Abstract
Aim
Our aim was to investigate muscle architecture and size of the rectus femoris (RF) and vastus lateralis (VL) in children and adolescents with cerebral palsy (CP) compared with age-matched typically developing participants.
Method
Muscle architecture and size were measured with ultrasound imaging in 18 participants with spastic CP (9 females, 9 males; age range 7.5–19y; mean age 12y [SD 3y 2mo]) within Gross Motor Function Classification System levels I (n=4), II (n=2), III (n=9), and IV (n=3) and 12 typically developing participants (10 females, 2 males; age range 7–20y; mean age 12y 4mo [SD 3y 11mo]). Exclusion criteria were orthopedic surgery or neurosurgery within 6 months before testing or botulinum toxin injections to the quadriceps within 3 months before testing.
Results
RF cross-sectional area was significantly lower (48%), RF and VL muscle thickness 30% lower, RF fascicle length 27% lower, and VL fascicle angle 3° less in participants with CP compared to the typically developing participants (p<0.05). Intraclass correlation coefficients were ≥ 0.93 (CP) and ≥ 0.88 (typical development), indicating excellent reliability.
Interpretation
These results provide the first evidence of altered muscle architecture and size of the RF and VL in CP, similar to patterns observed with disuse and aging. These alterations may play a significant role in the decreased capacity for force generation as well as decreased shortening velocity and range of motion over which the quadriceps can act.
Children with cerebral palsy (CP) have one of the most sedentary lifestyles across pediatric disabilities.1 Decreased activity leads to weakness, disuse muscle atrophy, and muscle shortening, which can further limit activity. The cycle of inactivity and disuse in children and adolescents with CP is often perpetuated by progressive development of contractures and weakness (atrophy), leading to further declines in activity and worsening disability over the lifespan.2–4 Because the function of a muscle is reflected in its structure, it would be reasonable to expect that muscle structure would be altered in CP in a manner that would parallel these secondary impairments. Muscle architecture refers to the arrangement of muscle fibers within a muscle, or bundles of fibers known as fascicles, and includes fascicle lengths and fascicle angles. Muscle fiber or fascicle length is proportional to the maximal excursion of the muscle and velocity of contraction and is indicative of the number of sarcomeres in series. Measures of muscle size, particularly physiological cross-sectional area, are directly proportional to the maximum force-generating capacity of the muscle and the number of sarcomeres in parallel.5
Fascicle or pennation angle is the positive angle between muscle fibers and the aponeurosis of the muscle. The larger the fascicle angle, the more contractile material can be packed within a set volume, thereby increasing the muscle’s capacity to produce force.6
The classic work of Williams and Goldspink in animal models clearly demonstrates that immobilization of muscle in shortened positions results in a decrease in the number of sarcomeres in series.8 Similarly, human models of disuse such as unloading,9,10 bed rest,11,12 and even aging13 result in decreases in muscle size, fascicle length, and fascicle angles, indicating a loss of sarcomeres in series and in parallel.5 Although it is plausible that children and adolescents with CP would demonstrate similar alterations in muscle architecture and size secondary to disuse, immobilization, and lower activity levels, research in this area is very limited. Until recently, measurement of muscle architecture required highly invasive techniques, such as intraoperative measurements or whole-muscle dissections in human cadavers.5,14 With the advent of musculoskeletal ultrasound imaging, muscle architecture can be measured directly and non-invasively. Research on muscle architecture in CP has been focused primarily on the gastrocnemius muscle. Results are inconclusive, with reports of decreased fascicle lengths15–17 and angles18 as well as no differences18–20 in CP compared with control groups or the uninvolved side in the case of hemiplegia. Some of the inconsistencies have been attributed to whether absolute versus fascicle lengths normalized to fibular length were reported. Ohata et al.21 measured combined rectus femoris (RF) and vastus intermedius muscle thickness as a measure of quadriceps thickness in a group of adults with CP who mostly had severe motor impairment. However, comparisons were not made with a typically developing or control population.
