Abstract
Let us assume that the purpose of any movement is to position our body in a more advantageous or rewarding state. For example, we might make a saccade to foveate an image because our brain assigns an intrinsic value to the information that it expects to acquire at the endpoint of that saccade. Different images might have different intrinsic values. Optimal control theory predicts that the intrinsic value that the brain assigns to targets of saccades should be reflected in the trajectory of the saccade. That is, in anticipation of foveating a highly valued image, our brain should produce a saccade with a higher velocity and shorter duration. Here, we considered four types of images: faces, objects, inverted faces, and meaningless visual noise. Indeed, we found that reflexive saccades that were made to a laser light in anticipation of viewing an image of a face had the highest velocities and shortest durations. The intrinsic value of visual information appears to have a small but significant influence on the motor commands that guide saccades.
Keywords: Optimal control, motor control, computational neuroscience, eye movements, saccades, kinematics, image value
Introduction
When we view a work of art, the face of a friend, or read this text, our brain shifts our gaze from one point to another, rapidly moving our eyes. Each movement is a saccade that positions the eyes so that the fovea can sample the currently most interesting part of the visual space. In performing these movements, the brain solves two kinds of problems: first, it selects where to look, and next, it programs the motor commands that move the eyes to that location.
Regarding the first problem, it has long been recognized that the scan sequence is not random (Yarbus AL, 1961) and that task demand, potential reward, uncertainty and risk, among other cognitive factors greatly influence where we look (Hayhoe and Ballard, 2005). For example, in viewing a scene consisting of a faces and non-face objects, we are naturally drawn to the face regions first and spend longer looking at faces compared to the rest of the scene (Cerf M. et al., 2008). This suggests that our brain may continuously assign a value (integrating various cognitive factors) to every part of the visible space forming a priority or salience map (Fecteau and Munoz, 2006;Gottlieb et al., 1998), and each saccade is our brain's attempt to direct our fovea to the region where currently, the value is highest. Because people are naturally drawn to faces, the implication is that faces may have an intrinsically higher value than other images.
The second problem, the problem of how to move the eyes during a saccade, was thought to be independent of the value that the brain might assign to the stimulus. Saccades are so short in duration (50–70ms) and so high in velocity (300–400°/s) that they were thought to be preprogrammed, ballistic processes, resulting in a stereotypical relationship between amplitude and velocity termed the “main sequence”(Bahill et al., 1975). However, recent results suggest saccade kinematics are not stereotypical. For example, monkeys that make a saccade to a remembered target location have higher saccade velocities and shorter durations when that target is also associated with a food reward (Takikawa et al., 2002). If an object is the target of a reaching movement, saccades that accompany the reach exhibit higher velocities and shorter durations (van Donkelaar et al., 2004;Snyder et al., 2002). If there is information that one needs to acquire at the visual target, saccades to that target exhibit higher velocities and shorter durations (Montagnini and Chelazzi, 2005). Finally, repeatedly making saccades to the same visual stimulus produces eye movements with smaller velocities and longer durations (Golla et al., 2008; Chen-Harris et al., 2008). It is possible that these manipulations (food, repetition, etc.) alter the implicit value that the brain assigns to the visual stimulus, and that in turn affects the saccade's trajectory. Indeed, one of the fundamental predictions of the optimal control framework is that the trajectory of saccades depends on the value of the visual stimulus. In this framework (Niv et al., 2007a), the trajectory of a saccade is affected by two kinds of costs: costs associated with the motor commands, and costs associated with the time that passes before the target is foveated. If the value of the stimulus is high, this second cost is also high, which should result in high velocity, low duration saccades.
Here, we attempted to test the prediction that the hypothetical intrinsic value associated with a visual stimulus affects control of saccades. To approach our problem, we considered reflexive (rather than voluntary) saccades, as they are thought to be a low-level orienting reflex. Instead of supplying the stimulus value externally by using money or food as reward, we tested whether visual images of social relevance alter the kinematics of the orienting reflex.
