Summary
Introduction
Meniscal damage is common in knee Osteoarthritis (OA) and predictive of structural progression, suggesting that their disruption plays a role in the development of OA. The bone marrow lesion (BML) is associated with pain and is a strong risk factor for structural progression. These lesions are associated with abnormal loading in a knee joint. Therefore, our hypothesis was that in those with symptomatic knee OA, large BMLs would be associated with ipsi-compartmental meniscal derangement.
Methods
This was a cross-sectional study of a subsample of the Osteoarthritis Initiative where one set of magnetic resonance (MR) images from each participant was scored for tibiofemoral BMLs and meniscal derangement. We performed chi-squared tests comparing the prevalence of large BMLs in those with ipsi-compartmental meniscal derangement and those without.
Results
160 Participants had a mean age of 61 (±9.9), mean BMI of 30.3 (±4.7) and 50% were female. 79% of medial and 39% of lateral menisci showed MRI (Magnetic Resonance Imaging) derangement. In those with medial meniscal MRI derangement, 44% had large medial BMLs while in those without medial meniscal derangement, 0% had large BMLs. Similar results were seen in the lateral compartment.
Conclusion
Medial and lateral MRI meniscal derangement are highly prevalent in symptomatic knee OA and BMLs are highly associated with ipsi-compartmental MRI meniscal derangement.
Keywords: Meniscus, Bone marrow lesion, MRI
Introduction
Osteoarthritis (OA) is the most common form of arthritis1 and is one of the most common causes of disability in the elderly2. Our limited understanding of the pathophysiology of this disease has been an obstacle in identifying effective treatments for knee OA.
Menisci are fibrocartilaginous structures located between the tibia and the femur and attached to the superior aspect of the tibial plateau (Fig. 1)3. A knee without menisci experiences twice the peak pressures with loading as compared to a knee with intact menisci4,5 since menisci play a large role in load distribution within this joint. Meniscal damage is common in knee OA6–11 and predictive of longitudinal ipsi-compartmental articular cartilage damage9. The fact that those with a partial or complete meniscectomy are at higher risk for developing knee OA7,10,12,13 suggests that a loss of the menisci’s ability to optimally distribute load within the knee is important to the development of knee OA.
Fig. 1.
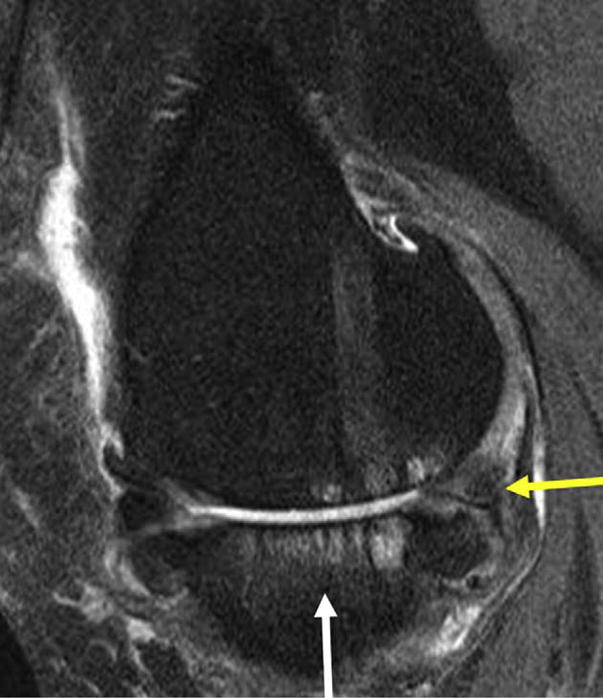
Sagittal view of the medial TF compartment. Posterior medial MRI meniscal derangement (yellow arrow). Medial tibial BML (white arrow).
The bone marrow lesion (BML), irregular hyperintense signal in the subchondral bone seen on T2 weighted fat-suppressed (FS) MRIs, is a feature associated with OA that has only been identified since MRIs have been used to image joints (Fig. 1)14–20. A great deal of attention has been focused on this feature in OA as it has been associated with pain in OA14,21,22 in two separate epidemiologic studies of people with established knee OA, and in another study it has been identified as a strong risk factor for longitudinal worsening of joint space narrowing and articular cartilage damage, consistent with structural progression in this disease16,17. BMLs probably represent a consequence of loading since knees with varus alignment have a higher prevalence of medial tibiofemoral (TF) BMLs while those with valgus alignment have a higher prevalence of lateral BMLs16.