The quadriceps and gastrocnemius are the primary muscles affected by disuse and unloading secondary to their antigravity nature.10,11 However, no data exist on thickness, cross-sectional area, or muscle architectural parameters, such as fascicle length and angle, of individual quadriceps muscles in CP. Therefore, the purpose of this prospective study was to compare muscle architecture and size of the RF and vastus lateralis (VL) in children and adolescents with CP versus those who are typically developing, to determine whether these parameters differ in CP in a similar pattern to that observed with disuse. We hypothesized that children and adolescents with CP would have decreased fascicle lengths and angles, as well as decreased measures of muscle size compared with those who were typically developing. A secondary purpose was to examine the intrasession reliability of the measures.
METHOD
Participants
Eighteen participants with spastic CP (age range 7.5–19y; mean age 12y [SD 3y 2mo]) and 12 age-matched typical developing participants (age range 7–20y; mean age 12y 4mo [SD 3y 11mo]) were recruited for this prospective study. The participant characteristics are summarized in Table I. Participants with CP were in Gross Motor Function Classification System (GMFCS) levels I to IV and had bilateral lower-extremity involvement. Exclusion criteria were orthopedic surgery or neurosurgery within 6 months before testing or botulinum toxin injections to the quadriceps within 3 months before testing. The rationale was that these interventions could result in structural changes within the muscle, such as changes in muscle volume and length, which have been reported after gastrocnemius recession.22 The study was approved by the institutional review board at Washington University in St Louis, Missouri, USA. Written consent was obtained from each participant over 18 years of age, and parental consent was obtained for children under 18 years.
Table I.
Characteristics of the children and adolescents with cerebral palsy (CP) participating in the study
| Diagnosis | Sex | Age | Body mass index, kg/m2 |
Femur length, cma |
Resting angle, ° |
Knee extension passive range of motion, ° |
Gross Motor Function Classification System level |
|---|---|---|---|---|---|---|---|
| Diplegia | F | 11y 4mo | 16.9 | 27.2 | 15 | − 18 | III |
| Diplegia | F | 15y 1mo | 18.1 | 34.0 | 28 | − 28 | III |
| Quadriplegia | F | 19y 1mo | 19.8 | 35.2 | 20 | − 20 | IV |
| Diplegia | M | 10y | 22.6 | 35.0 | 14 | − 14 | III |
| Diplegia | F | 7y 6mo | 17.9 | 25.2 | 6 | 0 | III |
| Diplegia | M | 7y 5mo | 20.0 | 29.4 | 9 | 0 | I |
| Triplegia | M | 11y 6mo | 15.6 | 31.0 | 20 | − 18 | IV |
| Diplegia | M | 13y | 19.3 | 29.2 | 22 | − 16 | III |
| Diplegia | M | 8y 1mo | 17.1 | 27.2 | 16 | − 10 | III |
| Triplegia | F | 10y 5mo | 13.6 | 28.5 | 22 | − 4 | II |
| Triplegia | M | 11y 8mo | 23.7 | 33.7 | 15 | − 12 | III |
| Quadriplegia | M | 9y 6mo | 15.0 | 30.0 | 15 | − 12 | IV |
| Diplegia | M | 10y 8mo | 12.9 | 32.4 | 8 | 0 | I |
| Diplegia | F | 13y 9mo | 16.4 | 37.4 | 10 | − 8 | III |
| Diplegia | F | 10y 9mo | 14.5 | 33.0 | 12 | − 12 | II |
| Diplegia | F | 14y 5mo | 18.2 | 37.5 | 6 | − 4 | I |
| Diplegia | F | 16y | 22.0 | 42.0 | 7 | + 4 | I |
| Quadriplegia | M | 15y 5mo | 19.5 | 40.0 | 26 | − 20 | III |
|
| |||||||
| CP group, mean (SD) |
9F, 9M |
12y (3y 2mo) | 17.9 (3.1) | 32.7 (4.7) | 15.1 (6.8)b | − 10.7 (8.7)b | I=4 II=2 III=9 IV=3 |
|
| |||||||
| Typically developing group, mean (SD) |
10F, 2M |
12y 4mo (3y 11mo) |
18.9 (3.0) | 36.0 (4.5) | 7.8 (1.8) | + 2.6 (3.3) | NA |
Femur length calculated as greater trochanter to lateral femoral condyle distance.
significant differences between groups (p<0.05). + indicates hyperextension; − indicates lack of full extension; F, female; M, male; NA, not applicable.
