Abstract
Lower extremity weakness has been documented in children with cerebral palsy (CP). However, the temporal characteristics of moment generation have not been characterized, and they may be important to function. This study tested ankle, knee, and hip flexion and extension moment generation capabilities in children with CP and in able-bodied children. Maximum voluntary isometric contractions (MVIC), the maximum rates of moment development and relaxation, and the time to produce and reduce the moments were quantified. Relationships between the temporal measures, Gross Motor Function Measure-66 (GMFM-66), and MVICs were also examined. Children with CP had significantly reduced MVICs, maximum development and relaxation rates, and increased times to produce and reduce moments. The maximum rates of moment development and relaxation at some joints were correlated with the GMFM-66 and MVICs. These results suggest that both the magnitude and temporal characteristics of moment generation need to be targeted during therapeutic interventions for children with CP.
Keywords: Cerebral Palsy, Joint Moment, Impairments, Lower Extremity, Weakness
Introduction
Maximum voluntary contractions, either isometric or isokinetic, are used to quantify force generating capacity, an important determinant of functional ability.7,8,13,16 The magnitude of force is critical, but force must also be generated in a timely manner in order to be useful for activities of daily living (ADLs).3 A number of populations with neurological impairments have decreased maximum voluntary contractions compared to able-bodied individuals.1 For example, children with cerebral palsy (CP) have significant amounts of lower extremity weakness, i.e. decreased maximum voluntary contractions, compared to their able-bodied peers.6,9,28 Even though these strength deficits have been correlated with functional activities such as gait and posture,5,10,13,16 most ADLs are performed using well-timed submaximal forces.23,24 Hence, the decrease in strength may not solely explain the functional difficulties, and other aspects of joint moment generation, in particular the temporal characteristics, should also be examined.3,6,17
The temporal characteristics of joint moment generation (i.e. the rates of force development) have been quantified in some populations with neurological impairments. Jayaraman et al.12 demonstrated that persons with incomplete spinal cord injury (SCI) had significantly decreased instantaneous plantarflexion moment (i.e. the moment available at 200 ms). Functionally, this altered moment profile would lead to an insufficient amount of plantarflexion moment available at the appropriate time in the gait cycle to safely ambulate in the community. Impaired rates of joint moment generation have also been shown in the upper extremities for persons with Parkinson's disease,4 and acute and chronic stroke.3,17 Different methods exist for characterizing the rates of joint moment development including instantaneous moment per time measures (e.g. Nm/s12) or the time taken to achieve a percent of maximal moment (e.g. the time from 10% to 70% of the maximum voluntary contraction17). The former is generally averaged over a brief time period to achieve an average rate of moment development, while the latter eliminates the effect of amplitude differences. Both measures have provided insight into moment generation capabilities that is not captured by solely quantifying maximum voluntary contractions.
While lower extremity weakness has been established in children with CP, the temporal characteristics of joint moment generation have not been examined. Given that children with CP have alterations in their muscular architecture11,15,18 and their ability to voluntarily activate a muscle,20,25 it would be expected that children with CP would also demonstrate alterations in their joint moment profiles, including changes to the temporal characteristics. Therefore, the first objective of this study was to examine the temporal characteristics of joint moment generation in the lower extremities of children with CP compared with able-bodied children by calculating maximum joint moment development and relaxation rates, and the time taken to produce or reduce the maximum voluntary isometric contraction (MVIC). This was completed using isometric contractions for six lower extremity degrees-of-freedom (DOF), specifically hip, knee and ankle flexion/extension. Isometric contractions were used, because they have proven to be a reliable measurement in subjects with neurological impairments, have been correlated to functional activity,1 and were easy to understand for the children participating. The second objective of this study was to examine the relationships between the temporal characteristics, MVICs, and the Gross Motor Function Measure – 66, a multi-component rating scale used to quantify motor capabilities of children with CP. We hypothesized that children with CP would not only have decreased MVICs as previously seen, but that they would also have altered temporal characteristics of moment generation, and that these measures would be correlated with function. Characterizing moment generation profiles in children with CP may provide insight into how alterations in moment generation capabilities may affect performance of functional activities such as posture and gait. Furthermore, this characterization may allow clinicians to develop more effective therapies to improve ADLs.