Meniscal damage6–10 and BMLs14–20 are both common in knee OA and predictive of progression of structural damage9,16,17. Since menisci have load distribution properties and BMLs are associated with abnormal loading within a knee, these phenomena may be associated with one another. Therefore, our hypothesis was that in those with symptomatic knee OA, large BMLs would be associated with ipsi-compartmental MRI meniscal derangement.
Methods
SAMPLE SELECTION
The Osteoarthritis Initiative (OAI) is a publicly available multi-center observational study of knee OA of 4796 participants, which is comprised of three groups, the progression (N = 1,389), the incidence (N = 3,285), and a non-exposed control group (N = 122). For this study, we specifically focused on the baseline assessments of the progression cohort, a group identified using the inclusion criteria of ages 45 – 79; and having at least one knee with both radiographic evidence of knee OA [Osteoarthritis Research Society International (OARSI) atlas osteophyte grades 1–3] and symptoms as defined by answering affirmative to the question “During the past 12 months, have you had pain, aching or stiffness in and around (the respective knee) on most days of the month? By most days, we mean more than half the days of a month”. Those with evidence of severe joint space narrowing in both knees were excluded.
These participants received assessments of knee-specific Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) pain, posteroanterior (PA) semi-flexed radiographs of bilateral knees, and 3.0 T MRIs of bilateral knees. Each MRI was obtained on one of four identical Siemens Trio 3 T MRIs at one of the four clinical sites, Memorial Hospital of Rhode Island (Pawtucket, RI,) Ohio State University (Columbus, OH), University of Pittsburgh (Pittsburgh, PA), and University of Maryland/Johns Hopkins University (Baltimore, MD).
From the 1389 participants in the progression cohort, a convenience sample of 160 were identified as all of those included in of one of the first data releases of the OAI, data set 0.B.1, blocked for sex, ethnicity, and clinical center, all of whom had complete baseline and year 1 follow-up MRIs. Baseline demographic data were obtained from data set 0.1.1, accessed from the website, http://www.oai.ucsf.edu/datarelease/.
KNEE SELECTION
We chose one knee to evaluate per participant. The goal of this selection process was to identify knees that were symptomatic with radiographic evidence of OA, minimizing the inclusion of those with end-stage radiographic OA. Eligible knees were those with symptomatic radiographic OA. For individuals with bilateral symptomatic radiographic OA, we selected the knee with radiographic appearances that offered the greatest opportunity for detection of progression, preferentially selecting those with a Kellgren/Lawrence (KL) score grades 2 or 3, with a greater anatomic axis varus angulation, and with medial TF OA.
The radiographic assessments from one unadjudicated reader (DH) were available and used to select the knee from a participant used in this study. In the 100 patients with unilateral symptomatic ROA, this knee was chosen for analysis, regardless of radiographic severity. For the remaining participants with bilateral symptomatic ROA, one knee was selected, favoring the knee with moderate disease. In patients with bilateral symptomatic ROA, if only one knee had KL grade 2 or 3 grade, then that knee was selected. If both knees had KL grade 2 or 3, then the knee with the greatest extent of each of the following features was selected, moving to the next feature if the knees were still ranked as equal:
❖ Greater anatomic axis varus angulation
❖ ≥2.0 mm Medial minimum Joint Space Width (JSW)
❖ Greater grade of medial Joint Space Narrowing (grades 1–3)
❖ The presence of any medial tibial or femoral osteophyte grade ≥ 2 with greater grade than lateral osteophytes
❖ The presence of any medial tibial or femoral osteophyte
❖ The right knee
If neither knee was KL grade 2 or 3, then the knee with the higher KL grade was chosen. If the patient had bilateral KL grade 0, 1 or 4 knees, then the process used to select among bilateral KL grade 2 or 3 knees was followed. Next, knees were listed on the basis of decreasing KL grade, and within KL grade by decreasing varus angulation.
