Abstract
Manufactured nanoparticles of aluminum oxide (nano-alumina) have been widely used in the environment; however, their potential toxicity provides a growing concern for human health. The present study focuses on the hypothesis that nano-alumina can affect the blood-brain barrier and induce endothelial toxicity. In the first series of experiments, human brain microvascular endothelial cells (HBMEC) were exposed to alumina and control nanoparticles in dose- and time-responsive manners. Treatment with nano-alumina markedly reduced HBMEC viability, altered mitochondrial potential, increased cellular oxidation, and decreased tight junction protein expression as compared to control nanoparticles. Alterations of tight junction protein levels were prevented by cellular enrichment with glutathione. In the second series of experiments, rats were infused with nano-alumina at the dose of 29 mg/kg and the brains were stained for expression of tight junction proteins. Treatment with nano-alumina resulted in a marked fragmentation and disruption of integrity of claudin-5 and occludin. These results indicate that cerebral vasculature can be affected by nano-alumina. In addition, our data indicate that alterations of mitochondrial functions may be the underlying mechanism of nano-alumina toxicity.
Keywords: manufactured nanoparticles, nano-alumina, blood-brain barrier, tight junctions
Introduction
Nanotechnology uses engineered materials or devices at the nanometer scale, typically ranging from 1 to ∼100 nm (Oberdorster et al. 2005). Various nanotechnology applications have been used for treatment, diagnosis, monitoring, and controlling of biological systems (Moghimi et al. 2005; Silva 2006). With respect to neuroscience, this technology has been employed for targeted drug delivery into the central nervous system (CNS) and the development of pharmacological, therapeutic, and diagnostic agents for CNS disorders (Uwatoku et al. 2003; Bianco et al. 2005; Olivier 2005; Silva 2006). Examples include “Trojan horse” drug carriers to the brain, application of gold nanoparticles for fluorescence resonance energy transfer measurements, and iron oxide nanocrystals with super-paramagnetic properties for magnetic resonance imaging (Koziara et al. 2003; Lockman et al. 2003; Uwatoku et al. 2003; Bianco et al. 2005; Moghimi et al. 2005; Olivier 2005; Silva 2006). Because of the diverse potential of nanoparticles, their occupational and public exposure will dramatically increase in the future. It is estimated that the production rates of engineered nanoparticles will increase to 58,000 metric tons per year by the year 2011 (Maynard 2007).
Because nanoparticles have high surface reactivity, they may have negative health and environmental impacts. Their small sizes facilitate cellular uptake and transcytosis across epithelial and endothelial cells into the blood and lymph circulation to reach potentially sensitive target sites such as brain, bone marrow, lymph nodes, spleen, and heart (Colvin 2003; Campbell et al. 2004). Nanoparticle access to the CNS and ganglia via translocation along axons and dendrites of neurons has also been observed (Oberdorster et al. 2005; Nel et al. 2006). Thus, specific types of nanoparticles can readily travel throughout the body, deposit in target organs, penetrate cell membranes, and lodge in mitochondria or nuclei. These events may trigger injurious responses at the cellular, subcellular, and protein levels. Overall, these facts necessitate the study of environmental impact and toxicity of nanoparticles.
Aluminum is relatively stable in the form of alumina (aluminum oxide) and can enter the body through drinking water, food intake, inhalation, and skin contact. In addition, specific medical interventions, such as dialysis or certain aluminum-containing drugs, may lead to aluminum accumulation in the tissues. Alumina is among the most abundantly produced chemical in nanosized particles, estimated to account for approximately 20% of the 2005 world market of nanoparticles (Rittner 2002). Aluminum can act as a disrupter of cell membranes and has been implicated as an etiological factor in a variety of neurode-generative diseases (Vorbrodt et al. 1994; Gault et al. 2005). For example, a number of studies have revealed aluminum deposits in the brains of Alzheimer’s disease patients, where it may potentiate the neurotoxicity and facilitate the disease process (Yokel and McNamara 2001; Becaria et al. 2002; Yokel et al. 2002; Service 2004; Kawahara 2005; Lukiw et al. 2005; Banks et al. 2006). There is evidence that exposure to aluminum may also contribute to an increase in oxidative stress, inflammatory events, and/or the breakdown of the blood-brain barrier (BBB) (Vorbrodt et al. 1994; Lockman et al. 2004; Yang and Watts 2005). These are important events because disruption of the BBB is associated with the development and/or progression of stroke, ischemia/reperfusion, hypoxia/reoxygenation and vascular dysfunction, and Alzheimer’s disease (Hawkins and Davis 2005; Weksler et al. 2005; Abbott et al. 2006).