Materials and Methods
Subjects
Twenty able-bodied (AB) subjects without neurologic or orthopedic injury (12 males and 8 females, mean age 11.6 ± 1.7 years, mass 41.4 ± 9.5 kg) and six children with CP (4 males and 2 females, mean age 12.5 ± 2.7 years, mass 45.4 ± 17.6 kg) were recruited for this study. All children were between 8 to 15 years of age, were able to comply with instructions, and demonstrated understanding of the joint moment visual biofeedback through a questionnaire and a series of videos specific to the task being performed. Additionally, children in the CP group were assessed to be at levels I-III on the Gross Motor Functional Classification System (GMFCS), and had not had surgery within the past year. The characteristics for the subjects with CP are summarized in Table 1. The experimental protocol was approved by the Institutional Review Board at Arizona State University. All parents/guardians of the children provided written informed consent, while each child provided written informed assent.
Table 1.
Characteristics of children with cerebral palsy
| Subject | Gender | Diagnosis | Age (Yr) | Mass (kg) | GMFCS | Surgeries |
|---|---|---|---|---|---|---|
| CP1 | F | Spastic Diplegia | 9.8 | 61.2 | II | None |
| CP2 | M | Spastic Hemiplegia | 8.8 | 25.0 | I | None |
| CP3 | M | Spastic Diplegia | 14.3 | 57.5 | II | None |
| CP4 | M | Spastic Diplegia | 15.2 | 64.6 | III | Heel cord lengthening (both), dorsal rhizotomy |
| CP5 | F | Spastic Diplegia | 14.7 | 34.3 | II | Heel cord lengthening (both), foot derotation (L) |
| CP6 | M | Quadraplegia, Mixed | 12.3 | 29.7 | I | Heel cord lengthening (both) |
| CP Average | 12.5 | 45.4 | ||||
| Able-Bodied Average | 11.6 | 41.4 | ||||
GMFCS - Gross Motor Functional Classification Scale, I - Move without restriction. Limitations in advanced skills. II - Walk without assistive devices; limitations walking outdoors and in community. III - Walk with assistive devices; limitations walking outdoors and in community.
Experimental Setup
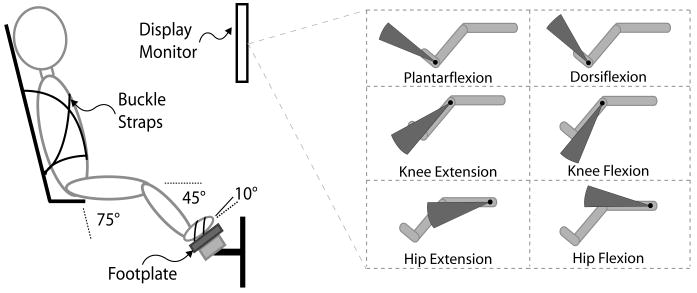
The experimental setup (Fig. 1) was designed for real-time monitoring and display of forces/torques for multiple DOF. A customized lower extremity footplate incorporating a six-axis force/torque sensor (MC36-1000, AMTI, Watertown, MA, USA) was integrated with a fully adjustable chair (Biodex System 3, Biodex Medical Systems, Inc., Shirley, New York, USA). The chair was placed in a semi-reclined position with the footplate positioned such that the subject's hip and knee were fixed at 75° and 45° flexion, respectively, while the ankle was at 10° plantarflexion. The angles for hip, knee, and ankle were chosen because they were approximately mid-range for range of motion for children with CP.16 Moreover, these values were similar to positions used successfully during maximum voluntary contraction testing by Wiley and Damiano28 and were not likely to be difficult for any of the children with CP to achieve. For testing, the trunk and pelvis were stabilized using buckle straps, and the foot of the side being tested was strapped to the footplate. The contralateral leg sat on a separate footrest to eliminate its interference with the leg being tested. Finally, visual biofeedback (described below) was displayed on an eye-level computer monitor approximately 60 cm in front of the subjects.
FIGURE 1.
Experimental setup and visual biofeedback. The inset demonstrates the visual biofeedback used during testing. The angular extent of the arc varied in real-time to indicate the current value of the amplitude and direction of a specific joint moment.