Procedures
Real-time ultrasound imaging (Voluson 730 Expert, GE Healthcare, Kretztechnik, Zipf, Austria) was used to examine muscle architecture and size of the RF and VL. The right lower extremity was chosen for measurement. Participants were supine with knees resting comfortably in extension near the natural resting position of 10 degrees. A towel roll or small pillow under the knee was used in some cases for positioning or to aid comfort and muscle relaxation. The resting angle of the knee was measured and recorded with a goniometer. Group means are listed in Table I. Participants were instructed to relax their muscles during scanning. When muscle contraction did occur, it was easily detected in real-time, and the image was discarded. RF images were taken at 50% of the distance between the anterior superior iliac spine and the superior border of the patella. VL images were taken at the midpoint between the most prominent portion of the greater trochanter and lateral femoral epicondyle. Each midpoint was clearly marked on the skin with a surgical pen to ensure proper placement of the probe across repeated scans. A generous amount of gel was applied to the skin to aid acoustic coupling and to eliminate compression or deformation of the muscle. Two-dimensional B-mode ultrasound imaging with a 6–12 MHz linear array transducer was used to measure (1) muscle thickness as the distance between the superficial and deep aponeuroses in the middle of the ultrasound image at a 90° angle from the deep aponeurosis; (2) fascicle angle as the positive angle between the deep aponeurosis and the line of the fascicle; and (3) fascicle length estimated as muscle thickness divided by the sine of the fascicle angle (Fig. 1).23 Images were taken with the probe oriented in the sagittal plane and perpendicular to the skin. Anatomical cross-sectional area of the RF was measured in three-dimensional volume mode with the probe oriented in the transverse plane (Fig. 2). Cross-sectional area of the VL was not measured because of the orientation of the muscle and limitations in transducer size. Three images were taken per muscle, and the average value from the three images was used for analysis. Group means were then calculated and independent t-tests were used to compare means between groups (two-tailed; α-level = 0.05).
Figure 1.
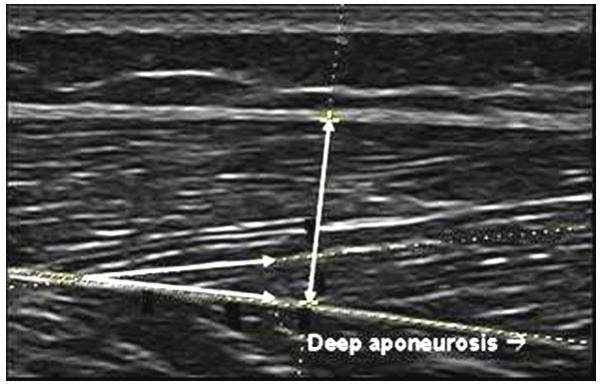
Muscle thickness was calculated as the distance between superficial and deep aponeuroses in the middle of the ultrasound image at a 90° angle from the deep aponeurosis as indicated by the vertical line. Fascicle angle was the positive angle between the deep aponeurosis and the line of the fascicle as indicated by the intersecting lines.
Figure 2.
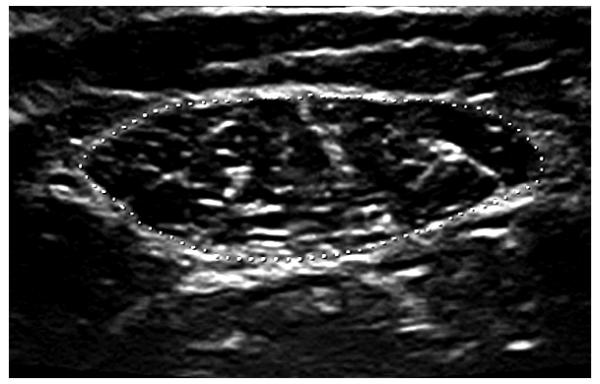
The muscle was traced along the inner edge of the muscle fascia (dotted line) to calculate the cross-sectional area.
Reliability
Intrasession reliability of ultrasound measurements were taken across three separate images using the intraclass correlation coefficient (ICC) Shrout and Fleiss24 model 3 for all participants in the study. A single examiner scanned all images, and another independent examiner performed all measurements.