Due to their potential toxic effects, manufactured nanoparticles are an emerging concern in vascular biology (Nel et al. 2006). Therefore, the aim of the present study is to evaluate the effects of nano-alumina on the integrity of the BBB. Our results indicate that nano-alumina can disrupt the BBB via alterations of cellular redox status and disruption of mitochondrial functions to a significantly greater extent than carbon nanoparticles. Nano-alumina-mediated endothelial toxicity was markedly attenuated by enhancing cellular glutathione levels.
Materials and methods
Cell system, animals, and treatment factors
A human brain microvascular endothelial cell (HBMEC) line was used in in vitro experiments. This cell line was recently developed (Weksler et al. 2005). It retains all morphological and functional characteristics of human brain endothelial cells. Cells were treated with nano-alumina (8–12 nm particle size, Alfa Aesar, Ward Hill, MA, USA) from 1 µM to 10 mM for up to 24 h. Alumina nanoparticles were extensively described in an earlier publication (Oesterling et al. 2008). Normal size alumina particles, nano-carbon particles, or carbon not converted into nanoparticles (Sigma-Aldrich, St. Louis, MO, USA) were used as the controls. In selected experiments, HBMEC were also exposed to 1 mM glutathione.
In animal experiments, Fisher 344 rats (354–411 g, Harlan, Madison, WI, USA) were intravenously infused with nano-alumina at the dose of 29 mg/kg as ∼0.6% dispersion in water with concurrent infusion of an equal volume of 1.8% saline via a second cannula. Two control rats (376 and 413 g) that received no treatment or water and 1.8% saline infusion were also studied. Rats were sacrificed 20 h postinfusion.
Cell viability and mitochondrial potential
Cell viability was assessed by the MTT conversion assay. Following treatment exposure, thiazolyl blue tetrazolium bromide (MTT) solution was added to the culture media and allowed to incubate for 2 h. Then, the cells were washed with PBS and the converted formazine was resolved in 200 µl DMSO. The absorbance was measured at 594 nm with 645 nm as the reference.
Mitochondrial membrane potential was assessed using MitoTracker® Red CMXRos (Invitrogen Corporation, Carlsbad, CA, USA) and a fluorescent dye JC-1. For the MitoTracker® Red CMXRos method, treated cultures were incubated with this dye for 15 min at 37°C in a cell culture incubator. The cells were then washed twice with normal medium, fixed in 10% formalin, and stained with FITC labeled phalloidin (Invitrogen) to detect cytoskeleton changes. The images were captured on the Olympus confocal microscope FluoView 300 with the filters proper for Texas-Red, FITC and DAPI.
The changes in mitochondrial membrane potential were quantified using JC-1. Once loaded into the mitochondria, JC-1 undergoes aggregate formation in the regions of high potential. The resulting spectral shift of the dye can be used to detect changes in mitochondrial activity. The green fluorescent JC-1 (5,5′, 6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcabocyanine iodine, Invitrogen-Molecular Probes, Eugene, OR, USA) exists as a monomer at low membrane potential. In polarized mitochondria, JC-1 forms red fluorescent aggregates that exhibit a broad spectrum and an emission maximum at 590 nm. Depolarization results in the dissociation of aggregates into monomers and a concomitant shift in fluorescence to an emission wavelength of about 525 nm. Treated endothelial cells were loaded for 20 min at 37°C with 5 µg/ml JC-1 dissolved in DMSO. Then, the cultures were washed and cellular fluorescence was measured in a fluorescence plate reader set at an excitation wavelength of 485 nm and emission wavelengths of 530 nm (JC-1 monomer) and 590 nm (JC-1 aggregates).
Superoxide production
Dihydroethidium (DHE, Invitrogen) was employed to detect cellular levels of superoxide. The method is based on the principle that DHE in a reaction with superoxide is oxidized to red fluorescent ethidium, which binds to DNA in the nucleus (Fink et al. 2004; Morten et al. 2006). To estimate superoxide production, cultures were incubated with 10 µM DHE for 45 min at 37°C. Then, the cells were washed and fluorescence was measured at excitation 480 nm and emission 567 nm using a fluorescence plate reader.
