Abstract
INTRODUCTION
Induction of immunity at one mucosal site protects other sites by disseminating sensitized lymphocytes. Parenteral nutrition (PN) reduces gut-associated lymphoid tissue mass and impairs respiratory anti-viral and antibacterial defenses. The effect of PN on lymphocyte mass in the lung is unknown but reduced mucosal lymphocytes were hypothesized to play a role in the reduced IgA-mediated immunity in both gut and lung. Ability to transfer & track cells between mice may allow study of diet induced mucosal immune function.
OBJECTIVE
1) Characterize lung T cell populations following PN feeding and 2) study distribution patterns of transferred donor lung T cells in recipient mice.
METHODS
1) Cannulated male Balb/c mice were randomized to receive chow or PN × 5 days. Lung lymphocytes were obtained via collagenase digestion and flow cytometric analysis identified total T (CD3+) and B (CD45/B220+) cells. In experiment 2, isolated lung T cells from chow-fed male Balb/c mice were pooled, labeled in vitro with CFSE fluorescent dye and 1.1×108 CFSE+ cells (3.1×106 T cells) transferred into chow-fed Balb/c recipients. Cells recovered from recipient lungs and intestinal lamina propria (LP) were analyzed by flow cytometry to determine CFSE/CD3+ T cells at 1, 2 & 7 days. Experiment 3: cells were transferred into PN-fed recipients.
RESULTS
Expt 1: PN significantly decreased lung T and B cell populations compared to chow feeding. Expt 2: No mortality occurred after cell transfer. Time-course analysis showed that CFSE+ T cell retention was highest on day 1 in lung and LP and dropped on day 2. Cells were gone by day 7. 98.1% of retained donor lung T cells migrated to recipient lungs and 1.9% to the intestine on day 1. Similar results were seen in a third experiment with transfer of cells to PN-fed recipients.
CONCLUSIONS
PN reduces pulmonary lymphocyte populations consistent with impaired respiratory immunity. Transferred lung T cells preferentially localize to recipient lungs rather than intestine with maximal accumulation at 24 hrs. Limited ‘cross-talk’ of transferred lung T cells to the intestine indicates mucosal lymphocyte traffic might be programmed to localize to specific effector sites.
Introduction
Nutrition impacts health and recovery from illness. Data also show that route of food delivery is an important variable affecting recovery from critical injury and illness1–6. More specifically, the health and function of the mucosal immune system, the principal arm of the specific immune system to protect mucosal surfaces, seems highly sensitive to alterations in both type and route of nutrition7–9. This relationship is highly relevant in modern clinical medicine as nutrition support is recognized as necessary in the care of hospitalized patients with severe illness or injury. Nosocomial infection at mucosal sites such as the lung remains a constant and ever-increasingly virulent threat.
Clinical studies demonstrate the importance of type and route of nutrition in severely injured trauma patients1–4. In this population, subjects fed enterally, developed significantly fewer infections, specifically pneumonia, than patients receiving parenteral nutrition (PN). One mechanism which explains the disparate infectious outcomes seen with varied type and route of nutrition is PN-induced alterations in the mucosal immune system thereby rendering patients more susceptible to infections at mucosal sites. Experimentally, exclusive parenteral feeding with an isocaloric, isonitrogenous solutions down regulates the mucosal immune system9.
Initially, rat models of sepsis first showed that exclusive parenteral feeding negatively impacted survival to an infectious challenge10–11. More recent work looking at specific effects of type and route of nutrition on mucosal immunity utilized mouse models7–9. In the mouse model, exclusive parenteral feeding (with concurrent lack of enteral stimulation) leads to a multiplicity of changes at both inductive and effector mucosal immune sites. These changes include depletion of multiple factors including lymphocyte populations9, specialized mucosal immune cellular adhesion molecules and molecular signaling molecules within inductive sites such as Peyer’s patches12–15. PN also depletes lymphocyte populations within the intestinal lamina propria effector site and reduces levels of intestinal polymeric immunoglobulin receptor (pIgR), the protein responsible for active transport of IgA across the epithelium to the mucosal surface16. Perhaps the most important and clinically relevant mucosal immune change induced by parenteral feeding is a decrease in luminal IgA levels at the intestinal and respiratory mucosal surfaces9,17. Functionally, PN without enteral stimulation inhibits the ability to generate new pulmonary mucosal immune responses18 or enact previously acquired responses upon antigenic re-challenge with respiratory pathogens19,20. PN significantly decreases survival in animals challenged with respiratory pathogens19.