MRI READING
The time point of interest for this study was the baseline visit. One rheumatologist (GHL) experienced in musculoskeletal MRI readings and centrally involved in the development of the Boston Leeds Osteoarthritis Knee Score (BLOKS)21 who was blinded to subject data and scored each knee MRI for BMLs and MRI meniscal derangement. BMLs and menisci were scored in separate reading sessions.
We defined a BML as an irregular hyperintense signal in the subchondral bone, proximal to the epiphyseal line, as seen on sagittal intermediate-weighted (IW), Turbo Spin Echo (TSE), FS, time to recovery (TR) of 3200 ms, time to echo (TE) of 30 ms, slice thickness of 3 mm, and field of view (FOV) of 160 mm (Table I). We also used Dual Echo in the Steady State (DESS) sequences to assist with localization of some lesions (Table I). BMLs were scored for size (0–3) at each of nine locations, medial and lateral patella, medial and lateral trochlea, medial and lateral weight-bearing femur, and medial, subspinous, and lateral tibia using BLOKS. Only those BMLs with greater than 25% of the surface area adjacent to the subchondral plate were included. We classified large BMLs as those with BLOKS score ≥ 2. To assess intra-rater reliability, a sample of 10 knee MRIs were read for BMLs twice by the same reader separated by at least 2 days for a weighted kappa of 0.88.
Table I.
MRI parameters
| 3-Plane | 2D TSE | 3D DESS | 2D TSE | |
|---|---|---|---|---|
| Weighting | T1W | Int | T2 | Int |
| Plane | 3-Plane | Coronal | Sag | Sagittal |
| Fat sat | No | No | WE | Yes |
| Matrix (phase) | 256 | 307 | 307 | 313 |
| Matrix (freq) | 512 | 384 | 384 | 448 |
| No. of slices | 21 | 41 | 160 | 37 |
| FOV (mm) | 200 | 140 | 140 | 160 |
| Slice thickness (mm) | 5 | 3 | 0.7 | 3 |
| Skip (mm) | 1 | 0 | 0 | 0 |
| Flip angle (°) | 40 | 180 | 25 | 180 |
| TE/TI (ms) | 5 | 29 | 4.7 | 30 |
| TR (ms) | 10 | 3850 | 16.3 | 3200 |
| BW (Hz/pixel) | 250 | 352 | 185 | 248 |
| Chemical shift (pixels) | 1.8 | 1.3 | 0 | 0 |
| NAV (NEX) | 1 | 1 | 1 | 1 |
| Echo train length | 1 | 7 | 1 | 5 |
| Phase encode axis | A/P, R/L | R/L | A/P | S/I |
| Phase partial fourier (8/8 = 1) | 1 | 1 | 0.875 | 1 |
| Readout partial fourier (8/8 = 1) | 1 | 1 | 1.000 | 1 |
| Slice partial fourier (8/8 = 1) | 1 | 1 | 0.875 | 1 |
| Options: | Elliptical k-space filter and large FOV filter | Elliptical k-space filter, elliptical sampling, large FOV filter | Elliptical k-space filter and large FOV filter | |
| Distance factor (%) | 50 | 0 | 0 | 0 |
| Phase oversampling | 0 | 20 | 0 | 4 |
| Slice oversampling | 0 | 0 | 10 | 0 |
| Phase resolution | 50 | 80 | 80 | 70 |
| Averaging technique | Short term | Short term | Short term | Short term |
| Gradient rise time | Fast | Fast | Fast | Fast |
| RF amplitude | Normal | Normal | Fat | Normal |
| X-resolution (mm) | 0.391 | 0.365 | 0.365 | 0.357 |
| Y-resolution (mm) | 0.781 | 0.456 | 0.456 | 0.511 |
| Calc time (min) | 2.7 | 3.4 | 11.2 | 4.7 |
| Scan time (min) | 0.5 | 3.4 | 10.6 | 4.7 |
We scored menisci using the same sequences used to evaluate BMLs in addition to the coronal IW 2D TSE, TR of 3850 ms, TE of 29 ms, slice thickness of 3 mm, and FOV of 140 mm. MRI meniscal derangement was defined as those with disruption of the overall morphology of the meniscus and diffuse hyperintense signal in the body of the meniscus. MRI meniscal derangement was graded at each of three locations (anterior, central, and posterior horns) in the medial and lateral meniscus. To assess intra-rater reliability, a sample of 10 knee MRIs were read for MRI meniscal derangement twice by the same reader separated by at least 2 days for a simple kappa of 0.87.
