54 was reacted with SmI2 in the presence of MeOH to effect concomitant formation of both 6- and 7-membered cyclic ether rings (the C and E rings, respectively) and give tricyclic intermediate 55 in high yield and with complete stereocontrol. Though the nearly identical nature of the functionalities attached to the C and E rings might have been expected to pose a problem of chemoselectivity, exposure to pTsOH resulted in selective lactonization, giving tetracycle 56 in a pleasing 79 % overall yield for the two-step process. The latter compound was an important building block for the Nakata synthesis of brevetoxin B (57).
Scheme 14.
A double carbonyl–alkene cyclization in the total synthesis of brevetoxin B (57) (Nakata et al., 2004).[37]
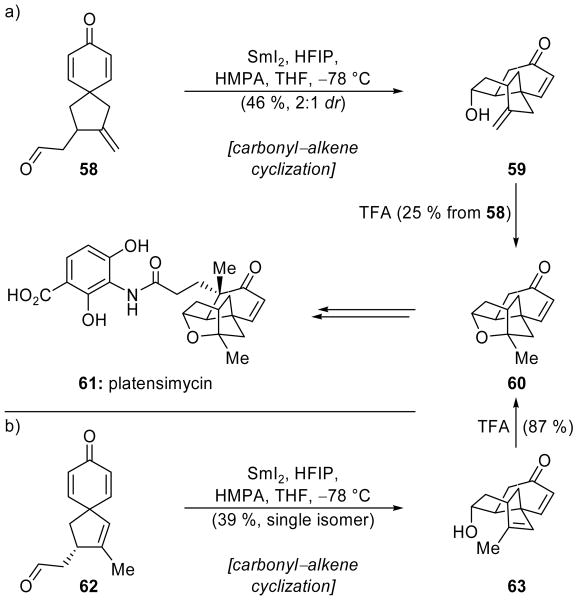
An intramolecular cyclization of an aldehyde onto an enone was employed in the first synthesis of (±)-platensimycin [(±)-61, Scheme 15a], disclosed by the Nicolaou group in 2006.[39] Spirocyclic aldehyde 58 was reacted with SmI2 in the presence of hexafluoroisopropanol (HFIP) and HMPA to deliver tricyclic product 59 in 46 % yield, and as a 2:1 mixture of epimers at the newly-formed hydroxyl moiety. HFIP is not commonly employed in SmI2-mediated reactions, but it was essential in this reaction in order to obtain an acceptable yield of product 59. This is believed to be due to its enhanced acidity (pKa = 9.3) as compared with more commonly used proton sources (e.g. MeOH or tBuOH) and the resulting enhanced activation of the dienone system of 58. Exposure of tricycle 59 to TFA resulted in intramolecular etherification, completing the construction of the platensimycin core (60) in 25 % overall yield from 58. A short series of manipulations then provided (±)-platensimycin [(±)-61].
Scheme 15.
Carbonyl–alkene cyclizations in a) racemic and b) enantioselective total syntheses of platensimycin (61) (Nicolaou et al., 2006, 2007).[39,40]
Interestingly, in one of the two asymmetric routes published shortly afterward by the Nicolaou team,[40] a similar SmI2-mediated ring closure of dienone 62 (Scheme 15b), differing from 58 only in the transposition of one olefin, proceeded in 39 % yield to deliver the desired product 63, now as a single stereoisomer. As before, TFA-promoted ring closure gave 60, and, thereby, platensimycin (61), but now in optically active form. This change in stereoselectivity, presumably caused by subtle differences in the conformations of the substrates 58 and 62, points to the potentially fickle nature of the stereocontrol in these reactions and the need for thorough experimentation, in order to uncover the optimal substrate and reaction conditions.
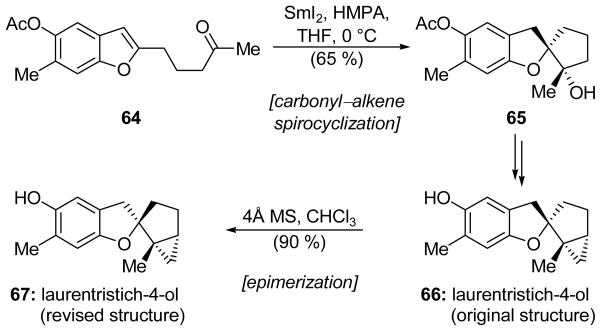
Li and colleagues developed a method for the stereoselective formation of 1-oxaspiro[4.4]nonanes that they employed in their total synthesis of laurentristich-4-ol (67, Scheme 16).[41] Thus, benzofuran system 64 was exposed to SmI2 and HMPA to afford spirocycle 65 in 65 % yield and as a single stereoisomer. Cyclopropanation and acetate cleavage yielded compound 66, possessing the originally proposed structure of laurentristich-4-ol. However, the 1H NMR spectrum of this substance did not match the published data and, furthermore, the compound was observed to undergo a slow isomerization in chloroform. This isomerization gave the epimeric substance 67, which was found to be identical to natural laurentristich-4-ol. This epimerization process presumably proceeded by way of a transient benzylic carbocation, and could be accelerated by the addition of 4Å molecular sieves.
Scheme 16.
Application of a carbonyl–alkene cyclization to a total synthesis and structural revision of laurentristich-4-ol (67) (Li et al., 2008).[41]
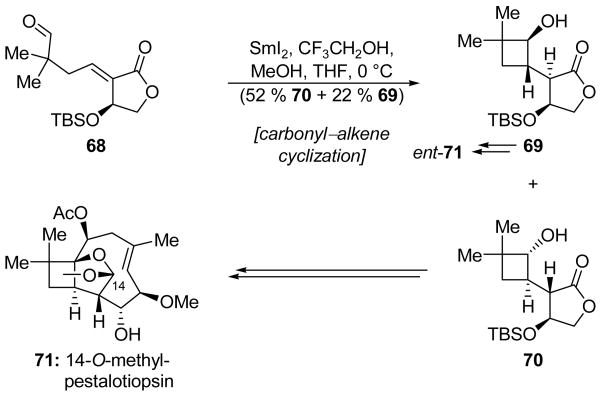
Procter and coworkers employed a carbonyl–alkene cyclization in their total synthesis of both enantiomers of 14-O-methyl pestalotiopsin A (71, Scheme 17).[42] Thus, aldehyde 68 underwent a SmI2-mediated cyclization in a THF/MeOH/CF3CH2OH mixture to give cyclobutane system 70 and the stereoisomeric product 69 in 52 % and 22 % yields, respectively. The use of CF3CH2OH as a cosolvent was critical to the success of this reaction, and in its absence only a 25 % yield of 70 was obtained. This effect was attributed to the ability of CF3CH2OH to both moderate the reduction potential of SmI2 and, by virtue of its greater acidity, rapidly quench the enolates corresponding to the products, thus avoiding the formation of elimination byproducts. Since the absolute configuration of natural pestalotiopsin A was not known at the onset of this campaign, the formation of diastereomeric products 69 and 70 was viewed as an opportunity to access both antipodes of the pestalotiopsin structure from one starting material. Indeed, 70 was advanced to 14-O-methyl pestalotiopsin A (71), and 69 to its enantiomer (ent-71). Unfortunately, the methyl mixed acetal at C14, installed subsequent to the aforementioned SmI2-mediated ring closure, has so far proved to be resistant to cleavage.
Scheme 17.
Synthesis of both enantiomers of 14-O-methyl pestalotiopsin (71) through a carbonyl–alkene cyclization (Procter et al., 2001, 2008).[42]
We previously highlighted the final step of Carroll and Little's synthesis of phorbol system 11 (see Scheme 4),[12] but we now return to an earlier stage of this synthesis that employs an intermolecular carbonyl–alkene addition. Thus, as shown in Scheme 18a, cyclohexanone system 72 and α,β-unsaturated ester 73 were coupled through the action of SmI2 to give hydroxyester 75 in 53–58 % yield, and as a single stereoisomer. The exclusive formation of this sterically congested product is proposed to be due to the ability of the SmIII ion, which is bonded to the initially formed ketyl radical species, to chelate with oxygen atoms of the benzoate ester, methyl ester, and ketal ring as shown in putative intermediate 74. It is worth noting that a seemingly innocuous change of the side chain benzoate to a nitrile, as shown in 76 (Scheme 18b), led to formation of 77 possessing a different relative stereochemistry, presumably because of the preference to adopt a less hindered transition state due to a lack of chelation between the SmIII ion and the nitrile functionality. This result was highly desirable since, unlike 75, the resulting product 77 possesses the desired relative stereochemistry, and, therefore, does not require a later-stage epimerization for the construction of phorbol system 11. However, difficulties in manipulating the nitrile moiety ultimately led to the decision to employ the route shown in Scheme 18a.
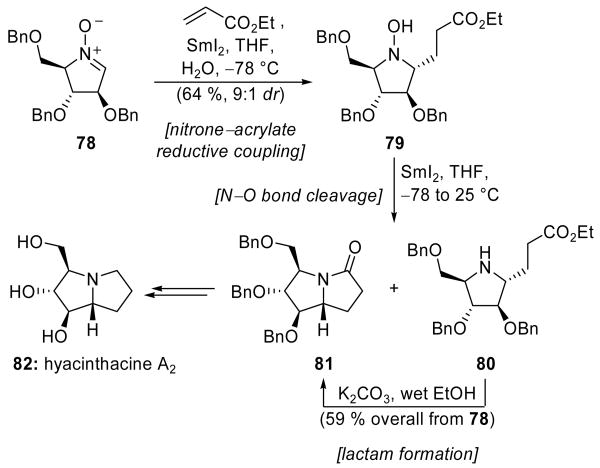
The carbonyl–alkene coupling has been extended to the use of related functionalities, such as nitrones and thioesters. Py and coworkers reported in 2002 an umpolung reaction whereby nitrones attacked carbonyls and α,β-unsaturated esters.[43] The application of this reaction to their total synthesis of hyacinthacine A2 (82)[44] is shown in Scheme 19. Thus, reductive coupling of cyclic nitrone 78 with ethyl acrylate, promoted by SmI2 at −78 °C in the presence of water, gave N-hydroxypyrrolidine 79. This reaction is thought to proceed through initial reduction of the nitrone to generate either a radical, or an organosamarium species, which then undergoes conjugate addition with ethyl acrylate. N-Hydroxypyrrolidine 79 could be isolated in 64 % yield, and as a 9:1 ratio of diastereomers; alternatively, subsequent warming of the reaction mixture in the presence of additional SmI2 led to cleavage of the labile nitrogen–oxygen sigma bond, giving a mixture of reduced product 80 and its lactamized form 81. Exposure of this mixture to K2CO3 in wet ethanol effected clean cyclization of amine 80, delivering lactam 81 in 59 % overall yield from 78. Reduction of the lactam of 81 and benzyl deprotection completed the total synthesis of hyacinthacine A2 (82).
Scheme 19.
SmI2-mediated nitrone–acrylate reductive coupling in the total synthesis of hyacinthacine A2 (82) (Py et al., 2005).[44]
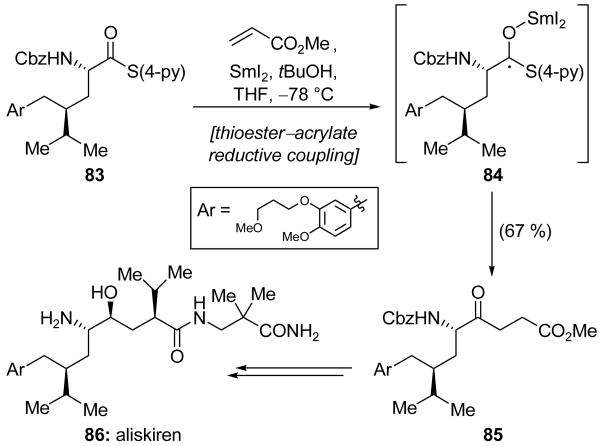
A novel thioester–acrylate reductive coupling was utilized as the key step in Lindsay and Skrydstrup's total synthesis of aliskiren (86, Scheme 20).[45] Thus, addition of SmI2 to a mixture of thioester 83, methyl acrylate, and tBuOH slowly gave, over the course of six days at −78 °C, coupled product 85 in 67 % yield. This remarkable process is equivalent to a conjugate addition of an acyl radical. However, as acyl radicals derived from α-amino acids are prone to rapid decarbonylation (a side reaction not observed in this process),[46] the intermediate in this transformation is proposed to be ketyl radical 84, which undergoes conjugate addition and then eliminates a thiolate anion to yield the observed ketone 85. The latter compound was successfully transformed into aliskiren (86).
Scheme 20.
SmI2-mediated thioester–acrylate reductive coupling in the total synthesis of aliskiren (86) (Lindsay and Skrydstrup, 2006).[45]
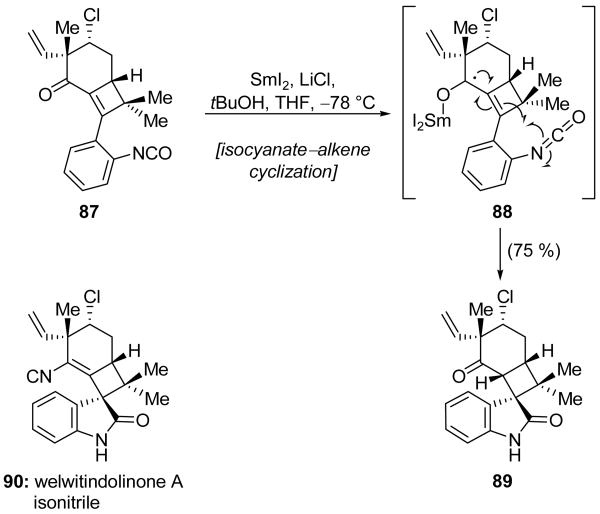
The Wood group reported a novel SmI2-mediated cyclization to construct oxindoles from isocyanates in the course of their studies toward the synthesis of welwitindolinone A isonitrile (90, Scheme 21).[47] Unlike a related intermolecular transformation,[48] this intramolecular variant required the addition of LiCl. This modification increases the reactivity of SmI2 towards carbonyl compounds, possibly through in situ formation of SmCl2 (a compound that is poorly characterized due to its low solubility), coordination of the chloride ion to SmI2, and/or activation of the carbonyl through chelation with the lithium ion.[49] In this manner, tricyclic enone 87 was converted into oxindole system 89 in 75 % yield. On the basis of earlier mechanistic studies, this reaction is believed to proceed by way of delocalized radical 88, which may attack the neighboring isocyanate moiety. Subsequent reduction of the resulting tetracyclic radical generates, after protonation, oxindole 89. Alternatively, radical 88 may first undergo a second reduction step to form a carbanion, which then attacks the isocyanate moiety. In spite of this mechanistic uncertainty, this methodology was utilized in the construction of many advanced intermediates for the synthesis of welwitindolinone A isonitrile (90). However, the synthetic route that ultimately led to the natural product did not employ this chemistry due to an inability to convert the ketone of 89 into the corresponding unsaturated isonitrile found in welwitindolinone A isonitrile (90).[50]
Scheme 21.
