Abstract
N, N-diethyl-m-toluamide (also known as DEET) is a broad-spectrum insect repellent that is used extensively against both human and animal pests, worldwide. Recent studies show that topical lipid formulations of DEET, such as LipoDEET, are highly effective in killing schistosome cercariae in the skin. Minimal systemic absorption, low manufacturing cost, and a wide range of activity against insects and schistosomes potentially makes compounds such as LipoDEET an excellent prophylactic agent against human and animal schistosomiasis in endemic areas, especially for travelers, until an effective vaccine is available.
An effective vaccine for human or animal schistosomiasis remains elusive, despite the high incidence of these infections in several parts of the world [1]. Schistosomiasis is a water-borne infection. The infective stages of the parasite, called cercariae, are released into fresh water by infected snails from several species such as Biomphalaria spp., Oncomelania spp. and Bulinus spp. Therefore, minimizing water contact and/or destruction of schistosome-bearing snails in endemic areas are probably the best ways to prevent this infection. Indeed, the St Lucia Island project, which involved a 15-year intense schistosomiasis control programme in St Lucia, is probably one of the greatest success stories of schistosomiasis control, indicating that it is possible to control and eradicate schistosomiasis in a well-contained region such as an island [2]. However, restricting people and animals from coming in to close contact with infected water sources in endemic are as in the mainland is an impracticable and daunting task [3]. Snail control in the mainland is probably a much more complicated preposition than that performed in an Island because of the vast area involved, cost of chemical or biological application, changing climatic conditions and several ecological problems [4]. Despite these hurdles, there are a few promising reports of partial success in the control of Schistosoma japonicum infection in mainland China and Philippines as a result of improved hygiene measures [5]. Another alternative to control schistosomiasis in endemic areas is mass chemotherapy with praziquantel, the only effective drug against schistosomiasis. Unfortunately, there are indications of potential drug resistance against praziquantel [6]. Added to this is the declining trend of reduced investment of pharmaceutical industries in drug research for tropical diseases mainly because of the high cost of drug development and registration [7]. Despite our enormous efforts directed towards schistosomiasis control during the past 50 years, schistosomiasis continues to be a global problem and there appears to be no decline in the incidence [8].
Infections with schistosomes occur when cercariae penetrate and enter into the body through intact skin. Therefore, blocking entry of cercariae into the skin could potentially control the infection. Over the years, several topical agents have been evaluated for their ability to block cercarial penetration into the skin [9–17]. Some of these compounds are highly effective, but none have been further developed for clinical application possibly because of potential toxicity, difficulty in manufacturing and/or difficulty in applying under field conditions.
Discovery of the cercaricidal property of DEET
During a routine drug screening experiment, we made an accidental, but interesting observation that N, N-diethyl-m-toluamide (also known as DEET) is a potent cercaricidal agent [18]. When cercariae were placed in cavity slides coated with 7.5% DEET, there was a dramatic reduction in the motility of cercariae and they shed their tails. Within five mins after exposure to DEET, degenerative changes were evident in their tegument and deeper parenchyma of the cercariae. Ten mins exposure to DEET resulted in 100% death of cercariae. This observation led us to investigate the potential of DEET as a topical agent to prevent cercarial penetration into the skin [18]. Application of 20% DEET in isopropanol to the abdominal skin or tail of mice one hour before exposure to 100–150 cercariae conferred 100% protection against infection. This confirmed the in vitro observations and the potential of developing DEET as a prophylactic agent against schistosomiasis. Subsequent studies by Secor et al. [19] and Twfeek [20] confirmed these findings. Further optimization studies showed that an application of 10% DEET in isopropanol to the skin 30 mins before infection confers 100% protection against S. mansoni infection in mice [18]. A preliminary study by Jackson et al.* on human subjects who visited Lake Malawi, a reservoir in Africa known for schistosomiasis, showed that application of 50% DEET over the whole body except scalp and genitalia prevented infection in 13 out of 15 individuals. The two infected individuals apparently had schistosomiasis before DEET application. It is not clear from their published report whether the two individuals were actually exposed to the infection or not in Lake Malawi. Nevertheless, this study suggests a potential for developing DEET as a prophylactic agent against schistosomiasis.