RESULTS
The two groups did not significantly differ by age, body mass index, or femur length (p>0.05). Children and adolescents with CP had greater knee resting angles and less knee extension range of motion than those who were typically developing (p<0.05). We were unable to obtain measurement of cross-sectional area of the RF in four typically developing participants because of inability to visualize the outer medial or lateral borders of the muscle within the scan window. Cross-sectional area of the RF was 48.5% lower in CP than in typically developing participants (t=5.32; p<0.001). Significant differences in muscle thickness were observed between the two groups for both the RF (t=5.01; p<0.001) and VL (t=2.90; p=0.007) with a 32% and 31% decrease respectively, in the group with CP. Fascicle length of the RF was decreased by 27% in the CP group compared with typically developing participants (t=3.25; p=0.003). Fascicle angle of the VL was on average 3° less in CP (t=2.75; p=0.010). No group differences in VL fascicle length (t=0.48; p=0.638) and RF fascicle angle (t=0.77; p=0.450) were observed. Group means and SDs and effect sizes are listed in Table II.
Table II.
Differences between groups in measures of muscle architecture and size
| Cerebral palsy | Typically developing | p | Effect size (Cohen’s d) |
|
|---|---|---|---|---|
| RF cross-sectional area, cm2a | 3.91 (1.51) | 7.58 (1.88) | 0.001 | 2.2 |
| RF muscle thickness, cm | 1.36 (0.38) | 2.01 (0.30) | 0.001 | 1.9 |
| RF fascicle length, cm | 7.08 (2.14) | 9.75 (2.29) | 0.003 | 1.2 |
| RF fascicle angle, ° | 11.58 (3.18) | 12.51 (3.36) | 0.450 | 0.3 |
| VL muscle thickness, cm | 1.22 (0.44) | 1.78 (0.61) | 0.007 | 1.1 |
| VL fascicle length, cm | 7.19 (2.24) | 7.52 (1.20) | 0.638 | 0.2 |
| VL fascicle angle, ° | 10.25 (3.38) | 13.54 (2.94) | 0.010 | 1.0 |
Data are provided as means (SD).
n=8 for the typically developing group; otherwise n=12 for this group. RF, rectus femoris; VL, vastus lateralis.
ICC values are listed in Table III. ICC values across three separate images were ≥0.93 for all measures in participants with CP and ≥0.88 in typically developing participants. ICC values of muscle thickness and cross-sectional area were ≥0.98 for both groups.
Table III.
Intrasession reliability coefficients
| ICC (3,k)a |
||
|---|---|---|
| Typically developing | Cerebral palsy | |
| RF cross-sectional area, cm2 | 0.99 (0.99–1.00) | 0.99 (0.98–1.00) |
| RF muscle thickness, cm | 0.98 (0.94–0.99) | 0.99 (0.98–1.00) |
| RF fascicle length, cm | 0.98 (0.95–0.99) | 0.96 (0.91–0.98) |
| RF fascicle angle, ° | 0.95 (0.78–0.99) | 0.97 (0.93–0.99) |
| VL muscle thickness, cm | 0.99 (0.97–1.00) | 0.99 (0.97–0.99) |
| VL fascicle length, cm | 0.88 (0.68–0.96) | 0.93 (0.84–0.97) |
| VL fascicle angle, ° | 0.96 (0.88–0.99) | 0.97 (0.93–0.99) |
Data are intraclass correlation coefficients (ICCs) with 95% confidence intervals.
ICC model (3,2) used for fascicle length and angles as average of two measurements; ICC model (3,1) used for cross-sectional area and muscle-thickness measurements. RF, rectus femoris; VL, vastus lateralis.
DISCUSSION
Muscle thickness, cross-sectional area, and fascicle length of the RF were observed to be decreased in this group of children and adolescents with CP compared with those who were typically developing, suggesting a loss of sarcomeres in parallel and in series. In contrast, muscle thickness and fascicle angle were decreased in the VL, suggesting a loss of sarcomeres in parallel only. To the best of our knowledge, the results of this study provide the first description of muscle architecture and size of the RF and VL in children and adolescents with CP. More importantly, effect sizes were large (Cohen’s d=1.0–2.2) where significant differences were observed (see Table II). Our results also demonstrate that ultrasound measures of muscle architecture and size of the RF and VL in CP are highly reliable across repeated scans within a single session using the described technique. This is noteworthy because ultrasound imaging provides a less invasive and lower-cost alternative to other methods, such as biopsies and magnetic resonance imaging, and it can be readily used in both clinical and laboratory settings.
