Abstract
Social stress in adolescence is correlated with emergence of psychopathologies during early adulthood. In this study, we investigated the impact of social defeat stress during mid-adolescence on adult male brain and behavior. Adolescent male Sprague-Dawley rats were exposed to repeated social defeat for five days while controls were placed into a novel empty cage. When exposed to defeat-associated cues as adults, previously defeated rats showed increased risk assessment and behavioral inhibition, demonstrating long-term memory for the defeat context. However, previously defeated rats exhibited increased locomotion in both elevated plus maze and open field tests, suggesting heightened novelty-induced behavior. Adolescent defeat also affected adult monoamine levels in stress-responsive limbic regions, causing decreased medial prefrontal cortex dopamine, increased norepinephrine and serotonin in the ventral dentate gyrus, and decreased norepinephrine in the dorsal raphe. Our results suggest that adolescent social defeat produces both deficits in anxiety responses and altered monoaminergic function in adulthood. This model offers potential for identifying specific mechanisms induced by severe adolescent social stress that may contribute to increased adult male vulnerability to psychopathology.
Keywords: adolescent stress, social defeat, anxiety, monoamine
Introduction
Adolescence is a crucial phase of maturation, when skills necessary for making the developmental shift from childhood to adulthood are acquired (Spear, 2000). Similar to humans, adolescent rats express increased social activity during this period, along with enhanced novelty-seeking and risk-taking (Primus & Kellogg, 1989; Spear, 2000; Douglas, Varlinskaya, & Spear, 2003; Varlinskaya & Spear, 2008). These behavioral changes in both humans and rats are accompanied by substantial re-organization of limbic monoamine systems thought to mediate emotive and motivational states (reviewed by Spear, 2000; Andersen, 2003), with such adolescent-typical neurobehavioral characteristics being most evident between postnatal days (P) 28 and P42 (Spear & Brake, 1983; Spear, 2000). However, these developmental changes in behavior and neurophysiology are believed to increase the vulnerability of adolescent organisms to the negative effects of stressful events (Spear, 2000; Anderson, 2003; Anderson & Teicher, 2008). With social activity at its peak during adolescence, there is a high probability that negative social experiences will have long-lasting detrimental effects that persist into adulthood. Indeed, numerous clinical studies have shown that repeated adolescent experience of social stress, often in the form of bullying, is correlated with greater incidence of stress-related psychiatric and addictive disorders in later life (Hoffman, Cerbone, & Su, 2000; Rossow & Lauritzen, 2001; Newman, Holden, & Delville, 2005; Wals & Verhulst, 2005; Gladstone, Parker, & Malhi, 2006). Therefore, it is important to understand the potential impacts that exposure to repeated adolescent social stress might have on neural systems associated with such adult psychopathologies.
Social defeat is considered an ecologically and ethologically relevant animal model of psychosocial stress that produces enduring behavioral and neurochemical sensitization in defeated individuals (Björkqvist, 2001; Miczek, Covington, Nikulina, & Hammer, 2004). This paradigm only employs males if mice or rats are used, as females do not fight each other in a resident-intruder situation (Bjorkqvist, 2001). In adult male rats, repeated social defeat evokes increased emotive and drug-seeking behavior (Miczek et al., 2004; Covington and Miczek, 2005; Rygula et al., 2005) comparable to that reported for adult recipients of human social defeat (Björkqvist, 2001), and is also associated with functional changes in stress-responsive limbic monoaminergic regions (Martinez, Calvo-Torrent, & Herbert, 2002; Lucas et al., 2004; Buwalda et al., 2005; Abumaria et al., 2006; Czeh et al., 2007). However, only one recent study (Vidal et al., 2007) has investigated the effects of repeated social defeat in adolescence on adult male brain and behavior. These authors employed a protocol that involved intermittent social defeat of male Wistar rats during late adolescence/early adulthood (P45 to P57), with subjects experiencing 5 bouts of defeat every third day. While this resulted in increased social avoidance in adulthood (P78), socially defeated rats had no changes in limbic monoamine content relative to controls. It remains uncertain if repeated social defeat experienced during the peak of adolescent neural re-organization (P28 to P42, Spear, 2000; Andersen, 2003) affects adult monoamine levels and the expression of stress-induced and anxiety-like behaviors.
The current study was designed to characterize a rat model of mid-adolescent social defeat using a resident-intruder paradigm. Adolescent male rats were subjected to repeated attacks from a larger aggressive adult male every day from P35 to P40. Following an initial behavioral validation, the model was used to test the hypothesis that exposure of male rats to repeated social defeat stress during this critical period of adolescent development leads to changes in the expression of anxiety behaviors and limbic monoamines in adults. Measures included behavioral responses to situations of both defeat context and generalized anxiety, along with assessment of monoamine levels in numerous stress-responsive limbic regions. We chose to examine these variables in the period from P58 to P63 (early adulthood), since the emergence of symptoms characterizing stress-related psychiatric and addictive disorders often occurs at the equivalent stage of human maturation (Andersen, 2003; Park, Paul Mulye, Adams, Brindis, & Irwin, 2006).
Method
Subjects
Male juvenile post-weaning Sprague-Dawley rats (P21, n = 60) were obtained from the University of South Dakota Laboratory Animal Services. Experimental subjects were housed in pairs within each treatment group (social defeat and control), and maintained at 22°C on a reverse 12 hr light/dark cycle (lights off at 09:00 hr) with free access to food and water. Animals were weighed weekly from arrival through to experimental endpoint. All testing procedures occurred between 10:00 and 15:00 hrs under red lighting. All procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Social Defeat Conditioning
At mid-adolescence (P35, Spear, 2000; Andersen, 2003), male rats in the socially-stressed group (n = 30, mass at P35 = 222.0 ± 9.04 g; mean ± SE) were exposed to repeated social defeat in the home cage of a larger adult male Sprague-Dawley rat (mass = 397.5 ± 14.5 g; mean ± SE), using a modified version of the resident-intruder paradigm (Miczek, 1979; Koolhaas, Meerlo, De Boer, Strubbe, & Bohus, 1997; Martinez et al., 2002). Similar to previous social defeat studies (e.g., Covington & Miczek, 2005; Czeh et al., 2007; Vidal et al., 2007), age-matched male controls (n = 30, mass at P35 = 222.5 ± 7.86 g; mean ± SE) experienced no social defeat or interaction, and were simply placed into a novel empty cage for the duration of each defeat trial to control for handling and novel environment stress. A control treatment group experiencing social contact with a non-aggressive adult male during mid-adolescence was not included, as natural formation of dominant-subordinate hierarchies and variance in intensity of this experience would limit effective use as a control comparison.
To encourage territoriality, each resident male (n = 6) was housed with a sexually receptive female in a large plastic cage (40 × 25 × 17.5 cm) for one week prior to and throughout the course of the social defeat procedure. Females had been anesthetized with ketamine/xylazine (50/10 mg/kg, ip.) and ovariectomized earlier. Female sexual receptivity was induced administration of 17β estradiol benzoate (5 μg/0.1 ml oil, sc., Sigma-Aldrich USA) followed 24 hrs later by progesterone (0.5 mg/0.1 ml oil, sc., Sigma-Aldrich USA; Farmer, Isakson, Coy, & Renner, 1996). Several different females were used to ensure that each resident was paired with a receptive female every day. Females were removed from resident cages at the start of each defeat session and replaced afterwards. Resident males were assessed for aggressive responses towards an adolescent (P36) male intruder two days before actual experiments began. All residents approached and investigated the intruder thoroughly by sniffing at the anogenital region. The intruder was often held immobile in a crouching position while its neck was groomed roughly by the resident (Miczek & De Boer, 2005). Intruders were then attacked and grappled with before being thrown on their backs and displaying submission (Blanchard & Blanchard, 1989; Blanchard, Sakai, McEwen, Weiss, & Blanchard, 1993; Miczek & De Boer, 2005), typically within 5 min of introduction. Intruder rats used for these screening trials were not included in any later experiments.
