Abstract
Context
Success of antiretroviral therapy depends on high rates of adherence, but few interventions are effective.
Objective
Determine if modified directly observed therapy (mDOT) improves initial antiretroviral success.
Design
Open-label randomized trial comparing mDOT and self-administered therapy with lopinavir/ritonavir soft gel capsules 800 mg/200 mg, emtricitabine 200 mg, and either extended release stavudine 100 mg or tenofovir 300 mg, all once daily.
Setting
23 U.S. AIDS Clinical Trials Group (ACTG) sites and one in South Africa between October 2002 and January 2006.
Participants
Plasma HIV RNA ≥2000 copies/ml and antiretroviral-naïve. 82 participants received mDOT and 161 self-administration. Participants were predominantly male (79%), median age 38 years, with 84 Latinos (35%), 74 non-Latino blacks (30%), and 79 non-Latino whites (33%).
Intervention
mDOT Monday through Friday for 24 weeks.
Main Outcome Measure(s)
Primary outcome was week 24 virologic success and secondary outcomes were week 48 virologic success, clinical progression, and adherence.
Results
mDOT had greater virologic success over 24 weeks [0.91 (95% CI: 0.81, 0.95)] than self-administered therapy [0.84 (95% CI: 0.77, 0.89)], but the difference [0.07 (lower bound 95% CI: −0.01)] did not reach the pre-specified threshold of 0.075. Over 48 weeks, virologic success was not significantly different between mDOT [0.72 (95% CI: 0.61, 0.81)] and self-administered therapy [0.78 (95% CI: 0.70, 0.84)], [−0.06 (95% CI: −0.18, 0.07); p=0.19)].
Conclusions
The potential benefit of mDOT was marginal and not sustained after mDOT was discontinued. mDOT should not be incorporated routinely for care of treatment naïve HIV-1 infected patients.
Keywords: HIV-1, antiretroviral therapy, adherence, randomized trial, directly observed therapy
Introduction
The success of modern antiretroviral therapy for the treatment of HIV-1 infection remains highly dependent on strict adherence to the regimen 1-3. Although rates of adherence in the developing world have recently been reported to be higher than those in the developed world4, challenges to adherence remain in both settings5. Unfortunately, relatively few interventions have been demonstrated to be effective 6.
The most consistently associated barriers to adherence have been forgetting to take doses 7, depression 7-10, active substance use 9, 11, 12, lack of social support 13, interrupted access to medications 14 and medication toxicities 15. Directly observed therapy (DOT) strategies can address many, but not all barriers to adherence 16. However, the results of randomized trials of DOT strategies to enhance antiretroviral therapy outcomes have been mixed 17-19. This heterogeneity may have been due to inadequacy of sample size, differences in DOT implementation, and populations studied. In addition, prior studies did not assess whether DOT entrained sustained adherence after the DOT was discontinued.
We modified DOT (mDOT) to be implemented only on weekdays and to focus on only one drug in the regimen. Our objectives were to test whether mDOT over the initial six months of a first antiretroviral regimen would result in improved virological outcomes after six months and whether it would entrain good adherence behavior after it was stopped.
Methods
Study Design
We conducted an open label three-arm randomized clinical trial comparing a once daily lopinavir/ritonavir soft gel capsule (sgc) 800 mg/200 mg regimen via self-administration with either once daily lopinavir/ritonavir sgc 800 mg/200 mg via mDOT or twice daily lopinavir/ritonavir sgc 400 mg/100mg via self-administration. The study aimed to answer two separate questions with a common comparison arm (once daily self-administered therapy). Here, we report on the comparison of the mDOT and self-administration strategies using once daily lopinavir/ritonavir.
The regimens all included emtricitabine 200 mg once daily and either extended release stavudine 100 mg once daily or tenofovir 300 mg once daily. At the outset (October 2002) the regimens exclusively included extended release stavudine, an investigational formulation that is not commercially available. In March 2004, participants were allowed to choose tenofovir as well, either as part of the initial treatment or to switch from stavudine to tenofovir during the study if desired by the patient and provider. Randomization to treatment strategy was stratified by screening plasma HIV-1 RNA of ≥100,000 copies/ml or not with dynamic balancing by main institution and assigned via permuted blocks in a 2:2:1 ratio. mDOT had the smaller sample size based on different primary endpoint considerations than for the once daily versus twice daily lopinavir/ritonavir comparison. Allocation was performed using an ACTG centralized-computer system. Due to the nature of mDOT, there was no masking of assignment.
Participants and Intervention
Individuals were eligible if they had plasma HIV-1 RNA level ≥2000 copies/ml, were treatment naïve (≤7 days prior antiretroviral treatment), with any CD4 count, weighed at least 40 kg, were ≥13 years of age, and were willing and able to participate in mDOT. mDOT was defined as observed and documented taking of lopinavir/ritonavir at least 5 days per week (Monday through Friday) and was conducted by any person in a medically related field (e.g., nurse, pharmacist, social worker) who was not a relative or close friend and could occur at any location. Participants were provided with a limited extra supply of medications for weekends, holidays, and emergencies. The choice of observer had to be approved by the site and could include site staff. The intervention had two phases: 1) active mDOT for the initial 24 weeks and 2) post-mDOT phase of self-administered therapy for weeks 24 to 48. The study did not prescribe actions to be taken by the observer or the site if non-adherence ensued. All participants provided informed consent, and the study was approved by the committees on the protection of human subjects or ethics boards at each site.
