Abstract
Purpose
Mesenchymal stem cells (MSCs) are multipotent cells in the bone marrow which have been found to migrate to tumors, suggesting a potential use for cancer gene therapy. MSCs migrate to sites of tissue damage, including normal tissues damaged by radiation. In this study, we investigate the effect of tumor radiation therapy on localization of lentivirus-transduced MSCs to tumors.
Methods and Materials
MSCs were labeled with a lipophilic dye to investigate migration to colon cancer xenografts. Subsequently, MSCs were transduced with a lentiviral vector to model gene therapy and mark infused MSCs. LoVo tumor xenografts were treated with increasing doses of radiation therapy to assess the effect on MSC localization, which was measured by quantitative PCR. MSC invasion efficiency was determined in an invasion assay.
Results
MSCs migrated to tumor xenografts of various origins, with few cells found in normal tissues. A lentiviral vector efficiently transduced MSCs in the presence, but not absence, of Polybrene. When LoVo tumors were treated with increasing doses of radiation, more MSCs were found to migrate to them than to untreated tumors. Irradiation increased MSC localization in HT-29 and MDA-MB-231, but not UMSCC1, xenografts. MCP-1 expression in tumors did not correlate with basal levels of MSC infiltration, however, MCP-1 was modestly elevated in irradiated tumors. Media from irradiated LoVo cells stimulated MSC invasion into basement membranes.
Conclusion
These findings suggest that radiation induced injury can be used to target MSCs to tumors, which may increase the effectiveness of MSC cancer gene therapy. Production of tumor-derived factors in response to radiation stimulates MSC invasion.
Keywords: gene therapy, lentivirus, migration, MSC, cancer, MCP-1
Introduction
Gene therapy approaches have been devised to specifically target cancer therapeutics to the tumor in order to minimize systemic toxicity. One such approach has used adenoviral vector transduction of tumor cells with yeast cytosine deaminase (yCD) as a way to target 5-fluorouracil (5FU) to the tumor 1. Tumor specificity is bestowed both by direct injection of vector into the tumor vasculature and by placing the expression of yCD under the control of a tumor-specific promoter. Unfortunately, intravascular adenoviral gene therapy can be associated with significant toxicity 2. Mesenchymal stem cells (MSCs) offer an attractive alternative to viral approaches. MSCs are adherent cells within the bone marrow which can give rise to multiple tissues including bone, cartilage, fat, and possibly other tissues 3, 4. These cells can be readily obtained and expanded in vitro while retaining their stem cell characteristics and in this regard represent an attractive cell population for use in gene therapy. Recently, it has been shown that MSCs migrate into tumors of brain, breast, and lung origin, and can be used to deliver cancer therapeutics specifically to the local tumor environment 5–8. MSCs were genetically modified by adenoviral vectors to express interferon-β or IL-2, resulting in decreased tumor growth and longer animal survival.
Therapeutic benefit of expressed anti-tumor genes in MSCs is dependent both on transgene expression in the MSCs and on achieving sufficient quantity of MSCs localized within tumors. To achieve long-term expression, an integrating vector such as lentiviral vectors based on HIV may be required. Enhanced MSC localization to tumors has been investigated by Sato et al. 9, who attempt to enhance localization by expression of EGFR in MSCs before infusion, to increase migration to EGF-producing gliomas.
It has been reported that MSCs migrate to normal tissue in response to damage, including damage caused by exposure to radiation 10, 11. The mechanism for this activity is not well defined but is thought to involve production of chemokines or growth factors by damaged tissue or vasculature, which attracts MSCs to the region. In this report, we investigated the effect of radiation therapy on localization of MSCs in tumors. Before infusion of gene-modified MSCs, we exposed subcutaneous tumors to radiation therapy and investigated the extent of MSC localization. We found an increase in MSCs in irradiated tumors compared to unirradiated tumors which was dependent on radiation dose. Irradiation of tumor cells induced production of soluble factors which acted to stimulate MSC invasion activity. These may include MCP-1, which was modestly increased in irradiated LoVo tumors. These data suggest that radiation therapy of tumors may work in conjunction with infusion of gene-modified MSCs to enhance cancer gene therapy.
Methods
Mesenchymal stem cells
MSCs lots were obtained either as a gift of K. D. Hankenson (UM) or from the Tulane Center for Gene Therapy. Cells were thawed and expanded in αMEM, 20% FBS, 2 mM GlutaMAX (all from Invitrogen, Carlsbad, CA), in T-162 flasks (Corning). Media was changed once every 3–4 d. MSCs were characterized by differentiation into adipocytes and osteocytes (data not shown).
MSCs were labeled with 2 μg/ml SP-DiOC18 (Molecular Probes, Eugene, Oregon) for 2 d at 37°C, which produced excellent cell visibility and minimal toxicity. Several hours before infusion into animals, dye-containing media was replaced with normal media. Just before infusion, MSCs were trypsinized and washed in media to remove free dye.
