Abstract
Children prenatally exposed to tobacco have been found to exhibit increased rates of behavior problems related to response inhibition deficits. The present study compared the brain function of tobacco-exposed (n = 7) and unexposed (n = 11) 12-year-olds during a Go/No-Go response inhibition task using an event-related functional MRI (fMRI) design. Prenatal alcohol exposure, neonatal medical problems, environmental risk, IQ, current environmental smoke exposure, and handedness were statistically controlled. Tobacco-exposed children showed greater activation in a relatively large and diverse set of regions, including left frontal, right occipital, and bilateral temporal, and parietal regions. In contrast, unexposed but not exposed children showed activation in the cerebellum, which prior research has indicated is important for attention and motor preparation. The diversity of regions showing greater activation among tobacco-exposed children suggests that their brain function is characterized by an inefficient recruitment of regions required for response inhibition.
Keywords: Cocaine, Executive Functioning, Functional Magnetic Resonance Imaging, Prenatal Exposure, Response Inhibition
1. Introduction
The prevalence rate of women who smoke cigarettes during pregnancy in the United States has decreased in recent years, yet 17% of mothers still report smoking while pregnant[66]. Subsequently, many children are exposed to nicotine, which is believed to be the main neurobehavioral teratogen in tobacco. Prenatal nicotine exposure reduces cholinergic neural transmission, elicits neural cell death, alters cell proliferation and differentiation, and damages serotonergic projections in the cerebral cortex[63, 76]. Children prenatally exposed to tobacco exhibit more behavior problems than their unexposed peers, including attention-deficit hyperactivity disorder (ADHD), antisocial behavior, and impulsive aggression[11, 12, 24, 51, 68, 71]. Such behavior problems typically involve response inhibition deficits[4, 44, 58], which tobacco exposed children have demonstrated[31, 36]. The inability to withhold a preplanned response may lead to greater distractibility and behavioral dyscontrol, negatively affecting behavioral adjustment[67]. Thus, children prenatally exposed to tobacco may exhibit higher rates of behavior problems due to response inhibition deficits.
The relations found between tobacco exposure and both brain development and behaviors associated with poor response inhibition suggests that tobacco exposure may be associated with differential brain function during response inhibition. Multiple brain regions, including the anterior cingulate, posterior cingulate, medial frontal, inferior frontal, superior frontal, superior parietal, superior temporal, and precentral gyrus appear to be important for response inhibition, which is often assessed using a Go/No-Go task[7-9, 15, 19, 28, 29]. Some of these brain regions, including the superior temporal gyri, appear to be affected by prenatal tobacco exposure[38]. At present, it is unknown whether tobacco-exposed children exhibit distinct patterns of brain function when attempting to inhibit a response.
Prenatal tobacco exposure tends to co-occur with other risk factors. For example, children exposed to tobacco are more likely to also experience prenatal alcohol exposure, sub-optimal neonatal health, low socioeconomic status, have lower IQ scores, and to be exposed to postnatal environmental tobacco smoke, each of which have been related to cognitive deficits in children[1, 23, 27, 54, 72, 75]. Furthermore, neonatal medical complications, environmental risk, and IQ have been related to somewhat distinct brain activation patterns in adolescents during cognitive tasks[18]. Handedness also has been related to differences in brain function during imaging tasks[42]. Collectively, prenatal alcohol exposure, environmental risk, IQ, ETS, and handedness need to be considered as covariates in examining the relation between prenatal tobacco exposure and brain function. Early adolescence is a particularly important time to examine these factors as the prefrontal cortex continues to develop[26], response inhibition skills have not yet peaked[48], and some children begin to engage in increasingly risky behaviors that may be associated with response inhibition deficits[6, 53].
The purpose of the present study is to examine the brain function of tobacco-exposed vs. unexposed children during a response inhibition (Go/No-Go) task. Brain function during response inhibition can be assessed by contrasting activation in the brain during No-Go trials with that during Go trials[59]. The development of functional MRI (fMRI) based on blood oxygen level dependant (BOLD) imaging provides a methodology to non-invasively observe differences in brain function during such a response inhibition task[2, 59].
Specifically, we hypothesized that brain regions associated with response inhibition would show differential activation between exposed and unexposed groups. The specific regions are the anterior and posterior cingulate; medial, inferior, and superior frontal; superior parietal; superior temporal; and precentral gyrus.
2. Methods
2.1 Participants
Eighteen 12-year-olds (M = 12.6 years, SD = 0.2), all African-American, were recruited from an ongoing longitudinal study of prenatal substance exposure and were paid $40 in vouchers for use at local stores for participation in this fMRI study. Seven children were prenatally exposed to cigarettes (3 boys, 4 girls; PTE group) and 11 were unexposed (5 boys, 6 girls; NE group). Participants were right handed with the exception of one left-handed participant in each group. None were taking stimulant medication or were prenatally exposed to cocaine, marijuana, opiates, or phencyclidine. Imaging was conducted at the Temple University Hospital imaging center in Philadelphia.
In the longitudinal study, participants were initially recruited at or prior to birth through hospital-based prenatal clinics or newly delivered women at a university affiliated hospital in Philadelphia. Prenatal cigarette exposure was assessed by maternal report at the time of birth, which has been shown to be a valid measure of maternal smoking during pregnancy[16]. Cocaine, cannibinoids, opiates, and phencyclidine exposures were screened using meconium assay and maternal report at birth. In addition, children were excluded from the longitudinal study if they were born prior to 32 weeks of gestation, required special care or oxygen therapy for more than 24 hours, exhibited congenital anomalies, or if their mothers were infected with HIV.