Materials and Methods
Subjects
12 subjects (6 female, mean age 27, range 21–44 years) were recruited from the Johns Hopkins medical school community. Author RS was one of the subjects. All subjects gave written consent to protocols approved by the Johns Hopkins Institution Review Board.
Experimental procedure
We used a single-axis scleral search coil system (Skalar Medical, Delft, The Netherlands) to record horizontal and vertical eye movements at 1000 Hz from either the right or the left eye (Robinson, 1963; Chen-Harris et al., 2008). Subjects sat in a dark room with their head restrained by a dental bite-bar. Raw coil signals were filtered in hardware (90-Hz low-pass Butterworth), digitized (1,000Hz), and saved on computer for later analysis. Saccade targets were presented with a red laser (~0.25° in visual angle) that was rear-projected onto a translucent screen located 1 m in front of the subject. The position of the beam was jumped using a galvo-controlled mirror, which had a step response of ~10ms. Images were presented via a projector (Sharp Notevision PG-M20X, 60 Hz refresh rate). The projector provided some ambient light in the room but otherwise the room had no other sources of light.
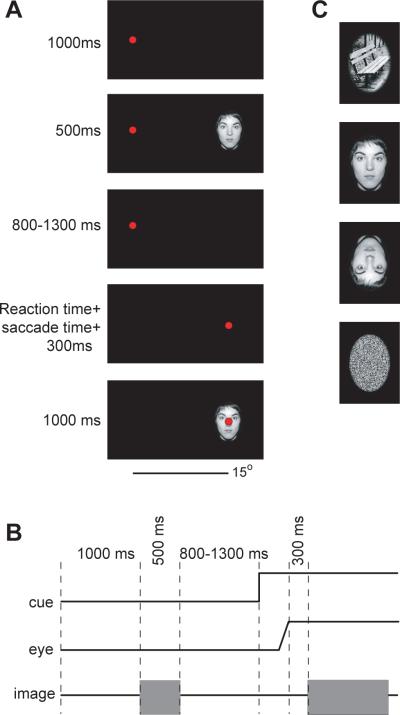
The idea of our experiment was to have people make reflexive saccades to foveate a laser light in a darkened room. However, we wanted to control the `expected reward' of each saccade. We did this by controlling the image that the subject would see after completion of the saccade. The trial sequence is shown in Fig. 1A and Fig. 1B. Participants made 15° horizontal saccades symmetric about the primary position between +7.5° and −7.5°. When the trial started at −7.5°, the target was 15° to the right and when the trial started at +7.5° the target was 15° to the left. Participants fixated a target (red laser) located at +/−7.5° for 1000ms. After this period, an image centered at 15° away with respect to fixation on the other side of midline was presented for 500ms. Subjects continued to maintain fixation. After a random delay of 800–1300ms the laser moved and subjects made a saccade crossing the midline to fixate the new target location. Around 300ms after completion of their saccade, the image was re-displayed at the location of the target (200 ms plus delay introduced by the projector, which was 104+/−7ms SD). Therefore, the saccade was `rewarded' with the image that the subject had initially seen in the periphery. In this way, we hoped that the expected value of each saccade could be controlled on a trial-by-trial basis via the image that first appeared in the periphery.
Figure 1.
Experimental procedures. A. A trial began with subjects fixating a red laser light. After a period of 1000ms, an image was displayed for 500ms centered at ±15° with respect to fixation. Subjects continued to maintain fixation at the red laser. After an additional 800–1300ms fixation period, the laser moved by 15°, and the subject made a saccade to the new target location. After an additional 300ms fixation period, the same image was displayed again. B. Timing of the events. C. On each trial, the image was randomly chosen from one of four image types: faces, objects, inverted faces, or random pixels.
