Abstract
A hallmark of acute relapsing fever borreliosis is severe bacteremia. Some Borrelia species, such as B. duttonii and B. crocidurae, associate with erythrocytes and induce aggregation recognized as erythrocyte rosetting. Erythrocyte rosettes contribute to disease severity by increased tissue invasiveness (such as invasion of CNS and encephalitis), hemorrhaging, and reduced blood flow in affected microcapillaries. Here we report that relapsing fever Borrelia binds to neolacto (Galβ4GlcNAcβ3Galβ4Glcβ1)–carrying glycoconjugates that are present on human erythrocytes. This interaction is of low affinity but is compensated for by the multivalency of neo-lacto-oligosaccharides on the erythrocyte cell surface. Hence, the protein–carbohydrate interaction is dependent on multivalent neolacto-glycans to mediate binding.
Keywords: adhesion, carbohydrate, glycosphingolipids, lacto-N-neotetraose, red blood cells
Relapsing fever (RF) is caused by Borrelia species such as B. hermsii, B. duttonii, B. crocidurae, B. recurrentis, and B. hispanica among others. The disease is a multisystemic infection characterized by flu-like symptoms, anemia, thrombocytopenia, splenomegaly, and hepatomegaly. A hallmark of infection is recurring high-grade fever and concomitant bacteremia reaching densities of up to 108 organisms per milliliter of blood. During acute infection, B. duttonii and B. crocidurae have been shown to associate with red blood cells (RBCs) in vivo and to induce intimate aggregates termed “erythrocyte rosettes” (1–4). Borrelia species capable of rosetting induce more severe symptoms, increased organ invasiveness, hemorrhaging, microemboli formation, and a delayed immune response (3, 4), suggesting that rosetting represents an important virulence factor. In vitro, rosette formation is influenced by both Borrelia species and temperature (1). However, the molecular mechanism that supports rosetting has not been described.
Carbohydrates are abundant surface-exposed structures on all eukaryotic cells. Given their ubiquitous nature and immense diversity, it is not surprising that some common motifs have been exploited by pathogens as binding sites for adherence to host cells and in addition to facilitate and/or promote entry into and invasion of the host cells (5, 6). In this study, RF Borrelia bound specifically to a subset of glycosphingolipids (GSLs) isolated from human erythrocytes. The GSL was characterized as Galβ4GlcNAcβ3Galβ4Glcβ1Cer (neolactotetraosylceramide) by proton NMR and by liquid chromatography-mass spectrometry (LC/MS) of the saccharide released by hydrolysis with Macrobdella decora ceramide glycanase.* As a natural model system, B. hispanica was found to bind to Neisseria meningiditis, which expresses neolactotetra glycans in its LOS/LPS. The results show that the Borrelia interaction with neolacto glycans is of low affinity and that the interaction necessitates multivalent neolacto epitopes to achieve high affinity. Here we present for the first time a molecular mechanism based on a lectin-glycan interaction that supports RF Borrelia-mediated erythrocyte rosetting.
Results
Variable Erythrocyte Rosetting of RF Borrelia Species.
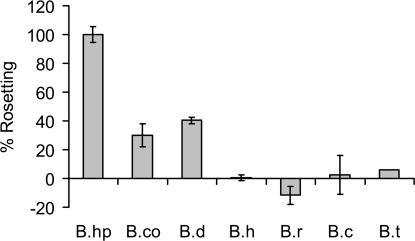
We developed an in vitro rosetting assay measuring hemoglobin release of captured erythrocyte rosettes, allowing detection of subtle changes in rosetting. Assay results correlate with rosetting assessed by microscopy. B. hispanica displayed the greatest capability followed by B. duttonii and B. coriaceae, whereas B. hermsii, B. turicatae, B. recurrentis, and B. crocidurae produced few or no rosettes (Fig. 1). Previous reports have indicated that B. duttonii (2) and B. crocidurae (1, 3, 4) are high rosetters, whereas B. hermsii (1, 3, 4) and B. recurrentis (unpublished) are nonrosetters. Results of the in vitro rosetting assay were confirmed by microscopy. Our results correlate well with previous reports, with the exception of B. crocidurae. We have used the same strain of B. crocidurae as in previous studies; thus it is unclear why this isolate has become a poor rosetter.
Fig. 1.
Varying rosetting of RF Borrelia: B.hp (B. hispanica CR1), B.co (B. coriaceae), B.d (B. duttonii 1120), B.h (B. hermsii HS1), B.r (B. recurrentis A11), B.c (B. crocidurae CR2A), and B.t (B. turicatae 071). Cells were harvested at comparable growth stages (late log). Percent rosetting was calculated as ratio of absorbance (405 nm) of released hemoglobin to spirochete number and normalized against the value for B. hispanica, defined as 100%. Samples were assayed in triplicate. Standard deviations are shown.
An Erythrocyte Carbohydrate Is Involved in Rosette Formation.
To narrow the search for erythrocyte components involved in rosette formation, we chemically modified the RBC surface and assessed the effect on rosetting. RBCs were subjected to oxidation by sodium periodate, which destroys most glycan epitopes (8). The treatment did not result in cell lysis or changes in cell morphology. After washing, RBCs were mixed with B. hispanica CR1 in an in vitro rosetting assay. RBCs treated with 5 mM sodium periodate had severe rosette reduction, and those treated with <2.5 mM had no effect (Fig. 2). These results suggest that a carbohydrate, presumably nonsialic acid, mediates erythrocyte rosetting.
Fig. 2.
Periodate oxidation of erythrocytes reduces rosetting. Human erythrocytes were treated with 0–5 mM sodium periodate before incubation with B. hispanica CR1. Absorbance reflects hemoglobin released from captured erythrocytes. Samples were assayed in triplicate. Standard deviations are shown.