Formation of welwitindolinone A isonitrile model 89 through SmI2-mediated isocyanate–alkene coupling (Wood et al., 2004, 2008).[47, 50]
6. Pinacol-Type Reaction
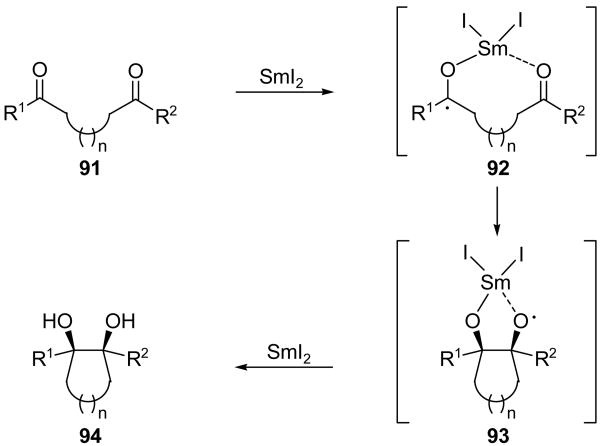
The pinacol reaction (Scheme 2e) is a reductive coupling of two carbonyl-containing functionalities to form a diol or other related species.[51] The SmI2-mediated pinacol reaction was first discovered by the Kagan group in 1983.[52] In 1988, the Molander group described its application as a ring-closing reaction.[53] The generally accepted mechanism for this process is shown using an intramolecular example in Scheme 22.[53] Thus, reduction of dicarbonyl compound 91 initially results in formation of ketyl radical 92, which attacks the other carbonyl compound to generate oxygen radical 93. Rapid reduction of the latter species and quenching of the resulting alkoxide delivers pinacol product 94. Whereas the intermolecular variant generally suffers from poor stereoselectivity, intramolecular examples generally provide a high degree of stereocontrol in favor of a cis diol product due to chelation of the initially formed ketyl radical species 92 (see Scheme 22).[53] Additionally, if an alkoxy group is present vicinal to one of the two carbonyl moieties, then the newly-formed diol is generally formed anti to this alkoxy substituent.[54] Both inter- and intramolecular variants are useful, and rings of various sizes, including macrocycles, may be formed through this process. Besides employing ketones and aldehydes, SmI2-mediated pinacol-type reaction may also couple a ketone or aldehyde to an oxime,[55] nitrile,[56] or hydrazone.[57]
Scheme 22.
Mechanism of the SmI2-mediated pinacol reaction.
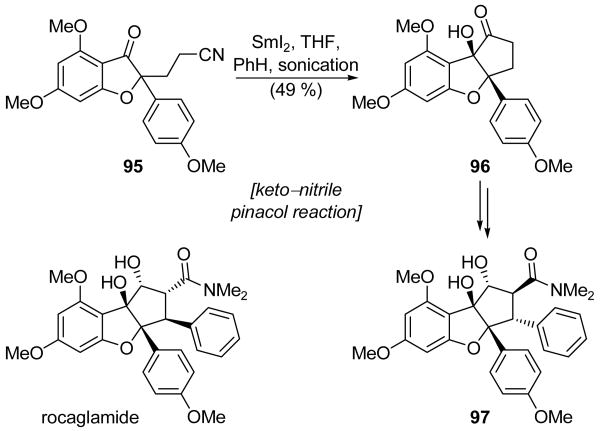
Kraus and Sy reported one of the earliest examples of a SmI2-mediated pinacol reaction between a ketone and a nitrile in the course of their total synthesis of a diastereomer of rocaglamide (i.e. 97, Scheme 23).[58] Thus, exposure of ketonitrile 95 to SmI2 under sonication provided α-hydroxyketone 96 in 49 % yield. Interestingly, other methods for performing the same transformation were tried without success. For example, neither Corey's method (Zn and TMSCl),[59] nor Hutchinson's protocol (Mg and TMSCl)[60] gave detectable amounts of the desired product 96, with both procedures resulting in simple ketone reduction instead. Furthermore, the choice of solvent was critical to the success of this reaction. Under optimal conditions, a 1:10 THF:benzene solvent mixture was used, and intermediate 96 was obtained in 49 % yield, along with 10 % of the secondary alcohol resulting from ketone reduction. However, when a higher percentage of THF was used, secondary alcohol formation was favored. α-Hydroxyketone 96 was advanced to compound 97, a diastereomer of natural rocaglamide.
Scheme 23.
Synthesis of rocaglamide diastereomer 97 through an intramolecular keto–nitrile pinacol reaction (Kraus and Sy, 1989).[58]
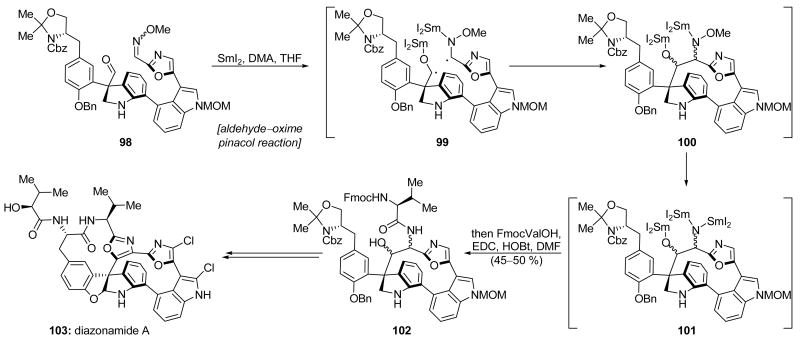
Nicolaou and coworkers employed a novel hetero-pinacol macrocyclization reaction as a key step in their second total synthesis of diazonamide A (103, Scheme 24).[61, 62] This was the first aldehyde–oxime pinacol reaction reported to construct a ring containing more than 7 atoms and the first SmI2-mediated reaction in which the resulting organosamarium intermediate (i.e. 101) was trapped by something more complex than a simple acylating agent. Thus, non-macrocyclic precursor 98 was exposed to SmI2 in the presence of dimethyl acetamide (DMA) to forge macrocyclic pinacol product 101, which was directly coupled (EDC, HOBt) with Fmoc-protected valine to deliver the desired product 102 in 45–50 % overall yield as an inconsequential mixture of stereoisomers. This reaction was proposed to proceed through the intermediacy of diradical 99 because the products of simple reduction of either the aldehyde, or the oxime are observed as byproducts in this reaction. A large excess of both SmI2 (9 equiv) and DMA (36 equiv) was required, and, in the presence of less DMA, the reduction of the N–O bond of intermediate 100 to afford 101 did not readily occur. The isolated product 102 was successfully transformed into diazonamide A (103).
Scheme 24.
An aldehyde–oxime pinacol macrocyclization in the second total synthesis of diazonamide A (103) (Nicolaou et al., 2001, 2003).[61]
7. Fragmentation Reactions
The fragmentation of cyclopropane and cyclobutane systems (Scheme 2f) is a useful means of installing complex ring systems or congested substituents,[63] and SmI2 has been employed for this purpose on several occasions. A different class of fragmentation reactions that has also been promoted by SmI2 is the cleavage of heterocycles containing weak heteroatom–heteroatom sigma bonds, such as the N–O and N–S bonds in isoxazoles and isothiazoles.
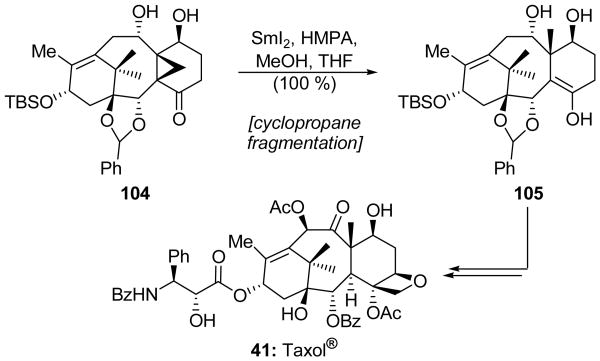
Kuwajima and coworkers used a SmI2-mediated cyclopropane fragmentation reaction to install a methyl group during their total synthesis of Taxol® (41).[64] Thus, as shown in Scheme 25, the congested cyclopropane-containing intermediate 104 was exposed to SmI2 in the presence of HMPA and MeOH, a reagent combination which induced a cyclopropane fragmentation to deliver enol 105 in quantitative yield. Interestingly, the enol group of the latter compound did not readily tautomerize to the corresponding ketone, presumably because protonation from the more accessible β-face of the enol would generate a large amount of strain. Related enols were found to be unstable to air, and the presence of the silyl and benzylidene protecting groups on 105 was essential in order to protect this functionality and allow subsequent manipulations leading to Taxol® (41).
Scheme 25.
Cyclopropane fragmentation in the total synthesis of Taxol® (41) (Kuwajima et al., 1998).[64]
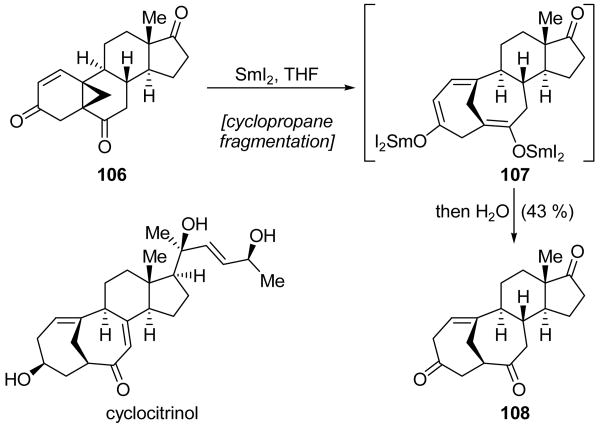
The Schmalz group called upon a SmI2-promoted cyclopropane fragmentation reaction in their synthesis of cyclocitrinol system 108 (Scheme 26).[65] Cyclopropanated steroid 106 was exposed to SmI2 in order to effect a fragmentation, thus constructing the bridging ring system of 107, which possesses two SmIII enolate functionalities. Upon quenching with water, cyclocitrinol system 108 was obtained in 43 % yield and as the only isolated product. It is interesting to note that although multiple products of this reaction may be envisioned, only one was observed to any appreciable extent, perhaps due to differences in the strain energies of the possible products.
Scheme 26.
Synthesis of cyclocitrinol system 108 through a cyclopropane fragmentation/ring expansion (Schmalz et al., 2007).[65]
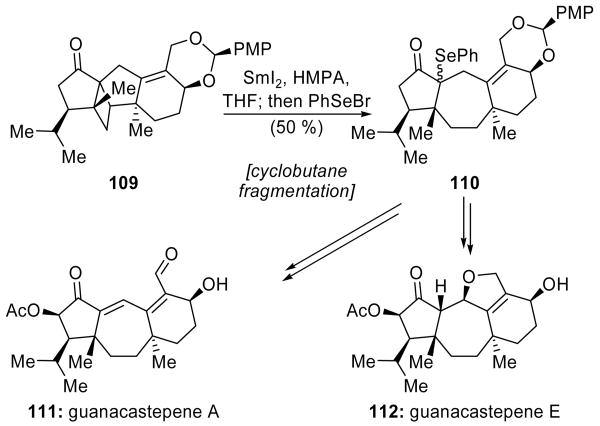
Shipe and Sorensen exploited a SmI2-mediated fragmentation of a strained cyclobutane system to form a 7-membered ring during their synthesis of guanacastepenes A (111, Scheme 27) and E (112, Scheme 27).[66] Cyclobutane system 109, the product of an intramolecular [2+2] photo-cycloaddition, was exposed to SmI2 that was activated by HMPA, and the resulting SmIII enolate was trapped with PhSeBr to give ring-expanded product 110 in 50 % yield, and as an inconsequential mixture of diastereomers. Alternatively, employing a dissolving metal reduction (Li/NH3) with an isopropylidene acetal in place of the benzylidene acetal resulted in a 46 % yield of the corresponding product after trapping with PhSeBr. Intermediate 110 was successfully elaborated to both guanacastepenes A (111) and E (112).
Scheme 27.