Of 33 children and their parents approached about participating in the present study of tobacco exposure, 26 agreed to participate. Eight children who agreed to participate were not included in the imaging study, however, as one was excluded because of the presence of a metal plate in his body, four were scheduled but did not keep their appointments, two became uncomfortable upon seeing the MRI scanner and decided not to participate, and one fell asleep during the task. Using a series of t-tests, no differences were found between children who participated and those who did not in age, gender, prenatal cigarette exposure, prenatal alcohol exposure, environmental risk, or IQ (effect sizes ranged from d = 0.02 for IQ to d = 0.74 for neonatal health, with a median effect size of d = 0.38). We also examined whether participants differed from the larger sample of subjects without cocaine exposure who were seen at the age 9 year visit in the longitudinal study (i.e., when the IQ assessment was most recently conducted) and who did not participate in the present study. Many potential participants were not approached because they either lived in a different city from where the imaging was being conducted (Philadelphia), had prenatal cocaine exposure, or were not 12-years of age at the time of imaging. Participants did not differ from the larger sample of non-participants (excluding those with cocaine exposure from the comparison) on IQ (t (93) = 0.47, p = .64), prenatal tobacco exposure (t (94) = 0.08, p = .94), prenatal alcohol exposure (t (94) = 0.15, p = .88), or gender (χ2 (1) = 0.52, p = .47) (effect sizes ranged from d = 0.04 for alcohol exposure to d = 0.24 for gender). They did, however, differ on environmental risk (t (93) = 2.14, p < .05; d = 0.57) and neonatal medical problems (t (90) = 3.73, p < .001; d = 0.99) as participants had more environmental risk and neonatal medical problems. The study was approved by the institutional review boards of the Drexel University College of Medicine and Temple University School of Medicine. Parental consent and participant assent were obtained at the time of the screening interview for the imaging study.
2.2 Measures of Covariates
2.2.1. Environmental risk
Several environmental risk variables were assessed by maternal interview at the 4, 18, 54, 84, 102, and 120 month visits of the longitudinal study. Each variable was standardized, reverse coded if necessary so that higher scores reflected greater risk, and then converted into a composite T-score[5]. This score was based on the following variables: maternal life stress (based on the Social Environment Inventory[56]; maternal social support network size (Norbeck Social Support Questionnaire[55]; number of regular caregivers (greater number = higher risk); regularity of child's schedule; stability of child's surroundings (Family Chaos Scale; R. Seifer, personal communication); single parent household (living alone with children = higher risk); maternal education; maternal race (non-European American = higher risk); and public assistance status (public assistance as main source of income = higher risk). The mean of the T-scores across each of the six visits was used to estimate environmental risk throughout each adolescent's lifetime.
2.2.2. Handedness
The brief handedness inventory[21] contains 4-items assessing whether participants usually use their right, left, or either hand when drawing, throwing a ball, erasing, and dealing cards. “Right hand” was assigned a score of 3, “either” a score of 2, and “left hand” a score of 1.
2.2.3. Intelligence
The Stanford-Binet Intelligence Scale, Fourth edition (SB-IV[69]) was administered at age 9-years. The subscales of Abstract/Visual Reasoning, Quantitative Reasoning, Short-term Memory, and Verbal Reasoning were summed to produce a composite IQ score.
2.2.4. Neonatal health problems
Prenatal and neonatal medical data were abstracted by nurses from hospital records shortly after birth. They were used to complete a neonatal medical risk scale consisting of 35 possible complications[35]. Variables included general factors (e.g., low birth weight, fetal anomalies, and feeding problems), respiratory complications (e.g., congenital pneumonia, apnea, and meconium aspiration syndrome), metabolic disorders (e.g., failure to gain weight and hypoglycemia), cardiac problems (e.g., murmur and cardiac anomalies), and CNS problems (e.g., CNS depression and seizures). The total neonatal health problems score had a range from 0 to 13 in the present sample.
2.2.5. Prenatal tobacco and alcohol exposure
Substance use information was obtained through a semi-structured interview conducted prenatally or in the mother's room on the maternity ward postnatally. The interview contained questions about the frequency and amount of cigarette and alcohol use, as well as use of cocaine, marijuana, opiates, phencyclidine (PCP), tranquilizers, amphetamines, and barbiturates. Maternal report of the average number of alcoholic beverages (i.e., 12 oz. beer, 5 oz. wine, or 1 shot of liquor) consumed throughout pregnancy was used as the covariate for alcohol exposure. Regarding tobacco exposure, children were assigned to the tobacco exposure group if their mothers reported any cigarette smoking during the pregnancy.
2.2.6. Postnatal environmental tobacco smoke exposure
Environmental tobacco smoke (ETS) exposure was assessed by a questionnaire give to participants and their mothers. Participants and their mothers were independently asked ”About how many hours per week usually are you around someone who a) smokes (cigarette, cigar, or pipe smoke) in your home?; b) smokes in small spaces other than your home (for example, in a car)?; and c) smokes in large indoor areas (such as a restaurant)? These items, adapted from Iribarren and colleagues[37], were then categorized as follows to reduce skewness: (0 = 0 hours/week exposure; 1 = 1 to 5 hours/week; 2 = 6 to 10 hours/week; 3 = 10 to 20 hours/week; 4 = more than 20 hours/week). Adolescent and mother scores were averaged for analyses.
2.3 fMRI Recording Apparatus
Scanning was performed using a 1.5 Tesla General Electric Scanner with an echo gradient-EPI sequence and an eight channel head coil. Participants were positioned supine on the gantry of the scanner with the head midline in the coil. In addition to instructions to limit head motion, foam pads within the head coil helped secure head fixation and prevent motion. Scanning began with a standard spin echo T1-weighted sequence positioned parallel to the line of the anterior and posterior commissures covering the whole brain. This yielded 24 axial slices of the brain for analyses as the high resolution structural images. Imaging parameters were matrix size = 256*256; TR = 3000 ms; TE = 60 ms; FOV = 22 cm; NEX = 1; slice thickness = 5 mm, with no skip. fMRI images were acquired using a T2*-weighted echo planar imaging (EPI) gradient echo sequence (matrix size = 64*64; TR = 2000 ms; TE = 50 ms; FOV = 22 cm; slice thickness = 5 mm, with no skip) covering the same brain regions and in the same plane as the T1-weighted sequence. The Go/No-Go stimuli were presented using MRI compatible 3D video goggles (Resonance Technologies, Inc.), which had 180,000-pixel resolution and a 30° field of view. All stimuli were delivered using Neurobehavioral Systems Presentation software (www.neurobs.com). An MR compatible response keypad was used to measure the responses participants made to the visual displays.