Analog voltage signals from the force/torque sensor were sampled at 500 Hz from a data acquisition card (PCI-6031E, National Instruments Corporation, Austin, TX) using customized software developed in LabVIEW™ (National Instruments Corporation, Austin, TX). The measured forces and torques, anthropometric measures, and limb configuration (i.e. joint angles) were used in static equilibrium equations to calculate isometric net joint moments for the hip, knee, and ankle. Force/torque data and calculated joint moments were stored on a personal computer for post-processing.
The biofeedback paradigm, based on net joint moments, provided a display of the net joint moment at a single joint in the form of a filled arc. The angular extent and direction of the arc fluctuated to represent the instantaneous amplitude and direction of the net joint moment (Fig. 1 inset). The software presented real-time visual biofeedback at a rate of 50 Hz. This biofeedback paradigm was designed so that it could be easily interpreted by subjects.
Experimental Protocol
Subjects were seated in the apparatus as described above. Before starting, each subject verbally indicated that they understood both the task at hand and the visual biofeedback. Subjects were given several minutes to become comfortable with the tasks. For testing, subjects were instructed to push in a specific direction (e.g. ankle plantarflexion) as hard and quickly as possible, hold for two seconds, and relax as quickly as possible. Auditory cues for starting and stopping were provided by the software at 1 and 3 seconds, respectively and were synchronized with data collection. Data was collected for a total of 5 seconds. Five second trials were selected to minimize the subject's fatigue. During each trial, subjects were provided with the visual biofeedback described above, and verbal encouragement. Each subject completed 3 trials for each of the 6 DOF of interest in the following order: plantarflexion, dorsiflexion, knee flexion, knee extension, hip flexion, and hip extension. Approximately 30 seconds of rest were given between trials. Both the right and left legs were tested.
Subjects with CP were also evaluated using the Gross Motor Function Measure – 66 (GMFM-66). The GMFM-66 is a reliable, functional measure developed for children with CP.22,27 It was administered by the same board-certified pediatric physical therapist for all subjects.
Data Analysis
The data collected were used to evaluate the subject's MVICs as well as the temporal characteristics of joint moment generation. To calculate MVICs, the raw data were filtered using a 250 ms moving window average, and the MVIC for each trial was calculated as the maximum value found during that trial. The largest value of the 3 trials was selected as each subject's MVIC. MVIC values for able-bodied children were related to body mass, therefore, MVICs were normalized to body mass for group comparisons.
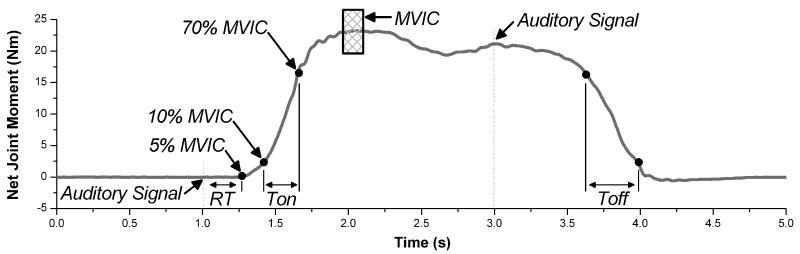
Temporal characteristics of joint moment generation were examined using 5 measures: reaction time (RT), time of moment generation (Ton), time of moment reduction (Toff), the maximum rate of moment development (MDmax), and the maximum rate of moment relaxation (MRmax). For these calculations, raw data were smoothed using a 25 ms moving window average. Three segments were defined on the moment profile: the initial segment (auditory sound to 5% MVIC), the ascending segment (10-70% MVIC while generating the moment), and the descending segment (70-10% MVIC while relaxing). The ascending segment limits of 10% and 70% have previously been shown to give consistent values.17 RT was the time of the initial segment. Ton and Toff were the times of the ascending and descending segments, respectively. MDmax was the maximum slope calculated for a best fit line of a 100 ms moving window on the ascending segment. MRmax was defined similarly for the descending segment.
Trials in which the child did not follow directions (i.e. the child started before the auditory cue, pushed in the wrong direction, or failed to stop pushing at the end of the trial) were excluded from analysis. For both groups, this represented approximately 15% of all trials. All temporal measures were averaged across the 3 like-trials. RT was further averaged across all DOF. The values for all subjects were right-left averaged, since subjects with CP did not display right-left differences. An example data trace with the segments depicted is provided in Fig. 2.