Vector and transduction
A second generation, self-inactivating lentiviral vector based on HIV was used 12. The expression cassette was driven by a CMV promoter and contained the following structure: luciferase-IRES-GFP-WPRE. The central polypurine tract/central termination sequence (cPPT/CTS) was included as described 13.
Vector was produced as described 14. Briefly, 293T cells were co-transfected with pHR’CMV.luc-I-GFP.WPRE.SIN-18 (transfer), pCMVΔR8.91 (packaging), and pMD.G (VSV-G envelope) plasmids using Lipofectamine2000 (Invitrogen) in OptiMEM (Invitrogen). The next day, media was replaced with fresh DMEM, 10% heat-inactivated FBS, 2 mM glutamine (all Invitrogen) and incubated overnight. Vector was collected, filtered (0.2 μm), and frozen at −80°C. Titer was determined by transduction of 293T cells with limiting dilutions of vector and assessing the proportion of transduced cells 4 d post-transduction by GFP flow cytometry.
MSCs for in vivo experiments were transduced at passage 3 using an MOI of 10. Hexadimethrine bromide (Polybrene; Sigma) was added to transduction cultures to 5 μg/ml to enhance transduction 15. Transduction was allowed to proceed 1 d before continuing culture in expansion media.
For determination of the efficiency of MSC transduction, MSCs were exposed to vector at MOIs of 0, 2, 6, 18, and 61 with or without Polybrene. Transduction proceeded for 1 d and the proportion of GFP+ cells was assessed 5 d post-transduction by flow cytometry.
Mice and tumors
Animal experiments were undertaken in accordance with University Committee on Use and Care of Animals procedures. Female nude mice (Harlan), 3–4 wks of age were injected subcutaneously in the flank with 1–5 × 106 LoVo (colon), UMSCC1 (head and neck), HT-29 (colon), or MDA-MB-231 (breast) cells in 100 μl serum-free media.
Mice were infused with 1–3 × 106 MSCs via the tail vein in a total volume of 100 μl.
Irradiation
Cells and mouse tumors were irradiated with a Philips 250 orthovoltage unit at approximately 2.5 Gy/min in the Irradiation Core of the University of Michigan Cancer Center. Dosimetry was carried out using an ionization chamber connected to an electrometer system, which is directly traceable to a National Institute of Standards and Technology calibration. Mice were either placed in a Lucite restrainer or anesthetized with ketamine/xylazine and positioned such that the apex of each flank tumor is at the center of a 2.4 cm aperture in the secondary collimator and irradiated with the rest of the mouse being shielded from radiation..
Histology
Tumors and normal tissues were collected and immediately frozen in OCT (Tissue Tek, Torrance, CA). Cryosections (6 μm) were cut on a Microm HM 500M cryostat. Slides were rinsed in PBS and mounted with FluorSave™ Reagent (Calbiochem) containing 0.75 μg/ml Dapi. Photographs were taken on an Olympus IX71 fluorescent microscope with an Olympus DP70 camera.
MCP-1 immunohistochemistry was performed on frozen sections using mouse anti-human MCP-1 primary antibody (BD Pharmingen) and goat anti-mouse IgG HRP-conjugated secondary antibody.
PCR
Tumors or skin tissue were collected and DNA extracted using a DNeasy kit (Qiagen). Real time PCR was done using a Quantitect Sybr-Green PCR kit (Qiagen) on an Applied Biosystems or MJ Research Opticon instrument. All samples were run in duplicate. For each sample, the relative amount of GFP (primers = GFP-F: 5′-AGATCCGCCACAACATCGAG-3′; GFP-R: 5′CCATGCCGAGAGTGATCCC-3′) and CCR5 (primers = CCR5-F: GCTGTGTTTGCGTCTCTCCCAGGA-3′; CCR5-R: 5′-CTCACAGCCCTGTGCCTCTTCTTC-3′) was determined by comparison to a standard curve made from a GFP+ cell line. The GFP value was divided by the CCR5 value to normalize to genomic copies. This value in irradiated tumors was then compared to the value in unirradiated tumors to determine the fold-change in response to radiation.
Immunofluorescence
Frozen tumor sections were fixed in acetone and washed in PBS before overnight incubation in 1/500 rat anti-muCD31 (Abcam #ab7388) and rabbit anti-luciferase (Abcam #ab21176) antibodies at 4°C. Slides were then washed and incubated for 1 hr at room temperature first in 1/2000 goat anti-rabbit-Alexa-594 (Invitrogen #A11012), washed with PBS, 1% BSA, and then incubated in rabbit anti-rat-Alexa-488 (Invitrogen #A21210).
Invasion assay
Invasion substrates consisting of naturally serum-free sea urchin embryo basement membranes and associated extracellular matrix (SU-ECM) were prepared, and invasion assays performed as previously described 16.