Evaluation of tight junction proteins
In studies on tight junctions, Western blotting is more sensitive than immunohistochemistry in the assessment of differences in total protein levels. On the other hand, immunostaining can visualize the integrity of tight junctions. Therefore, both Western blotting and immunofluorescent microscopy were employed to detect alterations in tight junction protein expression in the present study. Treated HBMEC cultures were lysed in the RIPA buffer (50 mM Tris–HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 0.1 mg/ml PMSF, 1 mM Na3VO4, 2 mM NaF, 10 µg/ml aprotinin, and leupeptin). Tight junction proteins, such as junctional adhesion molecule-A (JAM-A), and zonula occludens (ZO)-1 and ZO-2 were detected by Western blotting using specific antibodies. In animal experiments, the brains were fixed and sliced and then the expression of tight junction proteins, such as claudin-5 and occludin, was detected by immunostaining.
Statistical analysis
Dependent on the experimental design, one- or two-way ANOVA was used to compare responses among treatments. Treatment means were compared using Bonferroni’s least significant difference procedure and p < 0.05 was considered significant.
Results
Treatment with nano-alumina alters HBMEC morphology and viability
Confluent HBMEC cultures were treated with nano-alumina or carbon nanoparticles for 24 h and the cell morphology was examined with a phase-contrast microscope. As indicated in Fig. 1a, nano-alumina in a dose-dependent manner induced a marked cell shrinkage and formation of apoptotic-like bodies. In cells treated with nano-carbon particles, these effects were minimal. We also completed viability studies in HBMEC cultures exposed to nano-alumina, carbon nanoparticles, or the respective parent compounds of normal particle size. As shown in Fig. 1b, treatment with nano-alumina, 0.1 mM or higher, significantly decreased HBMEC viability as determined by the MTT conversion assay. Exposure to normal alumina or carbon did not affect HBMEC viability. At the highest concentration (10 mM), carbon nanoparticles also decreased HBMEC viability. However, these effects were significantly less pronounced as compared to those induced by nano-alumina.
Fig. 1.
Nano-alumina induces injury and death to HBMEC. a Confluent HBMEC cultures were treated with the indicated concentrations of nano-alumina (nano-Al) or carbon nanoparticles (nano-C) for 24 h. Nano-alumina-treated cells exhibit a marked cell shrinkage and apoptotic body formation (arrows). In nano-carbon-treated cells these changes are less apparent. b Cell viability was assessed by the MTT conversion assay. Cells were exposed to the indicated concentrations of nano-alumina, alumina, carbon nanoparticles (nano-carbon), or carbon for 24 h. Values are means±SEM. * Values in cultures treated with nanoparticles are significantly different as compared to the respective parent compounds. † Values in the nano-alumina group are significantly different as compared to the nano-carbon group
Nano-alumina induces mitochondrial dysfunction and induces oxidative stress in HBMEC
Confluent HBMEC were treated with nano-alumina or carbon nanoparticles for 24 h and the mitochondrial membrane potential was assessed using the dyes MitoRed CMXRos and JC-1. Mitochondria in the nano-alumina treated groups exhibited either a diffuse staining pattern (cells exposed to 10 µM nano-alumina; arrows) or were disrupted and fragmented (cells exposed to 1 mM nano-alumina; arrows), suggesting a progressive and dose-dependent loss of mitochondrial potential and function. The staining pattern of mitochondria was preserved in cultures treated with carbon nanoparticles (Fig. 2a).
Fig. 2.
Nano-alumina induces loss of mitochondrial membrane potential. a Confluent HBMEC were treated with the indicated concentrations of nano-alumina (nano-Al) and carbon nanoparticles (nano-C) for 24 h and mitochondrial potential was assessed by MitoRed CMXRos staining. Mitochondria in the 10 µM nano-alumina-treated groups exhibit diffuse staining pattern (arrows). In addition, staining pattern of mitochondria is disrupted and fragmented (arrows) in HBMEC exposed to 1 mM nano-alumina. b Mitochondrial membrane potential was quantified using a fluorescent dye JC-1. Values are means±SEM. * Statistically different as compared to control
Alterations of mitochondrial membrane potential were quantified using a fluorescent dye JC-1. As indicated in Fig. 2b, treatment with 10 µM nano-alumina but not with the same concentration of carbon nanoparticles resulted in a decrease in mitochondrial membrane potential. However, both types of nanoparticles at 1 mM were toxic and altered mitochondrial potential.