The current work further defines the effect of parenteral feeding on extra-intestinal mucosal immunity, specifically in regard to pulmonary lymphocyte populations, and lays the groundwork for potential cellular-based studies of PN-induced mucosal immune deficiencies.
Materials and Methods
Animals
The Animal Care and Use Committee at the University of Wisconsin-Madison, and the Middleton Veterans Administration Hospital (VAH) in Madison approved all protocols. Male Balb/c mice (Harlan, Indianapolis, IN) were housed in an American Association for Accreditation of Laboratory Animal Care-accredited facility on the VAH campus. Mice were housed in an environment controlled for temperature (70°F), humidity (20%) and light (12 hour light: dark) and fed ad libitum chow (LabDiet, PMI Nutrition International, St. Louis, MO) and water for 1 week prior to initiation of study protocol. After entry into study protocol, mice were housed individually in metal cages with wire grid floors to prevent coprophagia.
Experimental Design and Feeding Protocols
Experiment 1
Male Balb/c mice aged 11–13 weeks were randomized to Chow or parenteral nutrition (PN). Per experimental protocol, mice were anesthetized and centrally cannulated via the external jugular vein (catheter dimensions= 0.012″ I.D. and 0.025″ O.D.; Helix Medical, Inc., Carpinteria, CA). Catheters were tunneled subcutaneously over the back and exited mid tail. Mice were restrained by the tail, which has been shown not to induce significant physical or biochemical stress.
Immediately following placement the catheters were connected to infusion pumps and the mice recovered for 48 hours while receiving 4 mL/day of 0.9% saline to maintain open lines; mice had free access to food and water during this recovery period. Study diets were initiated after the recovery period. Chow mice received 0.9% saline at 4 mL/day and had free access to chow and water throughout the study. PN mice received solution at 4 mL/day (day 1), 7 mL/day (day 2) and 10 mL/day (days 3 through 5). The PN solution contains 6.0% amino acids, 34.9% dextrose (6002 kJ/L), electrolytes, and multivitamins, with a non-protein calorie/nitrogen ratio of 535.8 kJ/g Nitrogen. PN mice had free access to water only during the study period and were sacrificed after 5 days of feeding for analysis of pulmonary lymphocyte populations.
Experiment 2
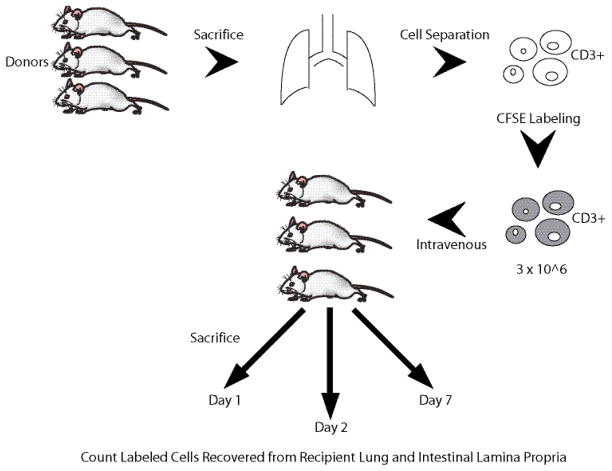
Chow-fed non-cannulated male Balb/c mice served as donors and recipients. Pooled pulmonary lymphocytes (3.1 × 106 T cells) from 4 donors were infused via the retrorbital plexus in anesthetized mice. Recipient mice were sacrificed at 1 (n=3), 2 (n=3), or 7 (n=2) days following cell transfer and the cells recovered (explained below) from the lungs and small intestine and counted (Figure 1).
Figure 1.