STATISTICAL ANALYSIS
We performed a cross-tabulation of medial MRI meniscal derangement and lateral MRI meniscal derangement. We then evaluated the prevalence of large medial tibio-femoral (TF) BMLs (including large BMLs in the medial weight-bearing femur and the medial tibia) stratified by the extent of medial MRI meniscal derangement by location: anterior, central, and posterior. We repeated the analyses evaluating the lateral TF BMLs as they related to lateral MRI meniscal derangement. We performed the chi-squared test to evaluate test significance of difference in prevalence of large BMLs among those with and without MRI meniscal derangement. We repeated the analyses in the lateral TF compartment, and then evaluated all BMLs [e.g., those with BLOKS score ≥ 1 (as opposed to only looking at large BMLs, those with a BLOKS score ≥ 2)]. We did evaluate age, sex, and BMI were as covariates in multivariate models.
Statistical analyses were performed using the SAS system for Windows (version 9.1; SAS Institute, Cary, NC). P-values ≤ 0.05 were considered statistically significant.
Results
160 Participants had a mean age of 61 (±9.9), mean BMI of 30.3 (±4.7) and 50% were female. 44/160 (27.5%) had large medial TF BMLs while 20/160 (12.5%) had large lateral TF BMLs. A detailed description of the prevalence of MRI meniscal derangement by region and type of damage is presented in Table II. The regions most frequently damaged were the medial central (67.5%) and medial posterior (73.5%) menisci. MRI meniscal derangement in at least one portion of the meniscus was present in 78.8% of medial and 39.4% of lateral meniscus with 29.4% of knees having both medial and lateral MRI meniscal derangement and 11.8% with neither meniscus showing MRI derangement (Table III).
Table II.
Prevalence of MRI meniscal derangement by location
| Medial meniscus | |
| Anterior MRI meniscal derangement | 63/160 (39.4%) |
| Central MRI meniscal derangement | 108/160 (67.5%) |
| Posterior MRI meniscal derangement | 117/160 (73.1%) |
| Lateral meniscus | |
| Anterior MRI meniscal derangement | 37/160 (23.1%) |
| Central MRI meniscal derangement | 38/160 (23.8%) |
| Posterior MRI meniscal derangement | 41/160 (25.6%) |
Table III.
Relationship of medial with lateral MRI meniscal derangement
| Any anterior, central, or posterior MEDIAL MRI meniscal derangement |
||||
|---|---|---|---|---|
| Present | Absent | Total | ||
| Any anterior, central, or posterior | Present | 29.4% (47/160) | 9.4% (15/160) | 38.8% (62/160) |
| LATERAL MRI meniscal derangement | Absent | 49.4% (79/160) | 11.8% (19/160) | 61.2% (98/160) |
| Total | 78.8% (126/160) | 21.2% (34/160) | ||
There was an increased prevalence of large medial TF BML if the medial MRI meniscal derangement occurred in the anterior, body, or posterior portions of the meniscus (Table IV). In fact, of those with MRI derangement of any portion of the medial meniscus, 34.9% had a large medial TF BML as compared with 0% of those without medial MRI meniscal derangement (Table IV). Similarly, the prevalence of large lateral TF BML was increased if lateral MRI meniscal derangement occurred in the anterior, body, or posterior portions of the meniscus (Table IV). When evaluating those with MRI derangement in any region of the lateral meniscus, 22.6% had a large lateral TF BML compared with 6.6% of those without lateral MRI meniscal derangement (Table IV).
Table IV.