A cyclobutane fragmentation/ring expansion in the total synthesis of guanacastepenes A (111) and E (112) (Shipe and Sorensen, 2002).[66]
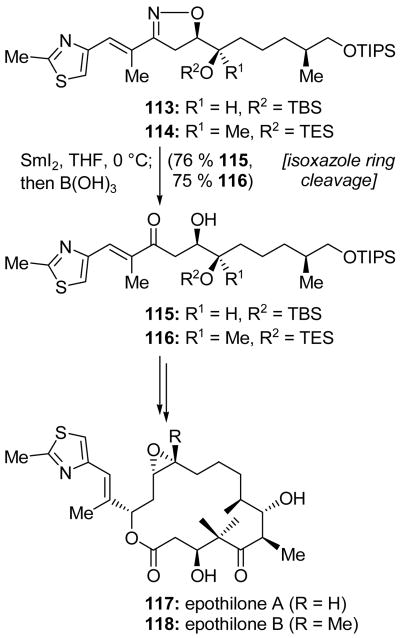
Bode and Carreira employed a different type of SmI2-induced fragmentation in their synthesis of epothilones A (117, Scheme 28) and B (118, Scheme 28).[67] They approached what they identified as the stereochemically and functionally most challenging portion of the epothilone structure with a nitrile oxide [3+2] cycloaddition to fashion isoxazoline 113. Subsequent SmI2-induced nitrogen–oxygen bond cleavage and boric acid-promoted imine hydrolysis gave β-hydroxyketone 115 in 76 % yield. This two step sequence is a useful surrogate for the aldol reaction. Hydroxyketone 115 was elaborated to epothilone A (117). Epothilone B (118) was synthesized in an analogous manner (114→116→118).
Scheme 28.
SmI2-promoted isoxazole ring cleavage in the total synthesis of epothilones A (117) and B (118) (Bode and Carreira, 2001).[67]
8. Elimination Reactions
Elimination reactions mediated by SmI2 are most commonly used in total synthesis to expel substituents vicinal to carbonyl-containing functionalities (Scheme 2g); indeed, every Reformatsky reaction is such an elimination wherein the intermediate enolate has been diverted for a purpose other than simple quenching. However, SmI2-induced eliminations may also occur farther away if the carbonyl-containing moiety is part of a conjugated system.[68] Furthermore, SmI2 has come to light as a means of selective protecting group cleavage.[69] In many ways, this latter group of eliminations is the same reaction as heterocycle fragmentations, exemplified by the isoxazoline cleavage in the Carreira epothilone synthesis (see Scheme 28).
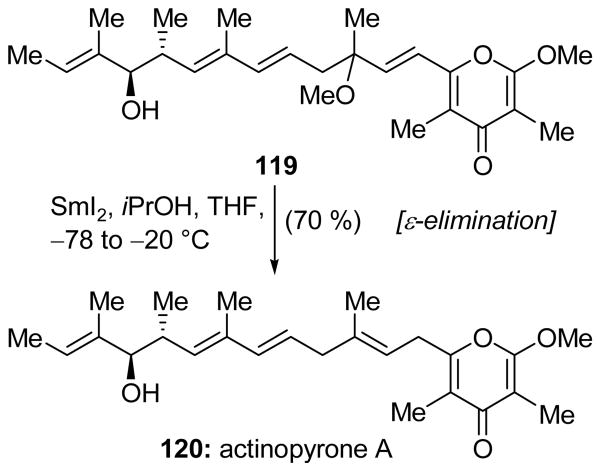
Tatsuta and coworkers utilized SmI2 in a reductive deconjugation reaction in the final step of their total synthesis of actinopyrone A (120, Scheme 29).[70] This was an insightful piece of engineering within their synthetic route, for the natural substance had been reported to be rather unstable,[71] likely due to the presence of the olefinic bond just one position away from conjugation with the pyrone system. Therefore, the Tatsuta team employed a fully conjugated system until the final step, during which elimination of a methoxy group from 119, induced by SmI2 in the presence of iPrOH, effected ε-elimination to give actinopyrone A (120) as an 88:12 mixture of E- and Z-isomers. Chromatographic separation delivered pure actinopyrone A (120) in a satisfying 70 % yield. To the best of our knowledge, this is the first example of a SmI2-mediated ε-elimination that is not driven by ring strain (e.g. epoxide opening).
Scheme 29.
Synthesis of actinopyrone A (120) through an ε-elimination of a methoxy group (Tatsuta et al., 2006).[70]
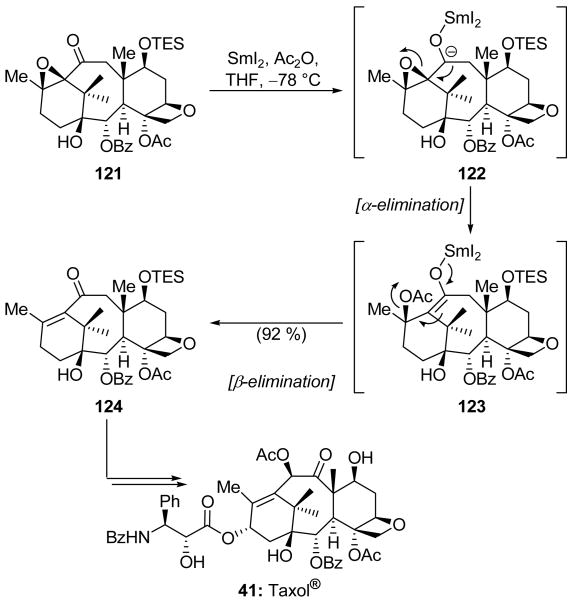
In their total synthesis of Taxol® (41), Danishefsky and coworkers used SmI2 in order to reductively eliminate an α,β-epoxyketone and generate the corresponding enone.[72] Thus, as shown in Scheme 30, treatment of epoxide 121 with SmI2 and Ac2O at −78 °C presumably effected two successive single-electron reductions of the ketone to form carbanion 122. Fragmentation of the neighboring epoxide ring afforded, after acetylation, β-acetoxy system 123. This unstable species suffered a second elimination reaction under the same conditions in order to deliver enone 124 in 92 % yield. The latter compound was successfully elaborated to synthetic Taxol® (41).
Scheme 30.
Epoxide elimination in the total synthesis of Taxol® (41) (Danishefsky et al., 1995).[72]
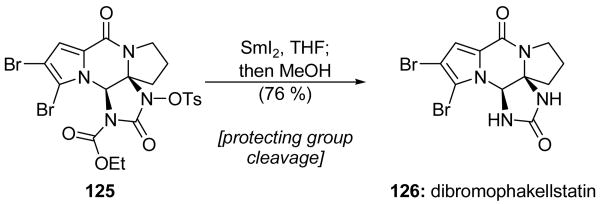
The action of SmI2 promoted the simultaneous cleavage of two nitrogen protecting groups in the Lindel group's total synthesis of dibromophakellstatin (126, Scheme 31).[73] Alkaline hydrolysis of their protected compound 125 (NaOEt) did not afford the desired product, but rather, gave cleavage of the cyclic urea system and displacement of the tosylamine moiety by ethoxide. In contrast, use of 2.5 equiv of SmI2 gave rapid and clean (95 % yield) deprotection of the tosylamine group. With 5 equiv of SmI2, slow carbamate cleavage also proceeded, thus providing dibromophakellstatin (126) in 76 % overall yield. Using even more SmI2 (7.5 equiv) resulted in a selective debromination at the pyrrole C2 position of dibromophakellstatin.
Scheme 31.
Synthesis of dibromophakellstatin (126) through SmI2-mediated double deprotection (Lindel et al., 2005).[73]
9. Cascade Reactions
Many of the most impressive examples of the use of SmI2 in total synthesis are those in which an entire sequence of reactions is promoted in cascade fashion.[6] These elegant cascade reactions can create significant molecular complexity by forming rings and/or casting multiple stereogenic centers. In this section, a selection of SmI2-promoted cascade reactions in total synthesis will be discussed in order to further highlight the power of this reagent in terms of efficiency and selectivity. To be sure, even more impressive applications will be conceived and executed in the future.
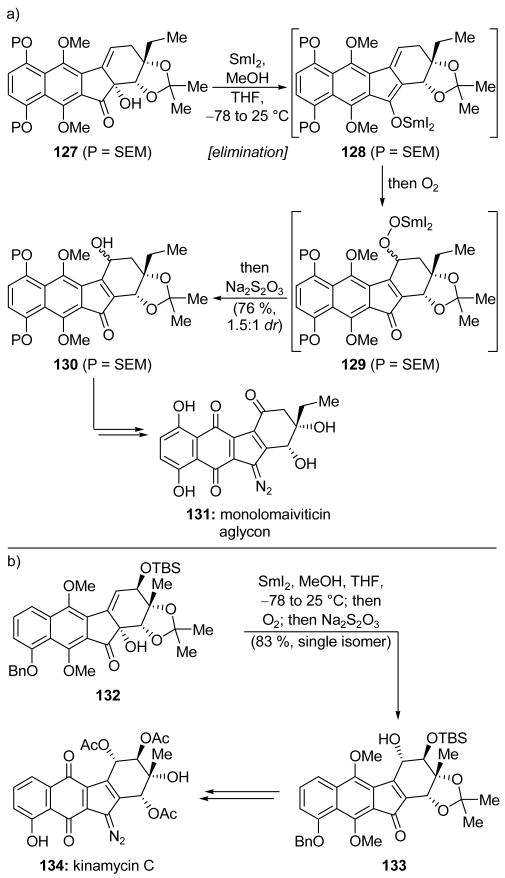
In a recent synthesis of the lomaiviticin aglycon monomeric unit 131 (Scheme 32a),[74] Nicolaou and coworkers developed an unusual SmI2-promoted isomerization. Exposure of α-hydroxyketone 127 to SmI2 in the presence of MeOH gave access to extended SmIII enolate 128, which was then reacted with O2 gas to generate hydroperoxide species 129. A Na2S2O3 quench then furnished the regioisomeric hydroxyketone 130 in 76 % yield, and as an inconsequential 1.5:1 mixture of diastereomers. Hydroxyketone 130 was successfully transformed into the lomaiviticin aglycon monomeric unit 131.
Scheme 32.
a) A SmI2-mediated isomerization in the synthesis of monolomaiviticin aglycon (131) and b) application to the total synthesis of kinamycin C (134) (Nicolaou et al., 2009).[74, 75]
This protocol was also applied to improving the Nicolaou synthesis of kinamycin C (134, Scheme 32b).[74, 75] Thus, exposure of α-hydroxyketone 132 to the newly defined reaction conditions (SmI2, MeOH; then O2; then Na2S2O3) gave the desired isomeric product 133 in 83 % yield, and as a single stereoisomer. This constitutes a marked improvement over the previously reported 4-step procedure, which generated the requisite alcohol 133 in 55 % overall yield from α-hydroxyketone 132.
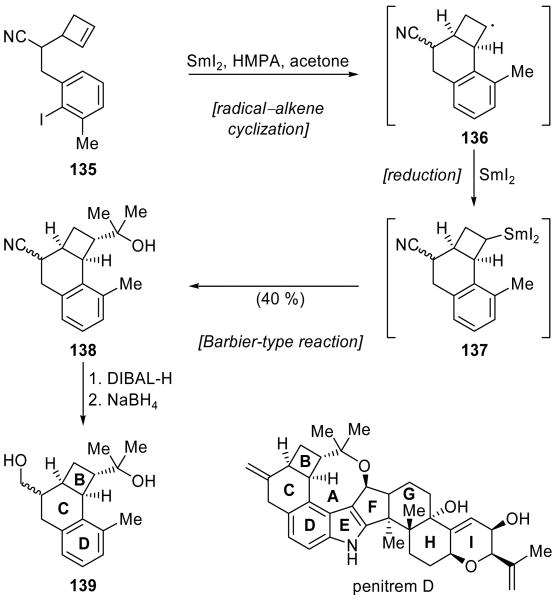
An unusual radical/ionic crossover reaction[5] was executed by Curran and coworkers in their synthesis of penitrem D model 139 (Scheme 33).[76] This cascade sequence commenced with generation of an aryl radical from iodide 135, which proceeded to attack the tethered cyclobutene system to form cyclobutyl radical 136. This transformation could be induced by nBu3SnH or SmI2 with comparable efficiency, but the ability of SmI2 to subsequently access an ionic reaction manifold allowed an impressive propagation of the cascade. Thus, further reduction of secondary radical 136 afforded organosamarium species 137, which underwent a Barbier-type reaction with acetone to give tertiary alcohol 138 in 40 % overall yield for the cascade sequence. The latter compound was obtained as a 1:1 mixture of nitrile stereoisomers, reflecting the diastereomeric mixture of starting cyclobutene 135. Quenching of radical intermediate 136 prior to the Barbier reaction represented a major side reaction, and the corresponding compound was also isolated in 40 % yield. Though the yield of desired product 138 was moderate, this example, nonetheless, highlights the potential for powerful cascade reactions that leverage the ability of SmI2 to access both radical and polar reaction manifolds. Reduction of nitrile 138 (1. DIBAL-H; 2. NaBH4) gave primary alcohol 139, the structure and stereochemistry of which was verified by X-ray crystallographic analysis to be that corresponding to the BCD system of penitrem D.
Scheme 33.