We now know that muscles are one of the most plastic tissues in the body, adapting to various negative and positive stimuli, such as disuse, immobilization, and increased activity. Decreased fascicle lengths and angles as well as muscle atrophy have been observed in response to disuse and even in response to aging.9–13 The results of the present study provide support for our hypothesis that muscle architecture and size in CP are similar to patterns observed with disuse, with evidence of decreased muscle size and fascicle length in the RF and decreased muscle size and fascicle angle in the VL. The similar findings in our study suggest that this may be the result of disuse from lower activity and mobility levels. In fact, studies over the past 15 years have documented progressive deterioration of functional skills and ambulation in CP, often beginning in adolescence and early adulthood.3,4 Because of the heterogeneity in CP and multitude of secondary impairments and comorbidities, a cause-and-effect relationship is speculative at this point.
The differences observed between the RF and VL in the group with CP compared with the typically developing participants appear to reflect the inherent functionality and morphology that is specific to each muscle. The RF is a biarticular, bipennate, strap-like muscle that is reported to have a larger ratio of fiber length to cross-sectional area than the other quadriceps muscles.5 Therefore, the architecture of the RF appears to be suited for greater velocity and displacement than the VL. The shorter fascicle lengths of the RF in our participants with CP could contribute to both decreased range of motion and velocity of the knee during gait. Preliminary evidence for this has been presented in abstract form.25 In contrast, the VL is a uniarticular, unipennate muscle that is reported to have a much larger physiological cross-sectional area than the RF,5 indicating that the primary role of this muscle is for force production. It has been suggested that an increase in fascicle angle allows more contractile material to be attached to the tendon or aponeurosis, resulting in a high correlation with measures of muscle thickness and cross-sectional area.6,26 Therefore, the decrease in fascicle angle and muscle thickness of the VL observed in our participants with CP indicates a loss of sarcomeres in parallel, which directly influences force production.
The issue of normalization of fascicle length must be considered in studies comparing different groups. The assumption underlying normalization is that there is a significant correlation between two variables, in this case fibular length and fascicle length. However, this issue remains controversial.16 In our study, femur length did not have a significant relationship to fascicle length in either muscle for the group with CP (r=0.19–0.29; p>0.05) nor the typically developing group (r=0.41–0.53; p>0.05). Furthermore, our two comparison groups had similar femur lengths and were of similar age and body mass index, thus negating the need for normalization.
Although it is assumed that muscles are relatively smaller in children with CP, surprisingly few reports have been published. Elder et al.27 reported decreased anatomical cross-sectional area of the gastroc-soleus obtained through magnetic resonance imaging in the affected side compared with the unaffected side of children with hemiplegic CP. Likewise, Mohagheghi et al.15 reported decreased muscle thickness of the gastrocnemius in children with hemiplegic CP. Ohata et al.21 reported decreased muscle thickness of the RF combined with the vastus intermedius in adults with CP with severe motor involvement (GMFCS level V) compared with those in GMFCS level III. However, comparisons were not made with a control population, nor were individual muscles measured. In addition to measures of muscle architecture, our study is the first to document decreased muscle thickness of the RF and VL as well as decreased cross-sectional area of the RF in children and adolescents with CP compared with a typically developing control group.
