Abstract
Historically herbs and spices have enjoyed a rich tradition of use for their flavor-enhancement characteristics and for their medicinal properties. The rising prevalence of chronic diseases world-wide and the corresponding rise in health care costs is propelling interest among researchers and the public for these food related items for multiple health benefits, including a reduction in cancer risk and modification of tumor behavior. A growing body of epidemiological and preclinical evidence points to culinary herbs and spices as minor dietary constituents with multiple anticancer characteristics. This review focuses on the anti-microbial, antioxidant, and anti-tumorigenic properties of herbs and spices, their ability to influence carcinogen bioactivation, and likely anticancer contributions. While culinary herbs and spices present intriguing possibilities for health promotion, more complete information is needed about the actual exposures to dietary components that are needed to bring about a response and the molecular target(s) for specific herbs and spices. Only after this information is obtained will it be possible to define appropriate intervention strategies to achieve maximum benefits from herbs and spices without eliciting ill-consequences.
Keywords: diet, herbs, spices, cancer prevention
Introduction
Archeologists discovered evidence that as early as 50,000 B.C. humans used the leaves of plants for flavoring meats and around 2300 B.C. for wine making (1). However, Alexander the Great's campaigns in Central Asia around 330 B.C. are often credited for introducing Asian, Persian, Indian, and Greek cultures and ideas, thus facilitating the dissemination and adoption of herbs and spices among many cultures (2, 3). The spice trade is known to have flourished during the second century A.D. along the trade routes known as the “Silk Road” which connected the East and the West (2). Early records indicate that herbs and spices were used as medicinals in ancient Egypt and Assyria and as food preservatives in ancient Rome and Greece (4). Herbs and spices continued to be used during the Middle Ages for flavoring, food preservation, and/or medicinal purposes. By the 1800s, new trade routes evolved and spice production and supplies increased, making herbs and spices more affordable, resulting in more widespread use among the European population (5).
Today, many ethnic cuisines are recognized for their reliance on “signature” herbs and spices. Turmeric in Indian cuisine; basil, garlic, and oregano in Italian and Greek cuisines; and lemongrass, ginger, cilantro, and chili peppers in Thai food represent some of the cultural diversity in the use of herbs and spices. Satia-Abouta et al (6) report that the cuisines of Asia, South-East Asia, and the Mediterranean are perceived by many to be healthier than the typical Western diet. Although it is logical to assume that the use of spices and herbs contributes to conflicts in scientific literature about the precise role of diet and cancer prevention, more detailed information is needed before firm conclusions can be drawn. The meta-analyses by Riboli and Norat (7) indicated cancer risk at various sites was associated with the intake of fruits and vegetables (which included onions and garlic). While herbs and spices were not specifically addressed in the analyses they did concluded other dietary protective factors may exist and contribute to individual variations in risk. For sure some evidence does point to herbs and spices as a likely important variable.
Herbs and spices used to season and preserve food may also contribute to eating behaviors. For instance, one of the highest rates of gastric cancer mortality in Europe occurs in Italy; yet rates are recognized to vary markedly across regions in the country (8). Results from a case-control study involving over 1,200 gastric cancer patients and more than 1,100 controls from 7 areas grouped into high and low risk areas, pointed to several categories of foods associated with gastric cancer risk (8). Specifically, Buiatti et al (8) determined that individuals who consumed more meats, salted fish, cold cuts and seasoned cheeses had the highest risk for gastric cancer, while those consuming more fresh fruit, raw vegetables, onion, garlic, and spices were associated with lower risk. In Asian countries, the consumption of curcumin, a component of curry powders, turmeric and mustard, along with low meat intake, have been reported to be factors linked to a lower incidence of colon cancer (9).
Today, strategies to improve health are a driving factor for market growth within the food and beverage industry (10). Interestingly, it has been reported that about 77% of U.S. households claim they are trying to reduce their risk of heart disease and cancer (11). Americans between the ages of 36 and 55 are increasingly interested in adopting healthy eating behaviors and are gravitating towards ethnic cuisines, such as Asian and Mediterranean, based on the perceived health benefits associated with these types of cuisines (12). Although some ethnic cuisines may be considered to be healthier than others, overall food consumption patterns, as well as food preparation techniques, are also likely equally important. According to a 1995 report (13) from the United States Department of Agriculture's (USDA) Economic Research Service (ERS), rising domestic use of spices reflects growing Hispanic and Asian populations within the U.S., as well as a trend toward the use of culinary herbs and spices to compensate for less salt and lower fat levels in foods, and a more general increase in the popularity of ethnic foods from Asia and Latin America. Between 1970 and 2005, the overall per capita consumption of spices has doubled, increasing from 1.6 pounds per year to 3.3 pounds per year; however in the case of garlic, as depicted in Figure 1, usage has increased more than six-fold (14).
Figure 1.
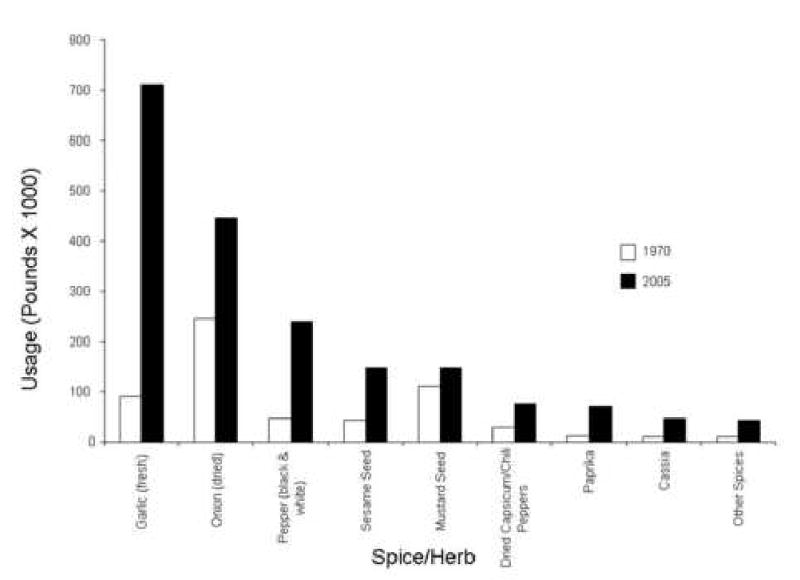
Changes in U.S. herb and spice use between 1970 and 2005 based on data from USDA's Economic Research Service Data (14). The “other spices” category includes basil, cardamom, capers, curry and curry powder products, dill, fenugreek, origanum, parsley, rosemary, savory, thyme, mixed spices, and other spices not individually reported by USDA.
There are no clear distinctions between culinary herbs and spices in much of the scientific and trade literature, with some plants considered to be both. While the U.S. Food and Drug Administration (FDA) do not provide standards of identity for spices (15), it does provide guidance concerning acceptable terminology used for food labeling purposes. FDA's general definition for a spice is an “aromatic vegetable substance, in the whole, broken, or ground form, whose significant function in food is seasoning rather than nutrition” and from which “no portion of any volatile oil or other flavoring principle has been removed” (15). The U.S. National Arboretum offers an alternative definition and describes spices as “flavorings (often of tropical origin) that are dried and culinary herbs are fresh or dried leaves from plants which can be used for flavoring purposes in food preparation” (16). Despite established definitions by FDA and the U.S. National Arboretum, herbs and spices are not only studied as intact products, but their active components are also examined. The interpretation of which is often complicated by the fact that these compounds frequently occur in multiple plants and their products (Table 1).
Table 1.