For experimental defeat trials, each adolescent experimental subject was transferred from its home cage to that of a resident, with the interaction video-recorded for later measurement of latency to defeat. Adolescent intruders were considered defeated after exhibiting 3 consecutive submissive postures in response to resident attacks. Adolescents were then confined behind a wire mesh barrier for 35 min, which prevented further physical contact from the resident but allowed transmission of auditory, olfactory and visual cues. Following this, adolescent rats were returned to their home cages. Adolescent subjects were exposed daily to social defeat over a 5 day period, and were confronted with a different resident male each time using a completely randomized design to control for individual variance in defeat intensity. Following social defeat conditioning, subjects and controls were left in their original pairs in their home cages and allowed to mature to early adulthood (P56). There were no differences in mass between defeated subjects and controls at any point during the entire study (data not shown).
Collection and measurement of plasma corticosterone
The effects of social defeat on plasma corticosterone levels of adolescent rats during the conditioning procedure were also examined. Two days prior to the start of social defeat, subjects and controls (n = 10 per treatment group) were restrained gently in dorsal recumbence, and a heparinized 28 G needle and syringe was used to extract 0.25 ml of blood from the jugular vein for measurement of plasma corticosterone levels. Blood samples were also taken immediately after defeat trials on the first and last days of social defeat, and 2 days following defeat procedures. All blood sampling was performed at the same time of day for each individual rat, and the mean ± SE time for blood collection across all conditions was 138.4 ± 29.1 s.
Following blood collection, samples were centrifuged at 1800 × g for 10 min and the plasma was drawn off and stored at −80°C. Measurement of plasma corticosterone was performed using a corticosterone enzyme-linked immunoassay kit, as per the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). Details of this assay have been published elsewhere (Forster et al., 2008). Briefly, 10 μl of plasma and 0.5 μl steroid displacement reagent were diluted with 990 μl of assay buffer for a 100-fold dilution, and samples, standards and assay controls were run in duplicate.
Sample plasma corticosterone levels were detected by absorbance at 405 nm (wavelength correction set at 595 nm) using an automated plate reader (Bio-Tek Instruments, Winooski, VT, USA), and were corrected for recovery using KinetiCalc Jr. software (Bio-Tek Instruments). Absorbance values were used to calculate both maximum binding percent (19.9 - 20.9 %) and percent of non-specific binding (2.4%); both values were within the range given by the manufacturer. The detection limit sensitivity of this assay was 27.0 pg/ml. Corticosterone levels obtained from this assay were expressed as ng corticosterone/ml plasma.
Testing adult male anxiety-like behavior
At the onset of early adulthood (P56, Spear, 2000; Andersen, 2003), previously-defeated male subjects and their controls (n = 10 per treatment group) were assessed for both defeat context and generalized anxiety. In the defeat context anxiety test, rats were exposed to cages in which adolescent defeat occurred, but with the larger resident male now absent. However, test cages contained resident olfactory cues in the form of mixed soiled bedding collected 2 days earlier from resident males that had previously defeated each subject. Previously defeated rats and controls were placed into separate cages containing resident bedding and video recorded for 10 min. Pilot experiments showed that the majority of behaviors produced by previously defeated rats in this context consisted of rearing, active exploration including locomotion, substrate sniffing, digging and motor patterns indicative of risk assessment (non-ambulatory scanning in the form of slow lateral head swaying while in a crouched posture, Blanchard & Blanchard 1989; Butler, Weiss, Stout, & Nemeroff, 1990; Abrams et al., 2005). The number of rears, duration of active exploration and duration of risk assessment behavior over each 10 min trial were scored from videotape by a scorer blind to treatment.
Two days after the defeat context test, the same defeated rats and controls were assessed for generalized anxiety behavior on the elevated plus maze (EPM). Both open and closed arms measured 50 cm long × 10 cm wide, and the entire apparatus was elevated 75 cm from the ground. Each animal was placed in the center of the EPM, and then video-recorded over the next 5 min for later behavioral scoring using Noldus Ethovision v 3.1 (Noldus Information Technology, Inc., Leesburg, VA, USA). We measured the number of open arm entries, time spent in the open arms, amount of rearing and distance moved in the entire maze. In addition, distance moved in each zone of the EPM (center, open and closed arms) was calculated.
Results from the EPM test indicated that previously defeated rats covered more distance than controls. Therefore, we conducted an additional experiment to assess the effects of adolescent social defeat on locomotion responses to an alternative novel and mildly anxiogenic situation. After maturing to early adulthood (P58), previously defeated and control rats (n = 20 per treatment group) were placed individually for 30 min into the center of a novel open field apparatus, consisting of a rounded opaque plastic enclosure (52 × 33.5 × 30 cm). The total distance moved by each animal over the 30 min period was calculated using Noldus Ethovision v 3.1. These animals were not included in the subsequent analyses of adult corticosterone and limbic monoamine concentrations.
Corticosterone and limbic monoamine concentrations in adulthood
At 9 weeks of age (P63), previously defeated male subjects and their controls (n = 10 per treatment group) were decapitated rapidly, with brains collected and frozen at −80°C prior to sectioning. Trunk blood was also collected for measurement of adult corticosterone levels using the assay described above. Brains were sliced coronally at −10°C into 300 μm serial sections, thaw-mounted on to glass slides, and refrozen at −80°C for later microdissection. Limbic and striatal terminal fields (medial prefrontal cortex [mPFC], nucleus accumbens [NAc] core and shell, caudate putamen [CPu], central nucleus of the amygdala [CeA], medial amygdala [MeA], dorsal and ventral hippocampal cornus ammon [CA], dorsal and ventral dentate gyrus [DG], dorsomedial hypothalamic nucleus [DMH] and paraventricular hypothalamic nucleus [PVN]), and monoaminergic cell body regions (substantia nigra pars compacta [SNc], ventral tegmental area [VTA], dorsal raphe nucleus [dRN] and median raphe nucleus [mRN]) were identified using published guides (Palkovits & Brownstein, 1988; Paxinos & Watson, 1997) and bilaterally microdissected with a 500 μm i.d. cannula using a dissecting microscope and freezing stage. The microdissected tissue was expelled into 60 μl of sodium acetate buffer (pH 5.0) containing 9.42 pg/μl of internal standard (dihydroxybenzylamine, DHBA). Samples were then frozen on dry ice to cause cell lysis, allowing release of soluble monoamine transmitters and their metabolites into the buffer upon thawing.
Samples were analyzed for levels of norepinephrine (NE), dopamine (DA), the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), and serotonin (5-HT) and the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) using high-pressure liquid chromatography (HPLC) with electrochemical detection. Details of this assay have been published elsewhere (Renner, Krey, & Luine, 1987; Watt, Forster, Korzan, Renner, & Summers, 2007). Briefly, 2 μl of a 1 mg/ml ascorbate oxidase solution was added to each sample, which was then centrifuged at 17,000 × g for 3 minutes. The supernatant was removed and 45 μl injected into an HPLC system (Waters 717 Plus Autosampler, Waters Associates, Inc., MA, USA) and analyzed electrochemically with a LC-4B potentiostat and a glassy carbon electrode (BioAnalytical Systems, IN, USA). The electrode potential was set at +0.6 V with respect to an Ag/AgCl reference electrode. The mobile phase consisted of 14 g citric acid, 8.6 g sodium acetate, 110 mg 1-octanesulfonic acid, 150 mg EDTA disodium salt, and 100 ml of methanol in 1L of deionized water. Flow rate was maintained at 1.4 ml/min.
The tissue pellet remaining from each sample was dissolved in 110 ml 0.4 M NaOH and protein content assayed using the Bradford method (Bradford, 1976). Sample monoamine concentrations were calculated with respect to peak height values obtained from standard runs set in the internal standard mode using CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Neurotransmitter and metabolite levels were expressed as pg amine/μg protein.