RF Borrelia Bind to a GSL Present on Human Erythrocytes.
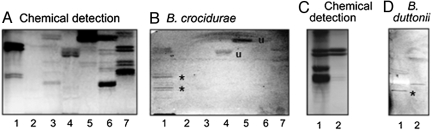
To further define the carbohydrate binding preferences of Borrelia spirochetes, the binding of radiolabeled spirochetes to GSLs was tested in the thin-layer chromatography binding assay. In initial experiments, mixtures of GSLs from various sources were used to expose the spirochetes to a large number of lectin-binding carbohydrates. Binding to the fast-migrating compounds in Fig. 3B (lanes 4 and 5), was judged as a nonspecific interaction caused by the large amounts of these compounds on the chromatograms, as no binding to any mono- or diglycosylceramides was obtained when using pure GSLs in defined amounts (see below).
Fig. 3.
Binding of Borrelia to GSL mixtures on thin-layer chromatograms. Chemical detection by anisaldehyde (A and C), and autoradiograms after adherence of 35S-labeled B. crocidurae CR2A (B) and B. duttonii 1120 (D). (A and B) Lane 1, nonacid GSLs of human neutrophils, 40 μg; lane 2, acid GSLs of human neutrophils, 40 μg; lane 3, acid GSLs of human small intestine, 40 μg; lane 4, acid GSLs of human small intestine, 40 μg; lane 5, nonacid GSLs of bovine intestine, 40 μg; lane 6, nonacid GSLs of porcine intestine, 40 μg; and lane 7, nonacid GSLs of human placenta, 40 μg. Lanes 3 and 4 represent samples from different individuals. (C and D) Lane 1, nonacid GSLs of human blood group O erythrocytes, 40 μg; lane 2, nonacid GSLs of human neutrophils, 40 μg. Autoradiography was performed for 48 h. Asterisk indicates band of interest; “u” indicates nonspecific band; other bands represent noise.
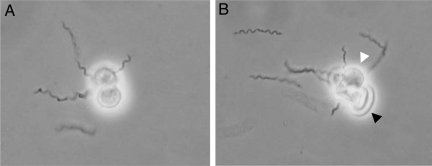
Distinct spirochete binding to two double bands migrating in the tetra- and hexaglycosylceramide regions in the nonacid fraction of human neutrophils (Fig. 3B), and a band in the tetra- to pentaglycosylceramide region in the nonacid fraction of human erythrocytes (Fig. 3D), were observed. These findings directed our interest toward neolacto-containing GSLs, because the tetraglycosylceramide of human neutrophils is neolactotetraosylceramide (14) and this compound also is present on human erythrocytes (15). Rosettes form when isolated neutrophils are mixed with RF Borrelia in vitro (Fig. 4), supporting the involvement of this glycan in the interaction between human cells and Borrelia. Neutrophils (Fig. 4A) or a combination of neutrophils and red blood cells (Fig. 4B) can make up the rosettes.
Fig. 4.
Neutrophil rosettes formed with B. hispanica. Neutrophils (A) or neutrophils and erythrocytes (B) from human blood form rosettes in the presence of Borrelia. Log-phase B. hispanica CR1 was resuspended in fresh BSK and mixed with neutrophils on a glass slide. Light micrographs at ×10–12 are shown. White arrowhead indicates a neutrophil; black arrow indicates an erythrocyte.
Isolation and Characterization of a Borrelia-Binding GSL of Human Erythrocytes, Neolactotetraosylceramide.
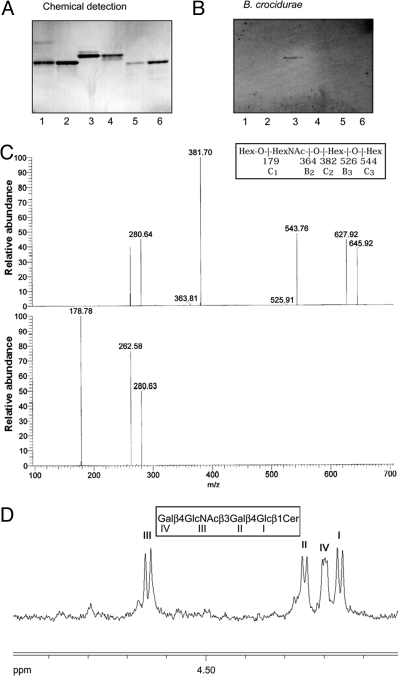
Starting with 800 mg of nonacid GSLs from human blood group O erythrocytes, a pure Borrelia-binding GSL (Fig. 5B, lane 3) was obtained after several separation steps as described in Materials and Methods. The GSL structure was established as follows. The carbohydrate sequence was established by LC/MS analysis of the saccharide obtained by hydrolysis with M. decora ceramide glycanase. The saccharide was detected as a [M-H]− ion at m/z 706, eluting at 29 min. The MS2 spectrum of the released saccharide (Fig. 5C) had prominent C-type ions (C2 at m/z 382 and C3 at m/z 544), whereas MS3 of the ion at m/z 382 gave a C1 ion at m/z 180. Both spectra had a 0,2A2 fragment ion at m/z 281, and a 0,2A3-H2O fragment ion at m/z 263, characteristic for GlcNAc substituted at C-4, i.e., type 2 carbohydrate chains (16), whereas no D1–2 ion at m/z 202, produced by a 3-substituted GlcNAc, i.e., a type 1 carbohydrate chain, was observed. Taken together, this allowed interpretation of the carbohydrate sequence as Hex-HexNAc-Hex-Hex, with a type 2 linkage between the terminal Hex and the HexNAc.