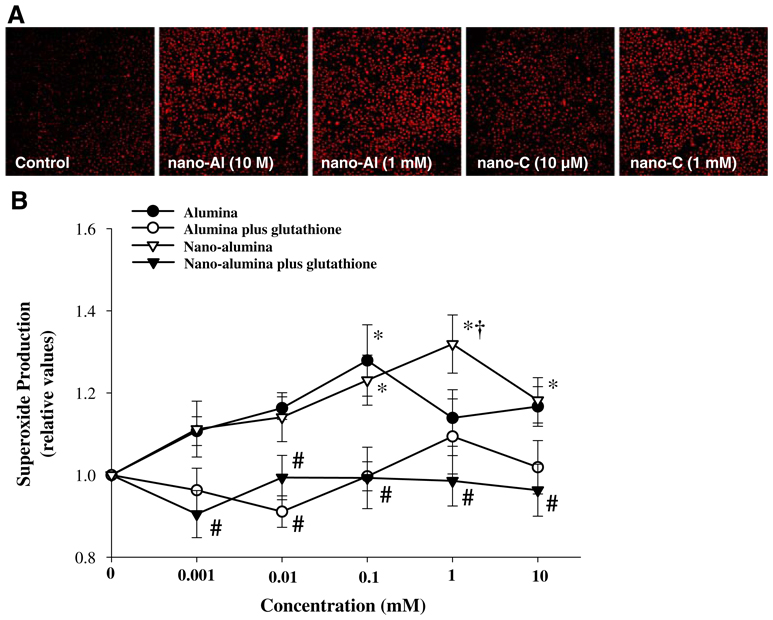
Alterations of mitochondrial potential can result in the induction of cellular oxidative stress. Therefore, confluent HBMEC were treated with nano-alumina or carbon nanoparticles for 24 h, followed by incubation with dihydroethidium (DHE, 10 µM) to assess superoxide production. As depicted in Fig. 3a, treatment of HBMEC with nano-alumina at 10 µM markedly increased cellular oxidation as compared to the effects of carbon nanoparticles. Nano-alumina-induced cellular oxidative stress was prevented by a concurrent treatment with glutathione (Fig. 3b).
Fig. 3.
Nano-alumina induces superoxide generation in HBMEC. a Confluent HBMEC cultures were exposed to the indicated concentrations of nano-alumina (nano-Al) or carbon nanoparticles for 24 h. Production of superoxide was measured using the DHE fluorescent assay and the intensity of red fluorescence is an indication of cellular oxidative stress. b Confluent HBMEC cultures were exposed to the indicated concentrations of nano-alumina or alumina for 24 h. In addition, selected cultures were treated with 1 mM glutathione at the same time as nano-alumina or alumina exposure. Production of superoxide was measured as in (a). The intensity of fluorescence was quantified and plotted using at least five images from three independent cultures. * Statistically different as compared to control (no treatment with alumina or nano-alumina). † Values in cultures treated with nano-alumina are significantly different as compared to alumina. # Values in the groups enriched with glutathione are significantly different as compared to the corresponding groups without added glutathione
Nano-alumina induces cytoskeleton re-arrangements and alterations of tight junction protein expression
In HBMEC treated with nano-alumina or carbon nanoparticles for 24 h, the cytoskeleton was visualized with FITC-conjugated phalloidin. As shown in Fig. 4b, exposure to nano-alumina at 10 µM resulted in rearrangements of F-actin with increased depositions at the cell–cell borders (arrows). Nano-alumina at the concentration of 1 mM generated loss of F-actin and induced formation of gaps between the cells (Fig. 4c, arrows). Carbon nanoparticles produced minimal changes in F-actin expression (Fig. 4d and e).
Fig. 4.