Study visits were scheduled to occur at 4, 8, 16, 24, 32, 40 and 48 weeks after randomization. Each visit included clinical assessments and laboratory testing, including measurement of plasma HIV-1 RNA levels (Roche UltraSensitive HIV-1 Monitor assay, run at a central laboratory). Plasma HIV-1 RNA assays were batched through visit week 8, but performed in real-time subsequently. An additional safety visit was scheduled for visit week 2. Alcohol and substance use were assessed using self-reports and excessive alcohol use was defined as averaging more than 2 drinks per day or binge drinking 20. Participants were to follow this schedule regardless of whether they were on their originally randomized study regimen.
Endpoints
The primary efficacy endpoint, virologic success, was defined as lack of virologic failure through week 24. Virological failure was defined as confirmed HIV-1 RNA level ≥200 copies/ml after two consecutive HIV-1 RNA values <200 copies/ml (confirmed viral relapse) or confirmed HIV-1 RNA level ≥200 copies/ml at or after visit week 24 without prior confirmed viral relapse. Secondary endpoints included virologic success through week 48, time to virologic failure, change in CD4 count from baseline at visit week 24 and 48, Grade 3 or 4 sign, symptom or laboratory measure that was at least one grade higher than baseline on the DAIDS toxicity scale 21, clinical events (AIDS-defining illnesses or death), adherence, and genotypic resistance. Adherence was measured using electronic monitors (MEMS, Aardex, Zug, Switzerland) which recorded the date and time of the bottle opening on the lopinavir/ritonavir bottles and by self-report using a standardized ACTG questionnaire 22. The adherence data from the MEMS were not provided to the participants, site staff, or the study team. Attitude toward mDOT was assessed in the mDOT participants at the outset and end of the active mDOT phase via a questionnaire developed for this study. Viral genotypic resistance testing (Monogram Biosciences, San Francisco, CA) was performed on pairs of samples from all participants with virologic failure at any time during follow-up: one stored at baseline and a second obtained at the time of virologic failure. Resistance was defined based on the Stanford algorithm version 4.2.6. (http://hivdb.stanford.edu/index.html).
Statistical Analyses
The primary analysis assessed whether the probability of virologic success was higher for mDOT compared with self-administered therapy through week 24 using Kaplan-Meier (KM) estimates from the survival distribution for the time to virologic failure and a one-sided Z-test for a difference in the probabilities of 0.075, taking an intent-to-treat approach. A one-sided test was chosen a priori because we were only interested in whether mDOT was better than self-administered therapy for virologic success. All other comparisons were two-sided. Time to virologic failure was defined as the time from randomization to the date of the initial failure sample. Participants whose last plasma HIV-1 RNA sample was an unconfirmed failure were considered virologic failure at the time of the last sample. For participants who missed visit week 24, if both visit week 16 and 32 samples had HIV-1 RNA≥200 copies/ml, the failure date was imputed at week 24. For participants without virologic failure, the failure time was censored at the date of their last plasma HIV-1 RNA sample. Secondary intent-to-treat analyses compared the probability of sustained virological success through week 48 using week 48 KM estimates and two-sided 95% confidence intervals around the difference in probability of virologic success. Time to event distributions were compared with a log rank test that was stratified by screening plasma HIV-1 RNA (<100,000 vs. ≥100,000 copies/ml). Modification of the effect of the intervention by mDOT phase was assessed in a Cox proportional hazards model of virologic success over the 48 weeks with an interaction term for study week ≤24 (active mDOT) or >24 (post mDOT).
Adherence for lopinavir/ritonavir was calculated separately for each individual over visit weeks 0-24 and weeks 24-48 using electronic monitor data as follows: (# of bottle openings ÷ # days of observation) × 100%. Days on which the participant was instructed not to take the medication (e.g., for toxicity) were excluded from the calculation. Percent adherence and CD4 count change from baseline were compared between treatment strategies using the Wilcoxon rank-sum test. Self-reported adherence was assessed with a Cochran-Mantel-Haenszel test. Changes in attitudes toward mDOT were compared with a signed-rank test. Analyses were performed using SAS version 9 (SAS Inc., Cary, NC) and S-plus version 6 (Insightful Corp., Seattle, WA).
An independent Study Monitoring Committee was impaneled by the ACTG and consisted of investigators not directly involved in the study. The committee performed three reviews, with blinded efficacy comparisons presented only at the first review soon after study initiation. The stopping guideline was based on the method of Lan and DeMets with an O'Brien-Fleming spending function. Reported p-values are nominal.
Sample Size Considerations
The sample size calculation required consideration of the other aim of the study. The aim comparing once-daily vs. twice daily lopinavir/ritonavir required 150 participants each. Thus, we targeted enrollment of 75 participants for the mDOT strategy to have 81% power to detect a difference of at least 0.075 between mDOT and once-daily self-administered therapy and probabilities of virologic success at week 24 of 0.91 and 0.70 for the two respective strategies.
Results
The study enrolled 403 participants into the three strategies. A total of 243 participants were randomized, 161 to self-administered therapy and 82 to mDOT. Enrollment began in October 2002 at 23 US sites (232 participants) and one site in Johannesburg, South Africa (11 participants) and ended in January 2005 with the last follow-up occurring in January 2006. Baseline characteristics appeared to be well-balanced between the strategies (Table 1). Figure 1 displays the flow of participants. All participants initiated treatment with their assigned lopinavir/ritonavir-based regimen and those randomized to mDOT all started mDOT. There was no significant difference in time to premature study discontinuation (p=0.59).