Examples of Bioactive Food Components in Commonly Used Culinary Herbs and Spices
| Herb/Spice | Bioactive Food Components |
|---|---|
| Allspice | Eugenol |
| Basil | Eugenol, apigenin, limonene, ursolic acid, methyl cinnamate, 1,8-cineole, α-terpinene, anthocyanins, β-sitosterol, carvacrol, cintronellol, farnesol, geraniol, kaempherol, menthol, p-coumaric acid, quercetin, rosmarinic acid, rutin, safrole, tannin, catechin, |
| Cardamom | Limonene, caffeic acid |
| Caraway | Carvone, limonene, α-pinene, kaempferol |
| Cinnamon | Cinnamic aldehyde, 2-hydroxycinnamaldehyde, eugenol |
| Cloves | Eugenol, isoeugenol, gallic acid |
| Coriander | Quercetin, caffeic acid, cineole, geraniol, borneol, 1,8-cineole, α-terpinene, β-carotene, β-pinene, β-sitosterol, cinnamic acid, ferrulic acid, γ-terpinene, kaempferol, limonene, myrcene, p-coumaric acid, p-cymene, quercetin, rutin, vanillic acid |
| Cumin | α-pinene, β-pinene, γ-terpinene, p-cymene, cuminaldehyde, carvone, 1,8-cineole, β-carotene, β-sitosterol, caffeic acid, carvacrol, carvaol, geranial, kaempferol, limonene, p-coumaric acid, quercetin, tannin, thymol |
| Dill | Carvone, limonene, isorhamnetin, kaempferol, myricetin, quercetin, catechin |
| Fennel | α-pinene, β-carotene, limonene, quercetin, benzoic acid, β-sitosterol, caffeic acid, cinnamic acid, ferulic acid, fumaric acid, kaempferol, myristicin, 1,8-cineole, p-coumaric acid, quercetin, rutin, vanillic acid, vanillin |
| Garlic | Allicin, diallyl disulfide, allyl isothiocyanate |
| Ginger | Zingiberone, zingiberene, ingerol, paradol, curcumin, shagoal |
| Lemongrass | Farnesol, geraniol |
| Licorice | Glycyrrhizin |
| Marjoram | Eugenol, limonene, ursolic acid, 1,8-cineole, α-pinene, α-terpinene, carvacrol, farnesol, geraniol, p-cymene, rosmarinic acid, sterols, thymol, apigenin |
| Mustard | Allyl isothiocyanate, β-carotene |
| Nutmeg | Caffeic acid, catechin |
| Onion | Quercetin, dipropyl disulfides |
| Oregano | Apigenin, luteolin, myricetin, quercetin, caffeic acid, p-coumaric acid, rosmarinic acid, carvacrol, thymol |
| Paprika | α-tocopherol, capsaicin, dihydrocapsaicin, lutein, β-carotene, ascorbic acid, Vitamin E |
| Parsley | Apigenin, luteolin, kaempferol, myricetin, quercetin, caffeic acid |
| Pepper, Black | Piperidine, piperine, limonene, α-pinene, β- pinene |
| Pepper, Red (also known as chili or cayenne pepper) | Capsaicin, α-tocopherol, lutein, β-carotene, ascorbic acid, Vitamin E |
| Peppermint | Limonene, menthol, eriodictyol, hesperitin, apigenin, luteolin |
| Rosemary | Carnasol, carnosic acid, cineole, geraniol, α-pinene, β-carotene, apigenin, limonene, naringin, luteolin, caffeic acid, rosmarinic acid, rosmanol, vanillic acid |
| Saffron | Crocetin, crocin, β-carotene, safranal, all trans retinoic acid |
| Sage | α-pinene, β-sitosterol, citral, farnesol, ferulic acid, gallic acid, geraniol, limonene, cineole, perillyl alcohol, β-carotene, catechin, apigenin, luteolin, saponin, ursolic acid, rosemarinic acid, carnosic acid, vanillic acid, caffeic acid, thymol, eugenol |
| Tarragon | Luteolin, isorhamnetin, kaempferol, quercetin, caffeic acid |
| Tea, Green | (-)-Epigallocatechin gallate, (-)-epigallocatechin, (-) – (+)-catechin, theophylline, gallic acid, theanine |
| Thyme | Thymol, carvacrol, cineole, α-pinene; apigenin, β-carotene, eugenol, limonene, ursolic acid, luteolin, gallic acid, caffeic acid, rosmarinic acid, carnosic acid, hispidulin, cismaritin |
| Turmeric | Curcumin, curcuminoids |
NCI's cancer Biomedical Informatics Grid™ (caBIG™), an open source, open access information network which is connecting the cancer research community and enabling the sharing of data and tools, recently funded the development of a nutrition ontology tool which integrates into the NCI Thesaurus (http://gforge.nci.nih.gov/projects/nutrition/). The NCI Thesaurus provides definitions, synonyms, and other information on nearly 10,000 cancers and related diseases, 8,000 single agents and combination therapies, and a wide range of other topics related to cancer and biomedical research. Standardized terminology is a key component of data sharing, and the recent inclusion of a nutrition ontology into the NCI Thesaurus will facilitate data-sharing between researchers from a variety of disciplines. Unfortunately, the nutrition ontology in the NCI Thesaurus is currently limited to foods and largely devoid of classically defined herbs and spices. Regardless, Table 2 provides an example of a classification framework for various phytochemicals found in herbs and spices which could be included in future versions of the nutrition ontology.
Table 2.
Classification Framework for Phytochemicals of Interest in Herbs and Spices
| Category | Class | Sub-Class | Examples of Compounds |
Examples of Herbs & Spices |
|---|---|---|---|---|
| Polyphenols | Flavonoids | Flavanols | (-)-Epi-gallocatechin gallate | Tea |
| Catechin | Nutmeg | |||
| Flavanones | Hesperitin | Peppermint | ||
| Naringenin | Rosemary | |||
| Eriodictyol | Peppermint | |||
| Flavones | Apigenin | Parsley | ||
| Thyme | ||||
| Luteolin | Oregano | |||
| Parsley | ||||
| Peppermint | ||||
| Rosemary | ||||
| Isoflavones | Diadzein | None | ||
| Genestein | None | |||
| Flavonols | Quercetin | Basil | ||
| Coriander | ||||
| Cumin | ||||
| Fennel | ||||
| Kaempferol | Basil | |||
| Coriander | ||||
| Cumin | ||||
| Fennel | ||||
| Isorhamnetin | Dill | |||
| Parsley | ||||
| Tarragon | ||||
| Myrcetin | Dill | |||
| Oregano | ||||
| Parsley | ||||
| Phenolic Acids | Hydroxy-benzoic acid derivatives | Gallic acid | Thyme | |
| Vanillic acid | Sage | |||
| Salicylic acid | Cumin | |||
| Hydroxy-cinnamic acid derivatives | Caffeic acid | Fennel | ||
| p-Coumaric acid | Cumin | |||
| Terpenes | Monoterpenes | Not applicable | Limonene | Cumin |
| Thyme | ||||
| Rosemary | ||||
| Caraway | ||||
| Mint | ||||
| Dill | ||||
| Celery seed | ||||
| Sage | ||||
| Coriander | ||||
| Fennel | ||||
| Marjoram | ||||
| Citral | Thyme | |||
| Sage | ||||
| Camphor | Thyme | |||
| Sage | ||||
| Rosemary | ||||
| Marjoram | ||||
| Fennel | ||||
| Coriander / cilantro | ||||
| Basil | ||||
| Menthol | Peppermint | |||
| Basil | ||||
| Perillyl alcohol | Spearmint | |||
| Sage | ||||
| Sesquiterpenes | Humulene, also known as caryophyllene | Turmeric (essential oil) | ||
| Coriander | ||||
| Diterpenes | Retinoids | Retinol | Paprika | |
| Red pepper | ||||
| Chili powder | ||||
| Triterpenes | Saponins | Glycyrrhizin | Licorice | |
| Tetraterpenes | Carotenoids | Mustard | ||
| Fennel | ||||
| Cumin | ||||
| Coriander | ||||
| Sage | ||||
| Vanilloids | Not applicable | Not Applicable | Curcumin | Turmeric |
| Ginger | ||||
| Mustard | ||||
| Gingerol | Ginger | |||
| Paradol | Ginger oleoresin | |||
| Capsaicin | Paprika | |||
| Red pepper | ||||
| Organosulfur Compounds | Disulfides | Not Applicable | Diallyl disulfide | Garlic |
| Thiosulfinates | Not Applicable | Allicin | Onion |
Consumption and Exposure Assessment Challenges
Efforts to assess dietary intake in general are complicated by measurement issues, such as recall bias, difficulty of estimating portion size and frequency of intake (7). Estimating typical herb and spice intake in sub-populations is even more problematic because they are generally consumed in conjunction with other foods and in trace amounts. Herbs and spices may not be a captured as a primary ingredient in a compositional analyses of food mixtures. Each year, the USDA's ERS publishes average U.S. per capita food availability (14), which takes into consideration a commodity's availability after subtraction of exports, industrial, and farm uses, and which may be considered a proxy for food intake, including spices. Some of the most commonly used herbs and spices for culinary purposes, as reported by the USDA, are presented in Figure 1.
Since regions around the world often have distinct ethnic cuisines, one can expect trends in spice and herb usage to vary substantially worldwide. A study which identified the most commonly used spices involving 36 countries found overlap between the most commonly used spices world-wide and the most common spices used in the U.S., such as onions, garlic, ginger, and some peppers (17). However, dietary exposure values by geographic region for the full range of herbs and spices, or their bioactive components, do not appear to exist. The difficulty in measuring herb and spice consumption arises from their use in food preparation in typically small amounts.
Concentrations of herbs and spices used in food preparation often vary based on individual preference, but the concentration of herbs and spices in finished foods frequently falls within the range of 0.5-1% (18). A recent attempt to evaluate human methyl eugenol exposure for the National Toxicology Program (NTP), which coordinates toxicology research and testing activities within the U.S. Department of Health and Human Services and in partnership with the National Cancer Institute (NCI), highlights many of the challenges associated with exposure assessment for herbs and spices in general. Methyl eugenol belongs to a family of chemicals which includes several naturally occurring compounds commonly found in the human diet, such as isoeugenol, eugenol, estragole, and safrole, which can be found in spices such as nutmeg and allspice, herbs such as basil and tarragon, in addition to fruits such as bananas and oranges (19).
As part of the NTP assessment, the Centers for Disease Control and Prevention (CDC) measured methyl eugenol exposure in a non-representative sample of adults in the U.S. as part of the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). According to Robison and Barr (19), methyl eugenol has a relatively short half-life so exposure assessment needs to include data related to when food was consumed relative to the collection of blood samples. Multiple samples obtained over a relatively short period of time may also be useful in the development of accurate human pharmokinetic models, which in turn can be compared to available toxicology data from rodent testing (19). Robison and Barr (19) called for longer term data collection through the more nationally representative NHANES samples which would allow for further analysis such as by racial/ethnic subpopulations and gender. Similar analyses would be needed for other key components in herbs and spices, such as those listed in Table 1, in order to establish robust research databases. Assessment of bioactive food components in herbs and spices are further complicated by known variations such as growing conditions and the actual varietals studied (20, 21).