Experiment 3
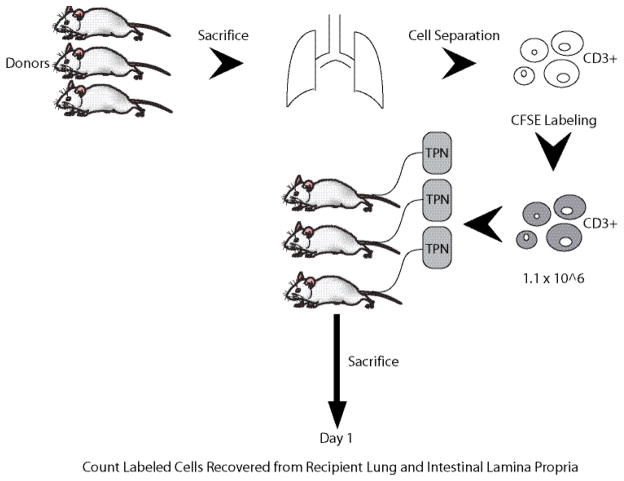
Chow-fed non-cannulated male Balb/c mice served as cell donors. Recipient animals (n=2) were intravenously cannulated and PN fed for 3 days before transcatheter infusion of the labeled cells (1.1 × 106 T cells). All recipients were sacrificed 1 day post-transfer based upon the results of experiment 2 (Figure 2).
Figure 2.
Lung cell Isolations
After 5 days of feeding, mice were anesthetized with a mixture of Ketamine/Acepromazine and exsanguinated by cardiac puncture. The chest was opened and the pulmonary vasculature flushed with phosphate buffered saline (PBS) via the right ventricle. All lung tissue was sharply removed and cut into small pieces. The lung tissue was then stirred and digested for 1h at 37°C in a solution of Dulbecco’s PBS with magnesium and calcium containing the following ingredients: collagenase type 1 (320 U/mL), DNAse type 1 (75 U/mL), heparin (25 U/mL), and β-mercaptoethanol (0.05mM). After 1 h of digestion the slurry was passed through a 40μm mesh. The filtered solution was spun at 1000 rpm for 10 min. The resultant cells were resuspended in 5 mL of Hank’s balanced salt solution and counted
For experiments 2 and 3 involving cell transfer into recipient mice the same isolation procedure was used to obtain donor cells. After counting, the cells were again spun and the pellet was resuspended in cold RPMI to yield a cell concentration of 20 million cells/mL and labeled with CFSE in proportions to yield a final concentration of 5 μM CFSE solution. Cells were incubated with CFSE for 15 min at 37°C and again pelletted and washed once with cold RPMI + 10% fetal bovine serum and again with cold PBS + 0.02% bovine serum albumin. For transfer, cells were resuspended in PBS + 0.02% bovine serum albumin at a concentration of 1 million cells/100μL and injected into the retrorbital venous plexus of isoflurane anesthetized animals in experiment #2 or via the intravenous catheter in awake animals in experiment 3. Harvesting of recipient lung cells to look for CFSE+ cells followed the same method as outlined above.
The following procedure was used to harvest CFSE+ lymphocytes present in the intestinal lamina propria. Following exsanguination the abdomen was opened and the pylorus divided. The entire small intestine was dissected free from the mesentery and removed. Twenty mL of cold HBSS was flushed through the intestinal lumen and the peyer’s patches were removed before the intestine was opened longitudinally and washed three times in a petri dish with cold HBSS. The intestine was cut into 1–2 cm long pieces and incubated with 25mL RPMI with 0.1M EDTA for 20 min at 37°C with constant agitation. The supernatant was discarded and the intestinal pieces were incubated with RPMI + 5% fetal calf serum for 20 min at 37°C with constant agitation. The supernatant was discarded and the intestinal pieces were incubated with 25 mL RPMI+5% FCS containing 320 U/mL collagenase Type I, for 1 hour at 37°C with constant agitation. The resultant slurry was passed through a glass wool column prewashed with HBSS. The cells were then spun at 1000 rpm for 10 min and the pellet resuspended in 2 mL HBSS and counted.
Flow Cytometric Analysis
Isolated lung and intestinal lamina propria cells were labeled with anti-CD45/B220 to label B cells and anti-CD3 to label T cells (antibodies purchased from BD Pharmingen, San Jose, CA). Cells were resuspended to a concentration of 20×106 cells/mL. Cells were incubated with the afore-mentioned labeled antibodies on ice and in the dark for 20 minutes. Cells were then washed twice with 1 mL of staining buffer and resuspended with 250 μL staining buffer + 250 μL 4% formaldehyde and stored in the dark at 4°C until analysis with a BD LSR II (Becton Dicksinson Biosciences, San Jose CA, USA).