Fig. 5.
Isolation and characterization of a Borrelia-binding GSL from human erythrocytes. Binding of Borrelia to GSLs on thin-layer chromatograms. Chemical detection by anisaldehyde (A), and autoradiogram after adherence of 35S-labeled B. crocidurae CR2A (B). Lane 1, H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) from human erythrocytes, 4 μg; lane 2, B5 pentaglycosylceramide (Galα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) from rabbit erythrocytes, 4 μg; lane 3, Borrelia-binding GSL isolated from human erythrocytes, 4 μg; lane 4, Forssman pentaglycosylceramide (GalNAcα3GalNAcβ3Galα4Galβ4 Glcβ1 Cer) from canine intestine, 4 μg; lane 5, iso-Forssman pentaglycosylceramide (GalNAcα3GalNAcβ3Galα3Galβ4Glcβ1Cer) from rat colon carcinoma, 2 μg; lane 6, para-Forssman pentaglycosylceramide (GalNAcβ3GalNAcβ3 Galα4 Galβ4Glcβ1Cer) from human erythrocytes, 4 μg. Autoradiography was performed for 48 h. (C) MS2 spectrum of the [M-H]− ion of m/z 706 of the saccharide derived from the Borrelia-binding GSL isolated from human erythrocytes (retention time 29 min) (upper chart) and MS3 spectrum of the [M-H]− ion at m/z 382 (lower chart). (D) Anomeric region of the 500 MHz proton NMR spectrum of the Borrelia-binding GSL fraction isolated from human erythrocytes (30 °C).
The proton NMR spectrum of the pure Borrelia-binding compound (Fig. 5D) reveals a single four-sugar structure having β-anomeric signals at 4.647 ppm, 4.249 ppm, 4.199 ppm, and 4.160 ppm, corresponding to sugar residues GlcNAcβ3, Galβ4, Galβ4, and Glcβ1, respectively, establishing the sequence Galβ4 GlcNAcβ3 Galβ4 Glcβ1Cer of neolactotetraosylceramide (17).
Thus, results from mass spectrometry and proton NMR spectroscopy established the Borrelia-binding GSL structure of human erythrocytes as Galβ4GlcNAcβ3Galβ4Glcβ1Cer, i.e., neolactotetraosylceramide, which previously has been identified on both human erythrocytes and neutrophils (14, 15).
Comparison of Differential Binding to GSLs Structurally Similar to Neolactotetraosylceramide.
The Borrelia binding activities of GSLs structurally related to Galβ4GlcNAcβ3Galβ4Glcβ1Cer were thereafter examined (exemplified in Figs. 5B and 6 and summarized in [Supporting Information (SI) Table S1]). Although the majority of the tested GSLs were nonbinding, neolactotetraosylceramide was recognized by all RF Borrelia species tested, B. duttonii (Figs. 3 and 6), B. crocidurae (Figs. 3 and 5), B. hermsii (Fig. 6), and B. recurrentis. The isomer lactotetraosylceramide (Galβ3GlcNAcβ3Galβ4Glcβ1Cer) was also bound by the four strains (exemplified in Fig. 6, lane 1). Thus, both type 1 (Galβ3GlcNAc) and type 2 (Galβ4GlcNAc) chains were recognized.
Fig. 6.
Comparison of Borrelia binding to pure GSLs structurally related to neolactotetraosylceramide. Binding of Borrelia to GSLs on thin-layer chromatograms. Chemical detection by anisaldehyde (A), and autoradiograms after adherence of 35S-labeled B. duttonii strain 1120 (B), and B. hermsii strain HS1 (C). Lane 1, lactosylceramide (Galβ4Glcβ1Cer) with nonhydroxy ceramide, 4 μg, and lactotetraosylceramide (Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 2, lactosylceramide (Galβ4Glcβ1Cer) with hydroxy ceramide, 4 μg, and B5 pentaglycosylceramide (Galα3Galβ4 GlcNAcβ3 Galβ4Glcβ1Cer), 4 μg; lane 3, H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3 Galβ4 Glcβ1 Cer), 4 μg; lane 4, B type 2 hexaglycosylceramide, (Galα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 5, A type 2 hexaglycosylceramide (GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 6, sialyl-neolactotetraosylceramide (NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 4 μg. Autoradiography was performed for 48 h.
Although binding to neolactotetraosylceramide and lactotetraosylceramide occurred consistently, occasional binding to other GSLs also occurred. For instance, B. recurrentis occasionally bound to the blood group H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) and the B5 pentaglycosylceramide (GalαGalβ4GlcNAcβ3Galβ4Glcβ1Cer) (Table S1). The B5 pentaglycosylceramide was also occasionally recognized by B. duttonii (Table S1). Thus, substitutions at the 2- and 3-positions of the terminal Gal of neolactotetraosylceramide were at least partly tolerated.
Neolacto-Bearing Neisseria Interacts with Borrelia.
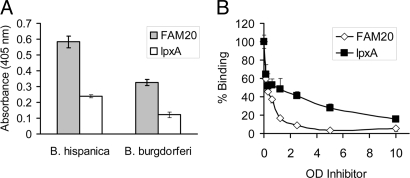
An abundant source of neolacto sequences is the lipooligosaccharide (LOS) of Neisseria meningiditis or N. gonorrheoae. To support our chromatogram binding results, we developed an enzyme-linked immuno sorbent assay (ELISA)–like assay whereby whole borreliae are immobilized in microtiter wells and attachment of biotinylated Neisseria is measured chromagenically. Wild-type Neisseria strain FAM20 attached to B. hispanica, whereas the LOS mutant ΔlpxA did not (Fig. 7A). Both strains attached poorly to the nonrosetting B. burgdorferi compared with the high-rosetting B. hispanica.