Lipid formulation of DEET that resists water
Schistosomiasis is acquired mainly through water contact. Therefore, a primary requisite for any topical anti-penetration agent is that it should be water-resistant. Unfortunately, repeated studies using 10–40% DEET in isopropanol failed to confer protection in mice when the skin was washed for 5–60 mins after application [21]. This indicated that DEET preparations in alcohol wash off easily, and therefore might not be suitable as a topical agent for the prevention of schistosomiasis. This finding prompted the screening of several lipid-based hydrophobic chemicals to identify a suitable vehicle that will retain DEET on the skin. Among the various preparations tested, a liposome-based formulation called LipoDEET [22] gave excellent anti-schistosomiasis properties [21]. Studies using a mouse model showed that a single skin application of 20% LipoDEET conferred 100% protection against all three species of human schistosomes, Schistosoma mansoni, S. japonicum and Schistosoma haematobium (Table 1). The livers of LipoDEET-treated animals were apparently normal with no granulomas or other lesions (Figure 1).
Table 1. Effect of DEET preparations on worm establishmenta.
| Agent (in 100 μl) |
Schistosoma mansoni (in mice)b |
Schistosoma japonicum (in mice)c |
Schistosoma haematobium (in hamsters)d |
|||
|---|---|---|---|---|---|---|
| Sample size# | Percentage establishment | Sample size# | Percentage establishment | Sample size# | Percentage establishment | |
| Isopropanol control | 10 | 78.47 ± 7.48 | 10 | 70.30 ± 11.06 | 5 | 46.00 ± 16.83 |
| 20% DEET | 10 | 0 ± 0 | 10 | 0 ± 0 | 10 | 0 ± 0 |
| Liposome control | 10 | 82.60 ± 4.67 | ND | ND | 5 | 38.00 ± 17.27 |
| 20% LipoDEET | 10 | 0 ± 0 | 10 | 0 ± 0 | 10 | 0 ± 0 |
| Ref. | [21] | –e | –f | |||
Worm establishment was determined by perfusion on Day 39–42 for Schistosoma mansoni, on Day 28–32 for Schistosoma japonicum and on Day 80–95 for Schistosoma haematobium. ND, no data available.
Mice were infected with 100 cercariae via the abdominal skin.
Mice were infected with 100 cercariae via the abdominal skin.
Hamsters were infected with 40 cercariae via the abdominal skin.
Data obtained from B. Salafsky et al. (2000) Topical application of a long-acting, safe, formulation of N-N-diethyl-m-toluamide (DEET) confers protection against Schistosoma japonicum. Abstract no. 507. 49th Annual meeting of the American Society of Tropical Medicine and Hygiene held 29th October to 2nd November 2000, in Houston, TX, USA.
Ramaswamy, K. and He, Y-X., unpublished data.
Figure 1.
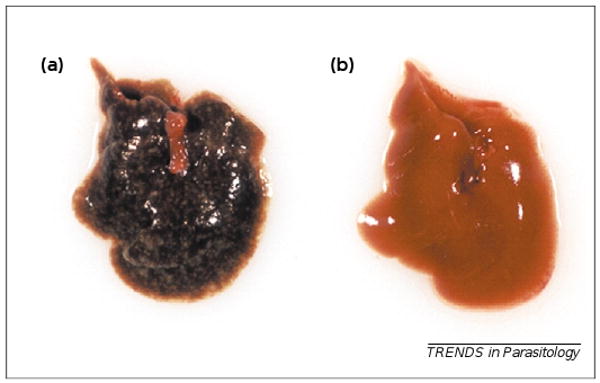
Effect of LipoDEET on liver pathology caused by Schistosoma japonicum. (a) Gross pathology of liver from a mouse 42 days post-infection with S. japonicum, showing several granulomatous foci. (b) Liver from a similarly infected mouse that had 20% LipoDEET applied topically to the skin 60 mins before infection.