The USDA's National Nutrient Data Base for Standard Reference (NNDB) serves as the one of the most commonly used references for food composition data in the U.S. The NNDB is often linked to other nutrient databases employed in large national surveys, such as NHANES. The NNDB also links to several therapeutic and research databases. Unfortunately, the inclusion of herbs, spices, and their active ingredients are only now beginning to be expanded in the NNDB and other USDA databases, such as the USDA's Database for the Flavonoid Content of Selected Foods. The available information on herbs and spices is limited in Release 19 of the USDA's NNDB and Release 2 of the USDA's flavonoid database in terms of the variety of products represented (e.g. fresh versus ground, commonly used varietals, etc.) and in the sense that other phytochemicals besides flavonoids are of interest for disease prevention, such as phenolic acids (e.g. salicylic acid and derivatives of hydroxybenzoic acid and hydroxycinnamic acid), terpenes, vanilloids, and organosulfur compounds. Table 2 provides examples of such compounds along with herbs and spices which contain such compounds. Additionally, there is evidence that the concentrations of bioactive components can differ by varietal and/or be influenced by processing (22-24). Parsley is the only product in the USDA flavonoid database for which data on both fresh and dried forms is available, but the database does not indicate which variety or varieties of parsley the data describes, e.g. Italian or “flat leaf” parsley vs. “curly” parsley, or a blend of both. Despite its limitations, the NNDB's flavonoid database is a promising first step and provides some valuable information regarding the chemical profile of several herbs and spices, especially since many herbs and spices contain one or more flavonoids.
While not everyone considers onions to be an herb or a spice, onions do provide flavoring and based on available data, they are one of greatest contributors of dietary flavonoids in the U.S., Japanese, Dutch, and Danish populations (25-28). Of the studies reviewed, only the study of flavonoid intake conducted in Japan (27) identified another herb or spice (parsley) as a significant contributor of flavonoids. Arai et al (27) reviewed 3-day food records and found that onions contributed 45.9% of total daily flavonoid intake, and 83.6% of quercetin intake; whereas parsley provided 2.4% of total daily flavonoid intake, primarily as myricetin. How this contribution relates to an overall phenotypic response remains to be established. Regardless, there is concern that such food frequency questionnaires (FFQ) do not adequately assess herb and spice intake and thus provides an incomplete assessment of dietary exposures and their ability to contribute to accurate exposure assessment for flavonoids and other compounds of interest (29).
Safety and Upper Limits
Herbs and spices are “Generally Recognized As Safe” (GRAS) by the FDA, at least at concentrations commonly found in foods; however, many herbs, spices, and their bioactive components, are being investigated for potential disease prevention and treatment at concentrations which may exceed those commonly used in food preparation. It is therefore imperative to identify any potential safety concerns associated with the use of various dosages which range from doses commonly used for culinary purposes to those used for medicinal purposes since there are often unclear boundaries between the various uses of herbs and spices. The NCI/NTP has reviewed several herbs and spices and their bioactive compounds (http://ntp.niehs.nih.gov/index.cfm?objectid=070073B1-B199-17BB-BCBF1BEEEA63AC7E) using the Ames Salmonella/Mammalian-Microsome Mutagenicity assay and/or the L5178Y TK+/- Mouse Lymphoma assay to identify the lowest effective dose (LED) that resulted in a positive increase in mutant frequency (30). The mouse lymphoma assay is one of the most sensitive assays (74%) but is one of the least specific (<45%), while the Ames assay has reasonable specificity (73.9%), low sensitivity (54%) (31). Kirkland et al (31) found that combinations of two to three genotoxicity assays resulted in sensitivities ≥90%. It should be noted that 75-95% of non-carcinogens gave positive results in at least one test. Many of the spices and herbs tested by the NCI/NTP had negative Ames test results, with the exception of kaempferol, (positive at 3.3 μg/plate) and quercetin (positive at 33 μg/plate) (30). There were no mouse lymphoma genotoxocity test results for capsaicin, curcumin, naringenin, or quercetin published by the NCI/NTP. The mouse lymphoma results were inconclusive or equivocal for saffron and cinnamic acid, and there were findings for caffeic acid (LED for nonactivated cells was 307 μg/mL), cinnamaldehyde (LED for nonactivated cells was 0.005 μg/mL and 0.049 μg/mL in S-9 activated cells), kaempferol (LED for nonactivated cells was 40 μg/mL), perillyl alcohol (LED was 200 μg/mL in S-9 activated cells), and sesamole (LED was 30 μg/mL in nonactivated cells and 26 μg/mL in S-9 activated cells). The NTP followed up with two year rodent carcinogenicity studies using some of these compounds, including quercetin, cinnamaldehyde, and turmeric. Although there was no evidence of carcinogenic activity associated with cinnamaldehyde, there was equivocal evidence of carcinogenic activity associated with turmeric in some rodent models consuming 10,000 ppm (which equates to approximately 1% of total diet) and some evidence of carcinogenic activity in male rats receiving 40,000 ppm quercetin (approximately 4% of total dietary intake) (32-34). It is difficult to translate the NTP's findings to humans since there was no evaluation of serum concentrations following ingestion. Despite this limitation, herbs, spices, and their bioactive components have not been an area of great concern since few studies have indicated potential safety concerns, and for those that do the dosage would typically be far beyond that occurring in t typical American diets.
Another source of data on the safety and efficacy of herbs and spices comes from the body of knowledge related to herbal medicine. The German Commission E Monographs (35) are probably the most widely known resource on herbal medicines. The German Commission E reviewed data on traditional uses, chemical data, experimental, pharmacological, and toxicology studies, clinical studies, field and epidemiological studies, patient case records, along with unpublished proprietary manufacturers' data. The Commission E published more than 400 monographs which are highly regarded and provide guidance to the public, health professionals, and industry on herbal products. There are two general categories of monographs. The first category of monographs consists of those that are negative or “unapproved,” for products where “no plausible evidence of efficacy” was available or when safety concerns outweighed potential benefits associated with the product's use. Basil, lemongrass, marjoram, nutmeg, and saffron were herbs and spices included in the unapproved monographs based either on documented and/or suspected risk or limited documentation of effectiveness for medicinal purposes. None of the purported uses of the unapproved herbs and spices in the Commission E monographs appears relevant to cancer prevention or treatment; however they raise some potentially relevant safety concerns. Although the Commission E monographs (35) indicate basil is safe when used for flavoring purposes in concentrations up to 5% of the total preparation, one of the risks associated with basil is a potentially mutagenic effect of estragole in the essential oil of basil. Although the Commission E monographs are based on a review of scientific and historical data, the monographs do not include references used to assess safety and efficacy. Lemongrass and nutmeg were also considered safe at doses used for flavoring purposes; however, safrole, found in the essential oil of nutmeg, has been shown to have mutagenic and carcinogenic effects, even though no mutagenic effects have been seen with essential oil of nutmeg (35). Nutmeg taken in amounts exceeding those typically obtained through diet alone has led to psychic disturbances (5 g nutmeg) and an atropine-like effect (9 teaspoons ground nutmeg per day). Hydroxyquinone in marjoram has also been found to be carcinogenic so marjoram is not recommended for extended usage; however there is no discussion of dose-response issues for marjoram in the English version of the Commission E monographs (35).
Several herbs and spices of culinary origin were included in the “approved” monographs, such as caraway oil and seed, cardamom seed, cinnamon bark, cloves, coriander seed, dill seed, fennel oil and seed, garlic, ginger root, licorice root, mint oil, onion, paprika, parsley herb and root, peppermint leaf and oil, rosemary, sage, thyme, turmeric root, and white mustard seed. The majority of the uses for these herbs and spices relate to dyspepsia and other gastrointestinal disturbances so do not pertain directly to cancer prevention, but the monographs do provide dosage guidelines, which indirectly may serve to guide researchers in establishing safe human dosages for herbs and spices under investigation for cancer treatment and prevention purposes. The recommendations for the various essential oils range from 3-6 drops per day for caraway and mint oils are 3-6 drops per day to 10-20 drops for rosemary (35). The recommended dose for seeds and other herbs and spices range from 1.5 g/day for caraway and cardamom seeds to 50 g/day of fresh onion or 20 g dried onion (35). While the typical doses recommended in the German Commission E monographs could be obtainable through diet alone in several instances, this might be easier to achieve with certain commonly used herbs and spices, such as garlic, onion, ginger, and parsley. There is little evidence documenting whether or not smaller amounts of a greater variety of herbs and spices which are known to have similar health effects would result in comparable health benefits. However, it is an area deserving further study.
Health Effects
To date, hundreds of compounds have been identified as potential modifiers of cancer, several of which are active ingredients in herbs and spices. Despite a rapidly growing body of experimental evidence supporting the cancer preventive properties of non-nutritive food components, such as herbs and spices, minimal data exists regarding actual dietary intake levels of herbs and spices in the U.S. and elsewhere. Consequently, researchers are limited in their ability to compare the effective exposures of culinary herbs, spices, and their bioactive components from experimental studies, which are primarily animal studies, using approximate human intake levels. Furthermore, information on the uptake, distribution, and excretion of most non-nutritive dietary components is sparse and little data related to human blood levels of these compounds has been published in the scientific literature (36). As seen in Figure 2, despite these limitations, research indicates that herbs and spices, or their bioactive components, may act alone or in concert to reduce cancer risk through their anti-microbial, anti-oxidant, and anti-tumorigenic properties, as well as their direct suppressive effect on carcinogen bioactivation. These properties will be discussed in greater detail below.