Results
Migration of MSCs to tumors
The ability of MSCs to migrate to human colon cancer tumors was investigated by infusion of MSCs labeled with the lipophilic membrane dye SP-DiOC18 to allow visualization in tissue sections by fluorescent microscopy. SP-DiOC18-labeled MSCs were viable and retained the dye for at least 3 d in vitro without noticeable ill effects (data not shown).
We were then studied migration of labeled MSCs to xenografts derived from human LoVo colon cancer cells and and U87 glioma cells. We observed labeled MSCs in LoVo tumors at both 3 (data not shown) and 8 d post-infusion (Fig. 1A). Frequently, MSCs were found in several clusters within the tumor, often, but not exclusively, at it outer third. Isolated cells were also seen scattered throughout the tumor. MSCs were found in U87 tumors, but at a lower frequency and rarely in clusters (data not shown).
Figure 1.
Migration of MSCs to tumors. (A) MSCs were stained with SP-DiOC18 before infusion. LoVo tumor sections from mice receiving labeled MSC infusion 8 d prior. Left column shows 3 different tumor sections with green MSCs located in clusters within the tumor. Right column is the corresponding Dapi picture of the same section showing nuclear staining. It can be seen that clusters are located both internally (top 2 pairs) and at the tumor periphery (bottom pair). (B) Analysis of other tissues for the presence of MSCs shows few to none in liver, spleen, muscle, and bone marrow. Small numbers are observed in the lung at 8 d post-infusion.
If MSCs are to be useful for delivery of cancer therapeutics, they need to selectively target tumors compared to normal tissues. We therefore analyzed multiple other tissues histologically for the presence of MSCs (Fig. 1B). Liver, muscle, spleen, bone marrow aspirates, and intact femur (not shown) displayed few, if any, MSCs by this technique. Small numbers of MSCs were observed in the lung. This was time dependent, with more observed very early after injection. However, by 7 d post-injection, MSCs in the lung were minimal. These data suggest that MSCs localize selectively to tumors.
Transduction of MSCs
Utilization of MSCs for targeted delivery of cancer therapeutics to tumors will require gene modification by ex vivo transduction in order to express the therapeutic gene of interest. Lentiviral vectors are suited to this task by way of their ability to efficiently transduce nondividing or slowly dividing cells 17. We investigated MSC transduction efficiency and expression following lentiviral transduction of a marker gene as a model of therapeutic gene therapy.
Transduction was tested in the presence or absence of Polybrene. In the absence of Polybrene, transduction was inefficient, with about 11±4% transduction at an MOI of 61 versus 89±2% in the presence of Polybrene (Fig. 2A). Lower MOIs led to barely detectible transduction in the absence of Polybrene. In the presence of Polybrene, however, a moderate MOI of 6 was able to transduce 54±9% of MSCs as assessed by flow cytometry for GFP (Fig. 2A). Transgene expression was observed at 4 d post-transduction and maintained in vitro for up to 12 wks without a noticeable reduction in expression intensity or proportion (Fig. 2B).
Figure 2.
MSC transduction. (A) MSCs were transduced with a lentiviral vector in the presence or absence of Polybrene at various MOIs from 2 – 61 and the proportion of GFP+ cells analyzed by flow cytometry. Efficient transduction was only observed in the presence of Polybrene. (B) GFP expression by MSCs in vitro was observed at 4 d post-transduction and maintained for up to 12 wks.
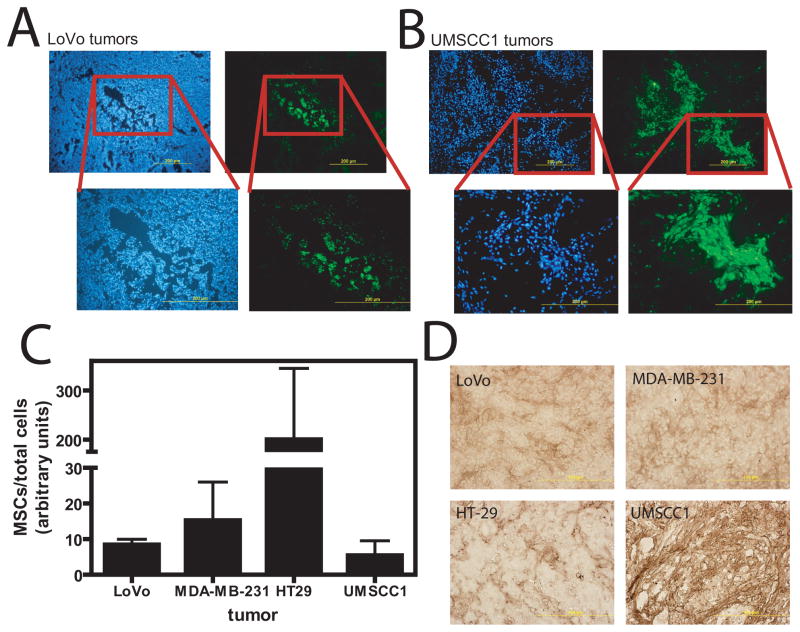
We next tested whether transduced MSCs retained the ability to migrate to, and express their transgene in, xenografts of various tumors. Tumors were removed 14 d following transduced MSC infusion and cryogenic sections prepared or tissue processed for PCR. Direct visualization of GFP fluorescence indicated the presence of transduced MSCs in colon cancer (LoVo) tumors (Fig. 3A). Likewise, analysis of UMSCC1 (head/neck) tumors shows transduced MSC localization (Fig. 3B). MSCs were found in clusters, which may indicate proliferation at the tumor site as has previously been suggested 7.