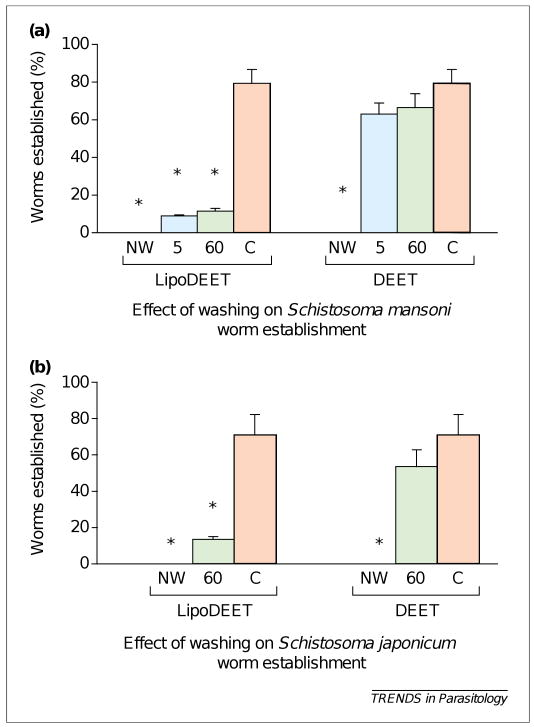
Pharmacokinetic studies suggested that LipoDEET is retained to the superficial layers of the skin and is not easily washed off with water. The loss of DEET activity was < 10% after washing the skin for 60 mins (applied with 20% LipoDEET) in water at 37°C [21]. Washing the skin for 60 mins with water after a single application of 20% LipoDEET still conferred >75% protection against S. mansoni infection and >80% protection against S. japonicum infection (Figure 2). This suggested that, in an endemic area, application of 20% LipoDEET to the skin before entering the water could substantially reduce the risk of infection. The anti-schistosomal activity is retained in the skin for up to 48 h if not washed with a detergent. Another interesting observation was that LipoDEET is minimally absorbed through the skin. Both high-performance liquid chromatography (HPLC) and tracer studies confirmed that only ∼7% of LipoDEET applied to the skin was systemically absorbed compared with 80% for DEET preparations in an alcohol vehicle [21]. Despite the fact that DEET has been used safely for the past 50 years by millions of people, worldwide, there are a few reports and experimental evidence of toxicity associated with systemic absorption of DEET in humans and animals, especially, if the chemical is misused [23–25]. The danger is more acute when DEET preparations containing alcohol were used [26,27]. Therefore, LipoDEET or a similar lipid-containing preparation of DEET that minimizes systemic absorption through the skin could be a safer formulation to reduce any possible DEET-associated toxicity [21,28].
Figure 2.
Effect of washing (for 5 mins and 60 mins) with water on the prophylactic activity of DEET and LipoDEET. Percentage of worm establishment in mice (10–15 mice were used per group) was determined by perfusion on Day 39–42 for Schistosoma mansoni (a) and on Day 28–32 for Schistosoma japonicum (b). The asterisks indicate significant p < 0.01 when compared with control mice that were not washed. Data in Figure 2a were obtained from Ref. [21]. Data in Figure 2b were obtained from B. Salafsky et al. (2000) Topical application of a long-acting, safe, formulation of N-N-diethyl-m-toluamide (DEET) confers protection against Schistosoma japonicum. Abstract no. 507. 49th Annual meeting of the American Society of Tropical Medicine and Hygiene held 29th October to 2nd November 2000, in Houston, TX, USA. Abbreviations: 5, 5 mins of washing with water; 60, 60 mins of washing with water; C, control; NW, not washed.