Results
Experiment 1 (Table 1)
Table 1.
PN/DES Induced Changes in Pulmonary Lymphocyte Populations
| Group | T Cells (CD3+) | B Cells (CD45+/B220+) |
|---|---|---|
| Chow (n=8) | 8.5 ± 0.4 × 105 | 1.4±0.2 × 106 |
| PN/DES (n=5) | 7.3 ± 0.2 × 105* | 0.7 ± 0.3 × 106* |
Results are mean ± std. dev.
indicates p<0.001 vs. Chow group
Parenteral nutrition for 5 days significantly reduced pulmonary T and B lymphocytes compared to chow fed animals. PN mice had 15% less T cells compared to chow mice (Chow: 8.5 ± 0.4 × 105, PN: 7.3 ± 0.2 × 105, p<0.001) and 50% less B cells compared to chow mice (Chow: 1.4 ± 0.2 × 106, PN: 0.7 ± 0.3 × 106, p<0.001).
Experiment 2 (Table 2)
Table 2.
Cell Recovery from Chow Donor to Chow Recipient Cell Transfer Experiment
| Day 1 | Day 2 | Day 7 | |
|---|---|---|---|
| Transferred Lung T cells Recovered from Recipient Lung | 5.2 ± 0.3 × 104 | 0.6 ± 0.1 × 104 | None detected |
| Transferred Lung T cells Recovered from Recipient Intestine | 1.0 ± 0.7 × 103 | 0.6 ± 0.2 × 103 | 109 ± 25 |
Results are mean ± standard deviation
More transferred/labeled cells were recovered from both the lung and small intestine at day 1 (lung: 5.2 ± 0.3 × 104, small intestine: 1.0 ± 0.7 × 103) than day 2 (lung: 0.6 ± 0.1 × 104, small intestine: 0.6 ± 0.2 × 103). None were detected in the lung at day 7 and very few were recovered from the small intestine (109 ± 25). At day 1, the transferred/labeled cells recovered from the lung represented 4.8% of all lymphocytes (native + transferred) recovered. More transferred cells were recovered from the recipient lungs than from the small intestine at all time points except day 7.
Experiment 3 (Table 3)
Table 3.
Cell Recovery from Chow Donor to PN Recipient Cell Transfer Experiment
| Day 1 | |
|---|---|
| Transferred Lung T cells Recovered from Recipient Lung | 1.2 ± 0.5 × 104 |
| Transferred Lung T cells Recovered from Recipient Intestine | 4.3 ± 4.0 × 103 |
Results are mean ± standard deviation
As in experiment 2, more transferred/labeled cells were recovered from the recipient lung than small intestine. The transferred/labeled cells represented 6.3% of all lymphocytes (native + transferred) recovered from the lung.
Discussion
This series of experiments serves two main purposes. Experiment 1 demonstrates for the first time that route of nutrition reduces pulmonary lymphocyte populations similar to changes observed previously in the murine intestine9. Experiments 2 and 3 show that isolated pulmonary T cells relocalize to the lung for 2 days after administration with little migration to the GI tract. These experiments also demonstrate that mice tolerate these transfers without problem.
Experiment 1 agrees with the overall hypothesis that parenteral feeding and/or decreased enteral stimulation (PN/DES) alters the mucosal immune system. Lymphocyte populations in the lung declined with PN/DES treatment similar to previously observed drops in the Peyer’s patches and intestinal lamina propria9. Overall, the PN/DES induced lymphocyte decrease at least partially explains alterations in mucosal immunity and the decrease in respiratory IgA levels through reduced cells available for IgA production. In addition, in the intestine at least, alterations in cytokines shift the profile from a Th-2 IgA-stimulating cytokine profile to a Th-1 IgA-inhibiting profile21–22. These alterations carry functional consequences in the respiratory tract. Five days of PN/DES decreases survival of mice previously immunized (while chow fed) and resistant to an intra-tracheal bacterial challenge with Pseudomonas.19 Likewise, animals immunized against influenza virus lose that immunity following PN/DES 20. The reduction in pulmonary lymphocytes with loss of IgA, at least partially explain these changes.