Nano-alumina induces F-actin re-arrangement in HBMEC. Confluent cultures were treated with the indicated concentrations of nano-alumina (nano-A; (a) and (b)) or nano-carbon (nano-C, (c) and (d)) for 24 h. Then, the cytoskeleton was visualized by staining with FITC-conjugated phalloidin. a Exposure to nano-alumina at 10 µM results in rearrangements of F-actin with depositions at the cell–cell boarders (arrows). b Nano-alumina at the concentration of 1 mM induces a loss of F-actin immunoreactivity and formations of gaps between the cells (arrows). d and e Exposure to carbon nanoparticles at 10 µM or 1 mM, respectively, produced minimal changes in F-actin expression
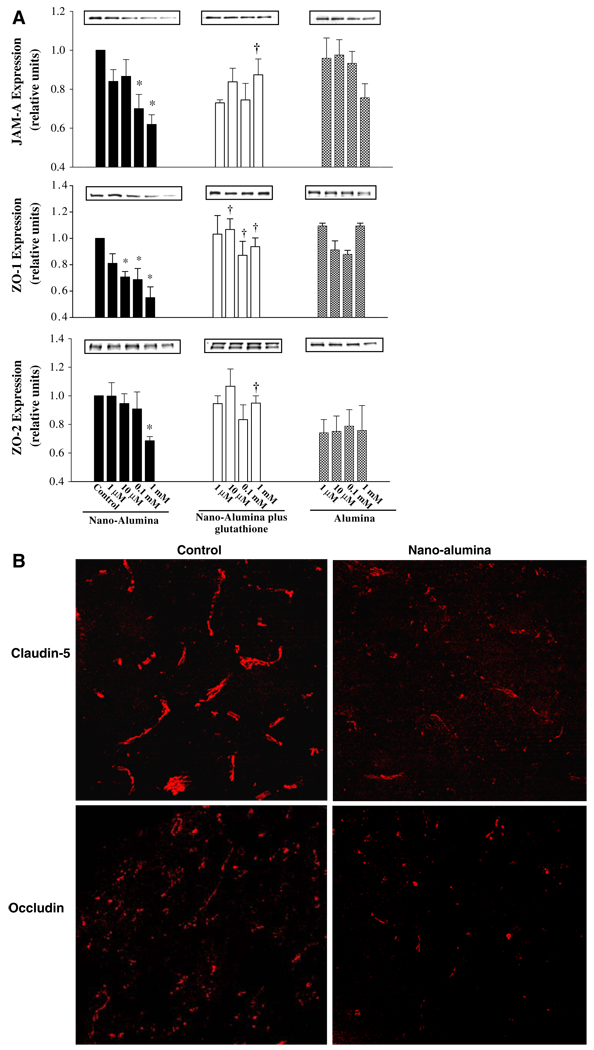
Cytoskeleton proteins are associated with tight junctions to create tight barrier properties of the brain endothelium. Therefore, we also evaluated the effects of nano-alumina on expression of tight junction proteins both in cell cultures and in animals. As illustrated in Fig. 5a, levels of the tight junction proteins JAM-A, ZO-1, and ZO-2 were markedly decreased in cultured HBMEC exposed to various concentrations of nano-alumina for 24 h. These effects were not significant in cells treated with alumina of normal particle size. Importantly, the effects of nano-alumina on tight junction protein expression were protected by concurrent treatment with glutathione. Alterations of tight junction protein expression were also confirmed in vivo. Figure 5b shows a dramatic loss of claudin-5 and occludin immunoreactivity along the cerebral vessels in rats infused with nano-alumina.
Fig. 5.
Nano-alumina reduces expression of tight junction proteins. a Confluent cultures were treated with indicated concentrations of nano-alumina or alumina for 24 h. In addition, selected cultures were co-treated with 1 mM glutathione. Expression of tight junction protein was analyzed by Western blotting. All experiments were repeated four times, the intensity of the blots were measured by densitometry and plotted.
* Statistically different as compared to non-treated controls. † Values in the nano-alumina plus glutathione group are statistically different as compared to the nano-alumina group. b Rats were intravenously administered with nano-alumina (8–12 nm; 29 mg/kg as ∼0.6% dispersion in water) and 20 h later the brains were sampled and stained for the presence of claudin-5 and occludin. Treatment with nano-alumina induced loss and fragmentation of claudin-5 and occludin immunoreactivity in cerebral vessels as compared to the sham-treated animals
Discussion
A growing use of nanotechnology for treatment, diagnosis, monitoring, and controlling of biological systems generates concerns related to the potential toxicity of nanocompounds to human health (Kralj and Pavelic 2003; Uwatoku et al. 2003; Service 2004; Bianco et al. 2005; Moghimi et al. 2005; Maynard et al. 2006; Kreuter 2007). There is also a lack of substantial data that can confirm or dismiss these concerns. In the present study, we hypothesized that exposure to nanoparticles of aluminum oxide (nano-alumina) can disrupt the integrity of the cerebral endothelium. Our studies indicate that nano-alumina can affect the cerebral vessels, alter mitochondrial membrane potential, induce cellular oxidative stress, and decrease the expression of tight junction proteins in brain endothelial cells. In contrast to nano-alumina, carbon nanoparticles induced less vascular toxicity.