When applied post-infection, LipoDEET was only partially effective in preventing worm establishment. Topical application of 20% LipoDEET to the abdominal skin 60–90 mins after exposure to infection with S. mansoni in mice resulted in a reduction in the number of worms established per mouse from 87.2 ± 5.7 to 68.6 ± 2.1. This post-exposure effect was slightly improved to 64.4 ± 4.8% worm recovery when 20% LipoDEET was applied 30–60 min after exposure to infection with S. mansoni. Because LipoDEET is minimally absorbed through the skin, it is possible that a higher concentration of DEET in the lipid formulation could enhance the therapeutic effect of LipoDEET. LipoDEET or other lipid-containing formulations of DEETare highly effective as prophylactic agents; however, their ability to limit the infection post-exposure seems to be only partial.
The major factors that influence the use of an anti-infective compound under field conditions include its cost of manufacture and the ease of bringing it to the end user. In this respect, DEET appears to be highly cost-effective. Manufacture of LipoDEET requires only minimal facilities and can be easily produced in large quantities in an endemic area. The estimated cost for manufacturing 450 g of LipoDEET is ∼US$1 and 450 g of LipoDEET can be applied to ∼12 5000 mm2 surface area.
The laboratory investigations to date clearly demonstrate that lipid formulation of DEET is highly effective as a prophylactic agent. However, under field conditions, DEET by itself might not be sufficient to control schistosomiasis in an endemic area, rather lipid formulations of DEET could be used synergistically along with other control measures such as restricted water contact, snail control and prophylactic chemotherapy.
DEET as a prophylactic agent for travelers
Although chemical research has produced several DEET analogs and other related compounds, none has been consistently used or is as widespread as DEET. Since its first commercial application in 1956, DEET has become one of the most broadly used insect repellents for human and veterinary indications [29]. Repeated studies show that DEET is highly efficient in repelling various biting insects including mosquitoes, sandflies, tsetse, lice, fleas and ticks, which transmit deadly diseases such as malaria, filariasis, leishmaniasis, trypanosomiasis, west Nile fever, spirochetosis, lime disease, yellow fever and dengue fever. A recent study showed that topical application of LipoDEET is highly effective against ticks [30]. In schistosomiasis-endemic regions, people are also exposed to many of these insect-borne diseases. Thus, given its broad insect repellency and anti-schistosomal activity, LipoDEET or a similar lipid-containing preparation of DEET could be of benefit for travelers visiting an endemic area where schistosomiasis and other insect-borne infections are highly prevalent, especially if minimal water contact is expected. However, travelers should be made aware that, unlike insects that create a constant menace reminding the user to apply DEET, the cercarial penetration is an ‘invisible’ danger, requiring application of lipid-containing DEET to all the parts of the body exposed to the water. This is probably one of the major limitations of using DEET against schistosomiasis. Given the cercaricidal effect, a topical application of LipoDEET could also be used against cercarial dermatitis, an allergic skin reaction caused by the cutaneous migration of schistosomula of zoonotic schistosomes in the human skin. Previous studies using ethyl butylacetylaminopropionate (IR3535), an insect repellent, formulated as an ointment was unsuccessful in preventing cercarial dermatitis in a group of individuals participating in an annual cross-lake race in Annecy, France [31]. Therefore, it is important to develop a DEET preparation that does not wash off easily with water. Hence, the liposome preparation could have an advantage because the liposome can be formulated to stay in the skin for a longer duration of time by manipulating the size and charge of the liposome. However, minimizing water contact is the best way of protecting individuals and animals from schistosomiasis.
Perspective
DEET probably has one of the broadest spectrums of insect repellent activity. Despite the routine use of DEET by millions of people, only few adverse conditions have been reported over the past 50 years. New topical formulations of DEET, such as LipoDEET, are potentially safe and are probably ideal for travelers to endemic areas. In addition to protection against schistosomiasis, LipoDEET is also effective against a variety of biting insects that transmit deadly diseases. These unique advantages of lipid formulations of DEET, combined with the possibility of low-cost manufacturing in endemic areas, make them an attractive agent for the field control of schistosomiasis.