Figure 3.
Migration of transduced MSCs to tumors. Lentivirus-transduced MSCs were infused into LoVo or UMSCC1 tumor-bearing mice and tumor sections analyzed at 14 d. (A) Lentivirus-transduced MSCs migrated to, and expressed in, LoVo tumors. (B) UMSCC1 tumors contained several large clusters of GFP+ MSCs. Left panels are Dapi-stained photos of the same section. Right panels are direct GFP fluorescence. Boxed regions define area of higher magnification. (C) Plot of the proportion of MSCs in different tumor types as determined by PCR. A greater proportion of MSCs were found in HT-29 tumors compared to LoVo (P<0.001), MDA-MB-231 (P<0.01), or UMSCC1 (P<0.01; Tukey’s Multiple Comparison test). (D) MCP-1 immunohistochemistry of tumor sections showing different degrees of MCP-1 expression depending on tumor type.
Analysis of the MSC content by quantitative PCR in LoVo, UMSCC1, MDA-MB-231 (breast), and HT-29 (colon) showed detection of transduced MSCs in all 4 tumors (Fig. 3C). There was variability in the degree of MSC targeting, as significantly greater MSCs were found in HT-29 tumors than in LoVo, UMSCC1, or MDA-MB-231 tumors (P<0.01; Tukey’s Multiple Comparison test). Of 23 mice bearing LoVo tumors, 21 (91%) demonstrated localization of MSCs by PCR.
Differences in MSC homing to various tumor types may be determined by expression of chemokines by tumors. MCP-1 has been implicated in attracting MSCs to tumors 18, 19. To test whether MCP-1 contributed to differences in tumor tropism, we analyzed MCP-1 expression in LoVo, UMSCC1, HT-29, and MDA-MB-231 tumors by immunohistochemistry. In LoVo and MDA-MB-231 tumors, MCP-1 staining was light and distributed somewhat evenly in the interstitial space throughout the tumors (Fig 3D). In HT-29 tumors, MCP-1 staining was slightly more intense, but concentrated in discrete areas of the tumor. UMSCC1 tumors stained intensely for MCP-1 throughout the tumor.
The data indicate that MSCs are efficient targets of lentiviral transduction in the presence of Polybrene and they maintain transgene expression in vitro for up to 12 wks. Transduction does not negatively impact MSC localization to tumors and cells retain the ability to migrate to multiple types of tumor xenografts, and express the transgene in vivo. HT-29 colon tumors attract significantly more MSCs than UMSCC1, LoVo or MDA-MB-231 tumors.
Radiation therapy increases localization of MSCs in tumors
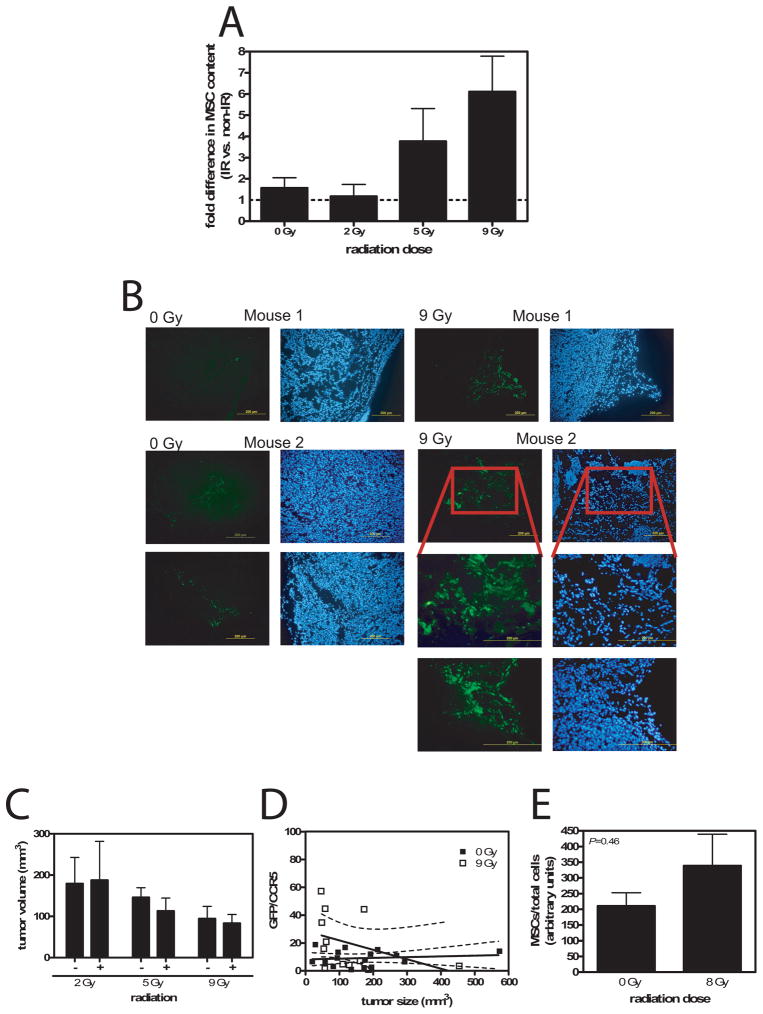
We next investigated the impact of tumor radiation therapy on MSC localization. Irradiation of tumors caused a dose-dependent increase in MSC localization in tumors, with 6.1-fold more MSC in tumors irradiated with 9 Gy compared to the matched control tumor on the same animal (Fig. 4A). Histological examination of tumors confirmed the quantitative PCR data (Fig. 4B). Although GFP fluorescent cells were observed in sections from both irradiated and unirradiated tumors, more MSCs were found in irradiated tumors, generally in larger clusters. Tumor irradiation also increased MSC localization in HT-29 and MDA-MB-231 tumors, but not UMSCC1 tumors (Table 1).