Data analysis
Latency to defeat (exhibition of 3 consecutive submissive postures by male adolescent subjects) was compared across the 5 days of trials using one way repeated measures ANOVA followed by Student-Newman-Keuls tests for multiple comparisons. Corticosterone levels across the defeat procedure were compared using two way repeated measures ANOVA, with one factor of treatment (defeat or control) and a repeated factor of time. Resulting significant main effects were further analyzed by appropriate one way ANOVA with Student-Newman-Keuls tests. For the defeat context anxiety test in adulthood, amount of rearing, duration of exploration and duration of risk assessment behavior were compared between previously defeated male subjects and controls using separate one way ANOVA. For the EPM test, amount of rearing, number of open arm entries, time spent in open arms and total distance moved in the entire EPM were compared between defeated subjects and controls using separate one way ANOVA. Distance moved in each EPM zone (center, closed and open) was compared between defeated rats and controls using a two way ANOVA, with independent factors of treatment and EPM zone, followed one way ANOVA with Student-Newman-Keuls tests when appropriate. Distance moved in the open field test was compared between defeated rats and controls using a one way ANOVA with Student-Newman-Keuls tests. Separate one way ANOVA were used to compare adult (P63) corticosterone levels obtained from trunk blood and concentrations of limbic monoamines and metabolites within each brain region. When significant differences in limbic variables were found between previously defeated rats and controls, a series of post hoc correlations between levels of each limbic variable from all subjects and expression of specific anxiety-like behaviors in the defeat context and EPM tests was performed using separate linear regressions, in order to provide further insight how neural changes induced by adolescent defeat may contribute to alterations in male adult anxiety-like behavior. All analyses were performed using SigmaStat 3.5, with the alpha level set at 0.05 throughout.
Results
Male adolescent behavioral and corticosterone response to repeated social defeat
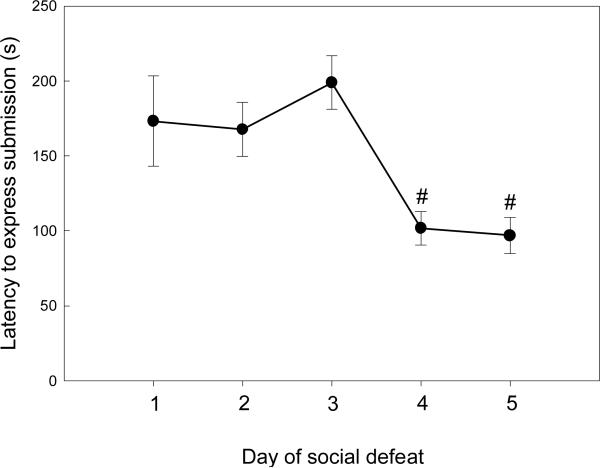
All male adolescent rats that were exposed to resident males were investigated, attacked and displayed submission by assuming a supine position with the forelimbs held out stiffly, often vocalizing audibly. The latency for subjects to assume 3 consecutive submissive postures in response to attacks changed during the course of the 5 day conditioning procedure (Figure 1; F(4,37) = 6.75, p < 0.001). While there were no differences in latency to submission across the first 3 days (p > 0.33), submission times decreased significantly by days 4 and 5 (p < 0.03).
Figure 1.
Latencies (mean ± SE s) for adolescent male rats to express submission and defeat towards an aggressive adult male over the five day course of social defeat trials. #Significantly different from days 1 through 3.
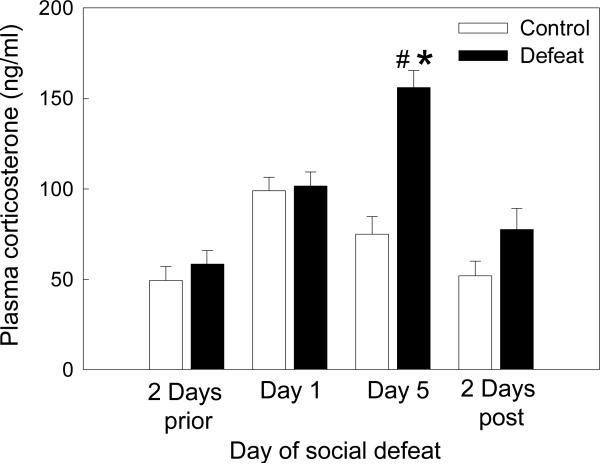
Comparison of plasma corticosterone levels over the course of the defeat process revealed significant main effects of treatment (F(18,59) = 12.71, p = 0.002) and time (F(33,59) = 17.16, p < 0.001), and a significant interaction between treatment and time (F(31,59) = 9.03, p < 0.001). Baseline corticosterone levels sampled two days before defeat conditioning did not differ between experimental subjects and controls (Figure 2, p = 0.688). Male rats subjected to defeat exhibited a significant increase in corticosterone from baseline levels following their first experience of social defeat (p = 0.01). A similar response was shown by controls after their first exposure to a novel cage (p = 0.004), with corticosterone levels increasing to the same degree as for defeated subjects (p = 0.687). By the last day of the conditioning procedure, corticosterone levels of controls had returned to baseline (p = 0.219). In contrast, corticosterone levels of defeated subjects after the final defeat were significantly higher than those of controls (p < 0.001). These final defeat-elicited corticosterone levels were not only significantly increased compared to their baseline concentrations (p < 0.001), but were also significantly higher than those evoked by the first defeat experience (p < 0.001). When sampled two days after the final defeat, corticosterone levels of both defeated subjects and controls were at baseline (p > 0.258), with no difference between the two treatment groups (p = 0.589).
Figure 2.
Plasma corticosterone responses (mean ± SE ng/ml) of adolescent rats during repeated exposure to social defeat. #Significant difference between socially defeated rats and controls (p < 0.05), *significant difference within socially defeated rats compared to all other time points (p < 0.05).
Anxiety-like behavior of adult males
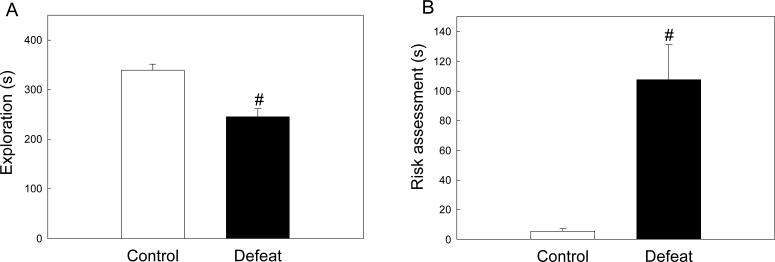
When re-exposed as adults (P56) to the defeat context, male rats defeated in adolescence showed markedly different behavioral responses from controls. While both groups displayed similar amounts of rearing (F(1, 19) = 0.108, p = 0.746), active exploration such as locomotion, digging and substrate sniffing was significantly reduced in previously defeated male rats compared to controls (Figure 3A, F(1, 19) = 21.797, p < 0.001). Previously defeated rats also exhibited significantly more risk assessment behavior than controls (Figure 3B, F(1, 19) = 18.642, p < 0.001) in the form of slow lateral head swaying without ambulation.
Figure 3.
Behavioral responses of previously defeated rats and controls to defeat-associated cues in adulthood. (A) Duration (mean ± SE s) of active exploratory behaviors, comprising locomotion, substrate sniffing and digging. (B) Duration (mean ± SE s) of behaviors indicative of risk assessment, comprising non-ambulatory scanning in the form of slow lateral head swaying while in a crouched posture. #Significant difference between treatment groups (p < 0.05).