The conclusions stated here are based largely on laboratory studies. Therefore, further field studies are warranted, in particular studies that evaluate the potential of lipid formulations of DEET in reducing the morbidity due to schistosomiasis in animals living in an endemic area. Similarly, the effects of DEET on post exposure to the infection have been tested only at lower concentrations of DEET. Given that the lipid formulations of DEET are minimally absorbed through the skin, it is possible that a higher concentration of DEET could be used with potentially greater success. Thus, it is becoming more apparent that LipoDEET or other lipid-containing preparations of DEET could be potentially developed into an excellent prophylactic agent against all major species of human and animal schistosomes.
Acknowledgments
Schistosoma mansoni and Schistosoma haematobium life cycle stages for this work were supplied through National Institute of Health–National Institutes of Allergy and Infectious Diseases contract NO1–55270. Schistosoma japonicum infected snails were obtained from Somei Kojima at the University of Tokyo, Japan.
Footnotes
Jackson, F., et al. (2002) Schistosomiasis prophylaxis using DEET. Abstract no. 263. 51st Annual Meeting of the American Society of Tropical Medicine and Hygiene held 10–14th November 2002, in Denver, CO, USA.
References
- 1.Bergquist R, et al. Blueprint for schistosomiasis vaccine development. Acta Trop. 2002;82:183–192. doi: 10.1016/s0001-706x(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 2.Jordan P. Schistosomiasis – the St Lucia project. Cambridge University Press; New York: 1985. [Google Scholar]
- 3.Chen XY. The challenges and strategies in schistosomiasis control program in China. Acta Trop. 2002;82:279–282. doi: 10.1016/s0001-706x(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 4.Morgan JA, et al. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123(Suppl):S211–S228. doi: 10.1017/s0031182001007703. [DOI] [PubMed] [Google Scholar]
- 5.Leonardo LR, et al. Difficulties and strategies in the control of schistosomiasis in the Philippines. Acta Trop. 2002;82:295–299. doi: 10.1016/s0001-706x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 6.Doenhoff MJ, et al. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist NR. Schistosomiasis: from risk assessment to control. Trends Parasitol. 2002;18:309–314. doi: 10.1016/s1471-4922(02)02301-2. [DOI] [PubMed] [Google Scholar]
- 8.Engels D, et al. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frischkorn CG, et al. Cercaricidal activity of some essential oils of plants from Brazil. Naturwissenschaften. 1978;65:480–483. doi: 10.1007/BF00702834. [DOI] [PubMed] [Google Scholar]
- 10.Greene LK, et al. Amoscanate as a topically applied chemical for prophylaxis against Schistosoma mansoni infections in mice. Am J Trop Med Hyg. 1983;32:1356–1363. doi: 10.4269/ajtmh.1983.32.1356. [DOI] [PubMed] [Google Scholar]
- 11.Haas W. Schistosoma mansoni: cercaricidal effect of 2-tetradecenoic acid, a penetration stimulant. Exp Parasitol. 1984;58:215–222. doi: 10.1016/0014-4894(84)90037-7. [DOI] [PubMed] [Google Scholar]
- 12.Grenan MM, et al. Hexachlorophene as a topically applied chemical for prophylaxis against Schistosoma mansoni infections in mice. Rev Inst Med Trop Sao Paulo. 1985;27:190–196. doi: 10.1590/s0036-46651985000400006. [DOI] [PubMed] [Google Scholar]
- 13.Miller RE, et al. Schistosoma mansoni: Salicylanilides as topical prophylactic against ceracarial penetration of mice. Exp Parasitol. 1986;61:359–368. doi: 10.1016/0014-4894(86)90191-8. [DOI] [PubMed] [Google Scholar]
- 14.Naples JM, et al. Schistosoma mansoni: cercaricidal effects of cedarwood oil and various of its components. J Trop Med Hyg. 1992;95:390–396. [PubMed] [Google Scholar]