For participants with CP, measures of muscle size of the RF (cross-sectional area and muscle thickness) and VL (muscle thickness) had excellent reliability, with ICC values of 0.99. ICC values for fascicle angle and lengths for both muscles were also excellent (0.93–0.97). In the study by Mohagheghi et al.,15 ICC values for the non-paretic and paretic gastrocnemius of children with hemiplegic CP were 0.81 and 0.91 for fascicle length, 0.88 and 0.85 for fascicle angle, and 0.93 and 0.94 for muscle thickness respectively. Compared with these data, our reliability coefficients were higher for the RF and VL and also comparable to the ICC values of 0.98 reported for muscle thickness of the quadriceps in adults with more involved CP.21 In addition, ICC values from the present study were comparable to values reported from repeated measures of muscle thickness, fascicle angles, and fascicle length of the VL in healthy adults, which ranged between 0.95 and 0.99.23
Limitations
There are several limitations of this study. First, we performed measurements at only one standardized site per muscle, and it has been shown that muscle architecture can vary throughout its length.26 Therefore, these measures may not represent the architecture of the entire RF and VL. Second, we performed all measurements at the resting angle of the knee for each participant. The differences in resting angles and passive limitations in knee extension in some participants with CP could have contributed to the differences in muscle architecture. The presence of knee flexion contractures was not excluded here, in an effort to be more inclusive in terms of age and level of involvement. Even with this limitation, we felt that using the resting angle was the most objective way to compare groups, because it has been shown that comparison of a standardized common angle requires shifting of the data relative to each participant’s resting angle for a more accurate comparison as a result of differences in total range of motion.17,20 Sex differences between groups (more females in the typically developing group), broad age range, and inherent heterogeneity of the CP population may have also contributed to an underestimation in the magnitude of the differences between groups. Perhaps a larger sample size would have provided greater power to detect group differences.
Clinical significance
The clinical implications of these results are noteworthy, because muscle architecture and size are the primary determinants of muscle function.14 Muscles with longer fascicle lengths and thus more sarcomeres in series can generate forces at higher shortening velocities and over a larger range of motion. Our results suggest that shorter fascicle lengths of the RF in children with CP may contribute to decreased muscle power, the product of force generation and velocity of contraction, as well as the range of motion over which the muscle can act during functional activities, such as gait. Perhaps power training or training for velocity of movement should be an important component of a strengthening program for the quadriceps in people with CP. Muscle size is directly proportional to the number of sarcomeres in parallel and, thus, maximum force generation. Therefore, the muscle atrophy observed in both muscles and the decreased fascicle angles of the VL may be contributing factors to the decreased force production of the quadriceps observed in CP.28 Future studies should be aimed at investigating the relationships between muscle function and muscle architecture and size in CP in order to better understand the physiological mechanisms underlying abnormal force production in CP, as well as to provide a rationale for new surgical and non-surgical interventions. In addition, the effect of decreased fascicle lengths of the RF on knee range of motion and velocity during gait should be examined further.
Conclusion
These results provide the first evidence of altered muscle architecture and size of the RF and VL in children and adolescents with CP, similar to patterns observed with disuse and aging. Decreased muscle size and fascicle lengths of the RF suggest a loss of sarcomeres in series (fiber shortening) and in parallel (muscle atrophy), whereas the decreased muscle size and fascicle angles of the VL indicate a loss of sarcomeres in parallel only. It is believed that these alterations are specific to the functional roles of each muscle and may play a significant role in the decreased capacity for force generation as well as decreased velocity of movement and range of motion over which the muscle can exert force. Furthermore, reliability coefficients were high, indicating that muscle architecture and size of the RF and VL can be reliably measured with ultrasound imaging. Lastly, these results highlight the importance of promoting activity and physical fitness in children with CP to counteract the accelerated loss of physical function, often beginning in adolescence and early adulthood.
ACKNOWLEDGMENTS
Dr Noelle Moreau was a postdoctoral fellow in the Movement Science Program at Washington University in St Louis, MO, USA, when this study was conducted and was supported by a National Center for Medical Rehabilitation Research/National Institutes of Health grant (T32HD007434-16). This project was also supported by a clinical research grant from the Section on Pediatrics of the American Physical Therapy Association to Dr Moreau. The authors would like to thank Chris Stanley for assistance with data collection and processing, and Dr Janice Brunstrom and Jennifer Miros PT, for their support and assistance with recruitment. We would also like to thank GE Healthcare and Tania Gordley, Applications Specialist for GE Healthcare Ultrasound, for their support and technical assistance with this project.