Fig. 7.
Neolactotetra saccharide-bearing Neisseria attach to immobilized RF Borrelia. N. meningitidis FAM20 (wild-type) and ΔlpxA (LOS mutant) were incubated with immobilized B. hispanica CR1 and B. burgdorferi B31 (A). Attachment of N. meningitidis FAM20 to immobilized Borrelia is only partially inhibited by the N. meningitidis ΔlpxA mutant (B). Standard deviations are shown.
Inhibition of biotinylated wild-type Neisseria attachment to B. hispanica was greater with unlabeled strain FAM20 than with ΔlpxA (Fig. 7B). The ability of strain ΔlpxA to partially block the interaction between Borrelia and neolacto-bearing Neisseria likely represents background due to the presence of other surface-exposed components. The low level of attachment of biotinylated FAM20 to B. burgdorferi (Fig. 7A) is due to nonspecific interactions, as it could not be inhibited by unlabeled FAM20.
Borrelia–Neolacto Interaction Is Specific and Requires Multivalent Neolacto Glycan Epitopes.
We attempted to analyze the affinity of neolacto glycans to Borrelia by RIA; however, no stronger binding of RF Borrelia compared with the negative control B. burgdorferi could be detected. The low density of the soluble glycan used in RIA prompted us to consider that multivalent interaction may be necessary.
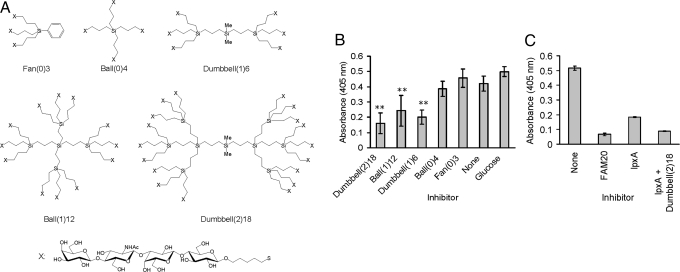
To assess whether multivalent neolactotetrasaccharides were more effective mediators of Borrelia interaction, we tested the ability of pure neolactotetrasaccharide clusters to inhibit attachment of biotinylated Neisseria to Borrelia. Clustered groups of 3, 4, 6, 12, or 18 neolactotetra epitopes were synthesized on carbosilane scaffolds, yielding glycoclusters structured as fan, ball, or dumbbell configurations (Fig. 8A). The glycoclusters bearing more neolactotetrasaccharide epitopes, Dumbbell (1)6, Ball (1)12, and Dumbbell (2)18, were effective inhibitors, whereas those with lower valencies, Fan (0)3, Ball (0)4, were not (Fig. 8B). Dumbbell (1)6, Ball (1)12, and Dumbbell (2)18 decreased Neisseria attachment to Borrelia by 52%, 43%, and 62%, respectively.
Fig. 8.
Synthetic neolactotetra glycoclusters inhibit attachment of neolactotetra-bearing Neisseria to immobilized Borrelia. Structures of glycoclusters bearing 3, 4, 6, 12, or 18 neolactotetra moieties synthesized on a carbosilane core (A). Attachment of Neisseria to Borrelia can be inhibited by glycoclusters bearing 12 or 18 but not 3, 4, or 6 neolactotetra moieties (B). Attachment of Neisseria to Borrelia is completely inhibited by Dumbbell (2)18 and N. meningitidis LOS mutant ΔlpxA (C). Standard deviations are shown. **, P <0.01.
Exogenous Neolactotetrasaccharide Complements the ΔlpxA Defect.
Attachment of labeled Neisseria (wild-type FAM20) to Borrelia could be completely inhibited by unlabeled FAM20 but could only partially be inhibited by the LOS mutant ΔlpxA (Fig. 8B). To investigate whether we could mimic wild-type inhibition, we complemented in trans the defect of ΔlpxA by adding neolactotetrasaccharide clusters exogenously. The combination of ΔlpxA and Dumbbell (2)18 was able to block Neisseria attachment to Borrelia to the same extent as the wild type (Fig. 8C). These results indicate that the difference in inhibition between wild-type and mutant Neisseria is caused by the neolactotetra sequence.
Discussion
Interaction of pathogens with red blood cells is a common phenomenon of bloodborne infections, including those caused by Plasmodium falciparum, Bartonella bacilliformis, Babesia spp. in addition to RF Borrelia spp. In the present study, an RF Borrelia-binding GSL was isolated from human erythrocytes and characterized by mass spectrometry and proton NMR as neolactotetraosylceramide. Spirochete binding to the isomeric compound lactotetraosylceramide was also observed, demonstrating that RF Borrelia does not discriminate between type 1 (Galβ3GlcNAc) and type 2 (Galβ4GlcNAc) chains. The terminal Galβ4GlcNAcβ sequence of neolactotetraosylceramide is a common core structure in carbohydrate chains of glycoproteins (18). Neolactotetraosylceramide has been described in several human tissues, such as erythrocytes (15), neutrophils (14), brain (19), stomach (10), and semen (20). This broad tissue distribution stands in sharp contrast to the distribution of lactotetraosylceramide, which only has been chemically identified in the human gastrointestinal tract (10, 21).