- 15.Abu-Elyazeed RR, et al. Field trial of 1% niclosamide as a topical antipenetrant to Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1993;49:403–409. doi: 10.4269/ajtmh.1993.49.403. [DOI] [PubMed] [Google Scholar]
- 16.Perrett S, et al. The plant molluscicide Millettia thonningii (Leguminosae) as a topical antischistosomal agent. J Ethnopharmacol. 1995;47:49–54. doi: 10.1016/0378-8741(95)01253-a. [DOI] [PubMed] [Google Scholar]
- 17.Ingram RJ, et al. Dimethicone barrier cream prevents infection of human skin by schistosome cercariae: evidence from Franz cell studies. J Parasitol. 2002;88:399–402. doi: 10.1645/0022-3395(2002)088[0399:DBCPIO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Salafsky B, et al. Evaluation of N,N-diethyl-m-toluamide (DEET) as a topical agent for preventing skin penetration by cercariae of Schistosoma mansoni. Am J Trop Med Hyg. 1998;58:828–834. doi: 10.4269/ajtmh.1998.58.828. [DOI] [PubMed] [Google Scholar]
- 19.Secor WE, et al. Short report: prevention of Schistosoma mansoni infections in mice by the insect repellents AI3-37220 and N,N-diethyl-3-methylbenzamide. Am J Trop Med Hyg. 1999;60:1061–1062. doi: 10.4269/ajtmh.1999.60.1061. [DOI] [PubMed] [Google Scholar]
- 20.Twfeek GM. The potential use of N, N-diethyl-m-toluamide (DEET) as a prophylactic agent in the control of schistosomiasis. J Egypt Soc Parasitol. 1999;29:763–776. [PubMed] [Google Scholar]
- 21.Salafsky B, et al. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of schistosomiasis. Am J Trop Med Hyg. 1999;61:743–750. doi: 10.4269/ajtmh.1999.61.743. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Patent No. 6,447,801 Lipodeet is a patented product.
- 23.Schoenig GP, et al. Evaluation of the chronic toxicity and oncogenicity of N,N-diethyl-m-toluamide (DEET) Toxicol Sci. 1999;47:99–109. doi: 10.1093/toxsci/47.1.99. [DOI] [PubMed] [Google Scholar]
- 24.Goodyer L, Behrens RH. Short report: the safety and toxicity of insect repellents. Am J Trop Med Hyg. 1998;59:323–324. doi: 10.4269/ajtmh.1998.59.323. [DOI] [PubMed] [Google Scholar]
- 25.Dorman DC, et al. Fenvalerate/N,N-diethyl-m-toluamide (Deet) toxicosis in two cats. J Am Vet Med Assoc. 1990;196:100–102. [PubMed] [Google Scholar]
- 26.Abdel-Rahman A, et al. Subchronic dermal application of N,N-diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Exp Neurol. 2001;172:153–171. doi: 10.1006/exnr.2001.7807. [DOI] [PubMed] [Google Scholar]
- 27.Briassoulis G, et al. Toxic encephalopathy associated with use of DEET insect repellents: a case analysis of its toxicity in children. Hum Exp Toxicol. 2001;20:8–14. doi: 10.1191/096032701676731093. [DOI] [PubMed] [Google Scholar]
- 28.Ross JS, Shah JC. Reduction in skin permeation of N,N-diethyl-m-toluamide (DEET) by altering the skin/vehicle partition coefficient. J Control Release. 2000;67:211–221. doi: 10.1016/s0168-3659(00)00210-8. [DOI] [PubMed] [Google Scholar]
- 29.Brown M, Hebert AA. Insect repellents: an overview. J Am Acad Dermatol. 1997;36:243–249. doi: 10.1016/s0190-9622(97)70289-5. [DOI] [PubMed] [Google Scholar]
- 30.Salafsky B, et al. Short report: study on the efficacy of a new long-acting formulation of N, N-diethyl-m-toluamide (DEET) for the prevention of tick attachment. Am J Trop Med Hyg. 2000;62:169–172. doi: 10.4269/ajtmh.2000.62.169. [DOI] [PubMed] [Google Scholar]
- 31.Caumes E, et al. Failure of an ointment based on IR3535 (ethyl butylacetylaminopropionate) to prevent an outbreak of cercarial dermatitis during swimming races across Lake Annecy, France. Ann Trop Med Parasitol. 2003;97:157–163. doi: 10.1179/000349803235001633. [DOI] [PubMed] [Google Scholar]