FIGURE 2.
Example joint moment profile. Raw data were filtered using a 25 ms moving window average. The following 3 segments were defined: initial segment (auditory sound to 5% MVIC), ascending segment (10% to 70% MVIC while generating force), and descending segment (70% to 10% MVIC while relaxing). Auditory signals, cueing the subject to start and stop pushing, were given at 1 and 3 seconds. The MVIC, calculated the maximum value of a 250 ms moving window average (applied to raw data), is indicated by the hatched rectangle. RT is the time from the auditory sound to 5% MVIC. Ton and Toff are the times taken for the ascending and descending segments, respectively.
Statistical Analysis
Group comparisons for age, body mass, and the calculated values for each DOF were analyzed statistically using independent samples t-tests, which were planned a priori. All dependent variables for both groups had normal distributions. For children with CP, relationships between the moment generation measures and the GMFM-66 were analyzed using Spearman's Rank Correlations. This conservative, non-parametric test was chosen because of the small sample of subjects with CP. All statistical analyses used SPSS 15.0 (SPSS Inc., Chicago, IL), and an alpha level ≤ 0.05 was used to determine significance. Data are presented as mean ± standard error of the mean.
Results
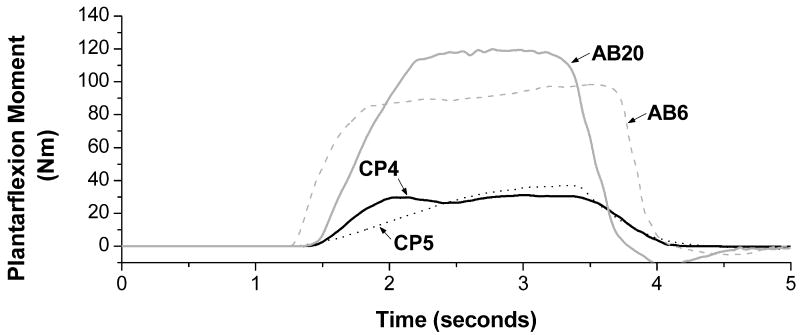
Raw data traces of a plantarflexion joint moment for four subjects (CP4, CP5, AB6, and AB20) are shown in Fig. 3. These data traces are representative of differences between groups.
FIGURE 3.
Example data traces. Data has been filtered using a 25 ms moving window average. Two subjects with CP (CP4 and CP5) and two able-bodied subjects (AB6 and AB20) were chosen for plotting and are representative of differences seen between the groups. The two subjects with CP have decreased MVICs as well decreased rates of moment development and relaxation.
Subject Characteristics
There were no statistical differences in age or body mass (p>0.05) between the two groups.
Reaction Time
RT was not statistically different in children with CP compared to the able-bodied children (0.54 vs.0.49 s, respectively).
Maximum Voluntary Contractions
Children with CP had decreased MVICs for all DOF except knee extension (p<0.05, Table 2).
Table 2.
Maximum voluntary contractions for the 6 DOF. The left hand portion of the table presents the normalized MVIC data from this study. The right hand portion presents MVIC data from previous studies on children with CP expressed as a percentage of maximum voluntary contractions recorded from able-bodied children in the that study. Children with CP had decreased MVICs for all DOF except knee extension.
| NORMALIZED MVIC (Nm/kg) | NORMALIZED MAXIMUM VOLUNTARY CONTRACTION (% OF VALUE FOR ABLE-BODIED CHILDREN) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOF | Able-bodied Children (n=20) |
Children w/ CP (n=6) |
p-value | Current Study |
Ross & Engsberg21 |
Damiano et al.7 |
Wiley & Damiano28 |
Thelen et al.26 |
Rose & McGill20 |
|
| PF | 2.18 ± 0.15 | 0.82 ± 0.20 | 0.000 | 38 | 40 | - | 39 | - | 48 | |
| DF | -0.81 ± 0.04 | -0.36 ± 0.10 | 0.000 | 44 | 56 | - | 41 | - | 26 | |
| KE | -0.67 ± 0.06 | -0.52 ± 0.18 | 0.281 | 78 | 55 | 63 | 63 | 43 | - | |
| KF | 0.71 ± 0.07 | 0.41 ± 0.13 | 0.045 | 58 | 56 | 38 | 68 | 51 | - | |
| HE | 2.51 ± 0.19 | 1.32 ± 0.21 | 0.003 | 53 | - | - | 44 | 59 | - | |
| HF | -1.66 ± 0.06 | -0.98 ± 0.18 | 0.013 | 59 | - | - | 57 | 76 | - | |
Data are mean ± standard error. MVICs are normalized to body mass (Nm/kg). Abbreviations are as follows: plantarflexion (PF), dorsiflexion (DF), knee extension (KE), knee flexion (KF), hip extension (HE), hip flexion (HF), maximum voluntary isometric contraction (MVIC), and degrees-of-freedom (DOF).