All RF Borrelia species tested, B. duttonii, B. crocidurae, B. hermsii, and B. recurrentis, had neolactotetraosylceramide binding capacity. In contrast, neolactotetraosylceramide was not recognized by B. burgdorferi, a related species causing Lyme disease and not demonstrating erythrocyte rosetting capacity. This distinction makes B. burgdorferi a useful control. Unlike RF Borrelia, B. burgdorferi is rarely found in blood, and therefore has little opportunity or advantage in interacting with RBCs. We chose these species to represent two rosetting (B. duttonii and B. crocidurae) and two nonrosetting (B. hermsii and B. recurrentis) isolates. During our investigation, it came to light that our B. crocidurae strain had lost reproducible rosetting competence (Fig. 1).
Interestingly, even nonrosetting species in this study (B. crocidurae, B. hermsii, B. recurrentis) retained the ability to bind to neolacto glycan. It is possible that these “nonrosetting” species rosette transiently or at a level below our limit of detection. Alternatively, the capacity to recognize these host glycans may serve an additional function. Multifunctional capabilities would fit with the profile of RF borreliae as bacteria with broad host range and broad tissue tropism despite a small genome. Rosette formation is likely a complex event governed by more than simple receptor-ligand recognition. A nonrosetting phenotype could therefore derive from loss of a separate but essential function, perhaps through repeated laboratory cultivation. Together, these observations suggest that neolacto glycan binding is necessary but not sufficient for erythrocyte rosetting.
The presence of neolactotetraosylceramide on human neutrophils makes the cells a potential target for rosetting. Nonetheless, neutrophil rosetting is not likely to be relevant in vivo. Opsonized neutrophils are potent killers of Borrelia; thus spirochetes would not survive an encounter for long. Neutrophil rosettes are not observed when blood is mixed with Borrelia in vitro, which is not surprising, because neutrophils in blood are outnumbered by RBCs by a factor of 1,000 and thus there is a low probability of neutrophils coming into contact with one another.
A requirement for multivalent interaction of neolacto glycan with Borrelia was established through assays with chemically synthesized glycoclusters and Neisseria bearing neolacto glycan. LOS mutant controls lack a lipid A biosynthesis enzyme encoded by lpxA, are Opa540 “on” and express reduced levels of iron-regulated proteins (7). The possibility of multivalency requirement was first brought to light through an inability to detect binding of radiolabeled neolactotetrasaccharides conjugated to human serum albumin (HSA) to RF agents over the negative control, B. burgdorferi, by RIA. The neolactotetrasaccharide:HSA ratio was ≈10:1 (IsoSep AB, Tullinge, Sweden), a low glycan density that, in solution, may not promote multivalent presentation in an RIA, which might explain lack of binding. In contrast, a higher local density of clustered epitopes was available on the thin-layer chromatograms as well as on the Neisseria cell surface, which could have mimicked solid-phase interactions inherently associated with high multivalency. An erythrocyte's ≈7-μm-diameter surface offers ample area for multiple epitope presentation along the spirochete's 20–30-μm length. Borrelia can potentially adjust the degree of adhesion by altering its surface contact area. This tunability is consistent with the in vitro observation that spirochetes can rapidly attach to and detach from RBCs, demonstrating reversible binding (Movie S1; note the strong, rolling adhesion between the single spirochete and RBC).
Neolacto recognition has previously been described for other microbes and microbial toxins, such as, dengue virus (22), S. pneumoniae (23), N. meningitidis (24), H. influenzae (24), H. pylori (25), and the heat-labile enterotoxin of E. coli (LT) (26). Dissociation constants have not been determined for any of these interactions, thus it is not known whether they represent low-affinity binding as in the case of RF Borrelia. The bacterial neolacto binding adhesins have not yet been identified either, but a comparison of crystal structures of LT and the neolacto-binding lectin from Erythrina cristagalli revealed that, despite very different overall topologies, the carbohydrate-binding sites of these proteins have a number of similar or conservatively replaced amino acids interacting with Galβ4GlcNAc (27). Whether the same structural features are used by the neolacto-binding adhesins of RF Borrelia should be the subject of further studies.
Formation of erythrocyte rosettes through Borrelia-erythrocyte adhesion is critical to pathogenesis of RF borreliosis. The potential to interfere with rosette formation hinges on identifying receptor-ligand(s) involved and elucidating the nature of their interaction. In this study, we have defined a neolacto glycan on human erythrocytes that adheres to RF Borrelia. Adherence requires multivalent epitope availability for tight association. These studies implicate neolacto glycan on erythrocyte membranes as a receptor for mediating attachment of RF Borrelia to RBCs during infection.
Materials and Methods
Bacterial Strains and Culture.
Borrelia strains used were as follows: B. hispanica CR1, B. duttonii 1120, B. coriaceae, B. crocidurae CR2A, B. hermsii HS1 serotype 7, B. recurrentis A11, B. turicatae 071, and low-passage B. burgdorferi B31. Borreliae were grown in BSK-II at 37 °C in 5% CO2. Neisseria meningitidis FAM20 serogroup C, serotype 2a (wild-type) and ΔlpxA (isogenic LOS mutant) (7) were kindly provided by Ann-Beth Jonsson, Uppsala University. N. meningitidis was grown in GC medium and heat-killed at 50 °C for 1 h. The cells were centrifuged at 3,000 g, washed three times with PBS, pH 7.4, and stored at −20 °C.
Red Blood Cells.
Blood, human ABO blood group O, was drawn into a citrate anticoagulant vacutainer (Becton Dickinson), washed three times with PBS using 30-min, 8,000-g centrifugations, and resuspended to 5% RBCs in RPMI, 10% FCS, and 2 mM glutamine. RBCs were stored at 4 °C up to 3 weeks.
Periodate Treatment of RBCs.