Table 1. Baseline Characteristics of Participants by Treatment Strategy.
| Characteristics | Self-administered | mDOT | Total | |
|---|---|---|---|---|
| n=161 | n=82 | n=243 | ||
| Sex | Male | 127 (79%) | 65 (79%) | 192 (79%) |
| Female | 34 (21%) | 17 (21%) | 51 (21%) | |
| Age (yrs) | Mean (SD) | 39.3 (10.4) | 38.7 (8.9) | 39.1 (9.9) |
| Median (Q1, Q3) | 38.0 (32.0, 45.0) | 38.0 (34.0, 44.0) | 38.0 (32.0, 45.0) | |
| Min, Max | 18.0, 66.0 | 23.0, 68.0 | 18.0, 68.0 | |
| * Race/ethnicity | White Non-Hispanic | 54 (34%) | 25 (30%) | 79 (33%) |
| Black Non-Hispanic | 51 (32%) | 23 (28%) | 74 (30%) | |
| Hispanic (Regardless of Race) | 54 (34%) | 30 (37%) | 84 (35%) | |
| Asian, Pacific Islander | 1 (1%) | 2 (2%) | 3 (1%) | |
| American Indian, Alaskan Native | 1 (1%) | 1 (1%) | 2 (1%) | |
| Other/unknown/more than one race | 0 (0%) | 1 (1%) | 1 (0%) | |
| Education Level | 11th grade or less | 32 (21%) | 18 (22%) | 50 (21%) |
| High school graduate or GED | 52 (34%) | 29 (36%) | 81 (34%) | |
| 2 yrs college/AA deg/Tech school training | 44 (28%) | 18 (22%) | 62 (26%) | |
| College graduate (BA or BS) | 17 (11%) | 12 (15%) | 29 (12%) | |
| Master's degree | 7 (5%) | 4 (5%) | 11 (5%) | |
| Doctorate/medical degree/law degree | 3 (2%) | 0 (0%) | 3 (1%) | |
| IV drug history | Never | 143 (89%) | 72 (88%) | 215 (88%) |
| Currently | 1 (1%) | 1 (1%) | 2 (1%) | |
| Previously | 17 (11%) | 9 (11%) | 26 (11%) | |
| Alcohol Use | None | 75 (48%) | 36 (44%) | 111 (47%) |
| Non-excessive use | 45 (29%) | 24 (30%) | 69 (29%) | |
| Excessive use | 37 (24%) | 21 (26%) | 58 (24%) | |
| NRTI Started | d4T XR | 100 (62%) | 54 (66%) | 154 (63%) |
| TDF | 61 (38%) | 28 (34%) | 89 (37%) | |
| History of AIDS Defining Diagnosis | Yes | 32 (20%) | 20 (24%) | 52 (21%) |
| No | 129 (80%) | 62 (76%) | 191 (79%) | |
| Hepatitis B surface antigen | Positive | 6 (4%) | 2 (3%) | 8 (3%) |
| Negative | 148 (95%) | 77 (96%) | 225 (96%) | |
| Indeterminate | 1 (1%) | 1 (1%) | 2 (1%) | |
| Hepatitis C antibody | Positive | 21 (13%) | 11 (13%) | 32 (13%) |
| Negative | 138 (86%) | 71 (87%) | 209 (86%) | |
| Indeterminate | 1 (1%) | 0 (0%) | 1 (0%) | |
| HIV-1 RNA (log10(cp/mL)) | Mean (SD) | 4.8 (0.6) | 5.0 (0.7) | 4.9 (0.7) |
| Median (Q1, Q3) | 4.8 (4.5, 5.2) | 4.8 (4.5, 5.5) | 4.8 (4.5, 5.3) | |
| Min, Max | 3.1, 6.6 | 3.4, 6.6 | 3.1, 6.6 | |
| (cp/mL) | <2000 | 1 (1%) | 0 (0%) | 1 (0%) |
| 2000-19,999 | 26 (16%) | 15 (18%) | 41 (17%) | |
| 20,000-54,999 | 47 (29%) | 17 (21%) | 64 (26%) | |
| 55,000-99,999 | 29 (18%) | 14 (17%) | 43 (18%) | |
| >=100,000 | 58 (36%) | 36 (44%) | 94 (39%) | |
| Screening HIV-1 RNA | < 100,000 | 79 (49%) | 41 (50%) | 120 (49%) |
| >= 100,000 | 82 (51%) | 41 (50%) | 123 (51%) | |
| CD4 cell count (cells/mm3) | Mean (SD) | 233 (179) | 212 (228) | 226 (197) |
| Median (Q1, Q3) | 218 (76.0, 336) | 137 (38.0, 337) | 199 (64.5, 336) | |
| Min, Max | 0.0, 869 | 2.5, 1509 | 0.0, 1509 | |
| (cells/mm3) | 0-50 | 35 (22%) | 23 (28%) | 58 (24%) |
| 51-100 | 14 (9%) | 10 (12%) | 24 (10%) | |
| 101-200 | 25 (16%) | 15 (18%) | 40 (16%) | |
| 201-350 | 51 (32%) | 15 (18%) | 66 (27%) | |
| 351-500 | 24 (15%) | 12 (15%) | 36 (15%) | |
| >500 | 12 (7%) | 7 (9%) | 19 (8%) | |
Baseline HIV RNA level and CD4 cell count were calculated as the geometric and arithmetic means, respectively, of pre-entry and entry evaluations.
self-reported
Figure 1. Disposition of Participants.