p-values in bold indicate significant differences (p<0.05) between able-bodied children and children with CP.
MDmax
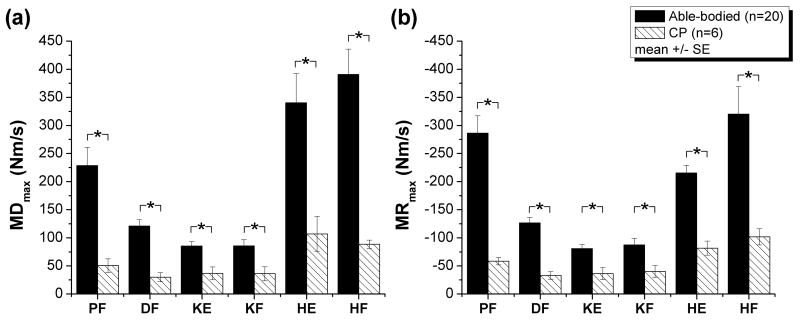
Children with CP had decreased MDmax for all DOF (p<0.05, Fig. 4a, Table 3).
FIGURE 4.
MDmax (a) and MRmax (b) for the 6 DOFs in children with CP compared to able-bodied children. Children with CP had reduced maximium rates of moment development and relaxation for all DOF. * indicate significant differences (p<0.05) between children with CP and able-bodied children. DOF abbreviations are defined in Table 2.
Table 3.
MDmax and MRmax for the 6 DOF. Children had significantly decreased rates of moment development and relaxation for all 6 DOF.
| MDmax (Nm/s) | MRmax (Nm/s) | |||||
|---|---|---|---|---|---|---|
| DOF | Able-bodied Children (n=20) | Children w/ CP (n=6) | p-value | Able-bodied Children (n=20) | Children w/ CP (n=6) | p-value |
| PF | 228.7 ± 32.1 | 50.7 ± 12.0 | 0.007 | -286.3 ± 31.1 | -58.4 ± 6.5 | 0.001 |
| DF | 121.1 ± 11.3 | 29.9 ± 8.1 | 0.000 | -126.6 ± 9.7 | -33.1 ± 6.8 | 0.000 |
| KE | 85.8 ± 11.2 | 36.2 ± 12.4 | 0.031 | -87.4 ± 11.7 | -40.3 ± 10.4 | 0.045 |
| KF | 85.6 ± 8.0 | 36.9 ± 11.5 | 0.005 | -80.7 ± 7.3 | -36.3 ± 10.6 | 0.006 |
| HE | 390.9 ± 44.9 | 88.7 ± 7.4 | 0.000 | -320.3 ± 48.9 | -101.8 ± 14.5 | 0.024 |
| HF | 340.4 ± 52.0 | 107.1 ± 31.0 | 0.025 | -215.7 ± 13.3 | -81.7 ± 12.6 | 0.000 |
Data are mean ± standard error. MDmax – maximum rate of moment development. MRmax – maximum rate of moment relaxation. Other abbreviations are defined in Table 2.
p-values in bold indicate significant differences (p<0.05) between able-bodied children and children with CP.
MRmax
Children with CP had decreased MRmax for all DOF (p<0.05, Fig. 4b, Table 3).