Carbohydrates on RBCs were subjected to periodate oxidation (8). A 1-ml quantity of stored RBCs was centrifuged at 8,000 g for 1 min. Cells were resuspended in 1 ml PBS or 1.25, 2.5, or 5 mM sodium m-periodate (Sigma-Aldrich) in PBS and incubated at room temperature with rocking. After centrifuging for 1 min at 8,000 g, cells were washed with 1 ml RPMI, 10% FCS, 2 mM glutamine (RFG), and resuspended in 1 ml RFG. Treated RBCs were used immediately.
Erythrocyte Rosetting Assay.
Log-phase Borrelia were harvested by centrifugation at 8,000 g and resuspended to ≈109 spirochetes/ml in warm RPMI, 10% FCS, 2 mM glutamine (RFG). Spirochetes and RBCs were separately preincubated at 37 °C for 15 min. Next, 20-μl spirochetes and 40 μl RBCs were mixed in 0.2 ml PCR strip tubes (ThermoScientific) and incubated at 37 °C for 15 min. Afterward, 40 μl liquid was removed from the tubes without disturbing the rosettes at the bottom. A 50-μl quantity of warm RPMI was added, and the contents were mixed and allowed to stand for an additional 15 min at 37 °C. A 50-μl quantity of liquid was removed without disturbing the rosettes. A 200-μl quantity of water was added to lyse the RBCs, after which 50 μl lysed RBCs was taken into microtiter wells containing 50 μl of water. Absorbance (405 nm) was measured.
Reference GSLs.
Total acid and nonacid GSL fractions were obtained by standard procedures (9). The individual GSLs were isolated by repeated chromatography on silicic acid columns of the native GSL fractions or acetylated derivatives thereof. The identity of the purified GSLs was confirmed by mass spectrometry, proton NMR spectroscopy, and degradation studies, as outlined elsewhere (10).
TLC.
Aluminum- or glass-backed silica gel 60 high-performance TLC plates (Merck, Darmstadt, Germany) were used for TLC and eluted with chloroform/methanol/water (60:35:8, by volume) as solvent system. The different GSLs were applied to the plates in quantities of 14 μg pure GSLs and 40 μg GSL mixtures. Chemical detection was performed with anisaldehyde (11).
Borrelia Radiolabeling.
Borrelia grown to log phase were inoculated (1:12–1:24 dilution) into fresh BSK-II containing 10 μl Redivue Pro-Mix L [35S] in vitro Cell Labeling Mix (Amersham) and grown as described above. After 3 days (late log), bacteria were centrifuged at 5,500 g for 15 min, washed once in 10 ml PBS, centrifuged at 5,500 g for 10 min, and resuspended in 5 ml blocking buffer (PBS, 2% BSA, 0.1% Tween 20, 0.1% sodium azide). Radiolabeled Borrelia were used immediately in the chromatogram binding assay.
Chromatogram Binding Assay.
Binding of radiolabeled Borrelia to GSLs on thin-layer chromatograms was performed as described (10). Mixtures of GSLs (40 μg/lane) or pure compounds (1–4 μg/lane) were separated on aluminum-backed silica gel plates. Dried chromatograms were soaked for 1 min in diethylether/n-hexane (1:5, by volume) containing 0.5% (wt/vol) polyisobutylmethacrylate (Aldrich Chemical). After drying, chromatograms were coated with blocking buffer to block unspecific binding sites. Coating was done for 1–2 h at room temperature. Thereafter 35S-labeled spirochetes diluted in blocking buffer to ≈2 × 108 spirochetes/ml (1–5 × 106 cpm/ml) were gently applied onto the chromatograms for 2 h at room temperature. After being washed five times with PBS and dried, the plates were autoradiographed for 1–5 days using XAR-5 x-ray film (Eastman Kodak).
Neutrophil Rosetting.
Neutrophils were isolated according to the Polymorphprep (Axis-Shield PoC AS, Oslo, Norway) protocol. Log-phase B. hispanica CR1 were resuspended in fresh, warm BSK. Neutrophils were prewarmed before mixing with Borrelia on a glass slide. A coverslip sealed with nail polish was used to avoid dehydration. Rosettes were visualized immediately by light microscopy.
Isolation of the Borrelia-Binding GSL of Human Erythrocytes.
Subfractions migrating as tetra- to pentaglycosylceramides, from a previous preparation from 800 mg of nonacid GSLs from human blood group O erythrocytes (12), were tested for spirochete binding using the chromatogram binding assay. Distinct binding to one subfraction was detected. Proton NMR (see below) demonstrated the presence of neolactotetraosylceramide, the para-Forssman pentaglycosylceramide and H5 type 2 pentaglycosylceramide in the Borrelia-binding fraction. The binding-active subfraction (3.0 mg) was acetylated and separated on a 10-g Iatrobeads column, eluted with a gradient of methanol in chloroform (0–2% vol/vol). After deacetylation, dialysis, and pooling, 0.5 mg of the pure binding-active GSL was obtained.
Ceramide Glycanase Digestion and LC/MS.
A 50-μg quantity of the Borrelia-binding GSL from human erythrocytes was dissolved in 100 μl 0.05 M sodium acetate buffer, pH 5.0, containing 120 μg sodium cholate, and sonicated briefly. Thereafter, 1 mU of ceramide glycanase from the leech Macrobdella decora (Merck Biosciences) was added. The mixture was incubated at 37 °C for 24 h. The reaction was stopped by adding chloroform/methanol/water to the final proportions 8:4:3 (by volume). The oligosaccharide-containing upper phase thus obtained was separated from ceramides and detergent on a Sep-Pak C18 cartridge (Waters, Milford, MA). The eluant containing the oligosaccharides was dried under nitrogen and under vacuum.