Figure 4.
Radiation increases MSC migration to tumors. (A) Radiation dose response of MSCs in LoVo tumors. A greater proportion of MSCs were found in tumors receiving a greater radiation dose (P=0.026 0 Gy vs 9 Gy, t test with Welch’s correction; N=2–11). (B) Photomicrograph examples of tumor sections taken from 0 Gy and 9 Gy irradiated LoVo tumors showing more readily observed MSC in irradiated tumors compared to unirradiated. Left panels are direct GFP fluorescence and right panels are DAPI-stained. (C) Graph of LoVo tumor volume for each irradiated group and their matched unirradated controls. There was no significant difference between groups (P=0.5–0.9, paired t test). (D) Plot of the proportion of MSCs in each tumor versus tumor size. Regression analysis showed no relationship between MSC content and size for neither 0 Gy (P=0.63) nor 9 Gy (P=0.21) irradiated tumors. There was no difference between these 2 groups (P=0.10). (E) MSCs in unirradiated or 8 Gy irradiated skin. MSC content trended higher in irradiated skin, but was not significant (P=0.46).
Table 1.
Effect of radiation on MSC content in different tumor types.
| tumor | 5 Gya | sizeb | 9 Gy | size |
|---|---|---|---|---|
| MDA-MB-231 (breast) | 62 | 0.28 | 1200 | 0.13 |
| HT-29 (colon) | 6.2 | 0.46 | 2.7 | 0.45 |
| UMSCC1 (head/neck) | n.d. | n.d. | 0.86 | n.d. |
average fold increase in MSCs in irradiated versus non-irradiated tumors;
average fractional difference in tumor size, irradiated versus non-irradiated; n.d.: not determined
A reduction in tumor size in response to radiation therapy, with equivalent numbers of MSCs localizing to both unirradiated and irradiated tumors might manifest as an apparent increase in MSC influx. However, there were no significant differences in tumor volume between the irradiated and unirradiated LoVo tumors at each radiation dose (P=0.5–0.9, paired t test; Fig. 4C). LoVo tumors did not regress during the 2 wk period following irradiation at up to 9 Gy. Likewise, reduction in tumor size did not explain the increase proportion of MSCs seen in irradiated HT-29 and MDA-MB-231 tumors (Table 1).
We also analyzed the relationship between MSC content and tumor size by constructing a plot of GFP/CCR5 versus tumor volume (Fig. 4D). Regression analysis indicated that the proportion of MSCs in the tumor was not related to tumor size (P=0.63 and P=0.21 for 0 Gy and 9 Gy tumors, respectively, that slope is non-zero). There was no difference between regression lines obtained from the 0 Gy data and the 9 Gy data (P=0.10).
We wished to investigate the selectivity of radiation to induce MSC migration in tumors compared to the normal tissues. To assess migration of MSCs to irradiated skin in the absence of underlying tumor, we irradiated the flank area of mice with 8 Gy before infusion of MSCs the next day. After 12 d post-MSC infusion, a section of irradiated skin and unirradiated skin were removed, and the number of MSCs quantified by quantitative PCR. We found 8 Gy irradiated skin contained 339±174 units MSCs/total cells, while 0 Gy irradiated skin contained 211±74 units MSCs/total cells (Fig. 4E). Although irradiated skin trended toward a higher number of MSCs, the difference was not significant (P=0.46).