Figure 2.
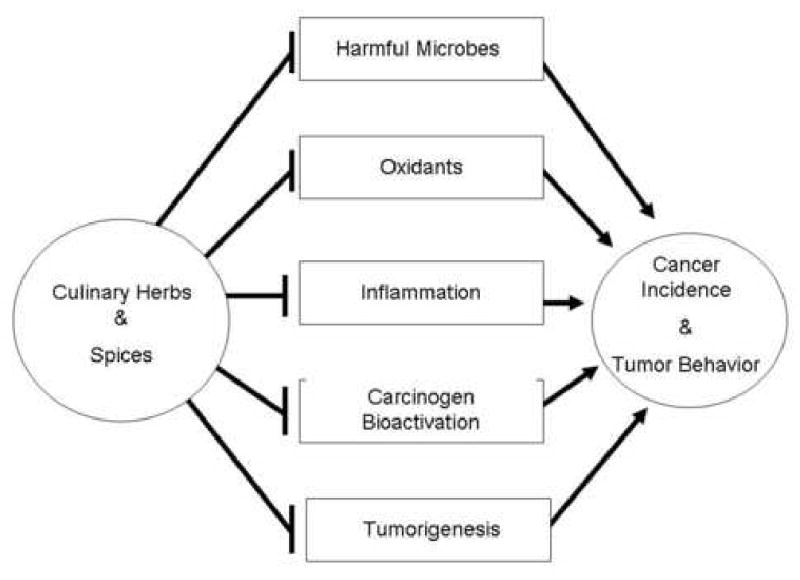
Herbs and spices can modify microbiota which can stimulate growth within organisms which protect against cancer as well as within microrganisms which may serve to increase cancer risk. Culinary herbs and spices generally serve as antioxidants but may also serve as prooxidants at higher exposures. Inflammation, tumorigenesis, and carcinogen bioactivation influence cancer risk and tumor behavior, but interventions which inhibit these processes can contribution to cancer prevention.
I. Antimicrobial and Antifungal Activity
Although historical records indicate that herbs and spices were used for flavoring, food preservation, and medicinal purposes in ancient times, it is uncertain how herbs and spices first became incorporated into food preparation and how widely herbs and spices were used in food and medicine until more modern times. Issues pertaining to the prevention of food-borne illness have long been an area of concern for scientists, public health officials, and the public; however, a growing number of researchers are investigating the links between the antimicrobial and antifungal properties of various foods and food components, such as culinary herbs and spices, and their role(s) in reducing chronic disease risk. Bacterial, viral, and parasitic mediated cancer deaths have been estimated to range from 20-25% in developing countries and 7-10% in industrialized countries (37). How culinary herbs and spices may influence the complications associated with these bacterial, viral, and parasitic challenges remains unknown.
Herbs and spices which possess antimicrobial activity include those containing simple phenols and phenolic acids, coumarins, terpenoids, and alkaloids (38, 39). A study in the late 1990s set out to investigate the hypothesis that herbs and spices, which have known antimicrobial properties, were incorporated into food preparation for their antimicrobial properties rather than for purely organoleptic purposes (17). Billing et al found that countries with hot climates use multiple spices on a regular basis (in >40% of meat-based recipes) versus countries with cooler climates (<5% of meat-based recipes) (17).
This finding is notable for two reasons: 1) prior to refrigeration, foods in warmer climates were likely to spoil more quickly than foods in cooler climates and 2) many spices work synergistically and display increased antibacterial capabilities when used in combination than when used alone (17). Use of chili peppers (also known as capsicums), garlic, onion, anise, cinnamon, coriander, cumin, ginger, lemongrass, and turmeric were positively correlated with mean annual temperature (17). There was also a significant positive correlation between mean annual temperatures and the proportion of meat-based recipes that incorporate spices which have a bacterial inhibition rate >75%, indicating that recipes from hotter regions of the world tend to have higher bactericidal potential (17).
A side-by-side comparison of the sensitivity of several bacteria to eight common antibiotics and extracts of garlic and clove found that the garlic extract displayed bactericidal activity similar to antibiotics with several Gram-negative bacteria (40). Gram negative bacteria which have been studied in relation to herbs and spices include Escherichia coli (E. coli),Salmonella, and Vibrio cholerae (V. cholerae). It is relatively well known that some herbs and spices have antibacterial effects on a wide range of species while others remain relatively specific (41). Liu and Nakano (41) assessed the growth inhibition of S. aureus, E. coli, Salmonella, V. cholerae, and Bacillus using three different concentrations (0.5%, 0.2%, and 0.1%) of five herbs and 17 spices. Overall, herbs and spices tended to inhibit gram-positive bacteria more than gram-negative bacteria (41). Based on these findings, further investigation of potential synergistic effects of herbs and spices when used in combination, as they are in meals, would be of interest.
The concentration of herbs and spices is important in these types of studies, as is the varietal and form of the herb and spice used, due to inherent differences in the types and amounts of bioactive ingredients contained in the various varietals and preparations. For instance, garlic did not show much inhibitory activity in Liu and Nakano's study (41) despite extensive literature indicating otherwise (40, 42). Liu and Nakano postulated that these differences may have been related to use of dried garlic powder in their study compared to other studies which relied on other forms of garlic (41). Essential oils extracted from herbs and spices have displayed varying bactericidal activity when compared to the known antimicrobial activity of their primary constituents, which indicates there may be unknown minor components in the essential oils with antimicrobial activity, or there may be synergistic or antagonistic effects between all the bioactive compounds combined (39, 43). Yano et al measured the inhibitory action of several herbs and spices on E. coli and V. parahaemolyticus and found that gram positive bacteria which form spores appear to be more sensitive to herbs and spices (44). Yano et al also found that the antibacterial activity of spice extracts against gram positive species of Bacillus was enhanced in some, but not all, cases in slightly acidic or salty conditions (44).
In most cases, the mechanism(s) of action for the antimicrobial actions of spices are not well understood (38, 45). Potential mechanisms for the antibacterial action of herbs and spices include: interference with the phospholipid bilayer of the bacterial membrane resulting in greater permeability, loss of cellular components, impaired enzyme systems needed for production of energy and structural components, and inactivation or destruction of genetic material (41). Human studies investigating potential mechanisms linking the antimicrobial nature of herbs and spices to the cancer continuum are limited but continued exploration of the antimicrobial nature of herbs and spice is warranted since both gram-positive and gram-negative bacteria appear to be affected by herbs and spices.
Several strains of Helicobacter species (H. pylori, H. cholecystus, H. pullorum, H. bilis, and H. hepaticus) may facilitate the invasion and progression of cancer, especially in the stomach, liver, gallbladder, and intestine (37, 46). H. pylori is susceptible to several essential oils, of which cinnamon bark oil and savory oil were the most effective bactericial inhibitors, with the minimum bactericidal concentration for each oil in a liquid medium equal to1 g/L at 1 hour and 0.04 g/L at 24 hours (39). Many of the essential oils with positive bactericidal activity were effective not only against the P1 strain, but other H. pylori strains as well, including a strain resistant to the two drugs which are part of current anti-H. pylori therapies (39). Garlic has been associated with low rates of peptic ulceration and reduced risk for gastric cancer in epidemiologic studies, yet when garlic oil capsules were given to patients with dyspepsia related to H. pylori in a pilot test, the garlic oil at the dose selected based on in vitro studies (4 mg garlic oil capsule with a meal four times per day for 14 days) did not eradicate or suppress H. pylori (47). Consequently, questions remain regarding the importance of dose-response relationships and the degree of bacterial overgrowth.
II. Antioxidant Actions
In the human body, oxidant-antioxidant balance is critical because it maintains cell membrane integrity and functionality, cell proteins, and nucleic acids (48). In healthy humans, free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels are controlled; however the concept of oxidative stress hypothesizes that exposure to adverse physiochemical, environmental, and pathological agents disrupts the body's natural balance, and if excess free radicals are not eliminated by antioxidants, they may damage crucial extracellular or cellular components. The utility of antioxidants in disease prevention has been challenged by negative consequences associated with antioxidant supplementation (vitamin E and selenium) (49). Examples of potential damage caused by oxidant-antioxidant imbalance include impaired cell functions, cell death, impaired immunity, and DNA damage which can cause mutations and ultimately contribute towards the development of chronic diseases, such as cancer (48, 50-52).