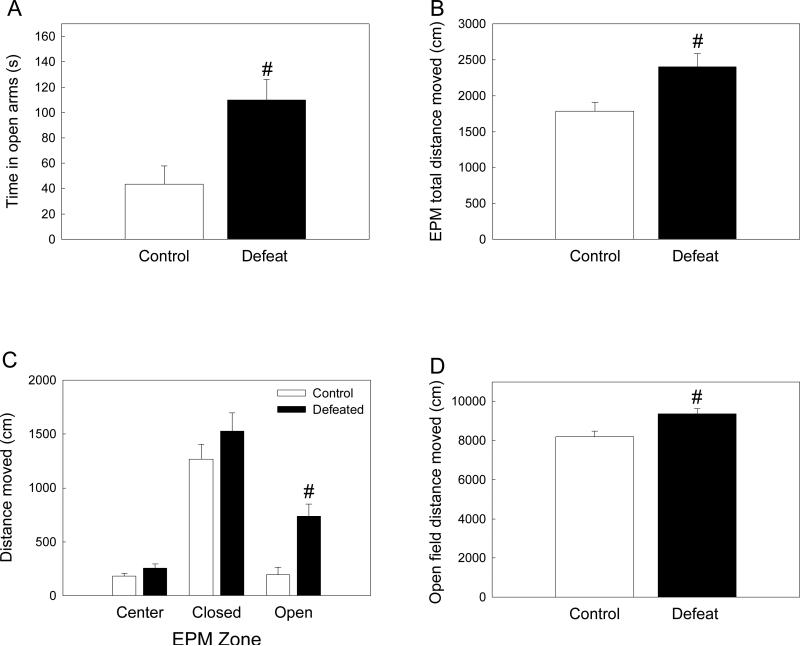
Previously defeated male rats also exhibited altered responses when assessed for generalized anxiety behavior on the EPM. While there was no difference between treatment groups in the number of open arm entries (F(1, 18) = 0.432, p = 0.520), rats defeated in adolescence spent significantly more time in the open arms of the EPM than controls (Figure 4A; F(1, 16) = 9.652, p = 0.007), and also covered more distance in the whole maze (Figure 4B, F(1, 19) = 7.957, p = 0.011). Two way ANOVA of distance moved in each EPM zone revealed significant main effects of treatment (F(18,49) = 13.05, p = 0.002) and zone (F(2,49) = 49.35, p < 0.001), the interaction between these two factors was not significant (F(2,49) = 2.53, p = 0.099). As shown in Figure 4C, previously defeated rats did not differ from controls in distance moved in either the center (p = 0.358) or closed arms (p = 0.122), but showed significantly greater levels of locomotion while in the open arms of the EPM (p < 0.001). There was no difference between treatment groups in amount of rearing (F(1, 19) = 0.138, p = 0.714).
Figure 4.
Behavioral responses of previously defeated rats and controls during elevated-plus maze (EPM) and open field testing in adulthood. (A) Time spent (mean ± SE s) in open arms. (B) Total distance moved (mean ± SE cm) within the entire EPM. (C) Distance moved (mean ± SE cm) within each EPM zone. (D) Distance moved (mean ± SE cm) during 30 min open field exposure. #Significant difference between treatment groups (p < 0.05).
Adult rats that had been defeated in adolescence also differed from controls in their response to a novel open field, with previously defeated male rats exhibiting higher amounts of locomotion as measured by total distance moved over the 30 min test (Figure 4D; F(1, 39) = 7.836, p = 0.008).
Corticosterone and limbic monoamine concentrations in adulthood
When sacrificed at P63, previously defeated and control male rats showed equivalent plasma corticosterone levels (defeated rats mean ± SE = 75.224 ± 7.576 ng/ml, control rats mean ± SE = 68.764 ± 6.737 ng/ml, F(1,15) = 1.417, p = 0.254). There were also no differences in levels of any monoamine or metabolite within the NAc core and shell, CPu, CeA, MeA, DMH, PVN, and hippocampal CA regions (see Table 1), nor within the VTA and mRN (Table 2).
Table 1.
Adult (P63) basal levels (mean ± SE pg/μg tissue) of monoamines (norepinephrine [NE], dopamine [DA], serotonin [5-HT]) and metabolites (DOPAC and 5-HIAA) in limbic terminal fields following social defeat in adolescence.
| NE | DOPAC | DA | 5-HIAA | 5-HT | |
|---|---|---|---|---|---|
| Medial prefrontal cortex (mPFC) | |||||
| Social defeat | 11.67±1.38 | 3.69±0.72 | 8.36±1.48* | 10.1±1.22 | 8.83±0.98 |
| Control | 10.91±0.61 | 3.79±0.44 | 12.87±1.07 | 9.43±1.09 | 9.66±0.95 |
| Nucleus accumbens (NAc) Core | |||||
| Social defeat | 14.63±2.31 | 26.73±1.92 | 148.19±21.09 | 7.55±0.55 | 13.96±2.18 |
| Control | 18.09±2.71 | 32.45±3.42 | 189.94±25.05 | 8.87±0.51 | 17.47±2.46 |
| Nucleus accumbens (NAc) Shell | |||||
| Social defeat | 12.75±1.15 | 26.87±2.77 | 129.82±10.65 | 8.38±0.39 | 14.75±1.32 |
| Control | 13.55±2.02 | 24.95±1.81 | 119.74±12.4 | 8.25±0.49 | 13.84±1.58 |
| Caudate putamen (CPu) | |||||
| Social defeat | 25.18±1.37 | 60.71±6.61 | 314.09±26.18 | 9.15±0.73 | 5.25±0.44 |
| Control | 22.09±3.42 | 51.82±6.64 | 314.42±13.48 | 7.71±1.04 | 4.43±0.63 |
| Central nucleus of the amygdala (CeA) | |||||
| Social defeat | 19.16±1.44 | 3.46±0.37 | 33.43±3.82 | 7.53±0.64 | 10.66±1.73 |
| Control | 17.37±1.85 | 2.52±0.25 | 27.04±4.64 | 8.21±0.93 | 12.04±1.65 |
| Medial amygdala (MeA) | |||||
| Social defeat | 18.54±0.88 | 0.98±0.28 | 2.63±1.07 | 10.98±0.39 | 14.63±1.13 |
| Control | 19.42±0.97 | 0.51±0.02 | 0.65±0.15 | 12.0±0.86 | 15.28±1.22 |
| Dorsomedial hypothalamus (DMH) | |||||
| Social defeat | 86.25±9.64 | 1.05±0.08 | 5.49±0.41 | 9.35±0.58 | 13.31±0.55 |
| Control | 91.07±10.24 | 1.23±0.13 | 5.15±0.46 | 10.59±1.05 | 12.05±0.83 |
| Paraventricular hypothalamic nucleus (PVN) | |||||
| Social defeat | 63.31±5.92 | 0.98±0.09 | 4.56±0.43 | 10.15±0.84 | 13.28±0.65 |
| Control | 64.29±6.53 | 0.81±0.08 | 4.22±0.39 | 9.04±0.38 | 12.72±0.75 |
| Dorsal cornus ammon (CA) | |||||
| Social defeat | 40.23±2.36 | - | - | 9.68±0.57 | 4.78±0.31 |
| Control | 37.38±1.83 | - | - | 9.64±0.85 | 5.39±0.26 |
| Ventral cornus ammon (CA) | |||||
| Social defeat | 27.77±0.84 | 0.47±0.03 | 0.57±0.08 | 13.75±0.57 | 9.96±0.53 |
| Control | 27.05±1.07 | 0.47±0.05 | 0.57±0.03 | 14.65±0.9 | 10.22±0.64 |
| Dorsal dentate gyrus (DG) | |||||
| Social defeat | 27.77±0.84 | 0.35±0.12 | 0.57±0.08 | 13.95±0.67 | 9.86±0.41 |
| Control | 27.05±1.07 | 0.48±0.05 | 0.57±0.03 | 14.25±0.88 | 10.02±0.65 |
| Ventral dentate gyrus (DG) | |||||
| Social defeat | 49.62±2.73* | - | - | 7.03±0.75 | 14.57±0.87* |
| Control | 37.36±1.89 | - | - | 7.26±0.63 | 11.68±0.95 |
Significant difference between previously defeated rats and controls (p < 0.05).