Subjects made six blocks of 40 saccades. We considered four types of images: faces, inverted faces, objects, and random pixels (Fig. 1C). One image type, selected at random, was presented on each trial. Thirty different images were used for each image type. Thus each image was used twice during the experiment. The images were constructed from the Psychological Image Collection of University of Stirling database (http://pics.psych.stir.ac.uk). All images were histogram equalized to have the same overall intensity values. The image size was 4.5° by 6.5° in visual angle.
Data analysis
The duration of saccades was determined by a 16°/s speed threshold. Abnormal saccades were excluded from analysis using global criteria that were applied to all subjects: 1) Saccade amplitude less than 11° (73% of the target displacement) and greater than 16°. 2) Saccade duration less than 50ms and greater than 150ms 3) saccade reaction time less than 50ms or greater than 500ms. 4) peak saccade velocity less than 150 °/s or greater than 550 °/s. For each subject, outliers for amplitude, duration, and peak velocity are those outside of 1.5 times the inter quartile range were also removed. Trials in which the subject broke fixation by reacting to flashing of the image were also excluded from analysis. Overall, ~10% of saccades were excluded from analysis.
Results
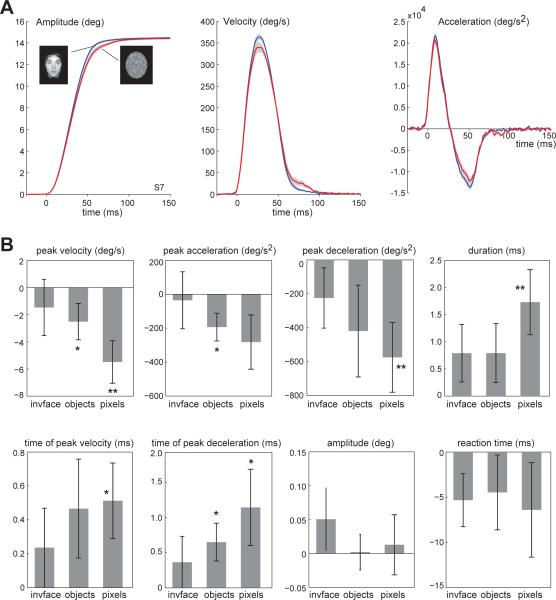
Reflexive saccades made in anticipation of viewing a face were generally faster and had a shorter duration than saccades for other images. Fig. 2A illustrates the average saccade trajectory of a single subject in the face and random-pixel trials. The saccades in the two types of trials were approximately the same amplitude (p=0.29, paired t-test), yet in the face trials the peak velocities were higher (p<0.05) and the durations were shorter (p<0.05, paired t-test).
Figure 2.
In anticipation of foveating an image of a face, vs. an image that contained random pixels, reflexive saccades tended to have higher velocities, shorter durations, higher accelerations, and lower decelerations. A. Average saccade trajectory of one subject for face trials (blue traces) and random-pixel trials (red traces). Gray region is SEM across trials. B. Within subject change in saccade parameters for the various images with respect to face (error bars are SEM across subjects, asterisk indicate p<0.05, one-sided t-test). For example, subjects on average had a 1.8ms longer duration saccade in random-pixel trials as compared to face trials. (*p<0.05 uncorrected comparison, **p<0.05 Bonferroni corrected comparisons)
These differences were also present in the population data. Fig. 2B illustrates within subject changes in saccade parameters with respect to face trials in the inverted face, object, and random-pixel trials. We found that saccade durations and peak velocities were significantly affected by image type (ANOVA with image type as the within subject factor, F(3,33)=3.4, p<0.05 for durations, and F(3,33)=3.6, p<0.05 for peak velocities). There was also a trend toward significance for peak deceleration and time of peak deceleration (F(3,33) = 2.73, p = 0.059 for peak deceleration, and F(3,33) = 2.68 p = 0.063 for time of peak deceleration). Post hoc pair-wise t-tests using the Bonferroni correction revealed that saccades in face trials had significantly higher peak velocities (5.48°/s, corrected t-test p = 0.01) and shorter durations (1.73 ms, corrected t-test p = 0.04) than saccades in random-pixel trials. In contrast, we did not observe an effect on saccade amplitudes (F(3,33)=0.77 p=0.52), endpoint variability (F(3,33)=0.201 p=0.895), or reaction times (F(3,33)=1.21 p=0.32). Figure 2B shows the result of pair-wise t-tests with and without Bonferroni corrections.