For LC/MS, the GSL-derived saccharides were separated on a column (200 × 0.180 mm) with 5-μm porous graphite particles (Hypercarb, Thermo Scientific) and eluted with a linear gradient from 0%B to 45%B in 46 min (Solvent A, 8 mM NH4HCO3, and Solvent B, 20% 8 mM NH4HCO3/80% acetonitrile [by volume]). Eluted saccharides were analyzed in the negative mode on an LTQ linear ion trap mass spectrometer (Thermo Electron), using Xcalibur software.
Proton NMR Spectroscopy.
1H NMR spectra were acquired on a Varian 600-MHz spectrometer at 30 °C. Samples were dissolved in dimethyl sulfoxide/D2O (98:2 by volume) after deuterium exchange.
Biotinylation of Neisseria.
A 1-ml quantity of N. meningitidis FAM20 (OD600 = 10) in PBS was incubated with 20 μg EZ-Link Sulfo-NHS-LC-Biotin (Pierce) at 4 °C for 2 h. Unlabeled biotin was removed by washing three times with PBS, 100 mM glycine with centrifugation at 7,000 g for 5 min. The bacteria were resuspended in PBS, pH 8.0, and stored at −20 °C.
Neisseria Attachment Assay and Inhibition.
Late-log Borrelia were centrifuged at 8000 g, washed three times in PBS, and resuspended to A600 = 0.1. Borrelia (100 μl) were coated on Nunc Polysorp immunomodule (Thermo Fisher) microtiter wells at room temperature for 2 h or at 4 °C overnight. Wells were blocked with 5% nonfat bovine dry milk (bovine milk does not contain lacto-N-neotetraose) in PBS for 2 h at room temperature, then washed three times for 5 min each with PBS. Air-dried wells were stored covered at 4 °C. Biotinylated Neisseria (OD = 0.075) was incubated in the wells for 15 min. After washing with PBS three times for 5 min each, wells were incubated with streptavidin-conjugated alkaline phosphatase (Jackson ImmunoResearch) diluted 1:1,000 in PBS for 1 h. After washing with PBS three times for 5 min each, the wells were incubated for 30 min with 1 mg/ml phosphatase substrate (Sigma) dissolved in 1 M ethanolamine, 0.5 mM MgCl2, pH 9.8.
For inhibition, the inhibitor was preincubated in the wells for 2 h before adding biotinylated Neisseria.
Synthesis of Neolactotetra Glycoclusters.
Neolactotetra glycoclusters Fan (0)3, Ball (1), and Dumbbell (1)6 were synthesized on carbosilane dendrimers and purified as described previously (13). Glycoclusters Ball (1)12 and Dumbbell (2)18 were synthesized and purified using the same techniques.
Supplementary Material
Acknowledgments.
This study was supported by the Swedish Research Council (Grant No. 12628 [to S.T.], 07922 [to S.B.], and 11218 [to T.B.]), the Swedish Cancer Foundation (to S.T., T.B.), Magnus Bergvalls Foundation (to S.T.), Infection and Vaccinology and MicMan, Swedish Foundation for Strategic Research (SSF) (to S.B.), and Insamlingsstiftelsen (to B.P.G.). We thank Ann-Beth Jonsson for providing the Neisseria strains; Rolf Sjöström, and Ingela Nilsson for technical assistance; Edmund Loh and Barbro Lindmark for donating and drawing blood; and Marie Andersson and Simon Birve for help with video microscopy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The glycosphingolipid nomenclature follows the recommendations of the IUPAC-IUB Commission on Biochemical Nomenclature (28). It is assumed that NeuAc, NeuGc, Gal, Glc, GlcNAc, and GalNAc are of the D-configuration, Fuc of the L-configuration, and all sugars are present in the pyranose form.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905470106/DCSupplemental.
References
- 1.Burman N, Shamaei-Tousi A, Bergstrom S. The spirochete Borrelia crocidurae causes erythrocyte rosetting during relapsing fever. Infect Immun. 1998;66:815–819. doi: 10.1128/iai.66.2.815-819.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mooser H. Erythrocyte adhesion and hemoglomeration by relapsing fever spirochetes. Z Tropenmed Parasitol. 1958;9:93–111. [PubMed] [Google Scholar]
- 3.Shamaei-Tousi A, Collin O, Bergh A, Bergstrom S. Testicular damage by microcirculatory disruption and colonization of an immune-privileged site during Borrelia crocidurae infection. J Exp Med. 2001;193:995–1004. doi: 10.1084/jem.193.9.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamaei-Tousi A, Martin P, Bergh A, Burman N, Brannstrom T, Bergstrom S. Erythrocyte-aggregating relapsing fever spirochete Borrelia crocidurae induces formation of microemboli. J Infect Dis. 1999;180:1929–1938. doi: 10.1086/315118. [DOI] [PubMed] [Google Scholar]
- 5.Esko JD. Microbial Carbohydrate Binding Proteins. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 6.Ofek I, Doyle RJ. Bacterial Lectins as Adhesins. New York: Chapman & Hall; 1994. [Google Scholar]
- 7.Albiger B, Johansson L, Jonsson AB. Lipopolysaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect Immun. 2003;71:155–162. doi: 10.1128/IAI.71.1.155-162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk P, Boren T, Haslam D, Caparon M. Bacterial adhesion and colonization assays. Methods Cell Biol. 1994;45:165–192. doi: 10.1016/s0091-679x(08)61851-8. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson KA. Preparation of total nonacid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- 10.Teneberg S, Leonardsson I, Karlsson H, Jovall PA, Angstrom J, et al. Lactotetraosylceramide, a novel glycosphingolipid receptor for Helicobacter pylori, present in human gastric epithelium. J Biol Chem. 2002;277:19709–19719. doi: 10.1074/jbc.M201113200. [DOI] [PubMed] [Google Scholar]