2.4 Design/Paradigm
Prior to entering the imager, children were told that they would be playing a game in which they would see a series of letters on their goggle screen. Children were told to press the button using their dominant hand for every letter except “V”, and that they were not to press the button whenever a “V” appeared. Children then put on the goggles and entered the imager. Once acclimated to the imager, children were asked to direct their gaze to the center of the field of view for 20 practice trials. To establish a prepotent response, each of the 20 practice letters was a letter other than “V”. Following the practice, participants viewed a cross hair on a white background for 18 sec, followed by the 160 letters (41 “V”s, 119 letters other than “V”; V′s were randomly interspersed among the other letters) that composed the Go/No-Go task. Letters were individually presented for 1000 msec with a 1000 msec inter stimulus interval (ISI). Total length of time for fMRI scanning was 5 min 38 sec.
2.5 Image Processing
The post-acquisition preprocessing and statistical analysis was performed using SPM2 (Statistical Parametric Mapping, Welcome Department of Cognitive Neurology, University College of London, UK), run under the Matlab® environment (The Mathworks, Inc., Natick, MA). Images were converted from DICOM format into ANALYZE (AnalyzeDirect, Inc., Lenexa, KY) format adopted in the SPM package. Slice timing correction was performed to compensate for delays associated with acquisition time differences among slices during the sequential imaging. A 3D automated image registration routine (six-parameter rigid body, sinc interpolation; second order adjustment for movement) was applied to the volumes to realign them with the first volume of the first series used as a spatial reference (i.e., immediately before subjects viewed the cross-hair for 18 sec). The functional volumes collected at this time are closest in sequence to the acquisition of the high resolution structural images. All functional and anatomical volumes were then transformed into the standard anatomical space using the T2 EPI template and the SPM normalization procedure[3, 32]. This procedure uses a sinc interpolation algorithm to account for brain size and position with a 12 parameter affine transformation, followed by a series of non-linear basic function transformations seven, eight, and seven nonlinear basis functions for the x, y, and z directions, respectively, with 12 nonlinear iterations to correct for morphological differences between the template and given brain volume. Next, all volumes underwent spatial smoothing by convolution with a Gaussian kernel of 8 cubic mm full width at half maximum (FWHM), to increase the signal-to-noise ratio (SNR) and account for residual intersession differences.
An event-related fMRI design was used. Individual subject-level statistical analyses were performed using the general linear model in SPM2, restricting analyses to correct trials. The two condition events and the baseline were modeled using a canonical hemodynamic response function. Response inhibition effects were examined via linear contrasts to the parameter estimates for the correct No-Go minus correct Go contrast, resulting in a contrast map for each participant. Group-level random effects analysis for main effects was accomplished by entering whole brain contrasts into multiple regressions with a constant statistical model.
Whole brain analysis on the BOLD signal assessed differences in activation between the exposed and unexposed groups using regressors controlling for prenatal alcohol exposure, neonatal health problems, environmental risk, IQ scores, current environmental smoke exposure, and handedness. A significant threshold based on spatial extent using a height of t ≥ 10 and cluster probability of an uncorrected p ≤ 0.001 was applied to the effects of interest and surviving voxels were retained for further analysis. Statistical parametric maps (SPM [1]) are created for visual representation of the areas in the brain wherein statistically significant differences between BOLD contrasts between two groups are presented and the regions are tabulated. Specific areas of the brain were named using a Talairach atlas software http://www.talairach.org/daemon.html, as well as expertise of a board certified neuroradiologist (SHF).
A region of interest (ROI) analysis of the brain regions was performed on eight predetermined regions identified in the literature as involved in response inhibition. These regions include the anterior cingulate, posterior cingulate, superior frontal, inferior frontal, medial frontal, prefrontal gyrus, superior temporal, & superior parietal. The BOLD activation in each of these regions was masked using WFU PickAtlas software (The Functional MRI Laboratory, Wake Forest University School of Medicine). The ROI analysis was conducted at the group level, and not at the individual level. All of the interpretations were done after a random effects analysis (RFX) of the group data. Cluster probability of an uncorrected value of p ≤ 0.001 was applied to the regions of interest and the surviving voxels were retained for further analysis. The volumes of these surviving clusters within the ROI's were calculated using MarsBar software.
3. Results
3.1 Covariates
As seen in Table 1, no significant differences were observed between the prenatal tobacco exposed group (PTE) and the non-exposed group (NE) on the measures of prenatal alcohol exposure, environmental risk, intelligence, neonatal health problems, handedness, or current smoke exposure. Nonetheless, these variables were included as covariates in analyses of the imaging data in the event that modest relations exist between these variables and brain function during response inhibition that could not be detected with current statistical power.
Table 1. Means (and standard deviations) of covariates.
| Tobacco-exposed (n = 7) |
Unexposed (n = 11) |
|
|---|---|---|
| Environmental Risk | 53.84 (10.0) | 51.80 (5.4) |
| Environmental smoke exposure (current) | 1.07 (1.2) | 1.14 (1.3) |
| Handedness (l=left; 3=right) | 2.68 (0.5) | 2.71 (0.6) |
| Intelligence | 90.50 (20.5) | 92.73 (11.3) |
| Neonatal Health Problems | 1.80 (2.5) | 0.89 (1.1) |
| Prenatal Alcohol Exposure | 0.04 (0.1) | 0.01 (0.0) |
| Prenatal Cigarette Exposure | 4.18 (3.4) | 0.00 (0.0)* |
Significant group difference at p < .05.