The biological effects of nano-alumina may be induced either by the size of nanoparticles, metal properties, or a combination of these two factors. Therefore, we included several control treatments, such as biologically inert carbon nanoparticles or alumina of normal particle size. The results of the present study indicate that the size of nanoparticles can be detrimental to their endothelial effects. For example, treatment with nano-alumina resulted in a dose-dependent decrease in HBMEC viability, while endothelial toxicity of alumina was less significant. Moreover, high concentrations of carbon nanoparticles also induced significant loss of cell viability. Thus, even nano-carbon that is usually considered inert can interrupt normal endothelial cell functions resulting in cell death. These observations are consistent with reports that nanoparticles can readily enter the cell membrane and accumulate in the cytoplasm, disrupt metabolism, and induce cell dysfunction or even cell death (Lockman et al. 2003; Braydich-Stolle et al. 2005; Yang and Watts 2005). To support this notion, it was recently demonstrated that various metal oxide nanoparticles can lead to dysfunction of the endothelium; however, this process appears to be dependent upon the particle composition (Gojova et al. 2007). Iron oxide (Fe2O3), yttrium oxide (Y2O3), and zinc oxide (ZnO) were all internalized into human aortic endothelial cells, but only Y2O3 and ZnO stimulated expression of ICAM-1, interleukin-8, or monocyte chemotactic protein-1. In addition, the expression of several adhesion molecules was demonstrated in human umbilical vein endothelial cells exposed to alumina nanoparticles (Oesterling et al. 2008).
The normal plasma aluminum concentration is around 0.1 µM, while among patients with kidney failure receiving dialysis treatment or workers in an aluminum manufacturing factory the levels can reach 3.7 µM (Becaria et al. 2002; Gault et al. 2005; Banks et al. 2006). Thus, the levels of alumina and nano-alumina used in the present study were within the pathological range. Generally, alumina can be absorbed through the pulmonary and digestive systems or perhaps by skin contact, and is probably excreted as aluminum citrate. Alumina can enter the brain through transferrin receptor endocytosis (Yokel and McNamara 2001; Becaria et al. 2002; Yokel et al. 2002). The brain elimination half-life of alumina has been estimated to range from 0.7 to 9 years (Baydar et al. 2005). Although the elimination time is not known for nano-alumina, they may be preserved even longer, since that they can easily enter cells and accumulate in cell organelles. The accumulated alumina in the brain can execute its toxicity through multiple direct or indirect mechanisms, such as mitochondrial dysfunction, oxidative stress, and cell death, as indicated in the present study. Aluminum toxicity has been observed in many aspects of cells and organs (Yokel and McNamara 2001; Becaria et al. 2002; Yokel et al. 2002; Gault et al. 2005; Kawahara 2005; Banks et al. 2006), including vascular endothelial cells (Oesterling et al. 2008). However, the novelty of our study is the demonstration that alumina is directly toxic to human brain endothelial cells.
Our data indicate that alterations of tight junction protein expression may provide an important mechanism of nano-alumina toxicity in the CNS. Tight junctions seal brain endothelial cells along the cerebral microvessels and are responsible for low permeability and high electrical resistance of the brain endothelium. They are formed by transmembrane proteins such as occludin, claudins, and JAMs. These proteins are linked to the actin cytoskeleton by tight junction accessory proteins, such as ZO-1 and ZO-2 (Hawkins and Davis 2005; Abbott et al. 2006). Tight junctions are crucial for maintaining the integrity of the endothelium and preventing paracellular transfer of bloodborn substances or cells into the brain. Our novel data indicate that exposure of HBMEC to nano-alumina results in disruption of both transmembrane tight junction proteins and tight junction accessory proteins. In addition, exposure to nano-alumina has a disruptive impact on the cytoskeleton and induces fragmentation and loss of occludin and claudin-5 in animal studies, indicating a profound disruption of the critical elements that normally regulate the integrity of the BBB.