Synthesis of the BCD ring system (139) of penitrem D through a radical–alkene cyclization/Barbier-type reaction cascade (Curran et al., 2004).[76]
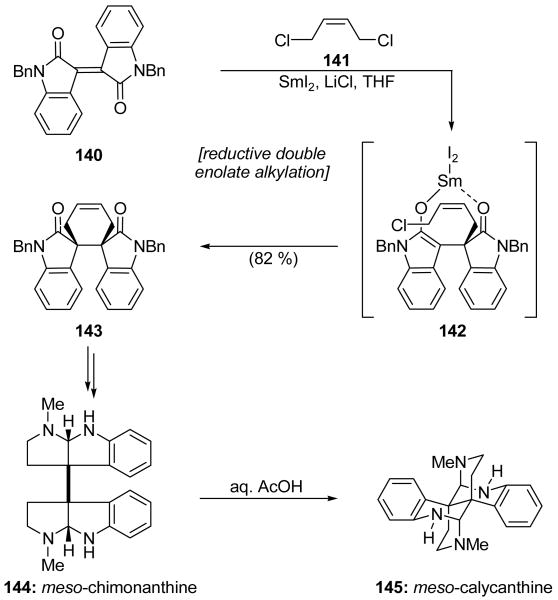
The Link and Overman total synthesis of meso-chimonanthine (144, Scheme 34) and meso-calycanthine (145, Scheme 34) employed a novel enolate alkylation cascade to cast a spirocyclic cyclohexene system.[77] Initially, the reaction of isoindigo 140 with dichloride 141 in the presence of SmI2 with or without HMPA did not yield any of the desired cyclohexene system 143, forming instead only the corresponding dihydroisoindigo. Double alkylation of the latter compound with dichloride 141, promoted by nBuLi or KHMDS, gave access to the desired product 143 contaminated with an isomeric cyclobutane system resulting from SN2′ ring closure. Remarkably, however, it was discovered that the use of LiCl as an additive enabled a clean reductive double alkylation of 140 to deliver in a single operation cyclohexene 143 in 82 % yield with nearly complete stereocontrol (>20:1 dr). This reaction is thought to proceed through the intermediacy of an initially-formed samarium dienolate, monoalkylation of which gives 142. An intramolecular alkylation then provides the observed product 143, with the samarium ion of the SmIII enolate serving to chelate the lactam oxygen and thus induce formation of the meso isomer (see 142). The role of LiCl is not clear, but its profound impact on the reaction pathway cannot be explained by a simple salt effect since the addition of KCl does not induce the same results. Possible explanations for the role of LiCl include aggregation resulting from the halide salt additive, a change in the coordination sphere of the samarium metal, or transmetallation from samarium to lithium.[49] Cyclohexene 143 was converted into meso-chimonanthine (144), which was then transformed upon exposure to aqueous acetic acid into meso-calycanthine (145) by following the published procedure.[78]
Scheme 34.
A reductive double enolate alkylation in the total synthesis of meso-chimonanthine (144) and meso-calycanthine (145) (Link and Overman, 1996).[77]
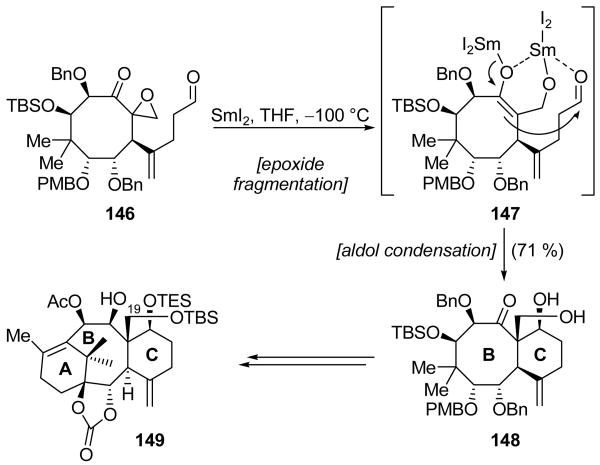
Having previously employed SmI2 for casting the 8-membered B ring of Taxol® (see Scheme 10), Mukaiyama and coworkers again turned to this reagent in their synthesis of 19-hydroxy taxoid 149 (Scheme 35), a compound which they hoped would enable the development of analogs with superior water solubility.[79] The desired sequence of events involved initial formation of SmIII enolate 147 through fragmentation of the epoxide ring of 146 followed by an intramolecular aldol cyclization to cast diol 148 with the stereochemistry shown. Though many different additives were investigated, it was discovered that optimal conditions, which gave diol 148 in 71 % yield, involved the use of SmI2 at −100 °C in the absence of additives. Two diastereomers were also isolated: one epimeric at the newly-formed quaternary center (10 % yield), and one with the opposite sense of stereochemistry at both of the newly-formed stereogenic centers (15 % yield). Interestingly, the final possible diastereomer, namely one epimeric at the secondary alcohol, was not observed under any conditions. Increasing the temperature of the reaction led to a decrease in the yield of the desired BC ring system 148, with no increase in the formation of the stereoisomeric products. The use of additives such as H2O, MeOH, iPrOH, and HMPA at −78 °C resulted in a reduced yield of diol 148 and a higher yield an epimeric product. Diol 148 was advanced to 19-hydroxy taxoid 149.
Scheme 35.
Synthesis of the C ring of a 19-hydroxy taxoid (149) through an epoxide fragmentation/aldol cyclization cascade (Mukaiyama et al., 2004, 2005).[79]
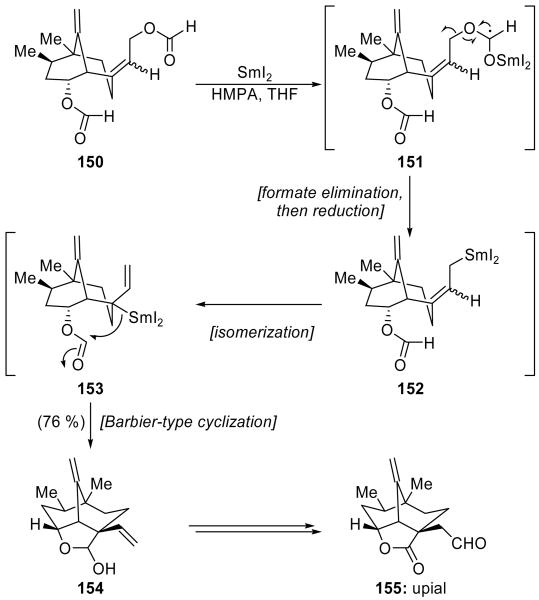
In their total synthesis of upial (155, Scheme 36),[80] Yamada and coworkers utilized an elimination–Barbier cyclization reaction sequence in order to construct the complex tricyclic core of the molecule. Originally developed for the synthesis of spirocyclic γ-butyrolactones,[81] the versatility and adaptability of this cascade sequence was demonstrated in this application. Thus, exposure of diformate 150 to SmI2 in the presence of HMPA produced hemiacetal 154 in a pleasing 76 % yield. The proposed mechanism for this reaction commences with formation of a ketyl radical selectively at the allylic formate, giving intermediate 151. Elimination of formate then yields a primary allylic radical, which is further reduced by SmI2 to afford organosamarium species 152. The latter is geometrically incapable of reacting through an intramolecular Barbier-type cyclization, and thus it isomerizes to tertiary organosamarium intermediate 153, cyclization of which delivers hemiacetal 154. The ionic nature of the cyclization is supported by deuterium labeling studies performed during the original cascade development. A short sequence of manipulations converted hemiacetal 154 into upial (155).
Scheme 36.
An elimination/Barbier cyclization cascade in the total synthesis of upial (155) (Yamada et al., 1993).[80]
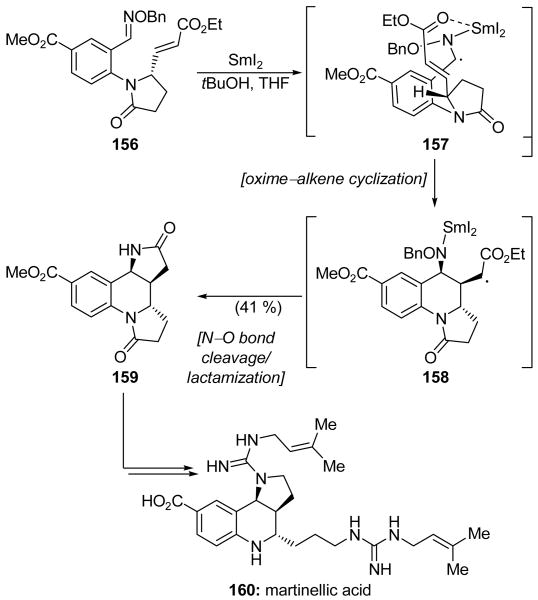
In the total synthesis of martinellic acid (160, Scheme 37),[82] Naito and coworkers used a radical addition–cyclization–elimination (RACE) reaction which could be promoted by nBu3SnH or SmI2 to establish the dihydropyrroloquinoline core of the natural product. Having previously developed the methodology for the RACE reaction between oxime ethers and α,β-unsaturated esters,[83] they exposed precursor 156 to nBu3SnH and AIBN in refluxing benzene to produce desired product 159 in 29 % yield, along with five related products in 26 % combined yield. While the desired product could be purified through careful chromatographic separation from the other substances, the use of SmI2 was investigated to determine whether the yield and selectivity of this cascade sequence could be further improved. Gratifyingly, exposure of precursor 156 to SmI2 in the presence of tBuOH produced markedly improved results, with the desired product 159 now isolated in 41 % yield and with only one other diastereomer obtained (10 % yield). To account for the significantly enhanced diastereoselectivity of the SmI2-mediated variant of the reaction, chelation of a SmIII ion between the ester and the nitrogen of the initially formed α-aza radical species (see 157) was proposed. This apparently enforced the desired cis orientation of the two functionalities during the oxime–alkene cyclization to generate 158, which underwent subsequent nitrogen–oxygen bond reductive cleavage and spontaneous lactamization to deliver the observed product 159. The latter compound was then converted into martinellic acid (160) in a straightforward manner.
Scheme 37.
A SmI2-promoted cyclization cascade in the total synthesis of martinellic acid (160) (Naito et al., 2008).[82]
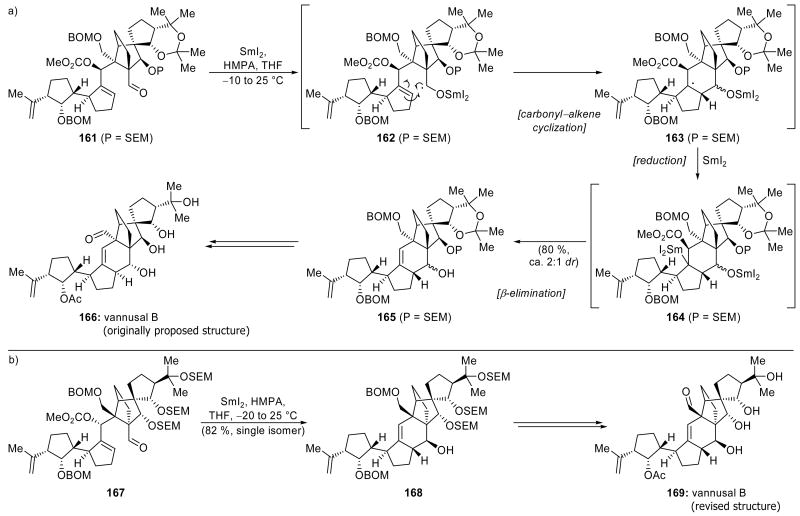
A SmI2-mediated cascade sequence involving a ketyl–olefin cyclization followed by a carbonate elimination was employed by the Nicolaou group in order to forge a key cyclohexene ring in their synthesis of the originally assigned structure of vannusal B (166, Scheme 38a).[84] This ring was formed through the reaction of aldehyde 161 with SmI2 in the presence of HMPA, providing the observed product 165 in 80 % yield, and as a ca. 2:1 mixture of secondary alcohol epimers. As this sequence generated the undesired stereochemistry at the ring fusion, the secondary alcohol was subsequently subjected to elimination in order to correct this problem. Thus, the mixture of epimeric products was inconsequential. The proposed mechanism of this reaction involves ketyl radical formation to give 162, which then reacts in a ketyl–olefin cyclization to produce tertiary radical 163. Further reduction gives transient organosamarium species 164, which undergoes elimination of the neighboring methyl carbonate group to deliver the observed product 165. Interestingly, the presence of the SEM protecting group on the hydroxyl group neighboring the aldehyde appears to be important for the efficiency of this process. Use of an acetonide as a protecting group produces the desired product in significantly lower yield, with the major product resulting from fragmentation of the bicyclo[2.2.1]heptane system followed by elimination of the carbonate. Correction of the ring fusion stereochemistry and completion of the synthesis provided a substance that possessed the assigned structure of vannusal B (166), but did not match the authentic natural material.
Scheme 38.
SmI2-mediated ring closure in the total synthesis of the a) originally proposed (166) and b) corrected (169) structures of vannusal B (Nicolaou et al., 2008, 2009).[84, 85]
Interestingly, the true identity of vannusal B was recently determined by the Nicolaou team through total synthesis.[85] In their synthesis of the corrected structure of vannusal B (169, Scheme 38b), the SmI2-mediated ring closure from aldehyde 167 was even more efficient since it led directly to the desired stereoisomer of cyclization product 168 in 82 % yield with complete stereocontrol. Importantly, since no inversions of stereocenters were required, only a few manipulations were needed in order to complete the synthesis of the corrected structure of vannusal B (169).
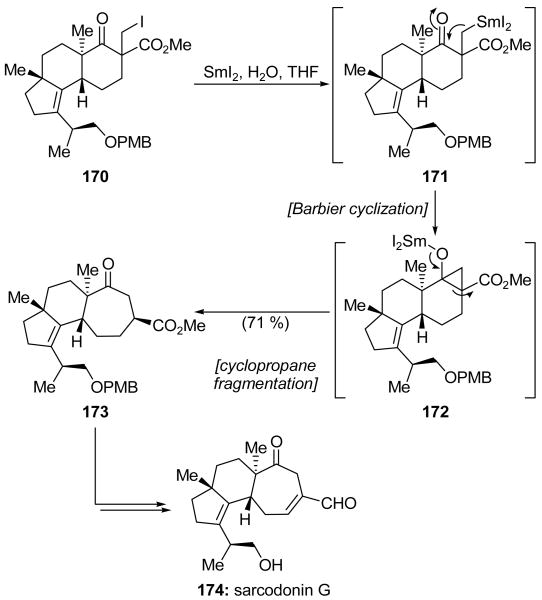
Piers and coworkers used SmI2 to perform a ring expansion and construct the 7-membered ring in the tricyclic core of sarcodonin G (174, Scheme 39).[86] Previous studies indicate that the ring expansion most likely occurs through a Barbier–cyclopropane fragmentation reaction pathway.[87] Initial reduction of the carbon–iodine bond of 170 produces a primary radical which can be subsequently reduced to give organosamarium intermediate 171. Barbier cyclization of the organosamarium onto the nearby ketone generates cyclopropane 172. It has also been proposed that this intermediate may arise from radical coupling of a ketyl radical generated at the ketone and one generated from the primary iodide.[88] In either case, fragmentation of 172 then delivers cycloheptanone 173, which was isolated in 71 % yield. Interestingly, the mixture of epimeric starting materials 170 was inconsequential, and the product was obtained as a single stereoisomer. A straightforward sequence of manipulations on cycloheptanone 173 gave sarcodonin G (174). This was the first application of this useful ring expansion cascade sequence in a total synthesis. Nakada and coworkers later employed the same methodology successfully in their total syntheses of cyathane diterpenoids allocyathin B2[89] and erinacine B.[90]
Scheme 39.