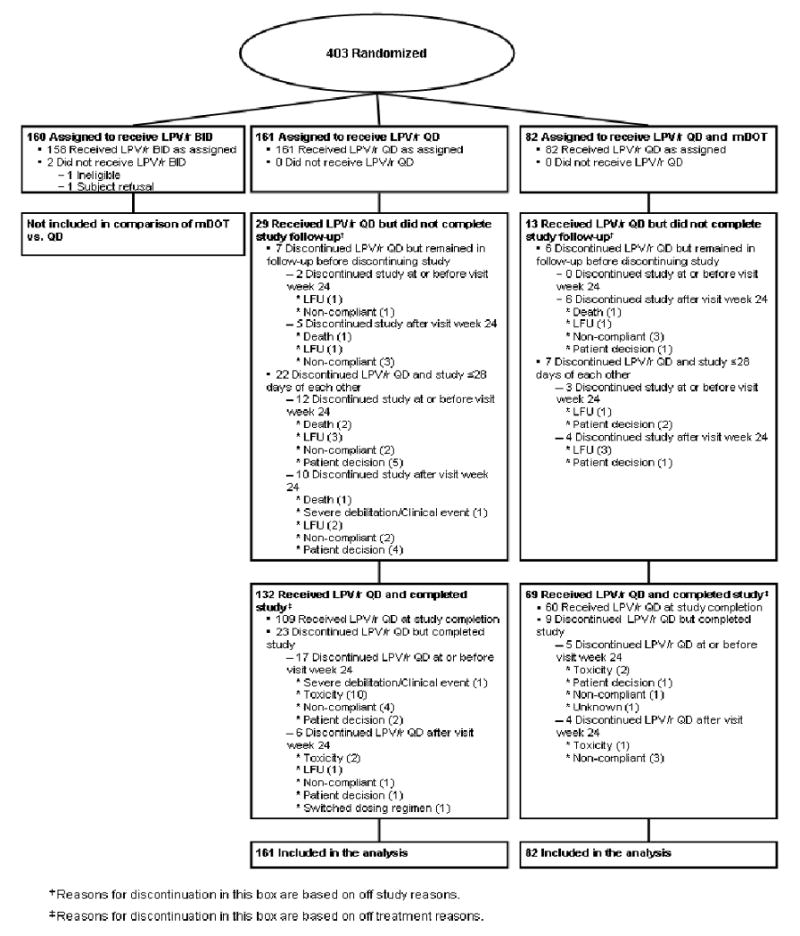
LPV/r QD- lopinavir/ritonavir 800 mg/200mg once daily
LFU-lost to follow-up
A higher proportion of mDOT participants remained on their originally assigned lopinavir/ritonavir dose schedule by week 24 compared to self-administered therapy participants [69 (84%) vs. 126 (78%), respectively]. However, there was no statistically significant difference in time to discontinuation of originally assigned lopinavir/ritonavir dose schedule (p=0.38). By week 48, 48 mDOT participants (73%) and 73 self-administered participants (68%) remained on their assigned lopinavir/ritonavir dose schedule, and the time to discontinuation through week 48 was likewise not significantly different (p=0.35).
Sixty-one participants (74%) remained on mDOT through visit week 24. Adherence to mDOT was high with 62 of the 82 (76%) mDOT participants attending >80% of intended mDOT visits with only 9 (11%) missing ≥30% of intended visits. Intended mDOT visits were missed at least once without prior arrangement with the site by 32 participants (39%). Fifty-four of the mDOT participants (68%) considered mDOT to either be somewhat or very helpful based on the mDOT attitude questionnaire. At the end of the active mDOT phase, the majority of the participants evaluable, 53 (83%), considered mDOT to either be somewhat or very helpful. Comparing attitudes at the end of active mDOT phase with baseline in the 61 participants with available data at both time points, attitude toward mDOT improved significantly (p=0.004).
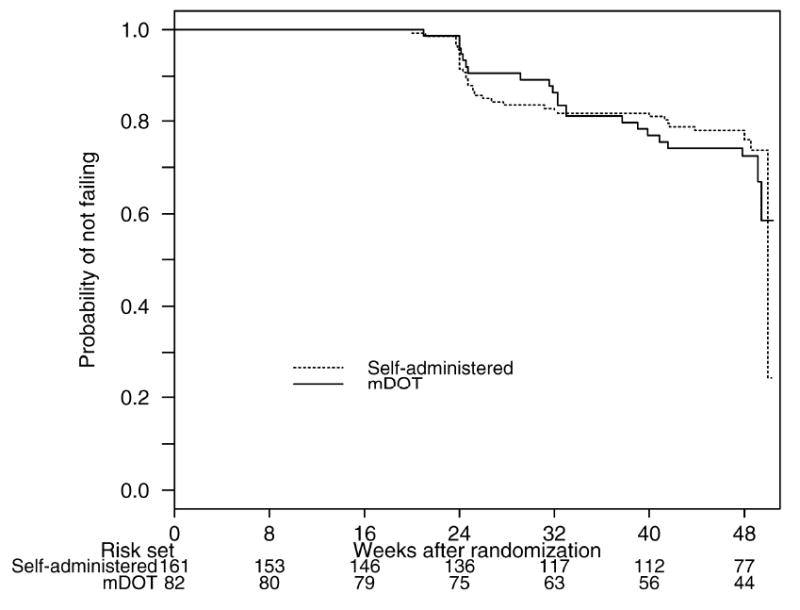
Figure 2 displays the virological outcomes. The proportion of participants with virologic success by week 24 was higher in mDOT [7 failures, K-M estimate 0.91 (95% CI: 0.81, 0.95)] than self-administered therapy [23 failures, K-M estimate 0.84 (95% CI: 0.77, 0.89)]. However, the difference of 0.07 (one-sided 95% lower confidence bound: -0.01) was not large enough to declare superiority based on an a priori specified absolute difference of 0.075, nor did the lower 95% confidence bound exclude no difference (p=0.56). Thus, mDOT cannot be declared superior to self-administered therapy in a treatment-naïve population.