3.2 Behavioral data
Tobacco-exposed children made 31% more commission errors than unexposed children, responding when they should have inhibited a response (Mexposed = 8.14, SD = 2.3; Munexposed = 6.20, SD = 5.0; Mann-Whitney U test, Z = 1.67, p = .05, 1-tailed). There were no group differences in either omission errors (i.e., failing to respond when they should have; Mexposed = 1.86, SD = 1.5; Munexposed = 2.70, SD = 2.6; p > .10) or reaction time (Mexposed = 566.3 msec, SD = 52.5; Munexposed = 592.9, SD = 73.9; p > .10).
3.3 Whole brain and ROI data
The whole brain analysis indicated that exposed children had more regions showing activation during response inhibition trials. Table 2 lists the brain regions and Figure 1 presents SPM glass brain representations showing brain activations based on group analysis. Of interest to the hypothesis that PTE affects response inhibition, while both groups showed activation in the right middle and right superior frontal gyri, only the PTE group showed activation in the left middle frontal, bilateral superior frontal, left middle temporal gyrus, and bilateral occipital regions.
Table 2. Whole brain analysis: Areas showing significant activation over resting during response inhibition trials (i.e., correct No-Go minus correct Go trials).
| Radiological Coordinates | |||||
|---|---|---|---|---|---|
| (3mm Range) | |||||
| Region | BA | X | y | z | Z score |
| Areas of activation for unexposed children | |||||
| Frontal lobe | |||||
| R superior frontal gyrus | 6 | -2 | 8 | 53 | 3.78 |
| R medial & superior frontal gyrus | 8 | 0 | 20 | 47 | 3.46 |
| R middle frontal gyrus | 6 | -18 | 11 | 57 | 3.29 |
| R middle frontal gyrus | 6 | -18 | -5 | 61 | 3.11 |
| Limbic lobe | |||||
| R cingulate gyrus | 24/32 | -2 | 8 | 38 | 3.16 |
| Parietal lobe | |||||
| R postcentral gyrus | 5 | -32 | -43 | 63 | 4.32 |
| R inferior parietal lobule | 40 | -50 | -40 | 54 | 4.15 |
| Cerebellum | |||||
| L posterior lobe, declive & uvula | - | 32 | -69 | -23 | 3.06 |
| Areas of activation for exposed children | |||||
| Frontal lobe | |||||
| L superior frontal gyrus | 6 | 8 | 26 | 54 | 3.00 |
| L superior frontal gyrus | 8 | 38 | 22 | 49 | 3.74 |
| R superior frontal gyrus | 6/8 | -4 | 26 | 52 | 3.84 |
| R superior frontal gyrus | 9 | -14 | 54 | 36 | 3.47 |
| L middle & superior frontal gyrus | 9/10 | 30 | 42 | 26 | 4.14 |
| L middle frontal gyrus | 10 | 34 | 57 | 19 | 3.51 |
| R middle frontal gyrus | 6 | -14 | 13 | 58 | 3.41 |
| R middle frontal gyrus | 6 | -36 | 5 | 53 | 3.76 |
| R middle frontal gyrus | 8 | -30 | 39 | 40 | 3.29 |
| R middle frontal gyrus | 9 | -44 | 10 | 36 | 4.05 |
| R precentral & middle frontal gyrus | 6 | -51 | 0 | 42 | 3.64 |
| Temporal lobe | |||||
| L middle temporal gyrus | 21 | 55 | -22 | -6 | 3.67 |
| L middle temporal gyrus | 21 | 59 | -18 | -14 | 3.01 |
| R fusiform gyrus | 37 | -50 | -49 | -16 | 3.09 |
| Parietal lobe | |||||
| R precuneus | 7 | -20 | -54 | 45 | 3.14 |
| Occipital lobe | |||||
| L cuneus & precuneus | 18/31 | 4 | -74 | 26 | 3.80 |
| L cuneus | 18/19 | 16 | -96 | 23 | 3.40 |
| R cuneus | 19 | -8 | -88 | 34 | 3.30 |
| R cuneus | 19 | 10 | -96 | 25 | 3.25 |
| Sub-lobar | |||||
| R thalamus, ventral lateral nucleus | -10 | -13 | 4 | 3.33 | |
Note. All areas significant at p ≤ .001 and with Z scores ≥ 3.00.
Note. BA = Brodmann's area.
Figure 1.
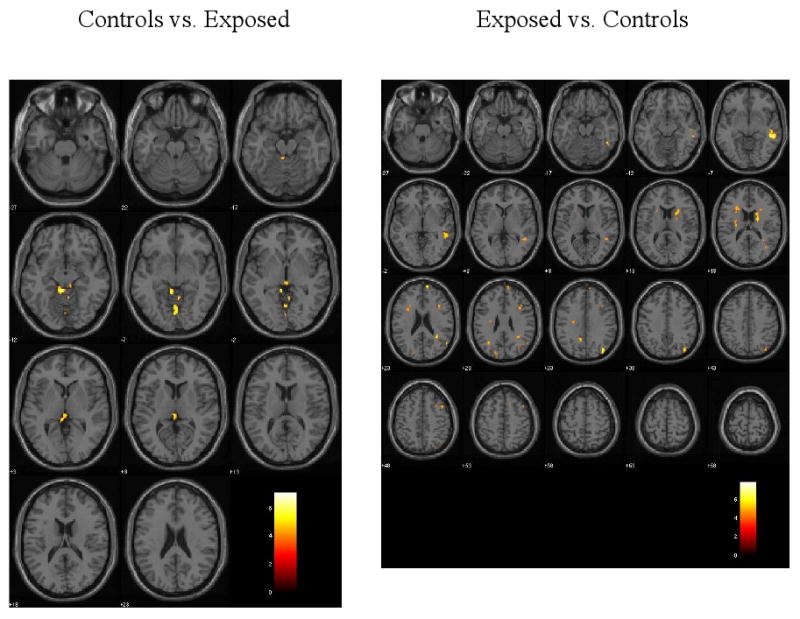
Results of fMRI whole brain group analysis overlayed onto anatomical images. Significant activation differences are shown for the No-Go minus Go contrast (i.e., response inhibition). The threshold is p < .001 with 10 or more contiguous voxels and a height threshold of T = 4.14. Images are in radiological coordinates.