SmI2-mediated ring expansion in the total synthesis of sarcodonin G (174) (Piers et al., 2000).[86]
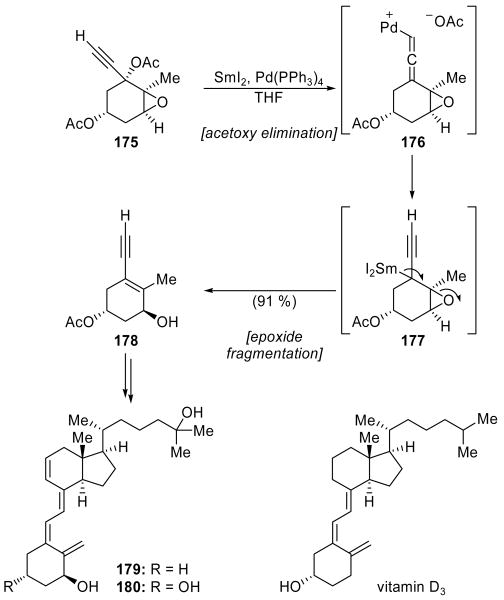
An unusual cascade reaction incorporating both palladium catalysis and SmI2-mediated reduction was featured in a total synthesis of two 9,11-dehydrovitamin D3 analogs (179 and 180, Scheme 40) reported by Aurrecoechea and coworkers.[91] Reaction of diacetate 175 with SmI2 in the presence of 2.3 mol % Pd(PPh3)4 produced the desired product 178 in 91 % overall yield. The first step in this cascade reaction, an acetoxy elimination developed by Inanaga and coworkers,[92] was proposed to proceed through formation of allenylpalladium intermediate 176 via oxidative addition of the Pd(0) catalyst to propargylic acetate 175. SmI2 can then serve to reductively regenerate the Pd(0) catalyst and form an allenic organosamarium intermediate, which presumably is in equilibrium with the isomeric propargyl organosamarium species 177. Epoxide opening, possibly assisted by another molecule of SmI2 serving as a Lewis acid, can then proceed to provide allylic alcohol 178. The latter compound was a useful building block for the synthesis of 9,11-dehydrovitamin D3 analogs 179 and 180. Prior to this publication, organosamarium species generated from organopalladium intermediates had not been demonstrated to undergo similar elimination or fragmentation chemistry; in previous studies, only protonation of the resulting organosamarium species had been observed.[92] This unique cascade serves as an elegant example of how SmI2 can be combined with other reagents to promote novel and advantageous reactions.
Scheme 40.
A palladium- and samarium-mediated cascade sequence in the synthesis of vitamin D3 analogs 179 and 180 (Aurrecoechea et al., 1989).[91]
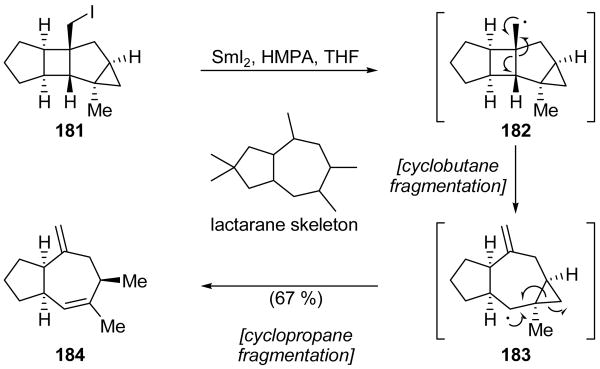
A cyclobutane/cyclopropane fragmentation sequence was developed by Lange and Corelli for the synthesis of sesquiterpene lactarane system 184 (Scheme 41).[93] Thus, the target compound was formed from tetracyclic iodide 181 in 67 % yield. The mechanism through which this transformation is proposed to occur commences with an initial reduction of the carbon–iodine bond by SmI2 in the presence of HMPA to produce primary radical 182. Fragmentation of the strained cyclobutane system of 182 then produces cycloheptyl radical 183, which is poised to fragment the nearby cyclopropane system and form a primary radical. Further reduction to an organosamarium species and quenching of the anion then yields the desired product 184. Attempts to produce a [6.3.0]bicycloundecane system (i.e. the 8-membered ring analog) using the same methodology were unsuccessful, resulting in only deiodination. However, a related cascade sequence suitable for the formation of [6.3.0]bicycloundecane systems had previously been disclosed by the Lange group.[94]
Scheme 41.
Synthesis of lactarane system 184 through a cyclobutane/cyclopropane fragmentation cascade (Lange and Corelli, 2007).[93]
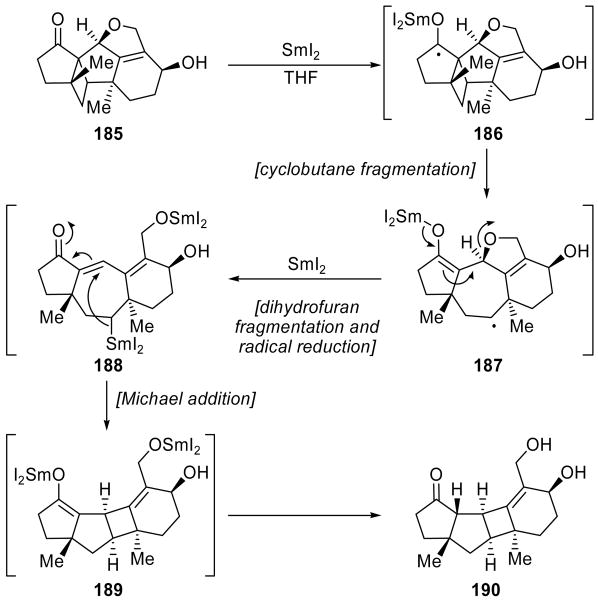
A remarkable cascade sequence mediated by SmI2 was encountered by Shipe and Sorensen during their model studies toward guanacastepenes A and E (111 and 112, see Scheme 27).[66] Thus, as shown in Scheme 42, reaction of SmI2 with pentacycle 185 produced the unexpected product 190 as the only isolable product. The proposed mechanism of this reaction entails formation of ketyl radical 186 followed by cyclobutane fragmentation as desired to produce cycloheptyl radical 187. Rupture of the dihydrofuran ring adjacent to the SmIII enolate and reduction of the radical then yielded diorganosamarium species 188, protonation of which would have rendered the desired product. Instead, an unexpected transannular conjugate addition onto the enone moiety occurred, producing the cyclobutane-containing ring system 189 which upon protonation gave the isolated product 190. This sequence of events suggested that intermediate 188 exists only transiently and, therefore, would not be a viable option in the real system. However, this realization led to the application of the cyclobutane fragmentation to the completion of the total synthesis of guanacastepenes A and E (111 and 112, see Scheme 27).
Scheme 42.
An unexpected cascade sequence during a guanacastepene model study (Shipe and Sorensen, 2006).[66]
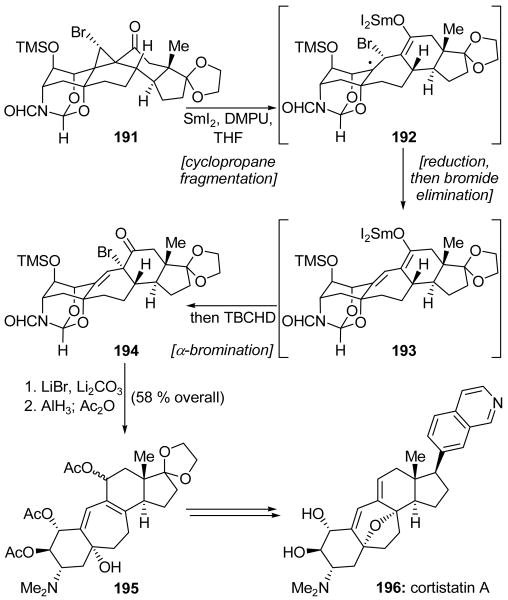
In their synthesis of the unusual steroid cortistatin A (196, Scheme 43), Baran and coworkers discovered a novel fragmentation/elimination/bromination cascade sequence mediated by SmI2.[95] Thus, treatment of bromoketone 191 with SmI2 in the presence of the additive DMPU followed a few minutes later by addition of 2,4,4,6-tetrabromo-2,5-cyclohexadienone (TBCHD)[96] delivered α-bromoketone 194. Though TBCHD is not a common brominating agent, it was found through a comprehensive search that its use gave superior yields and diastereoselectivities when compared with traditional reagents such as NBS. The reaction likely proceeded through initial formation of a ketyl radical followed by cyclopropane fragmentation to generate tertiary radical 192. The latter can undergo another single-electron reduction and bromide elimination to give extended enolate 193. Alternatively, a direct SmI2-mediated reductive bromide cleavage also might have generated 193. Finally, bromination at the α-position delivered the desired product 194. Subsequent elimination of the bromide (LiBr, Li2CO3), AlH3-promoted reduction, and acetylation (Ac2O) gave advanced intermediate 195 in an impressive 58 % overall yield from bromoketone 191. Pentacycle 195 was then elaborated in short order to cortistatin A (196).
Scheme 43.
A SmI2-promoted fragmentation/elimination/bromination cascade in the total synthesis of cortistatin A (196) (Baran et al., 2008).[95]
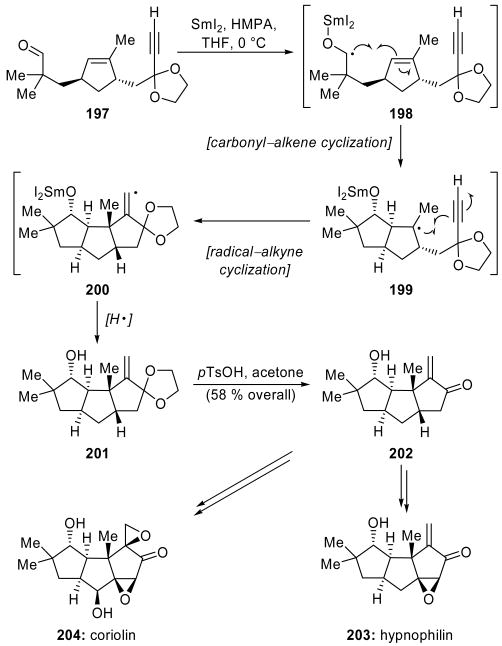
Curran and coworkers completed a total synthesis of hypnophilin (203, Scheme 44) and a formal synthesis of coriolin (204, Scheme 44) through the use of a SmI2-mediated radical cascade reaction.[97] In a model system, nBu3SnH/AIBN was found to be superior to other reaction conditions, including SmI2 in the presence of HMPA. These conditions, however, required an additional manipulation in the preparation of the precursor, namely the formation of a mixed phenylthio(trimethylsilyl)oxy acetal. Frustratingly, aldehyde 197 was not amenable to conversion to hemithioacetals or hemiselenoacetals, and, therefore, was not suitable for the cyclization conditions defined in the model study. Remarkably, whereas SmI2 and HMPA was an inferior reagent combination for the model cyclization, on aldehyde 197, it promoted the successful formation of the desired product 201 in high yield and diastereoselectivity. Acidic ketal cleavage produced tricyclic system 202 in 58 % overall yield for the two-step sequence. This cascade reaction proceeds through initial formation of ketyl radical 198 from aldehyde 197. A carbonyl–alkene cyclization generates the cis-fused ring of tertiary radical 199, which can then undergo a radical–alkyne cyclization. As only 1.3 equivalents of SmI2 were required, the resulting vinyl radical 200 is proposed to abstract a hydrogen radical from the solvent (THF) rather than undergo further reduction. This supposition is also supported by the absence of deuterium incorporation upon quenching with D2O. HMPA was found to be an essential additive in this reaction, as only an 11 % yield of the desired product was obtained without this co-solvent. Furthermore, substituting HMPA with the less bulky DMPU resulted in degraded stereocontrol. Tricyclic compound 202 was successfully converted into both hypnophilin (203) and coriolin (204).
Scheme 44.
A SmI2-promoted radical cascade in the total synthesis of hypnophilin (203) and formal synthesis of coriolin (204) (Curran et al., 1988).[97]
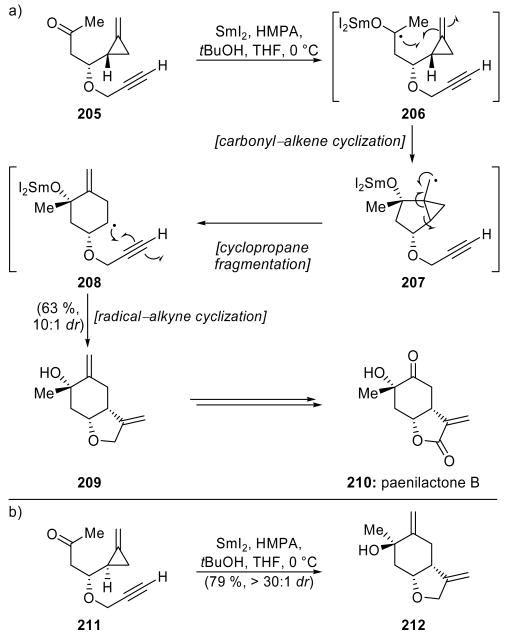
Another radical cascade fashioned two rings in the Kilburn group's synthesis of paeonilactone B (210, Scheme 45a).[98] Thus, treatment of methylenecyclopropyl ketone 205 with SmI2 in the presence of HMPA and tBuOH provided the desired fused bicyclic system 209 in 63 % overall yield and as a 10:1 mixture of tertiary alcohol epimers. Initial ketyl radical formation (206) followed by 5-exo-trig cyclization produced primary radical 207, which readily underwent cyclopropane ring fragmentation to afford cyclohexyl radical 208. A 5-exo-dig cyclization and subsequent reductive quenching of the resulting vinyl radical delivered the observed product 209. HMPA was an essential additive for this process, and in its absence, the yield dropped to 20 % and the diastereomeric ratio of the product was now 1.3:1 in favor of 212 (Scheme 45b). The use of DMPU was also ineffective, for while the resulting yield was acceptable (40 %) the undesired product epimer 212 predominated (1.5:1 dr). Tertiary alcohol 209 was elaborated in short order to paeonilactone B (210).