Many herbs and spices are the subject of ongoing scientific investigations related to antioxidant properties and health. Epidemiological evidence exists indicating there is a correlation between increased dietary intake of antioxidants and a lower incidence of morbidity and mortality (50). For instance, a population-based case-control study in approximately 500 newly diagnosed gastric adenocarcinoma patients and approximately 1100 control subjects in Sweden found that the total antioxidant potential of several plant-based dietary components was inversely associated with gastric cancer risk (51). The current body of scientific knowledge consists primarily of studies which employ one of four different analytical techniques to measure antioxidant capacity: the total peroxyl radical trapping parameter assay, the Trolox equivalent antioxidant capacity (TEAC) assay, the ferric reducing ability of plasma (FRAP) assay, and the oxygen radical absorbance capacity (ORAC) assay. The ORAC and TEAC assays measure the inhibition of free radical action and the FRAP assay measures the ability of a sample to reduce Fe 3+ to Fe 2+, which does not necessarily match its antioxidant capacity against free radicals (53). Cao and Prior found no correlation between serum ORAC and serum TEAC or between FRAP and TEAC (53). Although Cao and Prior found a weak but significant correlation between serum ORAC and serum FRAP results, another limitation of the FRAP assay is that it does not measure low molecular weight compounds, such as antioxidants which contain SH-groups (53). Furthermore, Pellegrini et al (54) demonstrated that between-study comparisons of the antioxidant capacity of various dietary components are complicated by the use of multiple analytical methods.
The largest published study to date which tested the antioxidant activity of foods from a nationally representative food evaluated the antioxidant activity in both water-soluble and fat-soluble fractions of 1,113 food samples from the USDA's National Food and Nutrient Analysis Program (NFNAP) using the FRAP method (55). This study found that of the top 50 foods with antioxidants, the top five antioxidants were dried spices (ground cloves, dried oregano, ground ginger, ground cinnamon, turmeric powder); however, compared to other categories of food products within this study, herbs and spices displayed the largest range in antioxidant capacity, 0.803-125.549 mmol/100 g (55). Another concern with these findings is that Halvorsen et al reported the antioxidant content as mmol/100 g, and even though data on the population-level exposure to herbs and spices is lacking, it would be much more unlikely a person would consume similar volumes of herbs and spices as they would other food categories reported in this study which were found to contain lower antioxidant levels but which are generally consumed in larger quantities on a regular basis, such as cereals and grains or fruits and vegetables (55).
A previous study by Dragland et al (21) also utilized the FRAP method to assess the antioxidant capacity of 18 fresh herbs and 38 commercially available dried spices in Norway. Oregano, sage, peppermint, and thyme contained the greatest antioxidant capacity for fresh herbs, while cloves, allspice, and cinnamon contained the highest levels of antioxidant activity in dried spices (21). Dragland et al (21) considered herbs or spices to be high in antioxidants if they contained >75 mmol/100 g, whereas >10 mmol/100g was considered to be a high antioxidant content in the study by Halvorsen et al (21, 55). In addition to differing in the criteria for what was considered “high antioxidant content,” the two studies, which had 10 spices in common, also demonstrated a wide range of values for similar spices. For instance, the measured antioxidant content for chili powder and ground mustard seed were quite similar between Halvorsen et al's study and Dragland et al (10.5 mmol/100 g and 10.4 mmol/100 g) while the values for ground cinnamon were found to range from 17.7 mmol/100g in Halvorsen et al's study to 53.0 mmol/100g and 98.4 mmol/100g in Dragland et al's study (21, 55). Dragland et al reported that antioxidant content of plants can differ between related varieties of plants, such as Mexican and Greek oregano, as well as growing seasons, which may also explain some of the variations in antioxidant content between similar spices in the two studies (21).
There are different versions of the ORAC assay, some of which assess the antioxidant capacity in the hydrophilic fractions of samples, while other versions evaluate both hydrophilic and lipophilic fractions. Ninfali et al (56) measured the antioxidant activity in the hydrophilic fractions of 15 fresh herbs along with six spices commonly consumed in Central Italy using the ORAC method. Thyme, sage, rosemary, and marjoram contained the greatest antioxidant capacity among the herbs, and among the spices, cumin and ginger had the highest ORAC scores. A previous study by Zheng and Wang (57) also evaluated the antioxidant activity in the hydrophilic fractions of 39 fresh herbs (but not spices) collected in September 2000 from the National Herb Garden in Washington, D.C. The herbs with the highest reported antioxidant capacity in Zheng and Whang's study were for Mexican and Greek oregano, marjoram, and dill (57). Wu et al (58) measured the antioxidant capacity of hydrophilic and lipophilic fractions of 16 dried spices from NFNAP samples. They found that the lipophilic ORAC values for four spices (clove, ginger, black pepper, and turmeric) were higher than the hydrophilic ORAC values, which indicated the essential oils in these spices contained a substantial amount of antioxidants (58). In addition to differences in antioxidative capacity attributable to hydrophilic versus lipophilic fractions, Shan et al (59) suggest that some of the variation in antioxidant capacity between studies may be attributed to genotypic and environmental differences within species, parts of the plants which are studied, time of year the samples were taken (especially for fresh products), and analytical methods used (59).
Researchers have found a positive linear correlation between phenolic compounds, primarily phenolic acids and flavonoids, and the antioxidant capacity of herbs and spices (57). Since increases in plasma antioxidant capacity in humans often greatly exceed the plasma concentrations of flavonoids or plant phenols following consumption of flavonoid-rich foods, which are usually in the nM to low μM range, the significance of these findings is questionable (60). Furthermore, plasma antioxidant capacity may be affected by other dietary constituents, such as carbohydrates and fats, which influence uptake, tissue mobilization, or metabolism. Because of the limited characterization of the herbs and spices studied in most cases, flavonoids may act synergistically with other bioactive food compounds to influence antioxidative capacity or may influence plasma concentrations of urate and ascorbate, which are powerful antioxidants (60).
Much remains to be done to understand the mechanisms of action for antioxidants, their impact on various types of tumors, the degree to which antioxidants are absorbed from foods, and what effective concentrations are needed in humans to reduce oxidative stress at the tissue or cellular level and how the antioxidant capacity of foods and food components relates to physiologic events in humans. To facilitate research in this area, there remains a need to collect additional data on the antioxidant capacity of herbs, spices, and their bioactive components from in vivo, in vitro, and clinical studies and establish more detailed research databases to serve as a repository for this data from a variety of disciplines to promote trans-disciplinary research and ultimately aid in fostering new discoveries. Such a database will become more robust as researchers learn more about the variation attributed to varietals of similar plants, processing (e.g. fresh vs. ground preparations, pre- and post-cooking), seasonal variation and growing conditions, etc. Databases on the physical properties of herbs and spices, along with data on intake levels, would also expand the understanding of the public health implications of herbs and spices at the population level, such as the effects of short-term versus long-term intake.
III. Inflammation
Since Virchow proposed a connection between inflammation and cancer in 1863, it has been estimated that approximately 15% of all cancers are linked to inflammation, including associations between cervical cancer and the human papillomavirus (HPV); liver cancer and hepatitis B or C; Barrett's esophagus, and esophageal cancer; and chronic inflammatory bowel disease and colorectal cancer (61). Inflammation alone will not cause cancer; mutations and epigenetic events from environmental exposures or alterations in immunity are also key contributors in the cancer process (37). Several proinflammatory mediators, such as cytokines, chemokines, prostaglandins (PGs), nitric oxide (NO) and leukotrienes disrupt normal signaling cascades within cells which contributes to the development of neoplasms (62). In vitro studies indicate several herbs and spices, or some of their bioactive components, can inhibit, and sometimes induce, several enzyme systems which are involved in pathways that regulate the inflammatory and immune response (63).
A depression in inflammation associated pathways, especially cyclooxygenase, has been associated with a reduced risk for breast, colon, lung, pancreatic, and head and neck cancers. The side effects of non-steroidal anti-inflammatory drugs (NSAIDS), especially the risk of cardiovascular events and gastrointestinal bleeding, make herbs and spices potentially appealing alternatives (64). Areas of ongoing cancer research include the clarification of the intracellular signaling mechanisms involved in NF-κB activation and the induction of COX-2 and iNOS, and how they impact different cell lines and cell types, as well as other factors within the cellular environment which influences regulation of the various pathways that influence inflammation and the cancer process (65). Cancer is generally characterized by unusually high iNOS expression and NO production, thus the potential for elimination of NO by NO-scavengers and/or compounds which inactivate iNOS, by food components may be a viable prevention strategy.
Cyclooxygenase (COX) has a critical role in the inflammatory process and has been shown to contribute to pain, swelling, and stiffness (66, 67). Likewise, the LOX pathway produces leukotrienes which, like PGs, are potent causal pain agents. One polyphenol compound, curcumin, inhibited COX-dependent arachidonic acid metabolism by 23%, displayed a strongest inhibitory effect on the peroxidase activity of ovine COX-1 (IC50 ∼50 μM), and significantly decreased induced COX-2 expression (20 μM), although the underlying mechanism remains poorly understood (68, 69). Very low concentrations of salicylic acid found in the blood following ingestion of aspirin (10-30 mg doses) have been shown to suppress COX-2 transcription and are thought to explain epidemiological observations which link aspirin use with reduced risk for colon cancer (70). Similar serum concentrations of salicylic acid have been found between people taking salicylate drugs and vegetarians not taking salicylate drugs, but are lower for non-vegetarians (71). Paterson et al evaluated the salicylate content by weight of several spices commonly used in Indian cooking and found that chili powder, paprika, and turmeric contained >0.1% salicylates and cumin contained >1.5% salicylates and also found that cooked dishes containing these spices contained pharmacological amounts of salicylic acid which were biologically available as evidenced by ∼20-fold increase in urinary metabolite excretion following test meals (23).