Table 3 presents significant (p ≤ .001) activation differences between exposure groups from the whole brain analysis. Consistent with findings from Table 2, exposed children showed greater activation in left frontal (including the superior and middle frontal gyri), bilateral temporal, bilateral parietal, and right occipital regions. In contrast, the unexposed children showed greater activation only in lower brain regions, particularly in the culmen.
Table 3. Whole brain analysis: Areas showing significant group differences in activation during response inhibition trials (i.e., correct No-Go minus correct Go trials).
| Radiological Coordinates | |||||
|---|---|---|---|---|---|
| (3mm Range) | |||||
| Region | BA | X | y | z | Z score |
| Greater activation for unexposed children | |||||
| Cerebellum | |||||
| R anterior lobe, culmen | -6 | -41 | -6 | 4.16 | |
| L anterior lobe, culmen | 6 | -51 | -3 | 3.49 | |
| Anterior lobe, culmen of vermis | 0 | -64 | 0 | 3.70 | |
| L brainstem, midbrain | 8 | -29 | -7 | 3.29 | |
| Occipital lobe | |||||
| Lingual gyrus | 2 | -72 | -3 | 3.98 | |
| Greater activation for exposed children | |||||
| Frontal lobe | |||||
| L superior frontal gyrus | 8 | 44 | 18 | 47 | 4.21 |
| L superior frontal gyrus | 9 | 10 | 60 | 28 | 3.31 |
| L anterior medial superior frontal gyrus | 10 | 12 | 63 | 19 | 3.88 |
| L middle frontal gyrus | 8 | 32 | 18 | 43 | 3.81 |
| L middle frontal gyrus | 8 | 36 | 17 | 25 | 3.57 |
| Temporal lobe | |||||
| L superior temporal gyrus | 39 | 54 | -59 | 26 | 3.98 |
| R superior temporal gyrus | 39 | -32 | -57 | 27 | 3.61 |
| L middle temporal gyrus | 39 | 53 | -59 | 25 | 3.98 |
| R middle temporal gyrus | 39 | -32 | -57 | 30 | 3.61 |
| L fusiform gyrus | 37 | 50 | -51 | -15 | 3.77 |
| L inferior temporal gyrus | 20 | 50 | -51 | -13 | 3.77 |
| R sub-gyral | 39 | -32 | -57 | 25 | 3.61 |
| Parietal lobe | |||||
| L superior parietal gyrus | 7 | 36 | -73 | 46 | 3.48 |
| L precuneus | 19 | 40 | -74 | 35 | 4.11 |
| R precuneus | 31 | -10 | -51 | 34 | 3.72 |
| Occipital lobe | |||||
| R precuneus | 31 | -20 | -78 | 28 | 3.60 |
| R cuneus | 18 | -20 | -78 | 32 | 3.60 |
Note. All areas significant at p ≤ .001 and with Z scores ≥ 3.00.
Note. BA = Brodmann's area.
The region of interest analysis was consistent with the whole brain analysis in finding that exposed children had greater superior frontal, inferior frontal, and anterior cingulate activation (see Table 4). Unexposed children did show greater activation in the medial frontal region and somewhat greater activation in the superior parietal region. Finally, a similar number of voxels was found to be activated for each group in the precentral gyrus and superior temporal regions, while neither group showed activation in the posterior cingulate.
Table 4. Region of interest analyses: Number of activated voxels by group during response inhibition (i.e., correct No-Go minus correct Go trials).
| Number of Activated Voxels | ||
|---|---|---|
| Region of Interest | Unexposed Group | Exposed Group |
| 1. Anterior cingulate | 0 | 16 |
| 2. Posterior cingulate | 0 | 0 |
| 3. Superior frontal | 246 | 756 |
| 4. Inferior frontal | 0 | 28 |
| 5. Medial frontal | 93 | 12 |
| 6. Precentral gyrus | 17 | 19 |
| 7. Superior temporal | 27 | 36 |
| 8. Superior parietal | 25 | 11 |
4. Discussion
The present study examined whether prenatal exposure to tobacco affects brain function in children during a response inhibition task. Tobacco-exposed children made more commission errors, consistent with prior studies indicating that tobacco-exposed children perform worse on tasks involving response inhibition[31, 36]. Tobacco exposure was also associated with a different pattern of brain activation among children in early adolescence while controlling for potential confounds. Specifically, tobacco-exposed children showed greater activation than unexposed children in a relatively large and diverse set of regions. Left frontal, right occipital, and bilateral temporal and parietal regions each showed greater activation among exposed than unexposed children. In contrast, children who were not exposed to tobacco showed greater activation than exposed children in only a few regions, primarily limited to medial regions of the cerebellum and the occipital lobe.
Greater activation in these multiple brain regions suggests that tobacco-exposed children have a less mature brain. Developmental neuroimaging studies indicate that with increased age children exhibit enhanced activation in a relatively small, focused number of brain regions for a given function, with decreased activation observed in other regions [28]. This pattern has been observed for response inhibition during Go/No-Go tasks. Younger children, including school-age children and preadolescents, have been found to exhibit greater activation in some of the same regions in which exposed children showed greater activation in the present study, specifically the superior [SFG] and middle frontal gyri [MFG][19, 67]. By shifting to an increasingly focal pattern of activation, the brain appears to be reorganizing itself to permit more efficient processing[28]. Tobacco-exposed children showed a more diffuse activation pattern during inhibition than did their unexposed peers in early adolescence. This diffuse pattern indicates that the greater activation among exposed children was evident even when their behavioral performance was the same as that of controls (i.e., when they correctly inhibited their responses).