Scheme 45.
a) A SmI2-mediated cascade in the synthesis of paeonilactone B (210) and b) clarification of the source of stereoinduction (Kilburn et al., 1998).[98]
Interestingly, exposure of the epimeric methylenecyclopropyl ketone 211 (Scheme 45b) to the same reaction conditions provided the epimeric product 212 in 79 % yield and as a >30:1 mixture of diastereomers. The stereochemical course of these reactions can be rationalized by a transition state in the carbonyl–alkene cyclization that places the bulky HMPA-bound alkoxysamarium species in a pseudoequatorial position. The use of the sterically less demanding additive DMPU in place of HMPA erodes the stereoselectivity of the reaction. A few years later, Kilburn and coworkers detailed the use of this cascade reaction on an allylic ether substrate, which they employed in their total synthesis of 6-epi-paeonilactone A[99] and construction of the eudesmane sesquiterpenoid skeleton.[100]
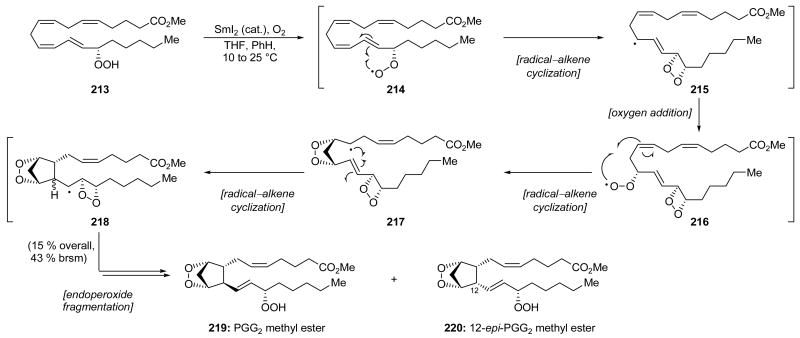
An unusual and impressive catalytic, oxidative SmI2-mediated cascade was reported by Corey and Wang in their bioinspired (and potentially biomimetic) synthesis of prostaglandin endoperoxides PGG2 methyl ester (219, Scheme 46) and 12-epi-PGG2 methyl ester (220, Scheme 46).[101] Thus, a THF/benzene solution of hydroperoxide 213 containing an excess of O2 was exposed to a catalytic amount of SmI2 that had been premixed with O2, providing 12-epi-PGG2 methyl ester (220) and PGG2 methyl ester (219) in 15 % overall yield (43 % brsm) as a 3:1 mixture (220:219) of chromatographically separable products. Though SmI2 is generally known as a single-electron reducing agent used in stoichiometric quantities and, indeed, has served such a purpose in all other examples within this Review, in this example, SmI2 served to catalyze an exquisitely controlled addition of O2 in a cascade sequence that installed two rings, including an unusual endoperoxide system. Pre-mixing SmI2 with O2 effected immediate decolorization to form what was presumed to be I2SmOOSmI2. This species is proposed to initially abstract a hydrogen radical from the hydroperoxide of 213, possibly through the intermediacy of I2SmO•, giving access to hydroperoxide radical 214. A 4-exo-trig radical–alkene cyclization gave access to allylic radical 215 which combined with one equivalent of O2 to afford hydroperoxide radical 216. A 5-exo-trig cyclization generated secondary radical 217 which underwent a second 5-exo-trig cyclization to form intermediate 218. Fragmentation of the strained 4-membered endoperoxide ring and abstraction of H• from another molecule of 213 completed the formation of PGG2 methyl ester (219) and 12-epi-PGG2 methyl ester (220) and allowed propagation of the chain reaction. In light of the significant increase in molecular complexity, the yield of this transformation is most impressive.
Scheme 46.
Synthesis of PGG2 methyl ester (219) and 12-epi-PGG2 methyl ester (220) through a SmI2-catalyzed oxidative cascade (Corey and Wang, 1994).[101]
10. Summary and Outlook
In the three decades since the first explorations of the synthetic utility of samarium diiodide (SmI2), its importance in organic synthesis has grown significantly. It is noteworthy that three separate total syntheses of Taxol® (41, see Schemes 10, 25, and 30) employed SmI2 for unrelated transformations. Access to this multitude of reaction types is what makes SmI2 chemistry both so rich in possibilies and, at times, so difficult to control. However, as demonstrated by the examples in this Review, careful fine tuning of substrate structure and reaction conditions—including solvents, co-solvents, additives, and temperature—can yield controlled and highly useful transformations with high efficiency and stereoselectivity. In particular, cascade sequences involving SmI2 offer virtually limitless possibilities, with access to both radical and ionic reaction manifolds and opportunities for catalytic and oxidative modes of action. To be sure, many more impressive and useful applications of this reagent in chemical synthesis will be invented and discovered in the future.
Acknowledgments
We gratefully acknowledge the National Institutes of Health (U.S.A.), the National Science Foundation (U.S.A.), and the Skaggs Institute for Research for supporting our research programs. S.P.E. thanks Novartis for financial support.
Abbreviations
- Ac
acetyl
- AIBN
2,2′-azobis(2-methylpropionitrile)
- Bn
benzyl
- BOM
benzyloxymethyl
- brsm
based on recovered starting material
- Bz
benzoyl
- cat
catalytic
- Cbz
benzyloxycarbonyl
- DIBAL-H
diisobutylaluminum hydride
- DMA
dimethyl acetamide
- DMPU
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
- dr
diastereomeric ratio
- EDC
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide
- equiv
equivalents
- Fmoc
fluorenylmethyloxycarbonyl
- HFIP
hexafluoroisopropanol
- HMPA
hexamethylphosphoramide
- HOBt
1-hydroxybenzotriazole
- hν
irradiation
- KHMDS
potassium hexamethyldisilazide
- LUMO
lowest unoccupied molecular orbital
- MOM
methoxymethyl
- MS
molecular sieves
- NBS
N-bromosuccinimide
- PGG2
prostaglandin G2
- PMB
para-methoxybenzyl
- PMP
para-methoxylphenyl
- py
pyridine
- RACE
radical addition–cyclization–elimination
- SEM
[2-(trimethylsilyl)ethoxy]methyl
- SOMO
singly occupied molecular orbital
- TBAF
tetra-n-butylammonium fluoride
- TBCHD
2,4,4,6-tetrabromo-2,5-cyclohexadienone
- TBDPS
tert-butyldiphenylsilyl
- TBS
tert-butyldimethylsilyl
- TES
triethylsilyl
- TFA
trifluoroacetic acid
- TIPS
triisopropylsilyl
- TMS
trimethylsilyl
- Ts
4-toluenesulfonyl
- Val
valine
Biographies
K. C. Nicolaou, born in Cyprus and educated in England and the United States, is currently Chairman of the Department of Chemistry at The Scripps Research Institute, where he holds the Darlene Shiley Chair in Chemistry and the Aline W. and L. S. Skaggs Professorship in Chemical Biology. He is also Distinguished Professor of Chemistry at the University of California, San Diego. The impact of his career on chemistry, biology, and medicine flows from his contributions to chemical synthesis, which have been described in numerous publications and patents. His dedication to chemical education is reflected in his training of hundreds of graduate students and postdoctoral fellows. His books, Classics in Total Synthesis I and II, and Molecules that Changed the World, co-authored with his students Erik J. Sorensen, Scott A. Snyder, and Tamsyn Montganon, respectively, are used around the world as teaching tools and sources of inspiration for students and practitioners of the art of chemical synthesis.
Shelby P. Ellery was born in Wausau, Wisconsin, in 1984. She received her B.S. in Chemistry from Cedar Crest College in 2006, where she carried out research under the guidance of Professor John Griswold. She is currently pursuing her Ph.D. in chemistry at The Scripps Research Institute in the laboratory of Professor K. C. Nicolaou, where she is working on total synthesis and new synthetic technologies. She also has been the recipient of a Novartis graduate fellowship in organic synthesis.
Jason S. Chen was born in Taipei, Taiwan, in 1979. He received his A.B. and A.M. degrees in 2001 from Harvard University, where he performed research under the supervision of Professor Matthew D. Shair. After spending two years at Enanta Pharmaceuticals (Watertown, MA) studying cyclosporine A analogs, he joined Professor K. C. Nicolaou's group at The Scripps Research Institute in 2003. He was a National Defense Science and Engineering Graduate (NDSEG) Fellow, and, in 2008, he completed his Ph.D. studies on the total synthesis and biological evaluation of uncialamycin. He is currently a research associate in Professor Nicolaou's laboratory.
Footnotes
Dedicated to Professor Henri B. Kagan
References
- 1.a) Namy JL, Girard P, Kagan HB. New J Chem. 1977;1:5–7. [Google Scholar]; b) Girard P, Namy JL, Kagan HB. J Am Chem Soc. 1980;102:2693–2698. [Google Scholar]
- 2.For selected reviews of SmI2-mediated reactions in organic synthesis, see: Soderquist JA. Aldrichimica Acta. 1991;24:15–23.Molander GA. Chem Rev. 1992;92:29–68.Molander GA. Org React. 1994;46:211–367.Imamoto T. Lanthanides in Organic Synthesis. Academic Press; London: 1994. p. 160.Molander GA, Harris CR. Chem Rev. 1996;96:307–338. doi: 10.1021/cr950019y.Skrydstrup T. Angew Chem. 1997;109:355–358.Angew Chem Int Ed Engl. 1997;36:345–347.Molander GA, Harris CR. Tetrahedron. 1998;54:3321–3354.Nomura R, Endo T. Chem Eur J. 1998;4:1605–1610.Krief A, Laval AM. Chem Rev. 1999;99:745–777. doi: 10.1021/cr980326e.Steel PG. J Chem Soc, Perkin Trans 1. 2001:2727–2751.Agarwal S, Greiner A. J Chem Soc, Perkin Trans 1. 2002:2033–2042.Kagan HB. Tetrahedron. 2003;59:10351–10372.Berndt M, Gross S, Hölemann A, Reissig HU. Synlett. 2004:422–438.Edmonds DJ, Johnston D, Procter DJ. Chem Rev. 2004;104:3371–3403. doi: 10.1021/cr030017a.Jung DY, Kim YH. Synlett. 2005:3019–3032.Gopalaiah K, Kagan HB. New J Chem. 2008;32:607–637.Rudkin IM, Miller LC, Procter DJ. Organomet Chem. 2008;34:19–45.
- 3.a) Shabangi M, Flowers RA., II Tetrahedron Lett. 1997;38:1137–1140. [Google Scholar]; b) Flowers RA., II Synlett. 2008:1427–1439. [Google Scholar]
- 4.For selected reviews of radical chemistry, see: Perkins MJ. Radical Chemistry. Ellis-Horwood; New York: 1994. p. 182.Curran DP. Aldrichimica Acta. 2000;33:104–110.Rozantsev EG, Loshadkin DV. Des Monomers Polym. 2001;4:281–300.Gansäuer A, Lauterbach T, Narayan S. Angew Chem. 2003;115:5714–5731. doi: 10.1002/anie.200300583.Angew Chem Int Ed. 2003;42:5556–5573. doi: 10.1002/anie.200300583.Hicks RG. Org Biomol Chem. 2007;5:1321–1338. doi: 10.1039/b617142g.
- 5.For selected reviews of radical/ionic crossover reactions, see: Bashir N, Patro B, Murphy JA. Advances in Free Radical Chemistry. Jai Press; Stamford: 1999. p. 123.Godineau E, Schenk K, Landais Y. J Org Chem. 2008;73:6983–6993. doi: 10.1021/jo801308j.
- 6.For selected reviews of cascade reactions in organic synthesis, see: Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. p. 631.Tietze LF, Beifuss U. Angew Chem. 1993;105:137–170.Angew Chem Int Ed Engl. 1993;32:131–163.Tietze LF. Chem Rev. 1996;96:115–136. doi: 10.1021/cr950027e.Pellissier H. Tetrahedron. 2006;62:1619–1665.Pellissier H. Tetrahedron. 2006;62:2143–2173.Ho TL. Tandem Organic Reactions. Wiley; New York: 1992. p. 512.Bunce RA. Tetrahedron. 1995;51:13103–13159.Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem. 2006;118:7292–7344. doi: 10.1002/anie.200601872.Angew Chem Int Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872.
- 7.For selected reviews of the Barbier reaction, see: Li CJ. Tetrahedron. 1996;52:5643–5668.Alonso F, Yus M. Recent Res Devel Org Chem. 1997;1:397–436.Ramón DJ, Yus M. Eur J Org Chem. 2000:225–237.Kouznetsov VV, Mendez LYV. Synthesis. 2008:491–506.