Ton
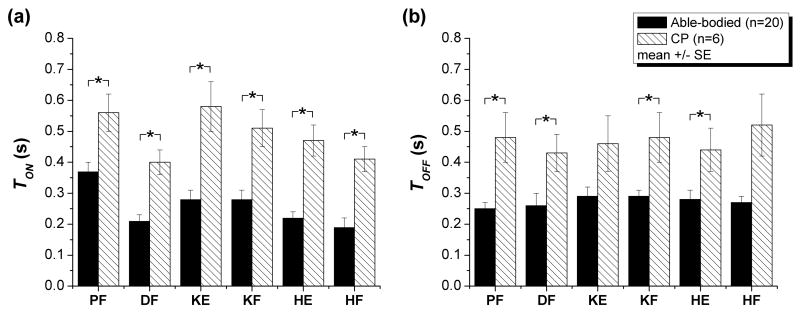
Children with CP had increased Ton for all DOF (p<0.05, Fig. 5a, Table 4).
FIGURE 5.
Ton (a) and Toff (b) for children with CP compared to able-bodied children for the 6 DOFs tested. Children with CP needed increased time to produce a joint moment (all DOF) and increased time to reduce a joint moment (plantarflexion, dorsiflexion, knee flexion, and hip extension). * indicate significant differences (p<0.05) between children with CP and able-bodied children. DOF abbreviations are defined in Table 2.
Table 4.
Ton and Toff for the 6 DOF. Children showed increased time taken to produce the joint moment for all 6 DOF, as well as increased time taken to reduce the joint moment for plantarflexion, dorsiflexion, knee flexion, and hip extension.
| Ton (s) | Toff (s) | |||||
|---|---|---|---|---|---|---|
| DOF | Able-bodied Children (n=20) | Children w/ CP (n=6) | p-value | Able-bodied Children (n=20) | Children w/ CP (n=6) | p-value |
| PF | 0.37 ± 0.03 | 0.56 ± 0.06 | 0.004 | 0.25 ± 0.02 | 0.48 ± 0.08 | 0.001 |
| DF | 0.21 ± 0.02 | 0.40 ± 0.04 | 0.000 | 0.26 ± 0.04 | 0.43 ± 0.06 | 0.037 |
| KE | 0.28 ± 0.03 | 0.51 ± 0.06 | 0.000 | 0.29 ± 0.02 | 0.48 ± 0.08 | 0.119 |
| KF | 0.28 ± 0.03 | 0.58 ± 0.08 | 0.001 | 0.29 ± 0.03 | 0.46 ± 0.09 | 0.004 |
| HE | 0.19 ± 0.03 | 0.41 ± 0.04 | 0.000 | 0.27 ± 0.02 | 0.52 ± 0.10 | 0.011 |
| HF | 0.22 ± 0.02 | 0.47 ± 0.05 | 0.000 | 0.28 ± 0.03 | 0.44 ± 0.07 | 0.054 |
Data are mean ± standard error. Ton – time from 10% to 70% on the ascending portion of the moment profile. Toff - time from 70% to 10% on the descending portion of the moment profile. Other abbreviations are defined in Table 2.
p-values in bold indicate significant differences (p<0.05) between able-bodied children and children with CP.
Toff
Children with CP had increased Toff for plantarflexion, dorsiflexion, knee flexion, and hip extension (p<0.05, Fig. 5b, Table 4).
Correlations
Spearman's rho values for all correlations are reported in Table 5. GMFM-66 scores were correlated with the MVICs for dorsiflexion and knee extension, as well as MRmax for plantarflexion and dorsiflexion. GMFM-66 scores were not correlated with MVICs or MRmax at other joints or with Ton, Toff, or MDmax at any joints. The MVICs at each joint were examined for correlations with the temporal measures at that specific joint. The MVIC was correlated with Ton for hip flexion; with MDmax for plantarflexion, knee extension, and hip flexion; and with MRmax for plantarflexion, dorsiflexion, knee extension, and knee flexion. MVICs were not correlated with Toff at any joint.
Table 5.