Examination of specific regions showed that both tobacco-exposed and unexposed children activated areas associated with response inhibition, including the premotor cortex (BA 6)[50, 57]. However, exposed children showed greater activation than unexposed children in several areas associated with response inhibition, including the SFG, fusiform gyrus, cuneus, and precuneus [7, 9, 13, 25, 45, 61]. ROI analysis also identified several regions in which exposed children showed greater activation, including the SFG, inferior frontal gyrus, and anterior cingulate. Similarly, the whole brain analysis found exposed children to exhibit greater activation in the left fusiform gyrus, a region closely associated with visual processing and letter identification [39, 40], and in the superior parietal lobe, a region associated with visual attention and attentional preparation[22].
In contrast, unexposed children showed greater activation primarily in the cerebellum, and particularly in the culmen. Cerebellar hypofunction has been associated with attention problems[17], raising the possibility that exposed children's attentional systems are challenged when completing a relatively simple Go/NoGo task. Activation of the culmen, which has been associated with motor response preparation and appears to be part of a circuit with premotor regions such as the SMA, has been associated with a more consistent response time during a Go/No-Go task[60]. In contrast, a more variable response time has been associated with activation of the SFG/MFG[60], consistent with our findings that tobacco-exposed children showed greater activation in superior and middle frontal regions. The greater activation of the SFG and MFG shown by the exposed group may indicate less efficient utilization of the premotor regions necessary to guide response selection and therefore elicit recruitment of higher-order prefrontal regions to guide basic motor response selection and inhibition[60]. Although there was not a significant group difference in response time in the present sample, the activation patterns are consistent with such an interpretation by Simmonds and colleagues. Tobacco exposed children also showed greater activation in the left superior parietal gyrus, which along with the MFG is implicated in working memory maintenance[77], and the middle temporal gyrus, which is associated with memory storage for rules[14]. The Go/No-Go task requires both inhibitory control and working memory as subjects must remember the rule dictating when to respond and when to refrain from responding while simultaneously encoding each new stimulus.
Imaging studies of children with prenatal tobacco exposure are still rare at present. Prior imaging research with tobacco-exposed children, however, has further implicated the MFG and orbitofrontal gyrus, which includes regions of the SFG (e.g., BA 10), as being affected by prenatal exposure. These regions, both of which showed greater activation among exposed children in the present study, were found to be thinner among tobacco-exposed adolescents, which may reflect a decrease in neuronal and glial growth.[70] A second imaging study also found greater activation in the MFG as well as several other regions among tobacco exposed children during an attention task, supporting the interpretation that exposed children exhibit a less efficient brain[38].
Right hemispheric dominance has frequently been observed during response inhibition tasks[13, 34, 41]. Tobacco-exposed children, however, exhibited greater activation in the left hemisphere, and particularly in left prefrontal regions, than did controls. This suggests that they have a more bilateral pattern of activation during response inhibition, and possibly use their left hemisphere in a compensatory manner. The left PFC, for example, has been implicated in behavioral correction following errors on a Go/No-Go task[33]; given that exposed children made more commission errors, this raises the possibility that their greater levels of left PFC activation were due to increased adjustment following error trials that preceded subsequent correct trials. Again, this may be interpreted as evidence of a relatively immature brain as prior research has found 9- to 12-year-old children to exhibit greater bilateral activation than adults during a Go/No-Go task[7], suggesting that greater use of the left hemisphere (vs. right hemispheric dominance) is associated with inefficient recruitment of brain regions during response inhibition.
Further support for the interpretation that tobacco-exposed children exhibit a relatively immature, inefficient brain when inhibiting a response comes from research on children with ADHD. As noted above, findings on ADHD may be relevant to tobacco-exposed children given their increased risk for ADHD symptoms[51, 68]. Children with ADHD have been found to have less white matter in their brains than their peers[44], suggestive of a less mature brain. Early adolescents with a history of ADHD, which is posited to include response inhibition as a core deficit[73], have been found to exhibit increased brain activation among a greater set of brain structures, including prefrontal regions, than controls during response inhibition, similar to exposed children in the present study[10, 59]. Moreover, children with ADHD have been found to exhibit greater activation in some of the same regions as those of tobacco-exposed children in the present study (e.g., the MFG and the precuneus)[59], although this is not a consistent finding[65]. Adolescents without ADHD also showed greater activation in a neural network that included the lingual gyrus and cerebellum[59], consistent with unexposed children in the present study. The similarity of findings between children with prenatal tobacco exposure and those with ADHD suggests that these two groups may exhibit common activation patterns during response inhibition. The finding that children who were prenatally exposed to tobacco and those with a history of ADHD both exhibit less cerebellar activation than controls also raises the possibility that increased activation of frontopolar and other regions might represent an adaptive mechanism to compensate for impairments in the cognitive processes mediated by the cerebellum. The present findings also parallel those of young adults prenatally exposed to marijuana who were found to exhibit increased activation in the prefrontal cortex but decreased activation in the cerebellum during response inhibition[64], raising the possibility that a similar mechanism is involved for tobacco and marijuana exposure.
Several possible mechanisms exist by which prenatal tobacco exposure may affect the efficiency of brain function and, consequently, response inhibition. First, prenatal nicotine exposure has been found to damage serotonergic projections in the cerebral cortex and striatum, resulting in decreased neuronal activity[63, 74]. Impaired serotonergic function has been associated with impaired inhibitory control[20, 47], raising the possibility that damage to the serotonergic system may impair the brain's ability to inhibit responses. A second mechanism concerns myelination, which enhances the speed of communication between nerve cells. Prenatal nicotine exposure has been found to cause fetal hypoxia[62], which in turn is associated with decreased myelination, both perinatally and during adolescence[43, 52]. Given that the extent of frontostriatal myelination is associated with more efficient performance during response inhibition[46], it is possible that tobacco-exposed children perform less efficiently because they possess fewer myelinated nerve fibers. Diffusion tensor imaging could be used in future studies of exposed vs. unexposed children to directly test this hypothesis. In addition to these potential mechanisms, prenatal nicotine exposure has been found to affect many other aspects of neurodevelopment (e.g., damage to nicotinic acetylcholine receptors that limits their ability to regulate neuronal migration; abnormalities in cell proliferation and differentiation; enhanced presynaptic release of not only serotonin but also of acetylcholine, dopamine, and noradrenaline[30]) that may also play a role in affecting response inhibition.