- 8.Molander GA, Etter JB. J Org Chem. 1986;51:1778–1786. [Google Scholar]
- 9.Kagan HB, Namy JL, Girard P. Tetrahedron. 1981;37:175–180. [Google Scholar]
- 10.a) Namy JL, Collin J, Bied C, Kagan HB. Synlett. 1992:733–734. [Google Scholar]; b) Molander GA, McKie JA. J Org Chem. 1991;56:4112–4120. [Google Scholar]; c) Curran DP, Fevig TL, Jasperse CP, Totleben MJ. Synlett. 1992:943–961. [Google Scholar]
- 11.a) Kito M, Sakai T, Shirahama H, Miyashita M, Matsuda F. Synlett. 1997:219–220. [Google Scholar]; b) Matsuda F, Kito M, Sakai T, Okada N, Miyashita M, Shirahama H. Tetrahedron. 1999;55:14369–14380. [Google Scholar]
- 12.Carroll GL, Little RD. Org Lett. 2000;2:2873–2876. doi: 10.1021/ol006301v. [DOI] [PubMed] [Google Scholar]
- 13.a) Collin J, Namy JL, Kagan HB. New J Chem. 1986;10:229–232. [Google Scholar]; b) Inanaga J, Yokoyama Y, Baba Y, Yamaguchi M. Tetrahedron Lett. 1991;32:5559–5562. [Google Scholar]; c) Machrouhi F, Hamann B, Namy JL, Kagan HB. Synlett. 1996:633–634. [Google Scholar]
- 14.Molander GA, Quirmbach MS, Silva LF, Jr, Spencer KC, Balsells J. Org Lett. 2001;3:2257–2260. doi: 10.1021/ol015763l. [DOI] [PubMed] [Google Scholar]
- 15.a) Skene WG, Scaiano JC, Cozens FL. J Org Chem. 1996;61:7918–7921. doi: 10.1021/jo960473h. [DOI] [PubMed] [Google Scholar]; b) Ogawa A, Sumino Y, Nanke T, Ohya S, Sonoda N, Hirao T. J Am Chem Soc. 1997;119:2745–2746. [Google Scholar]; c) Sumino Y, Harato N, Tomisaka Y, Ogawa A. Tetrahedron. 2003;59:10499–10508. [Google Scholar]
- 16.Lowe JT, Panek JS. Org Lett. 2008;10:3813–3816. doi: 10.1021/ol801499s. [DOI] [PubMed] [Google Scholar]
- 17.a) Fukuzawa Si, Nakanishi A, Fujinami T, Sakai S. J Chem Soc, Chem Commun. 1986:624–625. [Google Scholar]; b) Tabuchi T, Inanaga J, Yamaguchi M. Tetrahedron Lett. 1986;27:5763–5764. [Google Scholar]
- 18.a) Mazéas D, Skrydstrup T, Doumeix O, Beau JM. Angew Chem. 1994;106:1457–1459. [Google Scholar]; Angew Chem Int Ed Eng. 1994;33:1383–1386. [Google Scholar]; b) Skrydstrup T, Mazéas D, Elmouchir M, Doisneau G, Riche C, Chiaroni A, Beau JM. Chem Eur J. 1997;3:1342–1356. [Google Scholar]
- 19.Stork G, Suh HS, Kim G. J Am Chem Soc. 1991;113:7054–7056. [Google Scholar]
- 20.Trost BM, Neilsen JB, Hoogsteen K. J Am Chem Soc. 1992;114:5432–5434. [Google Scholar]
- 21.For selected reviews of the Reformatsky reaction, see: Furstner A. Synthesis. 1989:571–590.Ocampo R, Dolbier WR. Tetrahedron. 2004;60:9325–9374.
- 22.Molander GA, Hahn G. J Org Chem. 1986;51:1135–1138. [Google Scholar]
- 23.a) Moslin RM, Jamison TF. J Am Chem Soc. 2006;128:15106–15107. doi: 10.1021/ja0670660. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Moslin RM, Jamison TF. J Org Chem. 2007;72:9736–9745. doi: 10.1021/jo701821h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Tabuchi T, Kawamura K, Inanaga J. Tetrahedron Lett. 1986;27:3889–3890. [Google Scholar]; b) Inanaga J, Yokoyama Y, Handa Y, Yamaguchi M. Tetrahedron Lett. 1991;32:6371–6374. [Google Scholar]
- 25.Arhart RJ, Martin JC. J Am Chem Soc. 1972;94:5003–5010. [Google Scholar]
- 26.a) Mukaiyama T, Shiina I, Iwadare H, Sakoh H, Tani Y, Hasegawa M, Saitoh K. Proc Jpn Acad. 1997;73B:95–100. [Google Scholar]; b) Mukaiyama T, Shiina I, Iwadare H, Saitoh M, Nishimura T, Ohkawa N, Sakoh H, Nishimura K, Tani Y, Hasegawa M, Yamada K, Saitoh K. Chem Eur J. 1999;5:121–161. [Google Scholar]; c) Shiina I, Uoto K, Mori N, Kosugi T, Mukaiyama T. Chem Lett. 1995:181–182. [Google Scholar]; d) Shiina I, Iwadare H, Sakoh H, Tani Yi, Hasegawa M, Saitoh K, Mukaiyama T. Chem Lett. 1997:1139–1140. [Google Scholar]; e) Yamada K, Tozawa T, Saitoh K, Mukaiyama T. Chem Pharm Bull. 1997;45:2113–2115. doi: 10.1248/cpb.45.2113. [DOI] [PubMed] [Google Scholar]; f) Shiina I, Shibata J, Imai Y, Ibuka R, Fujisawa H, Hachiya I, Mukaiyama T. Chem Lett. 1999:1145–1146. [Google Scholar]; g) Shiina I, Shibata J, Ibuka R, Imai Y, Mukaiyama T. Bull Chem Soc Jpn. 2001;74:113–122. [Google Scholar]
- 27.Hamon S, Birlirakis N, Toupet L, Arseniyadis S. Eur J Org Chem. 2005:4082–4092. [Google Scholar]
- 28.a) Takeuchi S, Miyoshi N, Hirata K, Hayashida H, Ohgo Y. Bull Chem Soc Jpn. 1992;65:2001–2003. [Google Scholar]; b) Miyoshi N, Takeuchi S, Ohgo Y. Chem Lett. 1993:959–962. [Google Scholar]; c) Machrouhi F, Namy JL, Kagan HB. Tetrahedron Lett. 1997;38:7183–7186. [Google Scholar]
- 29.a) Molander GA, Kenny C. Tetrahedron Lett. 1987;28:4367–4370. [Google Scholar]; b) Molander GA, Kenny C. J Am Chem Soc. 1989;111:8236–8246. [Google Scholar]
- 30.Fukazawa Si, Nakanishi A, Fujinami T, Sakai S. J Chem Soc, Perkin Trans 1. 1988:1669–1676. [Google Scholar]
- 31.Hutton TK, Muir K, Procter DJ. Org Lett. 2002;4:2345–2347. doi: 10.1021/ol0260472. [DOI] [PubMed] [Google Scholar]
- 32.a) Banwell M, McLeod M. Chem Commun. 1998:1851–1852. [Google Scholar]; b) Banwell MG, Hockless DCR, McLeod MD. New J Chem. 2003;27:50–59. [Google Scholar]
- 33.Molander GA, George KM, Monovich LG. J Org Chem. 2003;68:9533–9540. doi: 10.1021/jo0347361. [DOI] [PubMed] [Google Scholar]
- 34.a) Hori N, Matsukura H, Matsuo G, Nakata T. Tetrahedron Lett. 1999;40:2811–2814. [Google Scholar]; b) Matsuo G, Hori N, Nakata T. Tetrahedron Lett. 1999;40:8859–8862. [Google Scholar]
- 35.Hori N, Matsukura H, Nakata T. Org Lett. 1999;1:1099–1101. [Google Scholar]
- 36.Fuwa H, Kainuma N, Tachibana K, Sasaki M. J Am Chem Soc. 2002;124:14983–14992. doi: 10.1021/ja028167a. [DOI] [PubMed] [Google Scholar]
- 37.Matsuo G, Kawamura K, Hori N, Matsukura H, Nakata T. J Am Chem Soc. 2004;126:14374–14376. doi: 10.1021/ja0449269. [DOI] [PubMed] [Google Scholar]
- 38.a) Fuwa H, Ebine M, Sasaki M. J Am Chem Soc. 2006;128:9648–9650. doi: 10.1021/ja062524q. [DOI] [PubMed] [Google Scholar]; b) Fuwa H, Ebine M, Bourdelais AJ, Baden DG, Sasaki M. J Am Chem Soc. 2006;128:16989–16999. doi: 10.1021/ja066772y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ebine M, Fuwa H, Sasaki M. Org Lett. 2008;10:2275–2278. doi: 10.1021/ol800685c. [DOI] [PubMed] [Google Scholar]
- 39.Nicolaou KC, Li A, Edmonds DJ. Angew Chem. 2006;118:7244–7248. [Google Scholar]; Angew Chem Int Ed. 2006;45:7086–7090. doi: 10.1002/anie.200603892. [DOI] [PubMed] [Google Scholar]
- 40.Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew Chem. 2007;119:4016–4019. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:3942–3945. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]
- 41.Chen P, Wang J, Liu K, Li C. J Org Chem. 2008;73:339–341. doi: 10.1021/jo7021247. [DOI] [PubMed] [Google Scholar]
- 42.a) Johnston D, Francon N, Edmonds DJ, Procter DJ. Org Lett. 2001;3:2001–2004. doi: 10.1021/ol015976a. [DOI] [PubMed] [Google Scholar]; b) Johnston D, Couché E, Edmonds DJ, Muir KW, Procter DJ. Org Biomol Chem. 2003;1:328–337. doi: 10.1039/b209066j. [DOI] [PubMed] [Google Scholar]; c) Hutton TK, Muir KW, Procter DJ. Org Lett. 2003;5:4811–4814. doi: 10.1021/ol0358399. [DOI] [PubMed] [Google Scholar]; d) Baker TM, Edmonds DJ, Hamilton D, O'Brien CJ, Procter DJ. Angew Chem. 2008;120:5713–5715. doi: 10.1002/anie.200801900. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:5631–5633. doi: 10.1002/anie.200801900. [DOI] [PubMed] [Google Scholar]
- 43.a) Masson G, Py S, Valleé Y. Angew Chem. 2002;114:1850–1853. doi: 10.1002/1521-3773(20020517)41:10<1772::aid-anie1772>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:1772–1775. doi: 10.1002/1521-3773(20020517)41:10<1772::aid-anie1772>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; b) Masson G, Cividino P, Py S, Valleé Y. Angew Chem. 2003;115:2367–2370. doi: 10.1002/anie.200250480. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:2265–2268. doi: 10.1002/anie.200250480. [DOI] [PubMed] [Google Scholar]; c) Riber D, Skrydstrup T. Org Lett. 2003;5:229–231. doi: 10.1021/ol027386y. [DOI] [PubMed] [Google Scholar]
- 44.Desvergnes S, Py S, Valleé Y. J Org Chem. 2005;70:1459–1462. doi: 10.1021/jo048237r. [DOI] [PubMed] [Google Scholar]
- 45.a) Lindsay KB, Skrydstrup T. J Org Chem. 2006;71:4766–4777. doi: 10.1021/jo060296c. [DOI] [PubMed] [Google Scholar]; b) Karaffa J, Lindsay KB, Skrydstrup T. J Org Chem. 2006;71:8129–8226. doi: 10.1021/jo061299s. [DOI] [PubMed] [Google Scholar]
- 46.a) Blakskjær P, Høj B, Riber D, Skrydstrup T. J Am Chem Soc. 2003;125:4030–4031. doi: 10.1021/ja029662+. [DOI] [PubMed] [Google Scholar]; b) Mikkelsen LM, Jensen CM, Høj B, Blakskjær P, Skrydstrup T. Tetrahedron. 2003;59:10541–10549. [Google Scholar]; c) Jensen CM, Lindsay KB, Taaning RH, Karaffa J, Hansen AM, Skrydstrup T. J Am Chem Soc. 2005;127:6544–6545. doi: 10.1021/ja050420u. [DOI] [PubMed] [Google Scholar]; d) Jensen CM, Lindsay KB, Andreasen P, Skrydstrup T. J Org Chem. 2005;70:7512–7519. doi: 10.1021/jo0505775. [DOI] [PubMed] [Google Scholar]
- 47.Ready JM, Reisman SE, Hirata M, Weiss MM, Tamaki K, Ovaska TV, Wood JL. Angew Chem. 2004;116:1290–1292. doi: 10.1002/anie.200353282. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:1270–1272. doi: 10.1002/anie.200353282. [DOI] [PubMed] [Google Scholar]
- 48.Kim YH, Park HS, Kwon DW. Synth Commun. 1998;28:4517–4524. [Google Scholar]
- 49.Fuchs JR, Mitchell ML, Shabangi M, Flowers RA., II Tetrahedron Lett. 1997;38:8157–8158. [Google Scholar]
- 50.a) Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J Am Chem Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s. [DOI] [PubMed] [Google Scholar]; b) Reisman SE, Ready JM, Weiss MM, Hasuoka A, Hirata M, Tamaki K, Ovaska TV, Smith CJ, Wood JL. J Am Chem Soc. 2008;130:2087–2100. doi: 10.1021/ja076663z. [DOI] [PubMed] [Google Scholar]
- 51.For selected reviews of the pinacol reaction, see: Ladipo FT. Curr Org Chem. 2006;10:965–980.Chatterjee A, Joshi NN. Tetrahedron. 2006;62:12137–12158.Hirao T. Top Curr Chem. 2007;411:53–76.