Prevalence of large BMLs stratified by the presence or the absence of region-specific MRI meniscal derangement
| Large medial BML prevalence | P-value | ||
|---|---|---|---|
| Anterior medial MRI meniscal derangement | Absent | 11/97 (11.3%) | <0.0001 |
| Present | 33/63 (52.4%) | ||
| Central medial MRI meniscal derangement | Absent | 2/52 (3.9%) | <0.0001 |
| Present | 42/108 (38.9%) | ||
| Posterior medial MRI meniscal derangement | Absent | 1/43 (2.2%) | <0.0001 |
| Present | 43/117 (36.8%) | ||
| Composite medial MRI meniscal derangement | Absent | 0/34 (0%) | <0.0001 |
| Present | 44/126 (34.9%) | ||
| Large lateral BML prevalence | P-value | ||
| Anterior lateral MRI meniscal derangement | Absent | 9/123 (7.3%) | 0.0003 |
| Present | 11/37 (29.7%) | ||
| Central lateral MRI meniscal derangement | Absent | 9/122 (7.4%) | 0.0004 |
| Present | 11/38 (29.0%) | ||
| Posterior lateral MRI meniscal derangement | Absent | 9/119 (7.6%) | 0.0013 |
| Present | 11/41 (26.8%) | ||
| Composite lateral MRI meniscal derangement | Absent | 6/98 (6.1%) | 0.0022 |
| Present | 14/62 (22.6%) | ||
Results evaluating all BMLs were similar to those evaluating large BMLs (not shown). The multivariate model adding age, sex, and BMI as covariates did not substantially alter these results illustrating that the bivariate results were unconfounded.
Discussion
MRI meniscal derangement, especially the medial meniscus, is very common among those with symptomatic knee OA, being present in nearly 80% of all knees in this study, with most of the damage occurring in the medial body and medial posterior menisci. The fact that 70% of the load within a knee passes through the medial TF compartment23,24 may predispose the medial meniscus to damage. Meniscal damage might itself add to the medial loading3,4 through its biomechanical consequences leading to a self-perpetuating cycle.
In addition, TF BMLs are highly associated with ipsi-compartmental MRI meniscal derangement, especially in the medial TF compartment. In fact, large medial BMLs were present only if some part of the medial meniscus was damaged. Conversely, we found no large medial BMLs in those knees without medial MRI meniscal derangement. We found a similar pattern in the lateral compartment where 22.6% of knees with lateral MRI meniscal derangement had large lateral BMLs as compared to only 6.1% of those knees without lateral MRI meniscal derangement having large lateral BMLs.
It is also interesting to note that the prevalence of large medial BMLs was much higher in knees with MRI derangement in the body and posterior portions of the medial menisci, relative to knees without meniscal derangement in these regions. These were the same regions of menisci that were the most commonly showing MRI derangement. Therefore, the areas of menisci that were most commonly damaged also were the most likely to be associated with large ipsi-compartmental BMLs.
In this study, we defined MRI meniscal derangement as those with disruption of the overall morphology of the meniscus and diffuse hyperintense signal in the body of the meniscus. This is a new definition of MRI meniscal derangement that has not been previously described. We used the definition of MRI meniscal derangement because this provided a straight-forward method of identifying pathology occurring in the meniscus, obviating the need to make the distinction between a meniscal tear and meniscal maceration which can often be difficult. In fact, reliability for this finding was high25 (intra-rater kappa of 0.87) in our study. We also felt that those knees with evidence of MRI meniscal derangement by our definition were likely the ones that were pathologic. In fact, in our study, we found that this measure was indeed associated with large BMLs, supporting our expectation. While this measure does not discriminate between meniscal tear and maceration, it is a reliable scoring method that has content validity.
A limitation to our study is that BMLs and MRI meniscal derangement have been assessed using some overlapping MRI sequences which could potentially bias our findings towards a positive association. This is an inherent limitation to any study where two radiographically defined features are being evaluated against one another. Methods of potentially minimizing this possible bias would be to have two different readers assess the different radiographic features who were not aware of the hypothesis. However, because we had limited resources to conduct this study, the same reader did perform both BML and MRI meniscal derangement readings and she was aware of the study hypothesis. To try and minimize this potential bias, the reader did perform the BMLs and the MRI meniscal damage assessments at different reading sessions. Further, when reading BMLs, the reader was blinded to the reading for MRI meniscal damage and vice versa. Also the fact that the image contrast level that allows for optimal viewing of the MRI sequences for BMLs is quite different than that used for reading MRI meniscal derangement reduced the likelihood of infusing bias into these study results.