Figure 2. Kaplan-Meier Curve of Sustained Virologic success by Treatment Strategy (Intent–to-Treat Analysis).
CD4 cell count improvements were similarly distributed with mDOT having a median increase from baseline to visit week 24 of 136 cells/mm3 (25th-75th percentile: 83 to192 cells/mm3) and self-administered therapy a median increase of 130 cells/mm3 (25th-75th percentile: 60 to 210 cells/mm3), and were not statistically significantly different (p=0.46).
In secondary analyses to week 48, the proportion with virologic success was lower in mDOT [20 failures, K-M estimate 0.72 (95% CI: 0.61, 0.81)] than self-administered therapy [33 failures, K-M estimate 0.78 (95% CI: 0.70-0.84)]. The 95% confidence interval on the difference of -0.18 to 0.07 did not exclude a difference of zero. However, in analysis of time to virologic failure, the hazard ratio for mDOT versus self-administered therapy differed by mDOT study phase- that is, the effect of the mDOT differed between the active mDOT phase and the post-mDOT phase (test for interaction, p=0.039). During the active mDOT phase, the hazard of virologic failure for the self-administered therapy was 1.79 (95% CI: 0.77, 4.17) times that of mDOT. During the post-mDOT phase, the hazard of virologic failure for self-administered therapy was 0.54 (95% CI: 0.25, 1.16) times that of mDOT. Although the treatment strategies cannot be considered different from each other over initiation to week 24 or from week 24 to week 48, the potential effect of mDOT was different over these time periods with the active mDOT phase favoring mDOT and the post-mDOT phase favoring self-administered therapy.
We also compared the rate of developing an AIDS-defining illness or dying between the treatment strategies. By week 24, 1 participant (1%) on mDOT developed an AIDS-defining illness / death compared with 11 (7%) on the self-administered therapy. Incident events in self-administered therapy included 2 cases of Kaposi's sarcoma, 2 cases of esophageal candidiasis, 1 case of wasting syndrome, and 1 case each of cytomegalovirus retinitis, toxoplasmic encephalitis, progressive multifocal leukoencephalopathy, disseminated Mycobacterium avium infection, and pulmonary tuberculosis, as well as 1 death from metastatic small cell carcinoma. The incident event in mDOT was cytomegalovirus colitis. The time to new AIDS- defining illness or death was significantly different favoring mDOT through week 24 (exact p=0.05), but not through week 48 (exact p=0.19).
Adherence was high for both strategies as assessed by both electronic monitors and self-report. At week 24, for the 80 mDOT participants (98%) with available electronic monitor data, median adherence for all days on study was 92.5% (25th-75th percentile: 78.4% to 95.7%) and for the 149 self-administered participants (93%) with available data, median adherence was 92.2% (25th-75th percentile: 80.0% to 96.3%), p>0.5. Similarly, at week 48, for the 62 participants (76%) on mDOT with available data, median adherence was 91.7% (25th-75th percentile: 1.6% to 98.3%) and for the 116 participants (72%) on self-administered therapy, median adherence was 91.3% (25th-75th percentile: 0% to 99.1%), p>0.5. Comparing self-reported adherence at week 24, a larger proportion of mDOT participants reported perfect adherence than self-administered participants (72% versus 62%), but at week 48, a larger proportion of self-administered participants reported perfect adherence than mDOT participants (72% versus 58%). However, these differences were not statistically significant (p=0.13, 0.25, respectively).
Table 2 shows Grade 3 or 4 signs, symptoms, or laboratory abnormalities that were one grade above baseline and present in ≥2% of participants. There were no statistically significant differences in the time to these events between treatment strategies (log rank, p-value: 0.62 through week 24, 0.96 through week 48).
Table 2. Selected Grade 3 or 4 Adverse Events by Treatment Strategy.