Several limitations of the present study deserve mention. First, observed differences could be due to factors other than tobacco exposure. For example, a recent study found about half of the association between prenatal smoking and child antisocial behavior to be attributable to correlated genetic effects, as prenatal tobacco exposure may be a proxy for partly heritable characteristics (e.g., maternal smoking and child antisocial behavior[49]). Second, the present sample size did not permit examination of potential gender by tobacco exposure interactions, or dose response effects. Third, the global pattern of differences observed were not all consistent with regions hypothesized to be involved in response inhibition and thus need replication. Fourth, participants did have more neonatal health problems and environmental risk than non-participants, possibly limiting the generalizability of the present findings.
In summary, the present findings are the first to examine the relation between response inhibition and brain function among children prenatally exposed to tobacco. While controlling for a number of potential confounds, children prenatally exposed to tobacco showed increased activation in a relatively large and diverse set of regions, including frontal, temporal, parietal, and occipital regions, while showing less activation in the cerebellum. Longitudinal research is needed to examine when such tobacco exposure effects emerge, whether these effects are stable over time given developmental changes observed in brain function during response inhibition tasks over adolescence[48], and whether these findings indicate only a delay in the maturation of response inhibition among tobacco exposed children, or a more permanent effect in their response inhibition proficiency.
Acknowledgments
This study was supported by grant #4100011451 from the Pennsylvania Department of Health (the Department specifically disclaims responsibility for any analyses, interpretations or conclusions) to the first author and by grant DA07109 from the National Institute on Drug Abuse to the last author. The sponsor agencies had no involvement in the study design; collection, analysis, and interpretation of data; writing of this report; or in the decision to submit this report for publication. The authors greatly appreciate the assistance of Sara Ragland in data collection and Monica Martinez in data processing.
Footnotes
Portions of this study were presented on June 13, 2006, at the Organization of Human Brain Mapping, Florence, Italy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National pregnancy and health survey. Drug use among women delivering live births, 1992. NIDA; Rockville, MD: 1996. [Google Scholar]
- 2.Altman NR, Bernal B. Pediatric Applications of fMRI. In: Faro SH, Mohamed FB, editors. Functional MRI: Basic principles and clinical applications. Springer; New York: 2006. pp. 394–428. [Google Scholar]
- 3.Ashburber J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkley RA. Behavioral inhibitions, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett DS, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure, gender and environmental risk. Journal of Developmental and Behavioral Pediatrics. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- 7.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. NeuroImage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 8.Booth JR, Burman DD, Meyer JR, Trommer BL, Davenport ND, Parrish TB, Gitelman DR, Mesulam MM. Brain-behavior correlation in children depends on the neurocognitive network. Human Brain Mapping. 2004;23:99–108. doi: 10.1002/hbm.20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth JR, Burman DD, Meyer JR, Zhang L, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 10.Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, Steinhausen HC. Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behavioural Brain Research. 1998;94:111–125. doi: 10.1016/s0166-4328(97)00174-5. [DOI] [PubMed] [Google Scholar]
- 11.Braun JM, Kahn RS, Foehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environmental Health Perspectives. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan PA, Grekin ER, Mortensen EL, Mednick SA. Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. American Journal of Psychiatry. 2002;159:48–54. doi: 10.1176/appi.ajp.159.1.48. [DOI] [PubMed] [Google Scholar]
- 13.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Human Brain Mapping. 2005;45:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunge SA. How we use rules to select actions: A review of evidence from cognitive neuroscience. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- 15.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women: Assessment of self-report against carbon monoxide (CO) Addictive Behaviors. 2001;26:1–9. doi: 10.1016/s0306-4603(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 17.Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: A resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- 18.Carmody DP, Bendersky M, Dunn SM, DeMarco JK, Hegyi T, Hiatt M, Lewis M. Early risk, attention and brain activation in adolescents born preterm. Child Development. 2006;77:384–394. doi: 10.1111/j.1467-8624.2006.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 20.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends in Cognitive Sciences. 2007;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Coren S. Measurement of handedness via self-report: The relationship between brief and extended inventories. Perceptual and Motor Skills. 1993;76:1035–1042. doi: 10.2466/pms.1993.76.3.1035. [DOI] [PubMed] [Google Scholar]
- 22.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 23.Day N, Cornelius M, Goldschmidt L. The effects of prenatal tobacco and marijuana use on offspring growth from birth through age 3 years. Neurotoxicology and Teratology. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- 24.Day NL, Richardson GA, Goldschmidt L, Cornelius M. Effects of prenatal tobacco exposure on preschoolers' behavior. Developmental and Behavioral Pediatrics. 2000;21:180–188. [PubMed] [Google Scholar]
- 25.de Zubicaray GI, Andrew C, Zelaya FO, Williams SCR, Dumanoir C. Motor response suppression and the prepotent tendency to respond: A parametric fMRI study. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. [DOI] [PubMed] [Google Scholar]
- 26.Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; New York: 2002. [Google Scholar]
- 27.DiFranza JR, Aligne A, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 28.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 29.Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman R, Casey BJ. A neural basis for development of inhibitory control. Developmental Science. 2002;5:9–16. [Google Scholar]