LIST OF ABBREVIATIONS
- RF
Rectus femoris
- VL
Vastus lateralis
References
- 1.Longmuir PE, Bar-Or O. Factors influencing the physical activity levels of youths with physical and sensory disabilities. Adapt Phys Activ Q. 2000;17:40–53. [Google Scholar]
- 2.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–40. doi: 10.2522/ptj.20050397. [DOI] [PubMed] [Google Scholar]
- 3.Bottos M, Feliciangeli A, Sciuto L, Gericke C, Vianello A. Functional status of adults with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. 2001;43:516–28. doi: 10.1017/s0012162201000950. [DOI] [PubMed] [Google Scholar]
- 4.Murphy KP, Molnar GE, Lankasky K. Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol. 1995;37:1075–84. doi: 10.1111/j.1469-8749.1995.tb11968.x. [DOI] [PubMed] [Google Scholar]
- 5.Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;179:275–83. [PubMed] [Google Scholar]
- 6.Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–44. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- 7.Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972;224:231–44. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116:45–55. [PMC free article] [PubMed] [Google Scholar]
- 9.Seynnes OR, Maganaris CN, de Boer MD, di Prampero PE, Narici MV. Early structural adaptations to unloading in the human calf muscles. Acta Physiol (Oxf) 2008;193:265–74. doi: 10.1111/j.1748-1716.2008.01842.x. [DOI] [PubMed] [Google Scholar]
- 10.de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–91. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer MD, Seynnes OR, di Prampero PE, et al. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol. 2008;104:401–07. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- 12.Reeves NJ, Maganaris CN, Ferretti G, Narici MV. Influence of simulated microgravity on human skeletal muscle architecture and function. J Gravit Physiol. 2002;9:153–54. [PubMed] [Google Scholar]
- 13.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol. 2003;95:2229–34. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–66. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. Differences in gastrocnemius muscle architecture between the paretic and non-paretic legs in children with hemiplegic cerebral palsy. Clin Biomech (Bristol, Avon) 2007;22:718–24. doi: 10.1016/j.clinbiomech.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. In vivo gastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev Med Child Neurol. 2008;50:44–50. doi: 10.1111/j.1469-8749.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheatwood AP. Changes in medial gastrocnemius architecture with spasticity and contracture. (Thesis) University of Southern California; 1995. [Google Scholar]
- 18.Malaiya R, McNee AE, Fry NR, Eve LC, Gough M, Shortland AP. The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J Electromyogr Kinesiol. 2007;17:657–63. doi: 10.1016/j.jelekin.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Shortland AP, Fry NR, Eve LC, Gough M. Changes to medial gastrocnemius architecture after surgical intervention in spastic diplegia. Dev Med Child Neurol. 2004;46:667–73. doi: 10.1017/s0012162204001124. [DOI] [PubMed] [Google Scholar]
- 20.Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol. 2002;44:158–63. doi: 10.1017/s0012162201001864. [DOI] [PubMed] [Google Scholar]
- 21.Ohata K, Tsuboyama T, Ichihashi N, Minami S. Measurement of muscle thickness as quantitative muscle evaluation for adults with severe cerebral palsy. Phys Ther. 2006;86:1231–39. doi: 10.2522/ptj.20050189. [DOI] [PubMed] [Google Scholar]
- 22.Fry NR, Gough M, McNee AE, Shortland AP. Changes in the volume and length of the medial gastrocnemius after surgical recession in children with spastic diplegic cerebral palsy. J Pediatr Orthop. 2007;27:769–74. doi: 10.1097/BPO.0b013e3181558943. [DOI] [PubMed] [Google Scholar]
- 23.Alegre LM, Jiménez F, Gonzalo-Orden JM, Martín-Acero R, Aguado X. Effects of dynamic resistance training on fascicle length and isometric strength. J Sports Sci. 2006;24:501–08. doi: 10.1080/02640410500189322. [DOI] [PubMed] [Google Scholar]
- 24.Shrout PE, Fleiss JL. Intraclass correlation: uses in assessing rater reliability. Psychol Bull. 1979;86:420–28. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 25.Moreau NG, Stanley C, Teefey S, Damiano DL. Rectus femoris fascicle length is related to dynamic measures of knee excursion during gait in cerebral palsy. Presented at the 13th Annual Meeting of the Gait and Clinical Movement Analysis Society; 2008. [Google Scholar]
- 26.Blazevich AJ, Gill ND, Zhou S. Intra- and intermuscular variation in human quadriceps femoris architecture assessed in vivo. J Anat. 2006;209:289–310. doi: 10.1111/j.1469-7580.2006.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elder GC, Kirk J, Stewart G, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–50. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- 28.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–07. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]