Subjects made equal number of leftward and rightward saccades. Equal numbers of each image type were presented for leftward and rightward saccades. In addition, we had half of the subjects wear the coil in the left eye to counterbalance any differences in the eye recorded. Analysis showed no difference between rightward and leftward peak velocity within subject (p = 0.75, 2 tailed paired t-test), duration (p=0.66), amplitude (p=0.66), and reaction time (p=0.82).
Collewijn et al. (1988) found that for saccades about the primary position, the temporal/abducting eye made saccades of higher amplitude, higher velocity, shorter duration, and less skewed than the nasal/adducting eye. However, we found no significant difference between temporal and nasal bound saccades (p=0.14), although the trend was in the direction suggested by Collewijn et al. (1988). Regardless, we had an equal number of temporal and nasal bound saccades, thereby counterbalancing any potential differences.
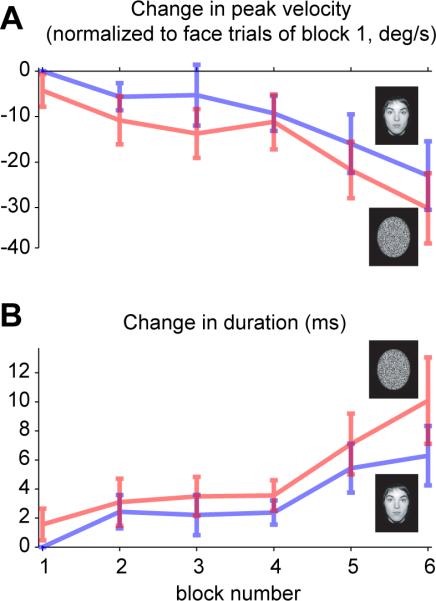
The data presented in Fig. 2 reflects average saccade kinematics as measured over six blocks of 40 trials. Our earlier work had suggested that the repetition of saccades tends to produce a fatigue-like effect so that set after set, the peak velocities tend to drop. We wondered whether the differences that we had seen in the pooled data (Fig. 2), i.e. the differences in saccade kinematics between face and random pixels, were present from the early trials, or were they due to a differing rate of fatigue. To answer this question, for each subject we found the average speed and duration of face saccades in block 1 and then compared the random pixel saccades to these measures. This difference with respect to face saccades of block 1 is plotted in Fig. 3. The data suggests that whereas repetition induced a fatigue-like effect on both face and random pixel saccades (ANOVA, main effect of block, peak velocity F(5,55)=4.6, p<0.01; duration F(5,55)=3.76, p<0.01), faces elicited a consistently faster saccade with a shorter duration (ANOVA, main effect of image type, peak velocity F(1,11)=9.7, p<0.05; duration F(1,11)=5.96, p<0.05), and this difference did not change markedly as a function of repetition (ANOVA, block by image type interaction, peak velocity F(5,55)=0.56, p=0.7; duration F(5,55)=0.64, p=0.7).
Figure 3.