Table 2.
Adult (P63) basal levels (mean ± SE pg/μg tissue) of monoamines (norepinephrine [NE], dopamine [DA], serotonin [5-HT]) and metabolites (DOPAC and 5-HIAA) in limbic monoaminergic cell bodies following social defeat in adolescence.
| NE | DOPAC | DA | 5-HIAA | 5-HT | |
|---|---|---|---|---|---|
| Substantia nigra pars compacta (SNc) | |||||
| Social defeat | 23.83±2.25 | 11.86±0.79* | 48.08±3.69 | 30.98±2.52 | 53.04±5.51 |
| Control | 21.26±1.66 | 8.26±0.96 | 45.84±4.78 | 26.11±1.32 | 51.57±4.89 |
| Ventral tegmental area (VTA) | |||||
| Social defeat | 39.14±4.65 | 15.29±2.66 | 47.4±8.33 | 26.64±3.36 | 37.43±2.64 |
| Control | 37.82±2.09 | 11.59±1.21 | 42.56±7.13 | 27.18±1.31 | 38.27±3.62 |
| Dorsal raphe nucleus (dRN) | |||||
| Social defeat | 38.33±3.34* | 3.36±0.56 | 23.88±1.84 | 45.82±3.49 | 40.57±5.23 |
| Control | 57.14±7.52 | 3.32±0.56 | 29.61±3.57 | 52.54±5.48 | 55.21±7.03 |
| Median raphe nucleus (mRN) | |||||
| Social defeat | 26.27±2.37 | 2.71±0.51 | 17.16±5.26 | 50.42±4.4 | 38.96±5.0 |
| Control | 26.14±1.41 | 3.22±0.39 | 24.34±3.74 | 50.41±2.1 | 39.01±3.06 |
Significant difference between previously defeated rats and controls (p < 0.05).
Medial prefrontal cortex (mPFC)
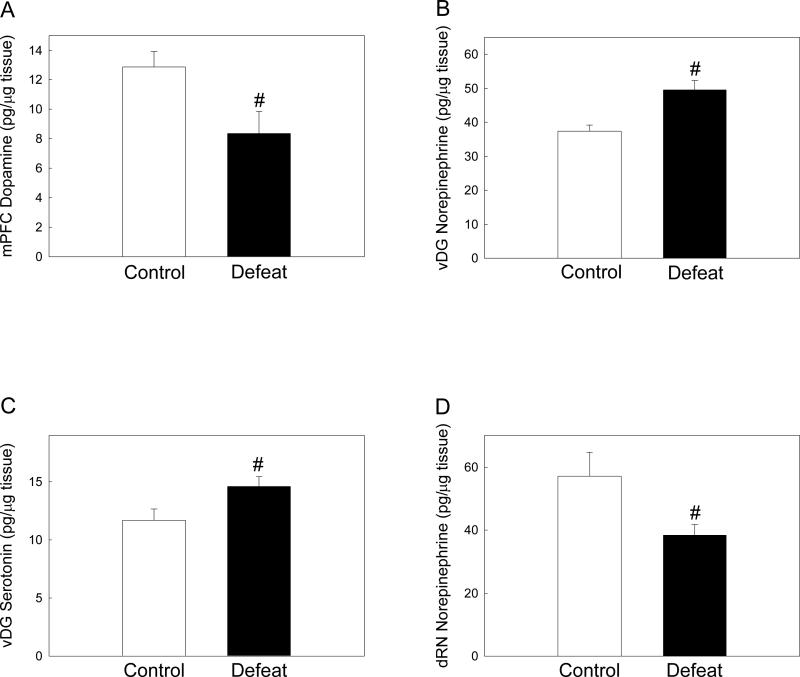
As adults, rats defeated in adolescence had reduced levels of dopamine in the mPFC when compared to controls (Figure 5A; F(1, 19) = 6.112, p = 0.024), but showed no difference in levels of the DA metabolite DOPAC (F(1, 19) = 0.0161, p = 0.900). Defeated and control rats had similar NE, 5-HIAA and 5-HT concentrations in the mPFC (Table 1).
Figure 5.
Adult levels (mean ± SE pg/μg tissue) of (A) medial prefrontal cortex (mPFC) dopamine, (B) ventral dentate gyrus (vDG) norepinephrine, (C) ventral dentate gyrus serotonin and (D) dorsal raphe (dRN) norepinephrine in previously defeated rats and controls. #Significant difference between treatment groups (p < 0.05).
Dentate gyrus (DG)
Adolescent defeat also affected adult monoamine concentrations in the dentate gyrus of the hippocampus. However, these effects were restricted to the ventral DG, with no differences between treatment groups noted in the dorsal DG (see Table 1). Previously defeated rats had significantly higher levels of NE (Figure 5B; F(1, 19) = 13.661, p = 0.002) and 5-HT (Figure 5C; F(1, 18) = 4.959, p = 0.04) in the ventral DG compared to controls. There was no difference in ventral DG 5-HIAA levels between treatment groups (F(1, 18) = 0.0574, p = 0.813), nor in levels of DA and DOPAC (Table 1).
Substantia nigra pars compacta (SNc)
Previously defeated rats exhibited significantly higher levels of DOPAC in the SNc compared to controls (Table 2; F(1, 11) = 7.369, p = 0.022). However, there were no differences in SNc DA levels (F(1, 12) = 0.109, p = 0.747), nor in levels of NE, 5-HT and 5-HIAA (Table 2).
Dorsal raphe nucleus (dRN)
Adult levels of NE in the dRN were significantly lower in rats defeated in adolescence than in controls (Figure 5D; F(1, 17) = 5.185, p = 0.037), but there were no differences in levels of any other monoamines or metabolites in this region (Table 2).
Correlations between limbic monoamine concentrations and adult anxiety-like behavior
Post hoc linear regressions revealed that differences in NE levels within the ventral DG accounted for many of the behavioral alterations exhibited by previously defeated male rats (Table 3). In the defeat context anxiety test, increased ventral DG NE resulting from adolescent defeat was positively correlated with expression of risk assessment behavior (R2 = 0.514, F(1,19) = 19.05, p < 0.001) and negatively correlated with amount of active exploration (R2 = 0.412, F(1.19) = 12.62, p = 0.002). Similarly, increased ventral DG NE was positively correlated with both time spent in open arms of the EPM (Table 3; R2 = 0.38, F(1,16) = 9.33, p = 0.008) and distance traveled in this zone (R2 = 0.53, F(1,15) = 15.6, p = 0.001). While decreased dRN NE levels resulting from adolescent defeat were not significantly correlated with defeat context behaviors in adulthood (Table 3), there was a negative correlation between dRN NE levels and both time in open arms of the EPM (R2 = 0.27, F(1,14) = 4.92, p = 0.045) and distance moved in these areas (R2 = 0.29, F(1,15) = 5.71, p = 0.031). There was a trend for decreased adult mPFC DA levels following adolescent defeat experience to be negatively correlated with both open arm time (R2 = 0.195, F(1,16) = 3.63, p = 0.076) and open arm distance traveled (R2 = 0.194, F(1,15) = 3.36, p = 0.083), but there was no significant correlation between mPFC DA levels and behaviors observed in the defeat context test (Table 3). No significant correlation was found between vDG 5-HT levels and behavior in either the defeat context or EPM tests in adulthood (Table 3).
Table 3.