- 52.a) Namy JL, Souppe J, Kagan HB. Tetrahedron Lett. 1983;24:765–766. [Google Scholar]; b) Souppe J, Danon L, Namy JL, Kagan HB. J Organomet Chem. 1983;250:227–236. [Google Scholar]
- 53.Molander GA, Kenny C. J Org Chem. 1988;53:2132–2134. [Google Scholar]
- 54.Chiara JL, Cabri W, Hanessian S. Tetrahedron Lett. 1991;32:1125–1128. [Google Scholar]
- 55.a) Hanamoto T, Inanaga J. Tetrahedron Lett. 1991;32:3555–3556. [Google Scholar]; b) Chiara JL, Marco-Contelles J, Khiar N, Gallego P, Destabel C, Bernabé M. J Org Chem. 1995;60:6010–6011. doi: 10.1021/jo970987w. [DOI] [PubMed] [Google Scholar]; c) Miyabe H, Toruda M, Inoue K, Tajiri K, Kiguchi T, Naito T. J Org Chem. 1998;63:4397–4407. [Google Scholar]
- 56.a) Molander GA, Kenny C. J Am Chem Soc. 1989;111:8236–8246. [Google Scholar]; b) Zhou LH, Zhang YM, Shi DQ. Synthesis. 2000:91–98. [Google Scholar]
- 57.a) Sturino CF, Fallis AG. J Am Chem Soc. 1994;116:7447–7448. [Google Scholar]; b) Sturino CF, Fallis AG. J Org Chem. 1994;59:6514–6516. [Google Scholar]
- 58.Kraus GA, Sy JO. J Org Chem. 1989;54:77–83. [Google Scholar]
- 59.Corey EJ, Pyne SG. Tetrahedron Lett. 1983;24:2821–2824. [Google Scholar]
- 60.Ikeda T, Yue S, Hutchinson CR. J Org Chem. 1985;50:5193–5199. [Google Scholar]
- 61.a) Nicolaou KC, Huang X, Giuseppone N, Rao PB, Bella M, Reddy MV, Snyder SA. Angew Chem. 2001;113:4841–4845. doi: 10.1002/1521-3773(20011217)40:24<4705::aid-anie4705>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:4705–4709. doi: 10.1002/1521-3773(20011217)40:24<4705::aid-anie4705>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Rao PB, Hao J, Reddy MV, Rassias G, Huang X, Chen DYK, Snyder SA. Angew Chem. 2003;115:1795–1800. doi: 10.1002/anie.200351112. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:1753–1758. doi: 10.1002/anie.200351112. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Snyder SA, Giuseppone N, Huang X, Bella M, Reddy MV, Rao PB, Koumbis AE, Giannakakou P, O'Brate A. J Am Chem Soc. 2004;126:10174–10182. doi: 10.1021/ja040091q. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Hao J, Reddy MV, Rao PB, Rassias G, Snyder SA, Huang X, Chen DYK, Brenzovich WE, Giuseppone N, Giannakakou P, O'Brate A. J Am Chem Soc. 2004;126:12897–12906. doi: 10.1021/ja040093a. [DOI] [PubMed] [Google Scholar]
- 62.For the first total synthesis of diazonamide A, see: Nicolaou KC, Bella M, Chen DYK, Huang X, Ling T, Snyder SA. Angew Chem. 2002;114:3645–3649. doi: 10.1002/1521-3773(20020916)41:18<3495::AID-ANIE3495>3.0.CO;2-7.Angew Chem Int Ed. 2002;41:3495–3499. doi: 10.1002/1521-3773(20020916)41:18<3495::AID-ANIE3495>3.0.CO;2-7.Nicolaou KC, Chen DYK, Huang X, Ling T, Bella M, Snyder SA. J Am Chem Soc. 2004;126:12888–12896. doi: 10.1021/ja040092i.
- 63.For selected reviews of fragmentation reactions in organic chemistry, see: Wakabayashi T, Watanabe K. J Synth Org Chem Jpn. 1980;38:853–861.Li JJ. Tetrahedron. 2001;57:1–24.Bradley D, Williams G, Blann K, Caddy J. Org Prep Proc Int. 2001;33:565–602.Williams DBG, Caddy J, Blann K. Org Prep Proc Int. 2003;35:307–360.
- 64.a) Morihira K, Hara R, Kawahara S, Nishimori T, Nakamura N, Kusama H, Kuwajima I. J Am Chem Soc. 1998;120:12980–12981. [Google Scholar]; b) Kusama H, Hara R, Kawahara S, Nishimori T, Kashima H, Nakamura N, Morihira K, Kuwajima I. J Am Chem Soc. 2000;122:3811–3820. [Google Scholar]; c) Kuwajima I, Kusama H. Synlett. 2000;10:1385–1401. [Google Scholar]
- 65.Sheikh SE, zu Greffen AM, Lex J, Neudörfl JM, Schmalz HG. Synlett. 2007;12:1881–1884. [Google Scholar]
- 66.a) Shipe WD, Sorensen EJ. Org Lett. 2002;4:2063–2066. doi: 10.1021/ol0259342. [DOI] [PubMed] [Google Scholar]; b) Shipe WD, Sorensen EJ. J Am Chem Soc. 2006;128:7025–7035. doi: 10.1021/ja060847g. [DOI] [PubMed] [Google Scholar]
- 67.a) Bode JW, Carreira EM. J Am Chem Soc. 2001;123:3611–3612. doi: 10.1021/ja0155635. [DOI] [PubMed] [Google Scholar]; b) Bode JW, Carreira EM. J Org Chem. 2001;66:6410–6424. doi: 10.1021/jo015791h. [DOI] [PubMed] [Google Scholar]
- 68.Otaka A, Yukimasa A, Watanabe J, Sasaki Y, Oishi S, Tamamura H, Fujii N. Chem Commun. 2003:1834–1835. doi: 10.1039/b303718e. [DOI] [PubMed] [Google Scholar]
- 69.a) Exon C, Gallagher T, Magnus P. J Am Chem Soc. 1983;105:4739–4749. [Google Scholar]; b) Vedejs E, Lin S. J Org Chem. 1994;59:1602–1603. [Google Scholar]; c) Chiara JL, Destabel C, Gallego P, Marco-Contelles J. J Org Chem. 1996;61:359–360. doi: 10.1021/jo970987w. [DOI] [PubMed] [Google Scholar]
- 70.a) Hosokawa S, Yokota K, Imamura K, Suzuki Y, Kawarasaki M, Tatsuta K. Tetrahedron Lett. 2006;47:5415–5418. [Google Scholar]; b) Hosokawa S, Yokota K, Imamura K, Suzuki Y, Kawarasaki M, Tastuta K. Chem Asian J. 2008;3:1415–1421. doi: 10.1002/asia.200800109. [DOI] [PubMed] [Google Scholar]
- 71.a) Yano K, Yokoi K, Sato J, Oono J, Kouda T, Ogawa Y, Nakashima T. J Antibiot. 1986;39:32–37. doi: 10.7164/antibiotics.39.32. [DOI] [PubMed] [Google Scholar]; b) Yano K, Yokoi K, Sato J, Oono J, Kouda T, Ogawa Y, Nakashima T. J Antibiot. 1986;39:38–43. doi: 10.7164/antibiotics.39.38. [DOI] [PubMed] [Google Scholar]
- 72.a) Masters JL, Link JT, Snyder LB, Young WB, Danishefsky SJ. Angew Chem. 1995;107:1886–1888. [Google Scholar]; Angew Chem Int Ed Eng. 1995;34:1723–1726. [Google Scholar]; b) Danishefsky SJ, Masters JJ, Young WB, Link JT, Snyder LB, Magee TV, Jung DK, Isaacs RCA, Bornmann WG, Alaimo CA, Coburn CA, Di Grandi MJ. J Am Chem Soc. 1996;118:2843–2859. [Google Scholar]
- 73.a) Jacquot DEN, Hoffman H, Polborn K, Lindel T. Tetrahedron Lett. 2002;43:3699–3702. [Google Scholar]; b) Jacquot DEN, Zöllinger M, Lindel T. Angew Chem. 2005;117:2336–2338. doi: 10.1002/anie.200462252. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:2295–2298. [Google Scholar]; c) Zöllinger M, Mayer P, Lindel T. J Org Chem. 2006;71:9431–9439. doi: 10.1021/jo061813u. [DOI] [PubMed] [Google Scholar]
- 74.Nicolaou KC, Nold AL, Li H. Angew Chem. accepted; Angew. Chem. Int. Ed., accepted. [Google Scholar]
- 75.Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A. J Am Chem Soc. 2007;129:10356–10357. doi: 10.1021/ja074297d. [DOI] [PubMed] [Google Scholar]
- 76.Rivkin A, González-López de Turiso F, Nagashima T, Curran DP. J Org Chem. 2004;69:3719–3725. doi: 10.1021/jo049873s. [DOI] [PubMed] [Google Scholar]
- 77.Link JT, Overman LE. J Am Chem Soc. 1996;118:8166–8167. [Google Scholar]
- 78.a) Hendrickson JB, Göschke R, Rees R. Tetrahedron. 1964;20:565–579. [Google Scholar]; b) Hall ES, McCapra F, Scott AI. Tetrahedron. 1967;23:4131–4141. doi: 10.1016/s0040-4020(01)97925-6. [DOI] [PubMed] [Google Scholar]
- 79.a) Matsuo Ji, Ogawa Y, Pudhom K, Mukaiyama T. Chem Lett. 2004;33:124–125. [Google Scholar]; b) Ogawa Y, Kuroda K, Matsuo Ji, Mukaiyama T. Bull Chem Soc Jpn. 2005;78:677–697. [Google Scholar]
- 80.a) Nagaoka H, Shibuya K, Yamada Y. Tetrahedron Lett. 1993;34:1501–1504. [Google Scholar]; b) Nagaoka H, Shibuya K, Yamada Y. Tetrahedron. 1994;50:661–688. [Google Scholar]
- 81.Shibuya K, Nagaoka H, Yamada Y. J Chem Soc, Chem Commun. 1991:1545–1546. [Google Scholar]
- 82.a) Shirai A, Miyata O, Tohnai N, Miyata M, Procter DJ, Sucunza D, Naito T. J Org Chem. 2008;73:4464–4475. doi: 10.1021/jo800560p. [DOI] [PubMed] [Google Scholar]; b) Miyata O, Shira A, Yoshino S, Takeda Y, Sugiura M, Naito T. Synlett. 2006;6:893–896. [Google Scholar]
- 83.a) Miyata O, Shirai A, Yoshino S, Nakabayashi T, Takeda Y, Kiguchi T, Fukumoto D, Ueda M, Naito T. Tetrahedron. 2007;63:10092–10117. [Google Scholar]; b) Rahaman H, Shirai A, Miyata O, Naito T. Tetrahedron Lett. 2008;49:5789–5792. [Google Scholar]
- 84.a) Nicolaou KC, Jennings MP, Dagneau P. Chem Commun. 2002:2480–2481. [Google Scholar]; b) Nicolaou KC, Tang W, Dagneau P, Faraoni R. Angew Chem. 2005;117:3942–3947. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:3874–3879. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Zhang H, Ortiz A, Dagneau P. Angew Chem. 2008;120:8733–8738. doi: 10.1002/anie.200804228. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:8605–8610. doi: 10.1002/anie.200804228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.a) Nicolaou KC, Zhang H, Ortiz A. Angew Chem; Angew Chem Int Ed. accepted. [Google Scholar]; b) Nicolaou KC, Ortiz A, Zhang H. Angew Chem; Angew Chem Int Ed. accepted. [Google Scholar]
- 86.Piers E, Gilbert M, Cook KL. Org Lett. 2000;2:1407–1410. doi: 10.1021/ol0057333. [DOI] [PubMed] [Google Scholar]
- 87.a) Hasegawa E, Kitazume T, Suzuki K, Tosaka E. Tetrahedron Lett. 1998;39:4059–4062. [Google Scholar]; b) Tsuchida H, Tamura M, Hasegawa E. J Org Chem. 2009;74:2467–2475. doi: 10.1021/jo802749g. [DOI] [PubMed] [Google Scholar]
- 88.Chung SH, Cho MS, Choi JY, Kwon DW, Kim YH. Synlett. 2001;8:1266–1268. [Google Scholar]
- 89.Takano M, Umino A, Nakada M. Org Lett. 2004;6:4897–4900. doi: 10.1021/ol048010i. [DOI] [PubMed] [Google Scholar]
- 90.a) Watanabe H, Takano M, Umino A, Ito T, Ishikawa H, Nakada M. Org Lett. 2007;9:352–362. doi: 10.1021/ol0628816. [DOI] [PubMed] [Google Scholar]; b) Watanabe H, Nakada M. J Am Chem Soc. 2008;130:1150–11151. doi: 10.1021/ja7102795. [DOI] [PubMed] [Google Scholar]
- 91.Okamura WH, Aurrecoechea JM, Gibbs RA, Norman AW. J Org Chem. 1989;54:4072–4083. [Google Scholar]
- 92.Tabuchi T, Inanaga J, Yamaguchi M. Tetrahedron Lett. 1986;27:5237–5240. [Google Scholar]
- 93.Lange GL, Corelli N. Tetrahedron Lett. 2007;48:1963–1965. [Google Scholar]
- 94.Lange GL, Gottardo C. J Org Chem. 1995;60:2183–2187. [Google Scholar]
- 95.Shenvi RA, Guerrero CA, Shi J, Li CC, Baran PS. J Am Chem Soc. 2008;130:7241–7243. doi: 10.1021/ja8023466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.a) Tanaka A, Oritani T. Tetrahedron Lett. 1997;38:1955–1956. [Google Scholar]; b) Saito A, Saito K, Tanaka A, Oritani T. Tetrahedron Lett. 1997;38:3955–3958. [Google Scholar]; c) Firouzabadi H, Iranpoor N, Shaterian HR. Bull Chem Soc Jpn. 2002;75:2195–2205. [Google Scholar]; d) Gupta N, Kad GL, Singh V, Singh J. Synth Commun. 2007;37:3421–3428. [Google Scholar]
- 97.Fevig TL, Elliott RL, Curran DP. J Am Chem Soc. 1988;110:5064–5067. [Google Scholar]
- 98.Boffey RJ, Santagostino M, Whittingham WG, Kilburn JD. Chem Commun. 1998:1875–1876. [Google Scholar]
- 99.Boffey RJ, Whittingham WG, Kilburn JD. J Chem Soc, Perkin Trans 1. 2001:487–496. [Google Scholar]
- 100.Watson FC, Kilburn JD. Tetrahedron Lett. 2000;41:10341–10345. [Google Scholar]
- 101.Corey EJ, Wang Z. Tetrahedron Lett. 1994;35:539–542. [Google Scholar]