Other tumor cell lines and types were also analyzed for the effect of radiation on attraction of MSCs (Table 1). HT-29 colon cancer tumors showed increased MSC localization in response to 5 and 9 Gy, compared to their matched, unirradiated, control tumors. Radiation caused regression of these tumors over 2 wks. At collection, they were about half the size of control tumors, and 5 and 9 Gy induced a 6.2 and 2.7-fold greater localization of MSCs, respectively. The breast cancer cell line, MDA-MB-231, had the greatest influx of MSCs following radiation. In response to 5 Gy, there were 62-fold greater MSCs present and after 9 Gy radiation, 1200-fold more MSCs were found. Tumors regressed after exposure to radiation, but not enough to account for the increase in MSC proportion. Interestingly, in experiments using UMSCC1 head and neck squamous cell carcinoma tumors, 9 Gy had no effect on the proportion of MSCs found in the tumors.
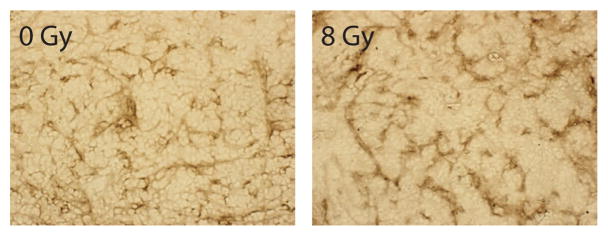
In vivo, LoVo tumors exposed to 8 Gy showed a modest increase in MCP-1 expression, as determined by immunohistochemistry (Fig. 5).
Figure 5.
MCP-1 expression in irradiated tumors. Sections of 0 or 8 Gy irradiated tumors were taken from mice 14 d post-irradiation and stained for MCP-1. In 8 Gy irradiated tumors, a modest increase in MCP-1 staining is observed.
Thus, the proportion of MSCs in tumors can be increased by pre-exposing them to radiation. The increased proportion of MSCs could not be attributed to differences in tumor size. MCP-1 expression in irradiated LoVo tumors was modestly increased and may promote greater MSC infiltration.
MSCs localize near the vasculature of irradiated tumors
The localization of MSCs within irradiated tumors was examined to determine whether there was a correlation with tumor vasculature. Mice bearing flank LoVo tumors were infused with MSCs transduced with luciferase by a lentiviral vector. Tumors were given 9 Gy, removed 14 d later, and processed for immunofluorescence. Co-labeling of sections with CD31 to detect vasculature and luciferase to detect MSCs showed that MSCs were predominantly located near vascular areas of the tumor (Fig. 6).
Figure 6.
MSCs localize near tumor vasculature. Sections of irradiated tumors taken from mice infused with luciferase-transduced MSCs were co-labeled with anti-CD31 and anti-luciferase antibodies. CD31 and luciferase labeling colocalized, indicating MSCs were predominantly found near tumor vasculature.
Irradiated tumor cells stimulate MSC invasion
Chemotaxis and invasion are separate cellular processes which influence the ability of cells to migrate, extravasate, and move into target tissues. MSCs have been shown to migrate to conditioned media from cancer cell lines or media supplemented with chemokines/growth factors such as EGF, PDGF, or SDF-1α5, 9. To investigate MSC invasion through intact basement membranes, rather than a coating of fibronectin, collagen, or Matrigel, we chose a model system based on sea urchin embryos. Sea urchin embryo basement membranes and associated extracellular matrix (SU-ECM) have been shown to contain an intact basement membrane that closely resembles mammalian basement membranes, with regard to both ultrastructure and molecular constituents 20. Furthermore, placement of the cells to be tested on their exterior surfaces allows them to encounter the apically-oriented surface of the basement membrane prior to the basal surface, much as blood-borne cells would encounter the endothelial basement membrane during extravasation. Also, SU-ECM invasion substrates have been found to reflect accurately the relative invasive potentials of a variety of different types of metastatic tumor cells, as well as stimulated and unstimulated normal cells 16, 21–24.
The SU-ECM-based assay was used to measure changes in the invasiveness of MSCs exposed to conditioned media. Media was conditioned by LoVo cells exposed to 0 or 9 Gy radiation. Fresh MSC media containing 20% FBS did not induce MSC invasion through SU-ECM and represents a negative control (Table 2). The peptide Ac-PHSRN-NH2 was added as a positive control and resulted in 27% invasion. When media from unirradiated LoVo cells was used, no MSC invasion was observed, similar to fresh media. Media that was conditioned in 9 Gy irradiated LoVo cells caused 20% invasion of MSCs, which was not statistically different from the positive control (P=0.093, t test). These findings suggest that radiation of tumor cells causes production of factors which act to increase the ability of MSCs to invade basement membranes.
Table 2.