To evaluate potential mechanisms by which nano-alumina can disrupt the integrity of tight junction proteins, we focused on oxidative stress-related reactions. As indicated, HBMEC treatment with nano-alumina results in a loss of mitochondrial potential and induction of oxidative stress. Mitochondria consume over 90% of the oxygen in the cell for ATP production; thus the disruption of the mitochondrial respiratory chain can directly lead to lower ATP production and higher superoxide escape. In addition, cellular enrichment with glutathione protected against nano-alumina-mediated oxidative stress and disruption of tight junction protein expression, suggesting that antioxidants may be applied as a counter-treatment against nanoparticle toxicity.
Cellular oxidative stress induced by nano-alumina may initiate reactions involved in disruption of tight junction proteins. It is known that alterations of cellular oxidation can activate redox-responsive signaling pathways, such as the Rho and Ras cascades. The role of the Rho pathway in phosphorylation and downregulation of tight junction proteins has recently been recognized (Persidsky et al. 2006). In addition, we determined that the Ras/MAPK signaling is involved in the disruption of tight junction proteins (Andras et al. 2005). Cellular oxidation is also known to be involved in the stimulation of matrix metal-loproteinases that can hydrolyze tight junction proteins (Yang et al. 2007). Finally, proteins, including tight junction proteins modified by oxidative processes, can be degraded by proteasome activity (Lui and Lee 2005).
In conclusion, the results of the present study indicate that nanoparticles can be toxic to brain endothelial cells. Treatment with nano-alumina altered mitochondrial potential, induced cellular oxidative stress, and decreased expression of tight junction proteins to a higher extent as compared to carbon nanoparticles or alumina of normal particle size. Thus, it appears that mitochondrial functions and the integrity of tight junctions are the key targets for nano-alumina vascular toxicity in the brain.
Acknowledgements
Supported in part by Kentucky Science and Engineering Foundation (KSEF-07-RDE-010), P42 ES 07380, MH63022, MH072567, and NS39254.
Contributor Information
Lei Chen, Molecular Neuroscience and Vascular Biology Laboratory, Department of Neurosurgery, University of Kentucky Medical Center, 593 Wethington Bldg., 900 S Limestone, Lexington, KY 40536, USA.
Robert A. Yokel, College of Pharmacy, University of Kentucky, Lexington, KY 40536, USA
Bernhard Hennig, College of Agriculture, University of Kentucky, Lexington, KY 40536, USA.
Michal Toborek, Email: michal.toborek@uky.edu, Molecular Neuroscience and Vascular Biology Laboratory, Department of Neurosurgery, University of Kentucky Medical Center, 593 Wethington Bldg., 900 S Limestone, Lexington, KY 40536, USA.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- András IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, et al. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- Banks WA, Niehoff ML, Drago D, Zatta P. Aluminum complexing enhances amyloid beta protein penetration of blood-brain barrier. Brain Res. 2006;1116:215–221. doi: 10.1016/j.brainres.2006.07.112. doi: 10.1016/j.brainres.2006.07.112. [DOI] [PubMed] [Google Scholar]
- Baydar T, Nagymajtenyi L, Isimer A, Sahin G. Effect of folic acid supplementation on aluminum accumulation in rats. Nutrition. 2005;21:406–410. doi: 10.1016/j.nut.2004.07.008. doi: 10.1016/j.nut.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Becaria A, Campbell A, Bondy SC. Aluminum as a toxicant. Toxicol Ind Health. 2002;18:309–320. doi: 10.1191/0748233702th157oa. doi: 10.1191/0748233702th157oa. [DOI] [PubMed] [Google Scholar]
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Becaria A, Lahiri DK, Sharman K, Bondy SC. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J Neurosci Res. 2004;75:565–572. doi: 10.1002/jnr.10877. doi: 10.1002/jnr.10877. [DOI] [PubMed] [Google Scholar]
- Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–1170. doi: 10.1038/nbt875. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- Gault PM, Allen KR, Newton KE. Plasma aluminium: a redundant test for patients on dialysis? Ann Clin Biochem. 2005;42:51–54. doi: 10.1258/0004563053026862. doi: 10.1258/0004563053026862. [DOI] [PubMed] [Google Scholar]