Correlations between altered adult limbic monoamine concentrations and expression of anxiety-like behavior following social defeat in adolescence.
| Adult behavior | Medial prefrontal cortex (mPFC) dopamine (DA) | Ventral dentate gyrus (DG) norepinephrine (NE) | Ventral dentate gyrus (DG) serotonin (5-HT) | Dorsal raphe nucleus (dRN) norepinephrine (NE) |
|---|---|---|---|---|
| Defeat context test | ||||
| Active exploration (s) | R2 = 0.11 | R2 = 0.514 (−) | R2 = 0.16 | R2 = 0.033 |
| F(1,19) = 2.22 | F(1,19) = 19.05 | F(1,18) = 3.23 | F(1,17) = 0.55 | |
| p = 0.15 | p < 0.001* | p = 0.09 | p = 0.47 | |
| Risk assessment (s) | R2 = 0.052 | R2 = 0.412 (+) | R2 = 0.082 | R2 = 0.095 |
| F(1,19) = 0.99 | F(1.19) = 12.62 | F(1,18) = 1.51 | F(1,17) = 1.68 | |
| p = 0.33 | p = 0.002* | p = 0.24 | p = 0.21 | |
| EPM test | ||||
| Open arm time (s) | R2 = 0.195 (−) | R2 = 0.38 (+) | R2 = 0.023 | R2 = 0.27 (−) |
| F(1,16) = 3.63 | F(1,16) = 9.33 | F(1,15) = 0.324 | F(1,14) = 4.92 | |
| p = 0.076 | p = 0.008* | p = 0.58 | p = 0.045* | |
| Open arm distance (cm) | R2 = 0.194 (−) | R2 = 0.53 (+) | R2 = 0.03 | R2 = 0.29 (−) |
| F(1,15) = 3.36 | F(1,15) = 15.6 | F(1,14) = 0.395 | F(1,15) = 5.71 | |
| p = 0.083 | p = 0.001* | p = 0.54 | p = 0.031* |
Significant correlation; (+) and (−) indicate positive and negative correlations respectively.
Discussion
When exposed to repeated aggression from an adult male, mid-adolescent male rats responded with the same supine submissive posture described for defeated adult male rats (Blanchard & Blanchard 1989; Blanchard et al., 1993; Miczek and De Boer 2005). Moreover, adolescent rats displayed decreases in the latency to exhibit submission by the fourth day of the defeat procedure, indicative of a conditioned or learned defeat response (Corrigan & Flannelly 1979; Siegfried, Frischknecht, & Waser, 1984; Potegal, Huhman, Moore, & Meyerhoff, 1993). Adolescent male subjects also showed sensitized corticosterone responses to social defeat, with plasma corticosterone levels elicited by the final day of defeat being even higher than those observed after the first defeat experience. This sensitized corticosterone response has not been observed in adult male rats subjected to repeated defeat (Covington & Miczek, 2005), and may be a function of differences between adolescent and adult HPA axis responses to stress (Gomez et al., 2002; Romeo et al., 2006; Wommack & Delville, 2007; McCormick, Smith, & Mathews, 2008). Defeat-induced changes in the corticosterone response did not extend beyond the defeat period, as plasma corticosterone levels assessed two days after the final defeat were equivalent to both control and pre-defeat levels. Overall, adolescent male rats subjected to repeated defeat episodes showed behavioral conditioning and HPA axis sensitization, indicating the highly stressful nature of this paradigm and highlighting the relevance of this model for studying long term effects of repeated social defeat during mid-adolescence on adult male brain and behavior.
Adult male anxiety-like behavior and responses to novel situations
Exposure to repeated social defeat in adolescence had a marked effect on context-dependent expression of anxiety-like behaviors by male rats in adulthood. Age-matched controls in our study were not exposed to social interactions with non-aggressive adult males, but were placed into novel empty cages during the defeat periods. It is therefore possible that the behavioral alterations we observed when comparing previously defeated rats with controls were a result of intense social encounters during adolescence rather than purely of aggression received. Future experiments incorporating controls that undergo non-aggressive social interaction during adolescence would be useful in elucidating this issue. In the present study, previously defeated male rats exhibited reduced active exploration compared to controls when re-exposed as adults to cues associated with adolescent defeat. The reduction in exploration was accompanied by increased non-ambulatory scanning, primarily in the form of slow lateral head swaying. Expression of such scanning behavior in the absence of a direct threat is thought to indicate increased vigilance and risk assessment reflecting heightened anxiety-like states (Blanchard and Blanchard, 1989, Butler et al., 1990; Abrams et al., 2005). Rats were not exposed to non-soiled bedding, thus it is not clear which cues (such as olfactory signals from resident bedding or other stimuli associated with the testing room) previously defeated rats were responding to specifically. The increase in adult male anxiety-like behavior as induced by adolescent social defeat compliments results obtained by Vidal et al. (2007), in which intermittent defeat in late adolescence caused increased avoidance in a social interaction test in adulthood. Together, these findings suggest that adolescent defeat has a lasting impact on behavioral expression by male rats in future contexts where defeat-associated cues are present. Similar increases in risk assessment and behavioral inhibition have been observed when male rats defeated as adults are re-exposed to defeat-associated cues several weeks after social defeat (Buwalda et al., 2005, Razzoli, Carboni, Guidi, Gerrard, & Arban, 2007), suggesting that this enduring effect may not be specific to experience of social defeat in adolescence.
In contrast to the defeat cue re-exposure, rats defeated in adolescence showed increased locomotion and exploration when exposed to two different tests of generalized anxiety. Previously defeated adult male rats spent more time in the open arms of the EPM when compared to controls, suggesting increased risk-taking behavior. In addition, previously defeated rats covered more distance than controls while in the open arms. Similarly, male rats defeated in adolescence showed higher locomotion when placed in a novel open field as adults. Interestingly, rats that exhibit high locomotion responses to novel environments also spend more time in open arms of an EPM (White, Kalinichev, & Holtzman, 2007; Ballaz, Akil, & Watson, 2007), and high novelty locomotion responses are thought to predict sensation-seeking or risk-taking traits (Dellu, Piazza, Mayo, Le Moal, & Simon, 1996). Overall, male rats defeated in adolescence appear to have retained characteristics of adolescent behavior into adulthood, since male adolescent rats (P45) seem to show greater locomotion and time spent in open arms of the EPM when compared to adults (McCormick et al, 2008). Such a behavioral response is concordant with the increased risk-taking and novelty-seeking profile shown by adolescent rodents (Laviola, Macri, Morley-Fletcher, & Adriani, 2003).
In a recent study, adolescent male rats exposed to daily isolation periods and constant replacement of cage mates from P30 to 45 did not exhibit increased EPM anxiety behavior when assessed immediately after the stress period, but did show a trend for enhanced anxiety-like behavior on the EPM in adulthood (McCormick et al., 2008). The disparity in adult behavioral responses to the EPM between our study and that by McCormick et al. (2008) may be partially accounted for by differences in the type of adolescent stress employed. In addition, the ability of paradigms such as the EPM to reveal differences in anxiety behavior in the current study may be confounded by the finding that adolescent defeat increased adult male locomotion responses to novelty. Future studies using alternative anxiety tests may be useful in further clarifying the exact effects of repeated adolescent defeat on generalized anxiety responses of male rats in adulthood.
Adult corticosterone and limbic monoamine concentrations
Adult male baseline levels of plasma corticosterone were not affected by the experience of adolescent social defeat when compared to non-defeated controls. This result is consistent with the lack of difference in plasma corticosterone levels between defeated rats and controls when assessed two days after the last defeat episode in adolescence. Similarly, adult male rats exposed to repeated defeat do not exhibit altered plasma corticosterone levels when sampled one week after the last defeat (Covington & Miczek, 2005), again suggesting similar effects of social defeat whether experienced in adolescence or adulthood. However, while baseline corticosterone levels may not be affected in the long term, evidence suggests that repeated adult social defeat can induce lasting changes to male HPA axis regulation (Buwalda et al., 1999), resulting in sensitized corticosterone responses to subsequent stressful situations of both a social (Razzoli et al., 2007) and non-social nature (Bhatnagar & Vining, 2003). It would be interesting to determine if repeated defeat restricted to mid-adolescence similarly sensitizes HPA axis responses of male rats to stress in adulthood.