An additional limitation to this study is its cross-sectional design; as a result, we cannot make any temporal inferences from our findings. However, these findings are hypothesis-generating. The fact that all the knees with large BMLs had some medial MRI meniscal derangement and that not all knees with medial MRI meniscal derangement had large BMLs raises the possibility that MRI meniscal derangement is upstream in the chain of pathological events that leads to the occurrence of BMLs. Another possibility is that BMLs and MRI meniscal derangement are independent consequences of TF impact forces (with larger forces being required to develop a large BML). These possible relationships need to be tested in longitudinal studies. Better clarification of the causal pathway involving meniscal and BML pathology will potentially be helpful in identifying potential treatments for this debilitating disease.
BMLs have been associated with pain in knee OA14,22,26. We and others11 have shown that those with knee OA have a high prevalence of meniscal damage. Our study has illustrated that MRI meniscal derangement is strongly associated ipsi-compartmentally with large BMLs. Interestingly, other studies suggests that meniscal damage is not a cause of knee pain6,11. This presents a possible opportunity for early intervention. Those people without pain but with MRI meniscal derangement may be the people who could be targeted for interventions that could someday prevent the occurrence of large BMLs and in turn, hopefully prevent the development of symptoms related to knee OA. As both BMLs and meniscal damage have been associated with longitudinal structural progression, these same interventions may also have the benefit of preventing structural progression9,16,17. Again, longitudinal studies are needed to test these hypotheses.
In short, we have found that medial and lateral MRI meniscal derangement are highly prevalent in symptomatic knee OA and BMLs are highly associated with ipsi-compartmental MRI meniscal derangement cross-sectionally. These findings are hypothesis-generating. The relationship between BMLs and MRI meniscal derangement need to be further investigated in longitudinal studies.
Acknowledgments
We are indebted to the participants and staff of the OAI Study. Without their help, this study would not have been possible. The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Conflict of interest
The authors of this manuscript have nothing to declare. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51(6):871–9. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 4.Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5(2):157–61. doi: 10.1016/0141-5425(83)90036-5. [DOI] [PubMed] [Google Scholar]
- 5.Song Y, Greve JM, Carter DR, Koo S, Giori NJ. Articular cartilage MR imaging and thickness mapping of a loaded knee joint before and after meniscectomy. Osteoarthritis Cartilage. 2006;14(8):728–37. doi: 10.1016/j.joca.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007;56(12):4048–54. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 7.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178–87. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 8.Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9(4):316–24. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A(1):4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg. 1948;30-B:664. [PubMed] [Google Scholar]
- 13.Jackson JP. Degenerative changes in the knee after meniscectomy. Br Med J. 1968;2(604):525–7. doi: 10.1136/bmj.2.5604.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 15.McAlindon TE, Watt I, McCrae F, Goddard P, Dieppe PA. Magnetic resonance imaging in osteoarthritis of the knee: correlation with radiographic and scintigraphic findings. Ann Rheum Dis. 1991;50(1):14–9. doi: 10.1136/ard.50.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, Lavalley MP, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54(5):1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 18.Kijowski R, Stanton P, Fine J, De Smet A. Subchondral bone marrow edema in patients with degeneration of the articular cartilage of the knee joint. Radiology. 2006;238(3):943–9. doi: 10.1148/radiol.2382050122. [DOI] [PubMed] [Google Scholar]
- 19.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215(3):835–40. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 20.Sowers MF, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-de-fined knee osteoarthritis. Osteoarthritis Cartilage. 2003;11(6):387–93. doi: 10.1016/s1063-4584(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The development and reliability of a new scoring system for knee osteoarthritis MRI: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2007 doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 22.Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, et al. Strong association of bone marrow lesions and effusion with pain in osteoarthritis. Arthritis Rheum. 2007;56(9 Suppl) (Abstract #2077) [Google Scholar]
- 23.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25(3):395–403. [PubMed] [Google Scholar]
- 24.Schipplein O, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 26.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]