- 11.Waldi D. Spray Reagents for Thin-Layer Chromatography. Berlin: Springer-Verlag; 1982. [Translated from German] [Google Scholar]
- 12.Teneberg S, Lonnroth I, Torres Lopez JF, Galili U, Halvarsson MO, et al. Molecular mimicry in the recognition of glycosphingolipids by Gal alpha 3 Gal beta 4 GlcNac beta-binding Clostridium difficile toxin A, human natural anti alpha-galactosyl IgG and the monoclonal antibody Gal-13: Characterization of a binding-active human glycosphingolipid, non-identical with the animal receptor. Glycobiology. 1996;6:599–609. doi: 10.1093/glycob/6.6.599. [DOI] [PubMed] [Google Scholar]
- 13.Yamada A, Hatano K, Koyama T, Matsuoka K, Esumi Y, Terunuma D. Syntheses of a series of lacto-N-neotetraose clusters using a carbosilane dendrimer scaffold. Carbohydr Res. 2006;341:467–473. doi: 10.1016/j.carres.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Macher BA, Klock JC. Isolation and chemical characterization of neutral glycosphingolipids of human neutrophils. J Biol Chem. 1980;255:2092–2096. [PubMed] [Google Scholar]
- 15.Siddiqui B, Hakomori S. A ceramide tetrasaccharide of human erythrocyte membrane reacting with anti-type XIV pneumococcal polysaccharide antiserum. Biochim Biophys Acta. 1973;330:147–155. doi: 10.1016/0005-2736(73)90219-8. [DOI] [PubMed] [Google Scholar]
- 16.Chai W, Piskarev V, Lawson AM. Negative-ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal Chem. 2001;73:651–657. doi: 10.1021/ac0010126. [DOI] [PubMed] [Google Scholar]
- 17.Levery SB, Nudelman E, Kannagi R, Symington FW, Andersen NH, et al. 1H-n. m.r. analysis of type-2 chain lacto-gangliosides. Carbohydr Res. 1988;178:121–144. doi: 10.1016/0008-6215(88)80106-x. [DOI] [PubMed] [Google Scholar]
- 18.Montreuil J. Spatial conformation of glycans and glycoproteins. Biol Cell. 1984;51:115–131. doi: 10.1111/j.1768-322x.1984.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa Y, Gasa S, Minami R, Makita A. Characterization of neutral glycosphingolipids from fetal human brain: Evidence for stage-specific expression of the globo, ganglio, and neolacto series in the central nervous system. J Biochem. 1987;101:1369–1375. doi: 10.1093/oxfordjournals.jbchem.a122005. [DOI] [PubMed] [Google Scholar]
- 20.Ritter G, Krause W, Geyer R, Stirm S, Wiegandt H. Glycosphingolipid composition of human semen. Arch Biochem Biophys. 1987;257:370–378. doi: 10.1016/0003-9861(87)90579-0. [DOI] [PubMed] [Google Scholar]
- 21.Bjork S, Breimer ME, Hansson GC, Karlsson KA, Leffler H. Structure of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J Biol Chem. 1987;262:6758–6765. [PubMed] [Google Scholar]
- 22.Aoki C, Hidari KI, Itonori S, Yamada A, Takahashi N, et al. Identification and characterization of carbohydrate molecules in mammalian cells recognized by dengue virus type 2. J Biochem. 2006;139:607–614. doi: 10.1093/jb/mvj067. [DOI] [PubMed] [Google Scholar]
- 23.Andersson B, Dahmen J, Frejd T, Leffler H, Magnusson G, et al. Identification of an active disaccharide unit of a glycoconjugate receptor for penumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983;158:559–570. doi: 10.1084/jem.158.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugosson S, Angstrom J, Olsson BM, Bergstrom J, Fredlund H, et al. Glycosphingolipid binding specificities of Neisseria meningitidis and Haemophilus influenzae: Detection, isolation, and characterization of a binding-active glycosphingolipid from human oropharyngeal epithelium. J Biochem. 1998;124:1138–1152. doi: 10.1093/oxfordjournals.jbchem.a022232. [DOI] [PubMed] [Google Scholar]
- 25.Miller-Podraza H, Lanne B, Angstrom J, Teneberg S, Milh MA, et al. Novel binding epitope for Helicobacter pylori found in neolacto carbohydrate chains: Structure and cross-binding properties. J Biol Chem. 2005;280:19695–19703. doi: 10.1074/jbc.M412688200. [DOI] [PubMed] [Google Scholar]
- 26.Teneberg S, Hirst TR, Angstrom J, Karlsson KA. Comparison of the glycolipid-binding specificities of cholera toxin and porcine Escherichia coli heat-labile enterotoxin: Identification of a receptor-active non-ganglioside glycolipid for the heat-labile toxin in infant rabbit small intestine. Glycoconj J. 1994;11:533–540. doi: 10.1007/BF00731304. [DOI] [PubMed] [Google Scholar]
- 27.Teneberg S, Berntsson A, Angstrom J. Common architecture of the primary galactose binding sites of Erythrina corallodendron lectin and heat-labile enterotoxin from Escherichia coli in relation to the binding of branched neolactohexaosylceramide. J Biochem. 2000;128:481–491. doi: 10.1093/oxfordjournals.jbchem.a022778. [DOI] [PubMed] [Google Scholar]
- 28.Chester MA. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids—recommendations 1997. Eur J Biochem. 1998;257:293–298. doi: 10.1046/j.1432-1327.1998.2570293.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.