| By Week 24 | By Week 48 | |||
|---|---|---|---|---|
| LPV/r QD (n=161) |
LPV/r QD/DOT (n=82) |
LPV/r QD (n=161) |
LPV/r QD/DOT (n=82) |
|
| Any Event | 55 (34%) | 30 (37%) | 62 (39%) | 31 (38%) |
| Any Sign/Symptom | 26 (16%) | 9 (11%) | 27 (17%) | 10 (12%) |
| Diarrhea/loose stools | 3 (2%) | 0 (0%) | 3 (2%) | 0 (0%) |
| Nausea and/or vomiting | 8 (5%) | 0 (0%) | 9 (6%) | 0 (0%) |
| Ache/pain/discomfort | 8 (5%) | 2 (2%) | 9 (6%) | 3 (4%) |
| Fever | 3 (2%) | 1 (1%) | 4 (2%) | 1 (1%) |
| Headache | 3 (2%) | 0 (0%) | 3 (2%) | 0 (0%) |
| Cognition | 3 (2%) | 1 (1%) | 3 (2%) | 1 (1%) |
| Mental status changes | 3 (2%) | 0 (0%) | 3 (2%) | 0 (0%) |
| Any Laboratory Abnormality | 38 (24%) | 22 (27%) | 44 (27%) | 22 (27%) |
| ALT (SGPT) [U/L] | 6 (4%) | 1 (1%) | 9 (6%) | 1 (1%) |
| AST (SGOT) [U/L] | 4 (2%) | 1 (1%) | 7 (4%) | 1 (1%) |
| GGT [U/L] | 0 (0%) | 0 (0%) | 3 (2%) | 0 (0%) |
| Lipase [U/L] | 8 (5%) | 6 (7%) | 8 (5%) | 6 (7%) |
| Neutropenia (ANC) [per mm3] | 4 (2%) | 9 (11%) | 4 (2%) | 9 (11%) |
| Creatine phosphokinase [U/L] | 9 (6%) | 4 (5%) | 11 (7%) | 4 (5%) |
| Glucose (non-fasting) (mg/dL) | 4 (2%) | 1 (1%) | 6 (4%) | 2 (2%) |
| Triglycerides (fasting) (mg/dL) | 4 (2%) | 1 (1%) | 4 (2%) | 1 (1%) |
| Triglycerides (non-fasting) (mg/dL) | 6 (4%) | 4 (5%) | 6 (4%) | 5 (6%) |
Only events present in 2% or more of the participants are listed.
Table numbers give the numbers of participants reporting at least one event at a given grade.
For each event, only the highest grade recorded for each subject that was at least one grade higher than baseline is counted, similarly, for category and overall totals.
Table 3 shows the proportion of virologic failures with protease and reverse transcriptase mutations at baseline and the proportion with new mutations that were not present at baseline. Among the 58 virologic failures (22 mDOT and 36 self-administered), 56 (97%) had specimens available for genotypic antiretroviral resistance testing. New protease mutations were invariably seen in those with other protease mutations at baseline. New reverse transcriptase mutations were seen in 8 (23%) self-administered participants with virologic failure and 6 (27%) mDOT participants with virologic failure. New reverse mutations invariably included M184V. The emergence of new resistance mutations was neither clinically nor statistically significantly different between treatment strategies.
Table 3.
Known Resistance Mutations in Participants with Virological Failure
| Self-administered (n=35) {n=161} |
mDOT (n=22) {n=82} |
||
|---|---|---|---|
| PI* | Number of participants with mutations at failure | 17 (49%) {11%} | 11 (50%) {13%} |
| Number of participants with new mutations at failure | 2 (6%) {1%} | 2 (9%) {2%} | |
| NRTI† | Number of participants with mutations at failure | 12 (34%) {7%} | 9 (41%) {11%} |
| Number of participants with new mutations at failure | 8 (23%) {5%} | 6 (27%) {7%} |
(% of failures with resistance data) {% of randomized participants}
New mutations conferring protease resistance at failure included I47V, I54V, Q58E, V77I.
New reverse transcriptase mutations conferring nucleoside analog resistance at failure included T69A, L74V, V75L, M184V L210W, K219E, K219Q, , , ,.
Discussion
This large randomized trial assessing the virological effect of directly observed antiretroviral therapy in a uniformly treated HIV-infected antiretroviral-naïve population failed to demonstrate a clear benefit. Although a 7% higher rate of virologic success with mDOT bordered on an a priori threshold of a clinically significant difference of 7.5%, the potential benefit suggested by the results is modest. The observed difference is consistent with a true difference of -1% or higher (based on 95% confidence interval). Interestingly, after mDOT was discontinued, there was some evidence that any potential benefit was not sustained. The virological findings are supported by the adherence results, which likewise did not show significant differences at week 24. While directly observed therapy may provide adherence benefits when it is ongoing, our study suggests that good adherence behavior is not entrained by 24 weeks of DOT. This finding makes mDOT administered by health professionals less attractive as an intervention.
There was a suggestion of a protective effect of mDOT on new AIDS defining events and deaths. This intriguing finding could possibly be explained in part by the lower, though not statistically significant, rate of virologic failure for mDOT over the initial weeks of the study. Additionally, perhaps the increased early consistent contact with health professionals conferred an as yet unexplained clinical benefit 16. Given that the number of events was small, this finding would need to be confirmed in other studies of variations of DOT strategies to conclude that mDOT reduces the incidence of clinical events.
Comparing our findings with other randomized trials testing mDOT interventions may help identify elements of mDOT programs with future promise. Wohl et al. studied a mixed treatment-naïve and -experienced population without selection for risk factors for nonadherence in whom community workers administered DOT for six months. Similar to our study, they found that DOT did not confer a significant advantage in either virologic suppression or adherence 18. Macalino et al. compared a daily mDOT strategy with self-administered therapy in a randomized trial of 87 active substance users followed for only three months 19. Unlike our study, they found a marginally significant benefit, although secondary analyses noted that the entire benefit was conferred upon experienced patients who had failed prior antiretroviral regimens. The clear difference between studies is that ours did not include experienced patients and suggests that implementing mDOT in an unselected treatment-naïve population may be providing an intervention to many who do not actually need it. Altice et al. conducted a trial of DOT in injection drug users (IDU) and found an advantage with respect to both virologic suppression and CD4 cell count changes 17. We had too few IDUs to make robust conclusions about this subpopulation.