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimers Dis. 2005;8:171–182. doi: 10.3233/jad-2005-8210. [DOI] [PubMed] [Google Scholar]
- Koziara JM, Lockman PR, Allen DD, Mumper RJ. In situ blood-brain barrier transport of nanoparticles. Pharm Res. 2003;20:1772–1778. doi: 10.1023/b:pham.0000003374.58641.62. doi: 10.1023/B:PHAM.0000003374.58641.62. [DOI] [PubMed] [Google Scholar]
- Kralj M, Pavelic K. Medicine on a small scale. EMBO Rep. 2003;4:1008–1012. doi: 10.1038/sj.embor.7400017. doi: 10.1038/sj.embor.7400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticles—a historical perspective. Int J Pharm. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Koziara J, Roder KE, Paulson J, Abbruscato TJ, Mumper RJ, et al. In vivo and in vitro assessment of baseline blood-brain barrier parameters in the presence of novel nanoparticles. Pharm Res. 2003;20:705–713. doi: 10.1023/a:1023492015851. doi: 10.1023/A:1023492015851. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12:635–641. doi: 10.1080/10611860400015936. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM. cAMP perturbs inter-Sertoli tight junction permeability barrier in vitro via its effect on proteasome-sensitive ubiquitination of occludin. J Cell Physiol. 2005;203:564–572. doi: 10.1002/jcp.20254. doi: 10.1002/jcp.20254. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Percy ME, Kruck TP. Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem. 2005;99:1895–1898. doi: 10.1016/j.jinorgbio.2005.04.021. doi: 10.1016/j.jinorgbio.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Maynard AD. Nanotechnology: the next big thing, or much ado about nothing? Ann Occup Hyg. 2007;51:1–12. doi: 10.1093/annhyg/mel071. doi: 10.1093/annhyg/mel071. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdörster G, et al. Safe handling of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- Morten KJ, Ackrell BA, Melov S. Mitochondrial reactive oxygen species in mice lacking superoxide dismutase 2: attenuation via antioxidant treatment. J Biol Chem. 2006;281:3354–3359. doi: 10.1074/jbc.M509261200. doi: 10.1074/jbc.M509261200. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterling E, Chopra N, Gavalas V, Arzuaga X, Lim EJ, Sultana R, et al. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol Lett. 2008;178:160–166. doi: 10.1016/j.toxlet.2008.03.011. doi: 10.1016/j.toxlet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, et al. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner MN. Market analysis of nanostructured materials. Am Ceram Soc Bull. 2002;81:33–36. [Google Scholar]
- Service RF. Nanotoxicology. Nanotechnology grows up. Science. 2004;304:1732–1734. doi: 10.1126/science.304.5678.1732. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- Silva GA. Neuroscience nanotechnology: progress, opportunities and challenges. Nat Rev Neurosci. 2006;7:65–74. doi: 10.1038/nrn1827. doi: 10.1038/nrn1827. [DOI] [PubMed] [Google Scholar]
- Uwatoku T, Shimokawa H, Abe K, Matsumoto Y, Hattori T, Oi K, et al. Application of nanoparticle technology for the prevention of restenosis after balloon injury in rats. Circ Res. 2003;92:e62–e69. doi: 10.1161/01.RES.0000069021.56380.E2. doi: 10.1161/01.RES.0000069021.56380.E2. [DOI] [PubMed] [Google Scholar]
- Vorbrodt AW, Dobrogowska DH, Lossinsky AS. Ultracytochemical studies of the effects of aluminum on the blood-brain barrier of mice. J Histochem Cytochem. 1994;42:203–212. doi: 10.1177/42.2.8288866. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway K, Leveque M, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PubMed] [Google Scholar]
- Yokel RA, McNamara PJ. Aluminium toxicokinetics: an updated minireview. Pharmacol Toxicol. 2001;88:159–167. doi: 10.1034/j.1600-0773.2001.d01-98.x. doi: 10.1034/j.1600-0773.2001.d01-98.x. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wilson M, Harris WR, Halestrap AP. Aluminum citrate uptake by immortalized brain endothelial cells: implications for its blood-brain barrier transport. Brain Res. 2002;930:101–110. doi: 10.1016/s0006-8993(02)02234-5. doi: 10.1016/S0006-8993(02)02234-5. [DOI] [PubMed] [Google Scholar]