A fatigue-like effect on saccade kinematic parameters over blocks. For each subject, we computed the average peak velocity and duration for face trials in block 1, and then compared the saccades to faces and random pixel during other blocks to these measures. A. Change in peak velocity. B. Change in duration. Error bars are SEM across subjects. (Without Bonferroni correction: *p<0.05, With Bonferroni correction **p<0.05)
Discussion
In our experiment, people made a reflexive saccade to foveate a point of light in a dimly lit room. After completion of the saccade, they were presented with an image centered on their fovea. We found that saccades that were made in anticipation of viewing a face had higher velocities and shorter durations than saccades that were made in anticipation of viewing an image consisting of random-pixels. It is important to note that the image types were not associated with an experimenter controlled value; rather, our intention was to ask whether there was some inherent property of the image that would affect saccade kinematics. Our results suggest that the brain assigns a value to the stimulus of the saccade, and this in turn affects the motor commands that orient the eyes toward that stimulus. While earlier work had found some evidence for the role of stimulus value in voluntary saccades of monkeys, for example, in anticipation of food (Takikawa et al., 2002; Chen-Harris et al., 2008), our results may be the first to demonstrate an effect of natural images.
Can images have an intrinsic value?
Instead of supplying the stimulus value externally by using money or food as reward, we tested whether visual images of social relevance altered the vigor of the orienting reflex. Visual images have been shown to elicit short latency responses in midbrain dopaminergic neurons (Dommett et al., 2005), and images can serve as positive reinforcement for animal behavior (Blatter and Schultz, 2006). Images conveying social information such as social status (Shepherd et al., 2006) and potential mate (Deaner et al., 2005) can modulate gaze behavior. Face images in particular are known to produce reward-like neuronal responses. Hayden and Platt (Hayden et al., 2007) found that the opportunity to look at another person is a valued commodity and that physical attractiveness is one dimension along which value rises. Indeed, attractive faces can activate the reward circuitry of the brain (Bray and O'Doherty, 2007;Kampe et al., 2001).
Small effect on kinematic parameters
The modulation that we were able to elicit with different image types was significant but quite small (5°/s in velocity and 1–2 ms in saccade duration). Foveating a target a few milliseconds earlier may not be crucial for survival. However, it is possible that our results are a reflection of a general framework of how the brain controls movements: the brain assigns a value to sensory stimuli and this value is reflected in the vigor with which movements are performed.
This view helps explain a number of previously published observations. For example, saccades accompanied by reaching movements (van Donkelaar et al., 2004;Snyder et al., 2002) or followed by a perceptual task (Montagnini and Chelazzi, 2005) are faster than saccades without subsequent tasks. Saccades made to repetitive stimuli become slower as the stimulus repeats (Straube et al., 1997;Chen-Harris et al., 2008). Predictive saccades (predictive in amplitude, direction, and timing) are slower than reflexive saccades (Bronstein and Kennard, 1987).
To explain these results, let us suppose that the stimulus that elicits the saccade holds more value if useful information is expected at the endpoint. Both predictive saccades and saccades to repeated targets offer little new information; potentially explaining why the accompanying saccades are slower. In contrast, saccades guiding a reaching movement or a perceptual task provide useful information that can help accomplish the task; potentially explaining why the accompanying saccades are faster.
However, the effect that we observed was quite small. For example, when saccades are accompanied with a reaching movement, velocities can be about 4% faster, while here image content had about a 1% effect. What might account for our smaller effect? One possibility is that we focused on reflexive saccades (driven by the sudden onset of external stimulus), whereas the effect of stimulus value may be much higher for voluntary saccades (the brain voluntarily chooses the target location of the saccade). The neural control of reflexive saccades is distinct from voluntary saccades (Johnston and Everling, 2008;Snyder et al., 2002), and it is likely that the effect of value on saccade velocities might be greater for voluntary saccades because voluntary saccades rely more heavily on basal ganglia structures, structures that in monkeys are modulated by the value of the stimulus (Hikosaka, 2007). Indeed, monkeys make faster voluntary saccades (by about 7%) to stimuli that produce more food (Takikawa et al., 2002). In contrast, our task was a low-level orienting reflex.
Another possibility is that our task relied on the intrinsic value of images, and not on any specific task that subjects needed to perform after observing the image. During each trial, the subject was led to anticipate a certain image by flashing that image for 500ms at 15° with respect to the fovea. After the image was removed, the saccade was elicited by a step change in a red laser dot. Therefore, the saccade was ultimately made in reaction to a jumping red target.