Our study has several strengths compared to other trials of mDOT strategies in HIV. First, the population was entirely antiretroviral-naïve and treated with a uniformly potent antiretroviral regimen. Second, we observed participants both during and after mDOT, allowing us to assess any lasting benefit of the intervention. Third, we measured adherence using an electronic monitor, a technique considered to be the most sensitive for non-adherence and the most accurate 23. However, this study has several potential limitations. First, we studied an experimental formulation of stavudine, which may have selected for a more adherent population. Our findings might have demonstrated a greater benefit in a less adherent population with potentially more to gain from mDOT. Second, our regimen included soft gel lopinavir/ritonavir which has been replaced by tablets. Yet, our findings are unlikely to have been substantially different with newer regimens because of the high rate of adherence and success with self-administered therapy. Third, the intervention did not include a transition phase of tapering of mDOT to allow the participants to develop a set of adherence strategies to replace mDOT. This addition might have improved outcomes for mDOT between weeks 24 and 48. Fourth, the relatively low AIDS defining illness and death rates, and the fact that this comparison was a secondary objective, limit our ability to make firm conclusions about clinical benefits of mDOT.
Although mDOT was appealing and accepted by the participants, the small effect and apparent need for sustained mDOT to maintain the benefit should limit enthusiasm for wide implementation. However, our findings do not exclude the possible utility of mDOT in populations enriched for non-adherence, such as those with a history of treatment failure. If tested in those populations, it may be important to avoid the abrupt discontinuation of mDOT, allowing instead a transition period for the gradual introduction of self-administered therapy skills. Further, if refined and retested in the future, mDOT strategies should consider incorporating adherence skills building into the design so as to increase the sustainability of the intervention's effect.
Acknowledgments
This study was sponsored by the National Institute of Allergy and Infectious Diseases' Division of AIDS and conducted by the AIDS Clinical Trials Group. Abbott Laboratories, Bristol-Myers Squibb, and Gilead Pharmaceuticals provided the medications and additional funding. The industry supporters monitored the development of the protocol and provided input into the design. They also reviewed earlier drafts of the manuscript prior to submission and suggested modifications. The decision to incorporate sponsors' and supporters' suggestions was exclusively the purview of the authors. Drs. Gross, Tierney, Andrade, Lalama, Flexner, and Mildvan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was presented in part at the 14th Conference on Retroviruses and Opportunistic Infections, February 2007. The authors wish to thank the study participants and the mDOT observers for their contribution. We would also like to acknowledge the study team members (listed below) and the site personnel who participated:
Jorge L Santana Bagur, MD and Olga Mendez, MD- Puerto Rico- AIDS CRS (Site 5401) CTU Grant # 5 A1069415
Judith A Aberg, MD and Karen Cavanagh, RN- NYU/NYC HHC at Bellevue (Site 401) ACTU Grants # AI27665 and #AI069532, GCRC Grant # RR00096
Kathleen Squires, MD and Bartolo Santos, RN-University of Southern California CRS (Site 1201) CTU Grant # 5U01AI069428
Andrea Weiss, RPh and Robin McKenzie, MD- Johns Hopkins Adult AIDS CRS (Site 201) CTU Grant #AI069465, GCRC Grant #RR025005
Carl J. Fichtenbaum, MD and Fran Hyc, RN, BSN- University of Cincinnati CRS (Site 2401) CTU Grant # AI069513
Martha Greenwald, RN, MSN and Mitchell Goldman, MD-Indiana University AIDS CTU (Site 2601) CTU Grant # AI25859
Karen Tashima, MD and Pamela Poethke, RN- The Miriam Hospital ACTG CRS (Site 2951) CTU Grant #AI069472
Cathi Basler, RN, MSN and Monica Carten, MD- University of Colorado Hospital CRS (Site 6101) GCRC Grant #RR00051, CTU Grant # AI69450, NIH Grant #AA54907
Cindy Firnhaber, MD and Ian Sanne, MD- Wits HIV CRS (Site 11101) CTU Grant # AI069463
Aleshia Thomas, RN- Hospital of the University of Pennsylvania (Site 6201) CTU Grant # AI032783, PENN CFAR Grant# AI045008
David Perlman, MD, Gwen Costantini, FNP, and Sondra Middleton, PA- Beth Israel Medical Center (Site 2851) CTU Grant # AI46370
Ann Conrad, RN and Kim Whitely, RN- MetroHealth CRS (Site 2503) CTU Grant # AI069501
Susan L. Koletar, MD and Mark D. Hite, RN- The Ohio State University AIDS CRS (Site 2301) CTU Grant # AI069474
Joseph Eron, MD, and David Currin, RN, CCRC-Wake County HHS CRS (Site 3206).
Richard Pollard, MD and Nancy Fitch, ANP-UC Davis Medical Center (Site 3852).