Effect of LoVo irradiation on MSC invasion.
| Medium | invasion (%)a |
|---|---|
| Fresh media | 0 |
| Ac-PHSRN-NH2 | 27.1±5.8 |
| LoVo-conditioned | 0 |
| 9 Gy IR LoVo-conditioned | 20.0±4.1 |
mean±SD
Discussion
This work describes migration of human MSCs to irradiated tumor xenografts derived from colon, breast, and head/neck cancer. When MSCs were infused via tail vein, they were found within subcutaneous tumors as early as 3 d post-infusion. Migration to other tissues was minimal or undetectable. As a potential carrier of cancer therapeutics in a gene therapy setting, we show that lentiviral vectors offer efficient and stable expression of a transgene in vitro and in vivo, without altering MSC migratory patterns. The number of MSCs localizing to tumors can be enhanced by treatment of tumors with radiation before MSC infusion. MSCs were found associated with vascular areas of the tumor following irradiation, which is consistent with reports that hypoxia induces apoptosis in MSCs 25. Irradiation of tumor cells induced production of factors that stimulated MSC invasion through intact basement membranes, thus providing a mechanism for the enhanced MSC numbers in tumors following radiation.
Although MSCs have been previously described to migrate to orthotopically implanted gliomas following intracranial or carotid artery injection, and lung and breast tumors within the lung following tail vein infusion 5, 8, the reason why they are attracted to tumors is largely unknown. In vitro evidence suggests MSCs migrate to SDF-1α, EGF, VEGF-A, and PDGF, which may be produced by untreated tumors 5, 9, 26, 27. When the relative amount of MSCs migrating to different tumors is compared, we find significant differences, even between tumors derived from the same tissue. For example, our analysis found greater MSC localization in HT-29 colon tumors than LoVo colon tumors.
A potential use of MSCs may be for gene therapy of cancer. While others have used adenoviral vectors to transduce MSCs, lentiviral vectors may offer distinct technical advantages as well as efficient transduction and long term transgene expression. In our studies, a high proportion of MSCs can be transduced at an MOI of 6–20. Transgene expression remained strong for up to 3 months in vitro and was maintained in vivo. Polybrene was a critical addition during transduction and little to no transduction could be obtained in its absence, but high levels in its presence. For ex vivo transduction in gene therapy, a similar compound may need to be used, such as protamine sulfate, to maximize potential therapeutic results.
The number of MSCs within a tumor will have direct effects on the efficacy of their use in cancer gene therapy. Sato et al. 9 increased MSC migration to tumors by transducing them with EGFR. In the current work we utilize natural MSC biology to increase their localization to tumors. MSCs have previously been shown to migrate to normal tissues damaged by radiation 10, 11. In normal skin, however, we did not find increased MSCs in response to radiation. The mechanism behind radiation-induced migration to normal tissues may be different than that in tumors. In our study, we find radiation causes production of soluble factors which directly stimulate MSC invasion, as opposed to other stromal or vascular elements contained within a tumor. A minimum of 5 Gy caused more MSCs to be found within tumors. We were able to achieve about a 6-fold increase in MSCs in LoVo and HT-29 tumors, but MDA-MB-231 breast tumors displayed substantially higher increases. A 62-fold and 1200-fold increase was observed following 5 and 9 Gy radiation, respectively. On the other end of the spectrum was UMSCC1 head/neck carcinoma cells, in which radiation produced no effect.
Radiation is associated with the release of several cytokines from exposed tissue. Endothelial cells, cancer cells, and other normal tissues have been found to secrete one or more of the following: G-CSF, GM-CSF, IL-8, IL-6, IL-1α, IL-11, MIP-1α, SCF, TGFβ, and TNFα28–31. Production of inflammatory cytokines following radiation may be responsible for attraction of MSCs as part of a wound healing response. A similar phenomenon has been observed in lung damaged by bleomycin 32. Elucidation of the signals responsible for attracting MSCs to irradiated tumors will be an important extension of the current study. Investigation of MCP-1 expression in irradiated tumors showed a modest increase, compared to unirradiated tumors. MCP-1, in combination with other factors such as CCR2, may partially explain increased MSC infiltration into irradiated tumors 19.
Since radiation is frequently a component of cancer therapy, it could work in combination with MSC cancer gene therapy to provide an opportunity to improve targeting of MSCs to tumors. Conformal radiation therapy allows precise targeting of tumor tissue while minimizing damage to normal, surrounding tissue. Specific targeting of radiation as well as preferential targeting of radiation-damaged tumors by MSCs should allow maximal numbers of MSCs to localize to the tumor. This is a particularly attractive strategy to consider with MSCs transduced with yCD. In this case, treatment of patients with 5FC (a benign prodrug used to treat fungal infections) would lead to increased and tumor-localized generation of 5FU, which has both cytotoxic and radiosensitizing effects. In addition, since MSCs have been reported to be radioresistant, continued radiation therapy following migration to tumors may provide prolonged benefit during a course of treatment 33, 34.
In conclusion, we have shown that MSCs migrate specifically to colon, breast, and head/neck tumors. Polybrene is necessary for efficient lentiviral transduction of MSCs and the transgene is expressed long term and in vivo. Radiation therapy of tumors increases the number of MSCs localizing to them, partially due to stimulation of MSC invasion. Irradiated tumors express modestly increased MCP-1, a chemokine implicated in MSC migration. Radiation can thus enhance the potential of MSCs for cancer gene therapy.