Reaction time and peak velocity
Many previous reports have focused on the relationship between target value and saccade reaction times (Watanabe et al., 2003;Madelain et al., 2007;Milstein and Dorris, 2007). These reports have generally not considered the effect of value on saccade kinematics. Here, we did not observe an effect of image type on reaction times. This could be because we were not able to induce a large enough range of stimulus values, or because we focused on reflexive rather than voluntary saccades. The correlation between peak velocity and reaction time in our experiment was very small (−0.2837 < r < 0.0859). The lack of correlation between saccade peak velocity and reaction have been observed in other tasks (Edelman et al., 2006). For example, repetition induced slowing of saccades produces up to 10% reduction in saccade velocities with little or no changes in reaction time. It is possible that for reflexive saccades, stimulus value more strongly affects saccade velocities as compared to reaction times.
Attention vs. reward
The different image types capture different amounts of attention and this can alter the motivation for the subsequent eye movements. Bindemann and Burton (Bindemann et al., 2007) showed that faces retain more attention than images of other categories (inverted images, objects). The question of whether saccade velocities are modulated because of changing attention or because of an intrinsic reward associated with that image is very difficult to answer (Maunsell, 2004). However, whether the attention or the reward system is engaged, both could translate to a value assigned to the upcoming movement.
Low level differences in the images
Our images were equalized for overall intensity, but not contrast or spatial frequency. This is because normalization for contrast and spatial frequency tends to make the images unrecognizable at the eccentricity that we presented them. Regardless, our experiment attempted to account for this potential confound by making the stimulus that guided the saccades a uniform laser light. That is, the saccade kinematics varied not because of the image on the fovea that elicited the saccade, but because of the memory of an image that would be presented after saccade completion. This may be analogous to the memory of a rewarding piece of food that is expected to be received after completion of a movement.
Although we have not ruled out the possibility that low-level features in visual memory could influence saccade kinematics, there is evidence that high level task demand rather than low level image features modulate saccade kinematics. For example, catch-up saccades during smooth pursuit to bright and dim targets showed similar main sequence relationships while a condition in which the target changed between bright and dim as a form of task feedback actually resulted in faster catch-up saccades (Ebisawa and Suzu, 1995).
Optimal control framework
It is reasonable that our brain should incorporate some concept of value in motor planning. Actions with more social priority such as looking to faces could benefit from being performed faster. Optimal control models incorporate the concept of value (Shadmehr and Krakauer, 2008). In these models, movement duration and velocity depend on the combined effect of two types of cost: a cost associated with the motor commands in which larger commands are penalized because they cause endpoint inaccuracy (encouraging slower movements), and a cost associated with passage of time in which longer duration movements are penalized (encouraging faster movements). The ratio of these two costs determines movement duration. Stimulus value increases the cost of time, encouraging faster movements with shorter duration. It is not enough to model movements simply with the constraint of endpoint variance (Harris and Wolpert, 1998). A much richer set of motor behavior can be explained with the incorporation of value of the action.
Neural correlates of value
Neural signals reflecting value and action selection in the context of eye movements have been found in many brain regions including the basal ganglia (Hikosaka et al., 2006), the posterior cingulated cortex (McCoy et al., 2003), and the amygdale (Belova et al., 2008). These signals can subsequently influence motor output: for example, the basal ganglia has direct projections to the superior colliculus which influences saccade kinematics. These signals are also important in implementing reinforcement learning of the optimal control policy with dopamine as a strong candidate for mediating reward based learning (Schultz et al., 1997;Niv et al., 2007b). Our work here may reflect the optimized behavior of responding with more vigor to biologically salient images.
In summary, our findings suggest that the brain assigns an internal value to our actions, even for low level orienting reflexes that orient the eyes in anticipation of viewing a natural image. Movements which carry more value are executed with more vigor, i.e., faster.