Nitric oxide (NO) is an inflammatory mediator implicated in cancer development which has been shown to be inhibited by bioactive components within herbs and spices (72, 73). Examples of bioactive compounds in herbs and spices which can suppress NO activity include: carnasol in rosemary, curcumin in turmeric, [6]-gingerol in ginger, and quercetin in basil, coriander / cilantro, cumin, and fennel (74-76). The suppression of NO likely reflects an activation of inducible nitric oxide synthase (iNOS) (72). Kim et al (73) examined the inhibition of NO in extracts from 48 fresh plants, several of which were herbs and observed that spearmint, basil, parsley, and portions of the garlic plant exhibited strong total inhibitory activity, which was defined as an inhibition ≥70%. Clove was the only spice tested found to be a poor candidate for further investigation due to a cytotoxic effect which was more pronounced than its NO-suppressing effect when the concentration exceeded 0.4% (73). Tsai et al (72) reported rosemary was superior in blocking NO formation, followed by decreasing efficacy with tarragon, cinnamon, oregano, basil, marjoram, allspice, and thyme. Of the spices tested by Tsai et al (72), only cinnamon displayed significant NO-scavenging ability; however, the scavenging was less than hemoglobin and thus its physiological significance is questionable. Overall, the ability of herbs and spices to block NO formation appears to be more physiologically relevant.
NO is closely linked to the nuclear transcription factor κB (NF-κB) pathway, which is often viewed as a “critical component to bridge inflammation and cancer” (62, 66, 77). NF-κB is a redox-regulated transcription factor, normally found in the cytoplasm as part of an inactive complex, but when it is activated by free radicals, inflammatory stimuli, carcinogens, tumor promoters, endotoxins, and radiation, it moves from the cytoplasm to the nucleus where it induces the expression of more than 200 genes. These genes not only affect inflammation, they also are associated with apoptosis, proliferation, and metastasis (66). Many of the target genes activated by the NF-κB pathway are key components for the establishment of many aggressive cancers (77). Quercetin, a bioactive compound found in many foods as well as herbs and spices such as basil, cumin, and fennel, has been shown to inhibit the NF-κB pathway in vivo and in vitro (66, 78). Comolada et al found discrepancies between in vivo and in vitro antiinflammatory effects in experimental models of rat colitis for quercetin and its glycosylated form (quercitrin), which is the form commonly found in the diet which may be due in part to quercetin's rapid absorption in the upper gastrointestinal tract before reaching effective concentrations at the target site, which was the colon (78). Other inhibitors of the NF-κB pathway include ursolic acid (found in basil and rosemary), gingerol (present in ginger), and curcumin at concentrations of 100 μmol, 5 μmol, and 40-60 μmol respectively (77, 79-81).
Peroxisome proliferators-activated receptors (PPARs) are transcription factors which belong to the nuclear receptor gene family. One PPAR sub-family, PPARγ, is thought to be involved with immune response, specifically by activating arachidonic acid metabolites (82). PPARs serve as a link between pro-inflammatory cytokines and gene transcription factors and can influence cellular differentiation, apoptosis, and inflammation (66). Liang et al (82) examined flavonoids, and found that flavones, flavonols, and isoflavones, but not flavanones and flavan-3-ols, were able to activate PPARγ, possibly because of the number and position of hydroxyl residues. Apigenin (a flavone) and kaempferol (a flavanol) in basil, coriander/cilantro, cumin, rosemary, and thyme activate PPARγ to inhibit COX-2 expression in a dose-dependent with an EC50 of approximately 5 μM and 10 μM respectively (82). Liang et al found that a higher concentration (IC50 was 50 μM) was needed to bind to a Gst-PPARγ in an in vitro competitive binding assay, indicating flavonoids might not bind directly to PPARγ (82). Liang et al also found a cytotoxic effect at 20 μM of apigenin in RAW264.7 cells and a corresponding decrease in PPARγ activation (82). Quercetin, a known LOX inhibitor, was found to block keratinocyte differentiation and directly inhibit PPARs and the expression of PPAR-regulated genes (83).
The non-steroidal anti-inflammatory drug (NSAID) activated gene-1 (NAG-1) protein, which can be induced by several dietary compounds, also has a broad range of activities related to inflammation, cancer, and differentiation and its expression may reduce TNF-α secretion in macrophages and several cancer cell lines (66). Components of spices and herbs which have been demonstrated to upregulate NAG-1 expression include capsaicin in red chili pepper (10-100 μM), curcumin in turmeric and ginger (0.01-0.1 μM), and 6-gingerol (10-100 μM) (66).
Unfortunately, human studies using herbs, spices, or their active components to reduce inflammatory diseases are limited at this point. Additional information is needed about the absorption, distribution, and metabolism of herbs and spices in humans. Nevertheless, a recent pilot study provided promising findings related to the ability of curcumin to reduce inflammatory markers in five patients with proctitis, ulcerative colitis, and Crohn's disease (84). Holt et al (84) administered 360 mg of curcumin as a tablet three times a day for one month followed by 360 mg four times a day for two more months. On completion of treatment, all patients with proctitis had a reduced number of stools and almost all were able to decrease or eliminate the need for medications and all of the participants with limited ulcerative colitis displayed improvements in their Crohn's Disease Activity Index, C-reactive protein levels, erythrocyte sedimentation rate, and serologic indexes of inflammation (84).
IV. Carcinogen Bioactivation
Phase I enzymes have an important role in the activation of procarcinogens; equally important in disease prevention are the phase II enzymes which are involved in the body's natural detoxification process and in drug metabolism and excretion. Some phase I cytochrome P450 (CYP) enzymes may activate pro-carcinogens to carcinogens, and some CYPs may assist with carcinogen removal from the body. CYP expression does vary between individuals, due in part to genetic mutations and polymorphisms, along with the influence of environmental factors, specifically the presence of inducers and inhibitors (85). Likewise, polymorphisms in phase II enzymes and altered gene expression patterns of these enzymes are related to the response to food components and may therefore influence cancer risk (86, 87).
Compounds in garlic, pepper, rosemary, turmeric, and cinnamon appear to influence phase I and phase II enzymes (85, 88-90). Multiple compounds in garlic, such as diallyl sulfide (DAS), diallyl sulfone (DASO), and diallyl sulfoxide (DASO2) may be involved in directly inhibiting CYP2E1 activity (85). Evidence from piperine, a component of black pepper, indicates an in vitro and in vivo dose-dependent response in some phase I enzymatic activity (CYP2B, CYP2C, CYP2E), although this response does vary by species and route of administration, in addition to the dose (85). Debersac et al (89) found that water soluble rosemary extract and essential oil of rosemary, with a high content of 1, 8-cineole (36.1%) induced CYP2B1, 2 and multiple phase II enzymes, such as hepatic glutathione-S-transferases (GST), quinine reductase (QR), and UDP-glucuronosyl-transferase (UGT), especially UGT1A6 which are involved in critical detoxification pathways. The cause of the strong induction of phase II enzymes remains largely unknown, however synergistic effects of a combination of phenolic compounds are thought to contribute to these findings (89). Another example of the widespread effects of herbs and spices comes from the observations that curcumin inhibited reactions catalyzed by CYP1A1, 1A2, and 2B1 in rat liver cells and induced phase II enzymes, especially GST and QR in the liver and kidney in rats, human melanoma cells, and glutathione S-transferase P1-1 (GSTP1-1) in K562 and Jurkat leukemia cells (88). Shen et al (91) recently demonstrated in vivo that curcumin (1000 mg/kg), along with transporter proteins and oxidative stress genes, could regulate phase I and II xenobiotic metabolizing enzyme genes in mouse liver and small intestine through Nrf2-dependent pathways.
In vitro and pre-clinical studies using animal models indicate herbs and spices potentially affect carcinogen bioactivation. It is premature to believe that these findings will translate into strategies for reducing cancer in humans (36, 92). Additionally, the regulation of phase I enzymes, such as CYPs, by herbs, spices, and their bioactive compounds varies significantly, depending on the CYP and the type of herb or spice studied and its dose, route of administration, target organ, and interspecies variation (85). The combination of new in vitro models for mechanistic research with the continuing development of new biomarkers and their increasing inclusion in epidemiological studies offers the opportunity to determine whether disease protection mechanisms may be realistic in humans.
V. Anti-tumorigenic Mechanisms
One of the defining characteristics of cancer is the loss of controlled growth regulation. Tumorigenesis can be activated by environmental carcinogens, inflammatory agents, and tumor promoters which modulate transcription factors, anti-apoptotic proteins, pro-apoptotic proteins, protein kinases, cell cycle proteins, cell adhesion molecules, COX-2, and growth signaling pathways (77). Some of the most critical pathways include the NF-κB pathway, receptor tyrosine kinase receptor (RTK) pathways, the mitogen-activated protein kinase (MAPK) pathways, inhibition of COX, and regulation of the cell cycle.
The NF-κB pathway has a central role in the anti-tumor properties of many herbs and spices because of the pathway's involvement in cell survival and proliferation in many types of malignancies (68, 77). The NF-κB pathway also promotes the expression of genes involved in angiogenesis and growth of invasive cancer cells (93). Although curcumin itself does not activate NF-κB, it is a very potent inhibitor of TNF-induced activation of NF-κB regardless of whether a low (0.1 nM) or high dose (10 nM) of TNF is employed (81).