Acknowledgments
We thank K. D. Hankenson (UM) and the Tulane Center for Gene Therapy for providing MSCs, and the Morphology Core of the UM Center for Organogenesis for help with histology. Some of the materials employed in this work were provided by the Tulane Center for Gene Therapy through a grant from NCRR of the NIH, Grant # P40RR017447. This work was supported by The Elsa U. Pardee Foundation (SPZ) and NIH grant 5R01CA080145 (TSL). SPZ was supported by a Kirschstein National Research Service Award (T32CA09676) and a LUNGevity Foundation – American Cancer Society Postdoctoral Fellowship in Lung Cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang M, Li S, Nyati MK, et al. Regional delivery and selective expression of a high-activity yeast cytosine deaminase in an intrahepatic colon cancer model. Cancer Res. 2003;63:658–663. [PubMed] [Google Scholar]
- 2.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 7.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 8.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 9.Sato H, Kuwashima N, Sakaida T, et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12:757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- 10.Chapel A, Bertho JM, Bensidhoum M, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 11.Francois S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 12.Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Follenzi A, Ailles LE, Bakovic S, et al. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 14.Zielske SP, Gerson SL. Cytokines, including stem cell factor alone, enhance lentiviral transduction in nondividing human LTCIC and NOD/SCID repopulating cells. Mol Ther. 2003;7:325–333. doi: 10.1016/s1525-0016(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 15.Zielske SP, Gerson SL. Lentiviral transduction of P140K MGMT into human CD34(+) hematopoietic progenitors at low multiplicity of infection confers significant resistance to BG/BCNU and allows selection in vitro. Mol Ther. 2002;5:381–387. doi: 10.1006/mthe.2002.0571. [DOI] [PubMed] [Google Scholar]
- 16.Livant DL, Linn S, Markwart S, et al. Invasion of selectively permeable sea urchin embryo basement membranes by metastatic tumor cells, but not by their normal counterparts. Cancer Res. 1995;55:5085–5093. [PubMed] [Google Scholar]
- 17.Zufferey R, Nagy D, Mandel RJ, et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer RM, Potter-Beirne SM, Harrington KA, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 19.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amemiya S. Electron microscopic studies on primary mesenchyme cell ingression and gastrulation in relation to vegetal pole cell behavior in sea urchin embryos. Exp Cell Res. 1989;183:453–462. doi: 10.1016/0014-4827(89)90404-7. [DOI] [PubMed] [Google Scholar]
- 21.Jia Y, Zeng ZZ, Markwart SM, et al. Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res. 2004;64:8674–8681. doi: 10.1158/0008-5472.CAN-04-0069. [DOI] [PubMed] [Google Scholar]
- 22.Livant DL, Brabec RK, Kurachi K, et al. The PHSRN sequence induces extracellular matrix invasion and accelerates wound healing in obese diabetic mice. J Clin Invest. 2000;105:1537–1545. doi: 10.1172/JCI8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livant DL, Brabec RK, Pienta KJ, et al. Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer Res. 2000;60:309–320. [PubMed] [Google Scholar]
- 24.Zeng ZZ, Jia Y, Hahn NJ, et al. Role of focal adhesion kinase and phosphatidylinositol 3′-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006;66:8091–8099. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Chen J, Cong X, et al. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 26.Fiedler J, Leucht F, Waltenberger J, et al. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;334:561–568. doi: 10.1016/j.bbrc.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 27.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Fedorocko P, Egyed A, Vacek A. Irradiation induces increased production of haemopoietic and proinflammatory cytokines in the mouse lung. Int J Radiat Biol. 2002;78:305–313. doi: 10.1080/09553000110104614. [DOI] [PubMed] [Google Scholar]
- 29.Gaugler MH, Squiban C, Claraz M, et al. Characterization of the response of human bone marrow endothelial cells to in vitro irradiation. Br J Haematol. 1998;103:980–989. doi: 10.1046/j.1365-2141.1998.01119.x. [DOI] [PubMed] [Google Scholar]
- 30.Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 31.van Valen F, Kentrup-Lardong V, Truckenbrod B, et al. Regulation of the release of tumour necrosis factor (TNF)alpha and soluble TNF receptor by gamma irradiation and interferon gamma in Ewing’s sarcoma/peripheral primitive neuroectodermal tumour cells. J Cancer Res Clin Oncol. 1997;123:245–252. doi: 10.1007/BF01208634. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MF, Lin CT, Chen WC, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys. 2006;66:244–253. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence TS, Davis MA, Maybaum J. Dependence of 5-fluorouracil-mediated radiosensitization on DNA-directed effects. Int J Radiat Oncol Biol Phys. 1994;29:519–523. doi: 10.1016/0360-3016(94)90448-0. [DOI] [PubMed] [Google Scholar]