Reference List
- Bahill AT, Clark MR, Stark L. The main sequence: a tool for studying human eye movements. Mathematical Biosciences. 1975;24:191–204. [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-Moment Tracking of State Value in the Amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindemann M, Burton AM, Langton SR, Schweinberger SR, Doherty MJ. The control of attention to faces. J Vis. 2007;7:15–18. doi: 10.1167/7.10.15. [DOI] [PubMed] [Google Scholar]
- Blatter K, Schultz W. Rewarding properties of visual stimuli. Experimental Brain Research. 2006;168:541–546. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- Bray S, O'Doherty J. Neural Coding of Reward-Prediction Error Signals During Classical Conditioning With Attractive Faces. J Neurophysiol. 2007;97:3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Kennard C. Predictive eye saccades are different from visually triggered saccades. Vision Res. 1987;27:517–520. doi: 10.1016/0042-6989(87)90037-x. [DOI] [PubMed] [Google Scholar]
- Cerf M, Harel J, Einhauser W, Koch C. Predicting human gaze using low-level saliency combined with face detection. Advances in Neural Information Processing Systems. 2008;20 [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive Control of Saccades via Internal Feedback. J Neurosci. 2008;28:2804–2813. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Binocular co-ordination of human horizontal saccadic eye movements. J Physiol. 1988;404:157–182. doi: 10.1113/jphysiol.1988.sp017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys Pay Per View: Adaptive Valuation of Social Images by Rhesus Macaques. 2005:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JEW, Overton PG, Redgrave P. How Visual Stimuli Activate Dopaminergic Neurons at Short Latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Ebisawa Y, Suzu K. Focal attentional level while tracking a smoothly moving target influences saccadic dynamics. 1995:1449–1450. [Google Scholar]
- Edelman JA, Valenzuela N, Barton JJ. Antisaccade velocity, but not latency, results from a lack of saccade visual guidance. Vision Res. 2006;46:1411–1421. doi: 10.1016/j.visres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends in Cognitive Sciences. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–144. doi: 10.1111/j.1460-9568.2007.05996.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc Biol Sci. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe M, Ballard D. Eye movements in natural behavior. Trends in Cognitive Sciences. 2005;9:188–194. doi: 10.1016/j.tics.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal Ganglia Orient Eyes to Reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. Brain and Cognition. 2008;68:271–283. doi: 10.1016/j.bandc.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Dolan RJ, Frith U. Psychology: Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Madelain L, Champrenaut L, Chauvin A. Control of sensorimotor variability by consequences. J Neurophysiol. 2007;98:2255–2265. doi: 10.1152/jn.01286.2006. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade Reward Signals in Posterior Cingulate Cortex. Neuron. 2003;40:1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- Milstein DM, Dorris MC. The influence of expected value on saccadic preparation. J Neurosci. 2007;27:4810–4818. doi: 10.1523/JNEUROSCI.0577-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnini A, Chelazzi L. The urgency to look: Prompt saccades to the benefit of perception. Vision Research. 2005;45:3391–3401. doi: 10.1016/j.visres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007a;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007b;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology. 2006;16:R119–R120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Calton JL, Dickinson AR, Lawrence BM. Eye-Hand Coordination: Saccades Are Faster When Accompanied by a Coordinated Arm Movement. J Neurophysiol. 2002;87:2279–2286. doi: 10.1152/jn.00854.2001. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol. 1997;77:874–895. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res. 2002;142:284–291. doi: 10.1007/s00221-001-0928-1. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Siu KC, Walterschied J. Saccadic output is influenced by limb kinetics during eye-hand coordination. J Mot Behav. 2004;36:245–252. doi: 10.3200/JMBR.36.3.245-252. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci. 2003;23:10052–10057. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbus AL. Eye movements during the examination of complicated objects. 1961:52–56. [PubMed] [Google Scholar]