- 30.Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Fried PA, Watkinson B, Gray R. A follow-up study on attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicology and Teratology. 1992;14:299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- 33.Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 34.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hobel C, Hyvarinen M, Okada D, Oh W. Prenatal and intrapartum high-risk screening. American Journal of Obstetrics and Gynecology. 1973;117:1–9. doi: 10.1016/0002-9378(73)90720-5. [DOI] [PubMed] [Google Scholar]
- 36.Huijbregts SCJ, Warren AJ, de Sonneville LMJ, Swaab-Barneveld H. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: An exploratory study. Journal of Abnormal Child Psychology. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iribarren C, Darbinian J, Klatsky AL, Friedman GD. Cohort study of exposure to environmental tobacco smoke and risk of first ischemic stroke and transient ischemic attack. Neuroepidemiology. 2004;23:38–44. doi: 10.1159/000073973. [DOI] [PubMed] [Google Scholar]
- 38.Jacobsen LK, Slotkin TA, Mecnl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- 39.James KH, Gauthier I. Letter processing automatically recruits a sensory–motor brain network. Neuropsychologia. 2006;44:2937–2949. doi: 10.1016/j.neuropsychologia.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 40.Joseph JE, Gathers AD, Piper GA. Shared and dissociated cortical regions for object and letter processing. Cognitive Brain Research. 2003;17:56–67. doi: 10.1016/s0926-6410(03)00080-6. [DOI] [PubMed] [Google Scholar]
- 41.Kelly AMC, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 42.Kloppel S, van Eimeren T, Glauche V, Vongerichten A, Munchau A, Frackowiak RS, Buchel C, Weiller C, Siebner HR. The effect of handedness on cortical motor activation during simple bilateral movements. Neuroimage. 2007;34:274–280. doi: 10.1016/j.neuroimage.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Kohlhauser C, Mosgoller W, Hoger H, Lubec B. Myelination deficits in brain of rats following perinatal asphyxia. Life Sciences. 2000;67:2355–2368. doi: 10.1016/s0024-3205(00)00816-x. [DOI] [PubMed] [Google Scholar]
- 44.Krain AL, Castellanos FX. Brain development and ADHD. Clinical Psychology Review. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 47.Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 48.Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 49.Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- 50.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: Findings from a high-risk sample of siblings. Journal of Clinical Child Psychology. 1998;27:352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- 52.Nagy Z, Lindstrom K, Westerberg H, Skare S, Andersson J, Hallberg B, Lilja A, Flodmark O, Lagercrantz H, Klingberg T, Fernell E. Diffusion tensor imaging on teenagers, born at term with moderate hypoxic-ischemic encephalopathy. Pediatric Research. 2005;58:936–940. doi: 10.1203/01.pdr.0000186516.85702.61. [DOI] [PubMed] [Google Scholar]
- 53.Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking an illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 54.NIH. National Pregnancy and Health Survey: Drug use among women delivering live births: 1992. Rockville, MD: 1996. [Google Scholar]
- 55.Norbeck J, Lindsey A, Carrieri V. The development of an instrument to measure social support. Nursing Research. 1981;30:264–269. [PubMed] [Google Scholar]
- 56.Orr ST, James SA, Casper R. Psychosocial stressors and low birth weight: Development of a questionnaire. Journal of Developmental & Behavioral Pediatrics. 1992;13:343–347. [PubMed] [Google Scholar]
- 57.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 58.Roussy S, Toupin J. Behavioral inhibition deficits in juvenile psychopaths. Aggressive Behavior. 2000;26:413–424. [Google Scholar]
- 59.Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: An event-related fMRI study. American Journal of Psychiatry. 2004;161 doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- 60.Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slotkin TA. Fetal nicotine or cocaine exposure: Which one is worse? Journal of Pharmacology & Experimental Therapeutics. 1998;285:931–945. [PubMed] [Google Scholar]
- 63.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: Serotonin receptors and cell signaling. Neuropsychopharmacology. 2006;31:2462–2475. doi: 10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- 64.Smith AM, Fried PA, Hogan JJ, Cameron I. Effects of prenatal marijuana exposure on response inhibition: An fMRI study of young adults. Neurotoxicology and Teratology. 2004;26:533–542. doi: 10.1016/j.ntt.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Smith AM, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 66.Office of Applied Studies. National Survey on Drug Use and Health: NSDUH Report Cigarette Use among Pregnant Women and Recent Mothers. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2007. [Google Scholar]
- 67.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder. American Journal of Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- 69.Thorndike RL, Hagen EP, Sattler JM. Technical Manual. Fourth. Riverside Publishing; Chicago: 1986. The Stanford-Binet Intelligence Scale. [Google Scholar]
- 70.Toro R, Leonard G, Lerner JV, Lerner RM, Peron M, Pike GB, Richer L, Veillette S, Pausova Z, Paus T. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33:1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- 71.Wakschlag LS, Pickett KE, Kasza KE, Loeber R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:461–467. doi: 10.1097/01.chi.0000198597.53572.3e. [DOI] [PubMed] [Google Scholar]
- 72.Weitzman M, Byrd RS, Aligne CA, Moss M. The effects of tobacco exposure on children's behavioral and cognitive functioning: Implications for clinical and public health policy and future research. Neurotoxicology and Teratology. 2002;24:397–406. doi: 10.1016/s0892-0362(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 73.Wodka EL, Mahone EM, Blankner JG, Gidley Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- 74.Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: Effects on CNS serotonergic systems. Brain Research. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 75.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environmental Health Perspectives. 2005;113:98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zahalka EA, Seidler FJ, Lappi SE, McCook EC, Yanai J, Slotkin TA. Deficits in development of central cholinergic pathways caused by fetal nicotine exposure: Differential effects on choline acetyltranserase activity and [3H]hemicholinium-3 binding. Neurotoxicology and Teratology. 1992;14:375–382. doi: 10.1016/0892-0362(92)90047-e. [DOI] [PubMed] [Google Scholar]
- 77.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive evidence against human hippocampal involvement in working memory maintenance of familiar stimuli. Cerebral Cortex. 2005;15:303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]