RTKs are involved in tumor pathogenesis when overexpressed (93). Turmeric has been identified as a spice that decreases expression of RTKs such as epidermal growth factor receptor (EGFR) and HER2(77). The activator protein-1 (AP-1) pathway is also linked to growth through regulation, cell transformation, apoptosis, cellular proliferation, repression of tumor-suppressor genes, as well as involvement in the stages of tumor metastasis (77). Quercetin, which is an active component in basil, coriander / cilantro, cumin, and fennel, as well as curcumin, and capsaicin have been shown to suppress AP-1 activation (77). Additionally, the mitogen-activated protein kinase (MAPK) pathways contribute to the development of several types of tumors by providing proliferation signals to cells (94). Coriander and fennel have been found to decrease expression of both MAPK and c-Jun N-terminal kinase (JNK), which is another component of MAPK pathways (77).
In vitro studies indicate herbs, spices, and their bioactive components can inhibit, and sometimes induce pathways that regulate cell division, cell proliferation, detoxification, in addition to the inflammatory and immune response (63). For instance, Shishodia et al (80) demonstrated that ursolic acid, a bioactive component in some herbs and spices, suppressed TNF-induced expression of genes regulated by NF-κB (cyclin D1, COX-2, and MMP-9) which are involved in tumor initiation, promotion, and metastasis. Cyclin D1 over-expression has been linked with breast, esophageal, head, neck, and prostate cancers (77). There are always concerns about how to interpret in vitro studies because of issues related to physiologically relevant concentrations and bioavailability (94, 95).
Another study which evaluated the impact of cumin seed as part of a diet fed to mice in varying concentrations (2.5% and 5%) found that compared to controls, those receiving the low dose of cumin had 28.6% fewer forestomach tumors and those consuming the higher dose of cumin had a 35.7% reduction in tumor incidence rate, although there was no correlation between cumin intake and hyperplasia or dysplasia used to calculate uterine cervix cancer incidence (96). Saffron, and some of its bioactive components, have also been shown to possess antitumor activity, but as observed in the research with cumin seeds, varied responses to saffron and its bioactive components have been observed in different types of cultured malignant cells (97). There also been a differential response noted between male and female rats with colon adenocarcinomas to crocin (a compound in saffron) which indicates the potential involvement of hormonal factors in tumorigenesis (98).
Future research should focus on identifying the key molecules in the cell signaling network which are affected by components of herbs and spices and elucidation of their mechanisms of action. Clarification is also needed regarding which types of cells respond to various herbs, spices, and their bioactive components and further exploration of the potential for gender-based differences in response.
Conclusions
Several issues surface when trying to unravel the health significance of herbs and spices. The lack of commonly accepted terminology, lack of standardized spice and herb extracts and/or preparations, a high degree of variability in the description of herbal and spice products used in studies, along with inconsistent and/or limited information regarding the chemical profiles or active ingredients, and the dearth of clinical interventions raise significant concerns about what is and is not known. Nevertheless, the evidence to date with herbs and spices is intriguing and warrants greater attention. More attention to exposures needed to bring about a biological effect and how susceptibility factors including nutrient-nutrient interactions and genetics influence a response is indicated. The identification of biomarkers of exposure, effect, and susceptibility will be crucial in identifying those who will benefit most from herbs and spices.
Acknowledgments
Grants, Sponsors, or Funding Sources: Division Cancer Prevention, National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The American Spice Trade Association. A timeline in spice history. [cited 2007 Jun 8]; Available from: http://www.astaspice.org/history/timeline.htm.
- 2.USC-UCLA Joint East Asian Studies Center. Along the Silk Road: People, Interaction & Cultural Exchange. 1993 [cited 2006 Dec 18]; Available from: http://www.isop.ucla.edu/eas/sum-inst/links/silkunit.htm.
- 3.Silk Road Study Group. Silk Road. 2000 [cited 2007 Jun 8]; Available from: http://gallery.sjsu.edu/silkroad/info.htm.
- 4.Parry JW. The story of spices. New York: Chemical Publishing Co, Inc; 1953. [Google Scholar]
- 5.McCormick. The history of spices. 2006 [cited 2007 Jun 8]; Available from: http://www.mccormick.com/content.cfm?ID=10111&StatusCD=P.
- 6.Satia-Abouta J, Patterson RE, Neuhouser ML, Elder J. Dietary acculturation: applications to nutrition research and dietetics. J Am Diet Assoc. 2002;102(8):1105–18. doi: 10.1016/s0002-8223(02)90247-6. [DOI] [PubMed] [Google Scholar]
- 7.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78 3:559S–69S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 8.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44(4):611–6. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 9.Mohandes KM, Desai DC. Epidemiology of digestive tract cancer in India V. large and small bowel. Indian J Gastroenterol. 1999;18:118–21. [PubMed] [Google Scholar]
- 10.ACNielsen. What's hot around the globe: insights on growth in food and beverages. 2006 [Google Scholar]
- 11.Sloan AE. Top 10 global food trends. Food Technol. 2005;59(4):20–32. [Google Scholar]
- 12.Uhl S. Flavor trends: ethnic and fusion cuisines. Food Product Design. 2000 [cited 2006 Aug 15]; Available from: http://www.foodproductdesign.com/articles/462/462_0500fa5.html.
- 13.Buzzanell PJ, Gray F. The spice market in the United States--recent developments and prospects. USDA Agriculture Information Bulletin. 1995 [cited 2006 Jul 24]; Available from: http://www.ers.usda.gov/Publications/AIB709.
- 14.United States Department of Agriculture Economic Research Service. Food availablility (per capita) Data System. 2007 feb 15; database on internet. [cited 2007 Apr 18]; Available from: http://www.ers.usda.gov/Data/FoodConsumption/
- 15.Food and Drug Administration. Chapter 5: Foods, Colors and Cosmetics, Sub Chapter 525: Condiment Industry. Compliance Policy Guide. 1980 Oct 1; [page on internet] updated. [cited 2007 Jun 8]; Available from: http://www.fda.gov/ora/compliance_ref/cpg/cpgfod/cpg525-750.html.
- 16.United States National Arboretum. Herb questions and answers. 2002 page on internet. [cited 2007 Jun 8]; Available from: http://www.usna.usda.gov/Gardens/faqs/herbsfaq1.html.
- 17.Billing J, Sherman PW. Antimicrobial functions of spices: why some like it hot. Q Rev Biol. 1998;73(1):3–49. doi: 10.1086/420058. [DOI] [PubMed] [Google Scholar]
- 18.Shelef LA. Antimicrobial effects of spices. Journal of Food Safety. 1983;6:29–44. [Google Scholar]
- 19.Robison SH, Barr DB. Use of biomonitoring data to evaluate methyl eugenol exposure. Environ Health Perspect. 2006;114(11):1797–801. doi: 10.1289/ehp.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11(11):1451–60. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- 21.Dragland S, Senoo H, Wake K, Holte K, Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr. 2003;133(5):1286–90. doi: 10.1093/jn/133.5.1286. [DOI] [PubMed] [Google Scholar]
- 22.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136 3:716S–25S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 23.Paterson JR, Srivastava R, Baxter GJ, Graham AB, Lawrence JR. Salicylic acid content of spices and its implications. J Agric Food Chem. 2006;54(8):2891–6. doi: 10.1021/jf058158w. [DOI] [PubMed] [Google Scholar]
- 24.Gnayfeed MH, Daood HG, Biacs PA, Alcaraz CF. Content of bioactive compounds in pungent spice red pepper (paprika) as affected by ripening and genotype. J Sci Food Agric. 2001;81:1580–5. [Google Scholar]
- 25.Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003;133(10):3248S–54S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 26.Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102(10):1414–20. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 27.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130(9):2243–50. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 28.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993;20(1):21–9. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 29.Rossi M, Negri E, Talamini R, Bosetti C, Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M, Giacosa A, La Vecchia C. Flavonoids and colorectal cancer in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1555–8. doi: 10.1158/1055-9965.EPI-06-0017. [DOI] [PubMed] [Google Scholar]
- 30.Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RH. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol. 2006;19(5):627–44. doi: 10.1021/tx0503552. [DOI] [PubMed] [Google Scholar]
- 31.Kirkland D, Aardema M, Henderson L, Muller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat Res. 2005;584(12):1–256. doi: 10.1016/j.mrgentox.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 32.National Toxicology Program. National Toxicology Program technical report on the toxicology and carcinogenesis studies of quercetin (CAS No. 117-39-5) in F344/N rats (feed studies) 1992 Report No.: NTP TR 409. [PubMed] [Google Scholar]
- 33.National Toxicology Program. National Toxicology Program technical report on the toxicology and carcingenesis studies of turmeric oleoresin (CAS No, 8024-37-1) (major component 79%-85% curcumin, CAS No 458-37-7) in F344/N rats and B6C3F1 Mice (feed studies). NIH. 1993 Report No.: NTP TR 427. [PubMed] [Google Scholar]
- 34.National Toxicology Program. National Toxicology Program technical report on the toxicology and carcinogenesis studies of trans-cinnamaldehyde (microencapsulated) (CAS No 14371-10-9) in F344/N rats and B6C3F1 mice (feed studies): NIH. 2004 Report No.: NTP TR 514. [PubMed] [Google Scholar]
- 35.Blumenthal M. The complete German Commission E monographs: therapeutic guide to herbal medicines. Austin, TX: American Botanical Council; 1998. [Google Scholar]