Brenda Jackson, RN & Rebecca Basham, BS- Vanderbilt University (Site 3652) CTU Grant # AI069439
Christine Hurley, RN; Mary Adams, RN- University of Rochester ACTG CRS (Site 1101) and AIDS Community Health Center (Site 1108) CTU Grant # AI069511, GCRC Grant # RR00044
Ann C. Collier, MD and Beck A. Royer, PA-C- University of Washington AIDS CRS (Site 1401) CTU Grant #AI069434
Kim Epperson, RN and Timothy Lane, MD- Moses H. Cone Memorial Hospital CRS (Site 3203) CTU Grant #AI069423
David Currin, RN and Sue Richard, PA- University of North Carolina AIDS CRS (Site 3201) CTU Grant #AI69423, CFAR Grant #AI50410, GCRC Grant #RR00046
Henry H. Balfour, Jr. MD and Christine Fietzer, RN- University of Minnesota ACTU (Site 1501)
Hector Bolivar, MD and Margaret A. Fischl, MD- University of Miami AIDS CRS (Site 901) CTU Grant #AI069477
Abby Olusanya, NP- UC Davis Medical Center ACTU (Site 3851) CTU Grant # AI069483
Robert Redfield, MD and Charles Davis, MD- University of Maryland CRS (Site 4651)
Jack Degnan, MPH and Dee Dee Pacheco- University of California San Diego AVRC CRS (Site 701) CTU Grant # AI69432
Karen Tashima, MD, and Joan Gormley, BSN-SSTAR Family Healthcare Center (Site 2954).
Sharon Riddler, MD, MPH and Carol Oriss, BSN, RN- Pittsburgh CRS (Site 1001) CTU Grant # AI 069494
Lorna Nagamine, RN and Scott Souza, PharmD- University of Hawaii at Manoa (Site 5201)
Nayef El-Daher, MD, and Carol Greisberger, RN, BS- McCree McCuller Wellness Center (Site 1107).
John Black, MD, and Beth Zwickl, RN, CS, MSN- Methodist Hospital of Indiana (Site 2602).
Timothy Flanigan, MD, and Joan Gormley, BSN- Rhode Island Hospital (Site 2953) CTU Grant # AI046381.
Marjorie Dehlinger DNSc-Division of AIDS, NIAID
Disclosures: RG reports that he has received grant support from Bristol-Myers Squibb and has grant support from Abbott for research unrelated to this study. AA has served on an advisory board for Abbott.TF has received grant support from Abbott and Gilead unrelated to this study. JS has served on advisory boards for Gilead, Tibotec, Roche, Bristol-Myers Squibb, and Merck regarding antiretroviral medications. He also reports having research grants from Gilead, Tibotec, Roche, and Merck. DM reports that she has received grant support from Abbott, Bristol-Myers Squibb, Boehringer-Ingelheim, Merck, Pfizer, Roche, Schering Plough, and Tibotec for research unrelated to this study, and has received honoraria from Bristol-Myers Squibb, Boehringer-Ingelheim, GlaxoSmithKline, and Tibotec.
Financial Support: This work was supported by the AIDS Clinical Trials Group via cooperative agreements with the investigators listed in the Acknowledgement section as well as K08 MH01584 and U18 HS016946 (RG), U01 AI68636 and U01 AI068634 (CT and CL), U01 AI069472 (TF), and AI46370 (DM)
References
- 1.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 2.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15(16):2109–2117. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 3.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006 Aug 9;296(6):679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 5.Mills EJ, Nachega JB. A wake-up call for global access to salvage HIV drug regimens. Lancet. 2007 Dec 8;370(9603):1885–1887. doi: 10.1016/S0140-6736(07)61790-5. [DOI] [PubMed] [Google Scholar]
- 6.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006 Dec 1;43 1:S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds NR, Testa MA, Marc LG, et al. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS Behav. 2004 Jun;8(2):141–150. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- 8.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):432–438. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 9.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003 May;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 10.Starace F, Ammassari A, Trotta MP, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002 Dec 15;31 3:S136–139. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- 11.Arnsten JH, Demas PA, Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002 May;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleeberger CA, Buechner J, Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18(4):683–688. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 13.Bouhnik AD, Chesney M, Carrieri P, et al. Nonadherence among HIV-infected injecting drug users: the impact of social instability. J Acquir Immune Defic Syndr. 2002 Dec 15;31 Suppl 3:S149–153. doi: 10.1097/00126334-200212153-00013. [DOI] [PubMed] [Google Scholar]
- 14.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006 Nov;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrieri MP, Leport C, Protopopescu C, et al. Factors associated with nonadherence to highly active antiretroviral therapy: a 5-year follow-up analysis with correction for the bias induced by missing data in the treatment maintenance phase. J Acquir Immune Defic Syndr. 2006 Apr 1;41(4):477–485. doi: 10.1097/01.qai.0000186364.27587.0e. [DOI] [PubMed] [Google Scholar]
- 16.Volmink J, Matchaba P, Garner P. Directly observed therapy and treatment adherence. Lancet. 2000;355(9212):1345–1350. doi: 10.1016/S0140-6736(00)02124-3. [DOI] [PubMed] [Google Scholar]
- 17.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007 Sep 15;45(6):770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006 Jun 1;42(11):1619–1627. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 19.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007 Jul 11;21(11):1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 20.United States Department of Agriculture and United States Department of Health and Human Services. Dietary Guidelines for Americans. Washington, D.C.: US Government Printing Office; 2005. Chapter 9-Alcoholic Beverages; pp. 43–46. [Google Scholar]
- 21.Anonymous. Tables for Grading Severity of Adult Adverse Experiences. Regulatory Compliance Center, Division of AIDS. Available at: http://rcc.tech-res.com/tox_tables.htm.
- 22.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]