There are a number of reasons why cell mass should decrease with PN/DES. First, PN significantly reduces expression of mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) on the high endothelial venules of Peyer’s patches12. MAdCAM-1 is the principal molecule responsible for ushering naive T and B cells into the GALT system for activation and subsequent distribution to intestinal and extra intestinal sites of function23–24. As a result, normal mechanisms to replace cells lost at sites of IgA production are not maintained and overall cell mass drops. Secondly, PN/DES significantly decreases the chemokine CCL-28 within the lung15. CCL-28 is a strong attractant for mucosal antibody producing cells 25. As a result of these lost signals, and probably others, cell populations drop in lamina propria sites of IgA production.
The cell transfer experiments are intriguing. The technique for lymphocyte isolation is relatively simple and is based upon techniques described by Mosley and Klein.26 These techniques have been successfully used previously in our laboratory9 for isolation of intestinal lymphocytes and were adapted for isolation of pulmonary lymphocytes. They demonstrate that transfer of cells is well tolerated by mice with no obvious deleterious clinical effects. Second, cells maximally concentrate in the lung within the first 24 hours with some drop off by 48 hours. All cells disappear by 7 days so that studies of migration and entry into the tissues should be limited to 48 hours. Third, between 4.8% (in chow animals) and 6.3% (in parenterally fed animals) of all infused T lymphocytes were recovered from the recipient lung. In the chow fed animals one would expect that the peripheral sites had a full compliment of cells so that a level of 4.8% “chimerism” seems reasonable. PN/DES mice accumulated approximately 30% more cells than the chow mice consistent with more space (because less native cells present) available for transferred cells. The pulmonary lymphocytes appear to migrate back to the lung with relatively little crossover to the gastrointestinal tract. This suggests limited cross talk once the cells have been sensitized in the Peyer’s patches or once cells have reached their final destination. We were somewhat surprised that intestinal recovery yields in experiment 3 were greater than in experiment 2. However the difference was not an order of magnitude and the variability is likely accounted for by the low n in each experiment (n=2 in experiment 3).
Additional experiments utilizing transfer of cells between parenteral animals (donor and recipient) and from parenteral to chow animals might provide additional indirect information about levels of, and/or functionality of, adhesion molecules on both lymphocytic and stromal cells. Either factor might somehow imprint them to preferentially return there. It is unlikely that the cells were simply cleared by the pulmonary tissue since these single cell suspensions of lymphocytes should easily pass through the pulmonary circulation. We have not examined presence of transferred cells in other organs simply because the functional endpoints In our work are respiratory and gut defenses..
Several important questions regarding this cell transfer process remain to be answered. It remains unknown where the recovered cells come to reside in the lung. Secondly, functionality of the transfused cells is unknown. Determination of the ability of these cells to normalize IgA production and/or generate an immediate functional impact (vs. a lag time to function) by producing cytokines must be studied. Such experiments are a necessary first step in the process to eventually attempt restoration of mucosal immunty in parenterally fed animals via transfer of chow cells.
Theoretically, altering the route of cellular transfer between experiments 2 and 3 introduced another variable. Experiment 2 was designed only to test recovery and labeling techniques for which we could not justify a survival surgical procedure such as central venous cannulation prior to the infusion. However, intravenous injection via the retrorbital plexus is a widely accepted technique. In experiment 3, the intravenous cannulation needed for parenteral nutrition provided intravenous access which permitted cellular transfer without the additional anestehetic required for retrobulbar injection. Theoretically this difference could affect the results possibly through cellular adhesion to the tubing but this did not justify additional invasive procedures in these preliminary experiments. Final experiments to test function of transferred cells will use cannulated mice to standardize the route of administration.
In summary, these experiments show the feasibility of gathering, labeling, transferring, and subsequently retrieving viable lymphocytes in an inbred mouse model. The work shows for the first time that PN detrimentally affects lung T & B cell populations similar to PN induced changes in the gastrointestinal tract. It confirms the relationship between type and route of nutrition and the mucosal immune system in both intestinal and extra intestinal tissues. Clearly, parenteral feeding with lack of enteral stimulation plays a significant factor in increasing susceptibility to infection. These cell transfer techniques provide investigative tools to better understand the mechanisms underlying mucosal immune disability associated with parenteral feeding and decreased enteral stimulation.