- 36.Dragsted LO, Strube M, Leth T. Dietary levels of plant phenols and other non-nutritive components: could they prevent cancer? Eur J Cancer Prev. 1997;6(6):522–8. doi: 10.1097/00008469-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56(2):69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 38.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergonzelli GE, Donnicola D, Porta N, Corthesy-Theulaz IE. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob Agents Chemother. 2003;47(10):3240–6. doi: 10.1128/AAC.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora DS, Kaur J. Antimicrobial activity of spices. Int J Antimicrob Agents. 1999;12(3):257–62. doi: 10.1016/s0924-8579(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 41.Liu ZH, Nakano H. J Fac Appl Biol Sci. Vol. 35. Hiroshima Univ; 1996. Antibacterial activity of spice extracts against food-related bacteria; pp. 181–90. [Google Scholar]
- 42.Sivam GP. Protection against Helicobacter pylori and other bacterial infections by garlic. J Nutr. 2001;131(3s):1106S–8S. doi: 10.1093/jn/131.3.1106S. [DOI] [PubMed] [Google Scholar]
- 43.Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol. 2003;36(3):162–7. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 44.Yano Y, Satomi M, Oikawa H. Antimicrobial effect of spices and herbs on Vibrio parahaemolyticus. Int J Food Microbiol. 2006;111(1):6–11. doi: 10.1016/j.ijfoodmicro.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 45.De M, De AK, Banerjee AB. Antimicrobial screening of some Indian spices. Phytotherapy Research. 1999;13:616–18. doi: 10.1002/(sici)1099-1573(199911)13:7<616::aid-ptr475>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 46.Yanagisawa N, Geironson L, Al-Soud WA, Ljungh S. Expression of matrix metalloprotease-2, -7 and -9 on human colon, liver and bile duct cell lines by enteric and gastric Helicobacter species. FEMS Immunol Med Microbiol. 2005;44(2):197–204. doi: 10.1016/j.femsim.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 47.McNulty CA, Wilson MP, Havinga W, Johnston B, O'Gara EA, Maslin DJ. A pilot study to determine the effectiveness of garlic oil capsules in the treatment of dyspeptic patients with Helicobacter pylori. Helicobacter. 2001;6(3):249–53. doi: 10.1046/j.1523-5378.2001.00036.x. [DOI] [PubMed] [Google Scholar]
- 48.Knight JA. Review: Free radicals, antioxidants, and the immune system. Ann Clin Lab Sci. 2000;30(2):145–58. [PubMed] [Google Scholar]
- 49.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18(9):567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc of Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 51.Serafini M, Bellocco R, Wolk A, Ekstrom AM. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002;123(4):985–91. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 52.Sies H. Biochemistry of oxidative stress. Angew Chem Int Ed Engl. 1986;25:1058–71. [Google Scholar]
- 53.Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44(6 Pt 1):1309–15. [PubMed] [Google Scholar]
- 54.Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res. 2006;50(11):1030–8. doi: 10.1002/mnfr.200600067. [DOI] [PubMed] [Google Scholar]
- 55.Halvorsen BL, Carlsen MH, Phillips KM, Bohn SK, Holte K, Jacobs DR, Jr, Blomhoff R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84(1):95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 56.Ninfali P, Mea G, Giorgini S, Rocchi M, Bacchiocca M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br J Nutr. 2005;93(2):257–66. doi: 10.1079/bjn20041327. [DOI] [PubMed] [Google Scholar]
- 57.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–70. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 58.Wu X, Beecher GR, Holden JM, Haytowitz DB, Genhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–37. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- 59.Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53(20):7749–59. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 60.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41(12):1727–46. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 61.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 62.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135 12:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 63.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37(910):937–42. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 64.Surh YJ, Na HK, Lee JY, Keum YS. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16 Suppl:S38–41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kundu JK, Surh YJ. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res. 2005;591(12):123–46. doi: 10.1016/j.mrfmmm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 66.Yoon JH, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46(5):585–96. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91(6):2046–50. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Surh YJ. Chemopreventive phenolic compounds in common spices. New York: Taylor & Francis; 2006. [Google Scholar]
- 69.Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25(9):1671–9. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 70.Xu XM, Sansores-Garcia L, Chen XM, Matijevic-Aleksic N, Du M, Wu KK. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc Natl Acad Sci U S A. 1999;96(9):5292–7. doi: 10.1073/pnas.96.9.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lawrence JR, Peter R, Baxter GJ, Robson J, Graham AB, Paterson JR. Urinary excretion of salicyluric and salicylic acids by non-vegetarians, vegetarians, and patients taking low dose aspirin. J Clin Pathol. 2003;56(9):651–3. doi: 10.1136/jcp.56.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai PJ, Tsai TH, Yu CH, Ho SC. Evaluation of NO-suppressing activity of several Mediterranean culinary spices. Food Chem Toxicol. 2007;45(3):440–7. doi: 10.1016/j.fct.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Kim OK, Murakami A, Nakamura Y, Ohigashi H. Screening of edible Japanese plants for nitric oxide generation inhibitory activities in RAW 264.7 cells. Cancer Lett. 1998;125(12):199–207. doi: 10.1016/s0304-3835(97)00513-2. [DOI] [PubMed] [Google Scholar]
- 74.Chan MM, Ho CT, Huang HI. Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett. 1995;96(1):23–9. doi: 10.1016/0304-3835(95)03913-h. [DOI] [PubMed] [Google Scholar]
- 75.Ippoushi K, Azuma K, Ito H, Horie H, Higashio H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci. 2003;73(26):3427–37. doi: 10.1016/j.lfs.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 76.Chen YC, Shen SC, Lee WR, Hou WC, Yang LL, Lee TJ. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J Cell Biochem. 2001;82(4):537–48. doi: 10.1002/jcb.1184. [DOI] [PubMed] [Google Scholar]
- 77.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71(10):1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35(2):584–92. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 79.Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21(14):27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 80.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63(15):4375–83. [PubMed] [Google Scholar]
- 81.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 82.Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496(1):12–8. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- 83.Thuillier P, Brash AR, Kehrer JP, Stimmel JB, Leesnitzer LM, Yang P, Newman RA, Fischer SM. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem J. 2002;366(Pt 3):901–10. doi: 10.1042/BJ20020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50(11):2191–3. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 85.Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of herbs with cytochrome P450. Drug Metab Rev. 2003;35(1):35–98. doi: 10.1081/dmr-120018248. [DOI] [PubMed] [Google Scholar]
- 86.Van Erk MJ, Teuling E, Staal YC, Huybers S, Van Bladeren PJ, Aarts JM, Van Ommen B. Time- and dose-dependent effects of curcumin on gene expression in human colon cancer cells. J Carcinog. 2004;3(1):8. doi: 10.1186/1477-3163-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai YY, McGlynn KA, Hu Y, Cassidy AB, Arnold J, Engstrom PF, Buetow KH. Genetic susceptibility and dietary patterns in lung cancer. Lung Cancer. 2003;41(3):269–81. doi: 10.1016/s0169-5002(03)00238-1. [DOI] [PubMed] [Google Scholar]
- 88.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223(2):181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 89.Debersac P, Heydel JM, Amiot MJ, Goudonnet H, Artur Y, Suschetet M, Siess MH. Induction of cytochrome P450 and/or detoxication enzymes by various extracts of rosemary: description of specific patterns. Food Chem Toxicol. 2001;39(9):907–18. doi: 10.1016/s0278-6915(01)00034-5. [DOI] [PubMed] [Google Scholar]
- 90.Sharma N, Trikha P, Athar M, Raisuddin S. Inhibition of benzo[a]pyrene- and cyclophoshamide-induced mutagenicity by Cinnamomum cassia. Mutat Res. 2001:480–481. 179–88. doi: 10.1016/s0027-5107(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 91.Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, Huang MT, Chan JY, Kong AN. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5(1):39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- 92.Knasmuller S, Steinkellner H, Majer BJ, Nobis EC, Scharf G, Kassie F. Search for dietary antimutagens and anticarcinogens: methodological aspects and extrapolation problems. Food Chem Toxicol. 2002;40(8):1051–62. doi: 10.1016/s0278-6915(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 93.HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20(4):239–49. doi: 10.1002/ptr.1841. [DOI] [PubMed] [Google Scholar]
- 94.Suresh D, Srinivasan K. Studies on the in vitro absorption of spice principles--curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol. 2007;45(8):1437–42. doi: 10.1016/j.fct.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 95.Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun YY, Hudson EA, Manson MM. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007;28(9):1274–304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 96.Gangadeep DS, Mendiz E, Rao AR, Kale RK. Chemopreventive effects of Cuminum cyminum in chemically induced forestomach and uterine cervix tumors in murine model systems. Nutr Cancer. 2003;47(2):171–80. doi: 10.1207/s15327914nc4702_10. [DOI] [PubMed] [Google Scholar]
- 97.Abdullaev FI. Cancer preventive and tumoricidal properties of saffron (Crocus sativus L.) Experimental Biology. 2002:20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Olmo DC, Riese HH, Escribano J, Ontanon J, Fernandez JA, Atienzar M, Garcia-Olmo D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr Cancer. 1999;35(2):120–6. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]