Adolescent defeat resulted in discrete regionally-specific changes to limbic monoamines in adulthood. These regions included the mPFC, hippocampus and dorsal raphe, which other studies have shown to be affected by social defeat of adult male rats (Miczek et al., 2004; Buwalda et al., 2005; Abumaria et al., 2006). In particular, adult levels of mPFC DA were reduced in previously defeated male rats compared to controls. This decrease in DA levels was restricted to the mPFC, with DA concentrations in other terminal fields remaining unaffected. While the exact mechanisms for this decrease in adult male mPFC DA levels following adolescent defeat are not known, threat of social defeat causes elevated extracellular DA release in the mPFC of adult male rats (Tidey & Miczek, 1996). Exposure to non-social stressors also induces similar increases in phasic mPFC DA release, which are heightened compared to release in subcortical areas in both adolescent (P45) and adult rats (Cenci, Kalen, Mandel, & Bjorklund, 1992; Lyss, Andersen, LeBlanc, & Teicher, 1999). Furthermore, repeated stress can alter DA receptor expression and function (Pani, Porcella, & Gessa, 2000). From this information, it is tempting to speculate that repeated exposure to social defeat caused continued increases in DA release in the mPFC of adolescent rats, which may have disrupted the developmental re-organization of the mesocortical DA system normally occurring at this time (Spear, 2000; Andersen, 2003) to alter feedback mechanisms and ultimately result in the reduced adult mPFC DA levels seen in our study.
Reduced DA activity is seen in the mPFC of rats that exhibit high locomotion responses to novelty (termed high responders), which in turn predicts acquisition of amphetamine self-administration (Piazza et al., 1991). Locomotion responses to novelty and amphetamine are also increased following selective DA depletion in the mPFC (Bubser & Schmidt, 1990; Ventura et al., 2004), as is cocaine self-administration (Schenk, Horger, Peltier, & Shelton 1991; Ventura et al., 2004). In our study, previously defeated male rats showed increased locomotion in the novel environments of the EPM and open field as adults, reminiscent of high responders. Post hoc regressions also indicated a trend for increased locomotion in the EPM test to be correlated with the decreased mPFC DA levels seen in previously defeated adult rats. Combined, these results suggest that adolescent defeat may increase the likelihood of drug-seeking in adult males. This possibility is made more likely by the fact that adult male rats previously exposed to repeated social defeat show cross-sensitized behavioral responses to psychomotor stimulants along with shortened self-administration latencies (Miczek et al., 2004).
Levels of monoamines in the hippocampus of adult males were also altered by experience of adolescent defeat. These changes were only seen in the ventral dentate gyrus (DG), with previously defeated rats having increased levels of both NE and 5-HT in this region compared to controls. While we did not observe any correlation between increased ventral DG 5-HT levels and the altered adult behaviors shown by previously defeated male rats, the ventral hippocampus has been implicated in mediating behavioral responses in anxiogenic situations (Bannerman et al., 2003; Bannerman et al., 2004), which appear to be regulated, in part, by increased serotonergic activity. For example, increased 5-HT release in the ventral hippocampus is observed during novelty-induced locomotion (Kobayashi, Ikeda, Haneda, & Suzuki, 2008) and novel EPM exposure (Rex, Voigt, & Fink, 2005). Expression of anxiety-like responses in the latter context also appears to be mediated by a balance in ventral hippocampal 5-HT1A receptor activation (File & Gonzalez, 1996; Nunes-de-Souza, Canto-de-Souza, & Rodgers, 2002). Interestingly, increases in extracellular 5-HT are also seen in the ventral hippocampus of adult mice subjected to acute social defeat (Keeney et al., 2006). Social defeat in adult rats also reduces hippocampal 5-HT1A receptor functionality (Buwalda et al., 2005), potentially leading to altered or inappropriate responses in subsequent anxiogenic contexts. Our results also show that exposure to adolescent defeat resulted in decreased NE levels in the adult dRN, which was also correlated with increased time spent and distance moved in the open arms of the EPM. Since the activity of serotonergic neurons is enhanced by NE in the dRN (Linner, Wiker, Arborelius, Schalling, & Svensson, 2004; Kusljic, Brosda, Norman, & van den Buuse, 2005), this finding suggests that NE-facilitated phasic firing of serotonergic neurons projecting to the ventral hippocampus could be reduced. Combined with the increased ventral DG 5-HT levels seen in adult rats exposed to adolescent defeat, this may result in altered 5-HT release in the ventral hippocampus and the expression of inappropriate behavioral responses during different anxious situations. Future studies would be useful in determining whether these alterations to adult anxiety-like behavior following adolescent defeat are paralleled by alterations to ventral hippocampal 5-HT release and 5-HT1A receptor functionality.
Increased NE activity in the ventral hippocampus is seen with repeated stress exposure, and appears to enhance aversive coping (Joca, Ferreira, & Guimaraes, 2007), which may partially account for the increases in adult ventral hippocampal NE levels observed after adolescent defeat. Interestingly, we also found that increased ventral hippocampal NE levels were significantly correlated with the alterations in adult behavioral responses shown by previously defeated rats to both defeat context and EPM exposure. These results suggest that adolescent defeat-induced increases in ventral hippocampal NE levels have an enduring effect on expression of adult anxiety-like behavior in male rats. Norepinephrine has also been shown to cause a long-term potentiation of synaptic plasticity in perforant paths entering the DG from the entorhinal cortex (Leranth & Hajszan, 2007). Thus, assuming the increased NE levels in previously defeated rats correspond to increased activity, the higher NE levels in the ventral DG may represent a compensatory mechanism to counteract inhibitory effects on DG cell excitability resulting from increased 5-HT levels in this same region (Leranth and Hajszan, 2007). While lesion studies suggest that the ventral hippocampus is critical for mediating anxiety responses (Bannerman et al., 2004), excessive neuronal activity in this region is thought to contribute to the hyper-responsiveness of the subcortical DA system underlying both increased responses to psychomotor stimulants and schizophrenic psychoses (Lodge & Grace, 2007). Given these results, it is possible that elevated levels of ventral DG NE in adult rats following adolescent defeat could contribute to hyperactivity in ventral hippocampal pathways mediating DA system activity. Combined with effects caused by lowered mPFC DA levels, this may result in heightened subcortical DA responses to stimuli associated with either stress or drugs of abuse. However, further experiments would be required to confirm such hypotheses in this model.
Potential applications to long-term effects of human male adolescent bullying
Human bullying is characterized by an imbalance of power in which the victim is less powerful than the aggressor and is unable to defend themselves adequately, and bullying does not occur in conflicts between people of equal or similar power (Björkvist, 2001; Nansel et al., 2001; Rigby, 2003). This power imbalance becomes even more noticeable over time, with the bullying increasing in severity (Björkqvist, 2001). The closest parallels of such hierarchical relationships in animal models arise from social defeat paradigms (Björkvist, 2001). Repeated interactions typically result in the formation of a dominant-subordinate hierarchy (Miczek et al., 2004), in which the dominant can be broadly regarded as the “bully” while the subordinate becomes the “victim” (Björkqvist, 2001).
Being bullied during adolescence represents a severe problem for many teenagers worldwide (Nansel et al., 2001; Rigby, 2003; Bond, Wolfe, Tollit, Butler, & Patton, 2007). Typically, adolescent bullying takes the form of physical, psychological or verbal abuse, with boys being more likely to receive physical victimization (Björkqvist, 2001; Nansel et al., 2001). In the short term, adolescent bullying can result in negative psychological maladjustments that include increased incidences of anxiety, depression, substance abuse and suicidal tendencies (Hawker & Boulton, 2000; Kaltiala-Heino, Rimpela, Rantanen, & Rimpela, 2000; Brunstein Klomek, Marrocco, Kleinman, Schonfeld, & Gould, 2007). Such consequences also appear to be long-lasting, with experience of repeated adolescent bullying being correlated with greater incidence of stress-related psychiatric and addictive disorders in adult males (Hoffman et al., 2000; Rossow & Lauritzen, 2001; Newman et al., 2005; Wals & Verhulst, 2005; Gladstone et al., 2006). Our results indicate that repeated social defeat disrupts normal adolescent neural development in male rats, producing alterations in adult anxiety responses and changing monoaminergic function in stress-responsive limbic regions implicated in human drug addiction and some psychiatric disorders. We suggest that our rat model offers potential for identifying specific mechanisms induced by severe human adolescent stress, such as bullying, that may contribute to increased vulnerability to psychopathology in adult males. In addition, this model would allow effective exploration of the restorative effects of various pharmacotherapies on defeat-evoked neural and behavioral alterations, given the approximately two week period between the end of the adolescent defeat experience and adult testing.