Acknowledgments
NIH Grant R01 GM53439
This material is also based upon work supported in part by the Office of Research and Development, Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Footnotes
This work originally presented February 12, 2008 at the American Society of Parenteral and Enteral Nutrition (A.S.P.E.N.)/Clinical Nutrition Week in Chicago, IL, USA.
References
- 1.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–11. doi: 10.1097/00000658-199205000-00013. discussion 511–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma--a prospective, randomized study. J Trauma. 1986;26:874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29:916–22. doi: 10.1097/00005373-198907000-00003. discussion 922–3. [DOI] [PubMed] [Google Scholar]
- 4.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazuki T, Ebisawa Enteral versus parenteral nutrition after gastrointestinal surgery: a systematic review and meta-analysis of randomized controlled trials in the English literature. J Gastrointest Surgery. 2008;12:739–755. doi: 10.1007/s11605-007-0362-1. [DOI] [PubMed] [Google Scholar]
- 6.Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 8.Kang W, Kudsk KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr. 2007;31:246–258. doi: 10.1177/0148607107031003246. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–51. doi: 10.1097/00005373-199507000-00006. discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 10.Kudsk KA, Carpenter BS, Peterson S, Sheldon GF. Effect of Enteral and Parenteral Feeding in Malnourished Rats with E. coli-Hemoglobin Adjuvant Peritonitis. J Surg Res. 1981;31:105–110. doi: 10.1016/0022-4804(81)90037-8. [DOI] [PubMed] [Google Scholar]
- 11.Kudsk KA, Stone JM, Carpenter BA, Sheldon GF. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma. 1983;23:605–609. doi: 10.1097/00005373-198307000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Zarzaur BL, Fukatsu K, Johnson CJ, et al. A temporal study in diet induced changes in Peyer patch MAdCAM-1 expression. Surg Forum. 2001;52:194–196. [Google Scholar]
- 13.Kang W, Gomez FE, Lan J, et al. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg. 2006;244:392–399. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez FE, Lan J, Kang W, et al. Parenteral nutrition and fasting reduces mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) mRNA in Peyer’s patches of mice. JPEN J Parenter Enteral Nutr. 2007;31:47–52. doi: 10.1177/014860710703100147. [DOI] [PubMed] [Google Scholar]
- 15.Hermsen JL, Gomez FE, Maeshima Y, Sano Y, Kang W, Kudsk KA. Decreased Enteral Stimulation Alters Mucosal Immune Chemokines. J Parenter Enteral Nutr. 2008;32(1):36–44. doi: 10.1177/014860710803200136. [DOI] [PubMed] [Google Scholar]
- 16.Sano Y, Gomez FE, Kang W, Lan J, Maeshima YL, Hermsen JL, Ueno C, Kudsk KA. Intestinal Polymeric Immunoglobulin Receptor is Affected by Type and Route of Nutrition. JPEN. 2007;31(5):351–357. doi: 10.1177/0148607107031005351. [DOI] [PubMed] [Google Scholar]
- 17.Renegar KB, Kudsk KA, DeWitt RC, Wu Y, King BK. Impairment of mucosal immunity by parenteral nutrition: Depressed nasotracheal influenza-specific secretory IgA levels and transport of parenterally fed mice. Ann Surg. 2001;233(1):134–138. doi: 10.1097/00000658-200101000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson CD, Kudsk KA, Fukatsu K, et al. Route of nutrition influences generation of antibody-forming cells and initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003;237:565–573. doi: 10.1097/01.SLA.0000059991.89316.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999;229:272–278. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223(6):629–638. doi: 10.1097/00000658-199606000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–7. doi: 10.1097/00000658-199905000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukatsu K, Kudsk KA, Wu Y, Zarzaur BL, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–322. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 23.Butcher EC. Lymphocyte homing to mucosal effector sites. In: Ogra PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, editors. Handbook of Mucosal Immunology. San Diego: Academic Press, Inc; 1994. pp. 507–522. [Google Scholar]
- 24.Connor EM, Eppihimer MJ, Morise Z, et al. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 25.Hieshima K, Kawasaki Y, Hanamoto H, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–3675. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- 26.Mosley RL, Klein JR. A rapid method for isolating murine intraepithelial lymphocytes with high yield and purity. J Immunol Methods. 1992;156:19–26. doi: 10.1016/0022-1759(92)90006-f. [DOI] [PubMed] [Google Scholar]