Acknowledgements
This work was supported by NIDA RO1 DA019921 (GLF), NIH R03 MH068364 (MJW), and NIH P20 RR15567, which is designated a Center of Biomedical Research Excellence (COBRE). We thank Jamie Scholl for valuable technical assistance with these experiments.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Abumaria N, Rygula R, Havemann-Reinecke U, Ruther E, Bodemer W, Roos C, Flugge G. Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus. Cell Mol Neurobiol. 2006;26:145–162. doi: 10.1007/s10571-006-9024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ. The CCK-system mediates adaptation to novelty-induced stress in the rat: a pharmacological evidence. Neurosci Lett. 2007;428:27–32. doi: 10.1016/j.neulet.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(Suppl):S3–14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Bond L, Wolfe S, Tollit M, Butler H, Patton G. A comparison of the Gatehouse Bullying Scale and the peer relations questionnaire for students in secondary school. J Sch Health. 2007;77:75–79. doi: 10.1111/j.1746-1561.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brunstein Klomek A, Marrocco F, Kleinman M, Schonfeld IS, Gould MS. Bullying, depression, and suicidality in adolescents. J Am Acad Child Adolesc Psychiatry. 2007;46:40–49. doi: 10.1097/01.chi.0000242237.84925.18. [DOI] [PubMed] [Google Scholar]
- Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, De Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, Tilders FJ, Bohus B, Koolhaas JM. Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–520. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, De Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Kalen P, Mandel RJ, Bjorklund A. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. doi: 10.1016/0006-8993(92)90711-h. [DOI] [PubMed] [Google Scholar]
- Corrigan JG, Flannelly KJ. Ultrasonic vocalizations of defeated male rats. Journal of Comparative and Physiological Psychology. 1979;93:105–115. [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res. 1996;711:84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- File SE, Gonzalez LE. Anxiolytic effects in the plus-maze of 5-HT1A-receptor ligands in dorsal raphe and ventral hippocampus. Pharmacol Biochem Behav. 1996;54:123–128. doi: 10.1016/0091-3057(95)02108-6. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. European Journal of Neuroscience. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Malhi GS. Do bullied children become anxious and depressed adults?: A cross-sectional investigation of the correlates of bullying and anxious depression. J Nerv Ment Dis. 2006;194:201–208. doi: 10.1097/01.nmd.0000202491.99719.c3. [DOI] [PubMed] [Google Scholar]
- Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143:2852–2862. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- Hawker DS, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: a meta-analytic review of cross-sectional studies. J Child Psychol Psychiatry. 2000;41:441–455. [PubMed] [Google Scholar]
- Hoffmann JP, Cerbone FG, Su SS. A growth curve analysis of stress and adolescent drug use. Subst Use Misuse. 2000;35:687–716. doi: 10.3109/10826080009148417. [DOI] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Rimpela M, Rantanen P, Rimpela A. Bullying at school--an indicator of adolescents at risk for mental disorders. J Adolesc. 2000;23:661–674. doi: 10.1006/jado.2000.0351. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Haneda E, Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci. 2008;28:6272–6280. doi: 10.1523/JNEUROSCI.1656-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Kusljic S, Brosda J, Norman TR, van den Buuse M. Brain serotonin depletion by lesions of the median raphe nucleus enhances the psychotomimetic action of phencyclidine, but not dizocilpine (MK-801), in rats. Brain Res. 2005;1049:217–226. doi: 10.1016/j.brainres.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T. Extrinsic afferent systems to the dentate gyrus. Prog Brain Res. 2007;163:63–84. doi: 10.1016/S0079-6123(07)63004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linner L, Wiker C, Arborelius L, Schalling M, Svensson TH. Selective noradrenaline reuptake inhibition enhances serotonergic neuronal activity and transmitter release in the rat forebrain. J Neural Transm. 2004;111:127–139. doi: 10.1007/s00702-003-0084-9. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lyss PJ, Andersen SL, LeBlanc CJ, Teicher MH. Degree of neuronal activation following FG-7142 changes across regions during development. Brain Res Dev Brain Res. 1999;116:201–203. doi: 10.1016/s0165-3806(99)00069-3. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, De Boer SF. Aggressive, defensive and submissive behavior. In: Wishaw IQ, Kolb B, editors. The Behaviour of the Laboratory Rat: A Handbook with Tests. Oxford University Press; New York: 2005. pp. 344–352. [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr., Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Overpeck M, Pilla RS, Ruan WJ, Simons-Morton B, Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. Jama. 2001;285:2094–2100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ML, Holden GW, Delville Y. Isolation and the stress of being bullied. J Adolesc. 2005;28:343–357. doi: 10.1016/j.adolescence.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nunes-de-Souza RL, Canto-de-Souza A, Rodgers RJ. Effects of intrahippocampal infusion of WAY-100635 on plus-maze behavior in mice. Influence of site of injection and prior test experience. Brain Res. 2002;927:87–96. doi: 10.1016/s0006-8993(01)03335-2. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and guide to microdissection of the rat brain. Elsevier Sciences Publishing Co., Inc.; New York: 1988. [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Park MJ, Paul Mulye T, Adams SH, Brindis CD, Irwin CE., Jr. The health status of young adults in the United States. J Adolesc Health. 2006;39:305–317. doi: 10.1016/j.jadohealth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd ed. Academic Press; New York: 1997. [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus). Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Carboni L, Guidi A, Gerrard P, Arban R. Social defeat-induced contextual conditioning differentially imprints behavioral and adrenal reactivity: a time-course study in the rat. Physiol Behav. 2007;92:734–740. doi: 10.1016/j.physbeh.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Renner KJ, Krey LC, Luine VN. Effect of progesterone on monoamine turnover in the brain of the estrogen-primed rat. Brain Res Bull. 1987;19:195–202. doi: 10.1016/0361-9230(87)90085-2. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci. 2005;22:1185–1189. doi: 10.1111/j.1460-9568.2005.04251.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Rossow I, Lauritzen G. Shattered childhood: a key issue in suicidal behavior among drug addicts? Addiction. 2001;96:227–240. doi: 10.1046/j.1360-0443.2001.9622275.x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Siegfried B, Frischknecht HR, Waser PG. Defeat, learned submissiveness, and analgesia in mice: effect of genotype. Behav Neural Biol. 1984;42:91–97. doi: 10.1016/s0163-1047(84)90484-9. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- Vidal J, Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Wals M, Verhulst F. Child and adolescent antecedents of adult mood disorders. Curr Opin Psychiatry. 2005;18:15–19. [PubMed] [Google Scholar]
- Watt MJ, Forster GL, Korzan WJ, Renner KJ, Summers CH. Rapid neuroendocrine responses evoked at the onset of social challenge. Physiol Behav. 2007;90:567–575. doi: 10.1016/j.physbeh.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Locomotor response to novelty as a predictor of reactivity to aversive stimuli in the rat. Brain Res. 2007;1149:141–148. doi: 10.1016/j.brainres.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack JC, Delville Y. Stress, aggression, and puberty: neuroendocrine correlates of the development of agonistic behavior in golden hamsters. Brain Behav Evol. 2007;70:267–273. doi: 10.1159/000105490. [DOI] [PubMed] [Google Scholar]