Spearman's rho reported for correlations between (a) Gross Motor Function Measure – 66 and (b) maximum voluntary isometric contractions (MVIC).
| (a) | (b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DOF | MVIC | MDmax | MRmax | Ton | Toff | MDmax | MRmax | Ton | Toff |
| PF | 0.77 | 0.77 | -0.94 | 0.60 | -0.26 | 0.83 | -0.83 | 0.54 | 0.03 |
| DF | 0.81 | 0.43 | -0.94 | 0.49 | -0.71 | 0.75 | -0.93 | 0.35 | -0.23 |
| KE | 0.89 | 0.61 | -0.60 | 0.26 | 0.54 | 0.81 | -0.83 | -0.09 | 0.49 |
| KF | 0.66 | 0.77 | -0.71 | -0.70 | -0.54 | 0.77 | -0.89 | -0.26 | -0.20 |
| HE | 0.66 | 0.71 | -0.77 | -0.71 | -0.54 | 0.71 | -0.77 | -0.09 | 0.14 |
| HF | 0.14 | 0.14 | -0.26 | 0.26 | -0.03 | 0.83 | -0.77 | -0.26 | 0.83 |
Values in bold indicate significant correlations (p<0.05). Abbreviations are defined in Table 2.
Discussion
We hypothesized that children with CP would not only have significant impairments in maximal isometric contractions (as shown by earlier research studies), but they would also demonstrate significant impairments in the temporal measures of moment generation at the hip, knee, and ankle. We tested this hypothesis by asking able-bodied children and children with CP to complete a series of isometric contractions by pushing as hard and quickly as possible and then relaxing as quickly as possible. Children with CP demonstrated differences in their moment generation profiles including: (1) decreased MVICs; (2) decreased maximum rates of moment development and relaxation; and (3) increased time needed to generate and reduce moments. Further, a few temporal measures were correlated to the GMFM-66 and MVICs.
Maximum Voluntary Isometric Contractions
As expected, children with CP showed decreases in MVICs at all three joints when compared to able-bodied children. It is important to note that the short trial length selected (5 seconds) may not have been a sufficient amount of time to allow some subjects to reach their maximal values, and as such, the absolute values should be regarded with caution. However, despite this and the small number of children with CP, the MVICs expressed as percentages of able-bodied children were similar to values calculated by previous studies (Table 2). Additionally, like Wiley and Damiano,28 the greatest deficits were plantarflexion, dorsiflexion, and hip extension (38, 44, and 53%, respectively). Unlike previous studies,7,13 the knee extension MVIC was not significantly different in children with CP when compared to able-bodied children. This was likely a result of the small sample size, as well as the high functional level of this group. However, the knee extension MVIC was correlated with the GMFM-66 score as reported by Damiano et al. (2000).7
Temporal Measures
In addition to significant strength decreases, children with CP also demonstrated significant changes in temporal aspects of their moment generation capabilities. Changes in the temporal characteristics of the moment generation profile could be related to both a change in the amount of moment generated over a given time period (i.e. ΔM) or to a change in time to generate a given amount of force (ΔT). We have shown that both aspects are altered in children with CP, thereby compounding the deficits in force generation. MDMAX and MRmax provided information regarding the amount of force generated in a given time. The change in the ΔM of the slopes are apparent in the example data traces provided in Fig. 3, as well as the significant differences for MDmax and MRmax found at all joints. Like the MVICs, MDmax, expressed as percentages of able-bodied children, indicated that the largest deficits were in plantarflexion, dorsiflexion, and hip extension (22, 25, and 23%, respectively). When averaged across all 6 DOFs, children with CP had only 28% of MDmax and 32% MRmax when compared to able-bodied children. Furthermore, the change in the ΔT is apparent in the values of Ton and Toff which documented changes in time that were independent of the maximum joint moment. Regardless of how much joint moment was being generated, children with CP had significantly increased times compared to able-bodied children to generate and relax the available joint moment. When averaged across all 6 DOFs, it took children with CP 89% longer to generate the moment and 71% longer to reduce the moment. Thus, for ADLs, not only do children with CP have decreased joint moments available, but the rates of moment generation and relaxation are also greatly reduced. Although tested in an isometric environment, these deficits may have implications for the ability of children with CP to respond to and recover from dynamic perturbations that exist during ADLs. Functional implications are further suggested through the correlations between MDmax, MRmax, GMFM-66, and the MVICs.
Some aspects of the temporal data presented here are consistent with results reported in the literature. Lee et al.14 showed that with electrical stimulation children with CP had slower muscle contractile characteristics compared to able-bodied children. Furthermore, these findings in children with CP are consistent with studies on other neurologically impaired subjects. Temporal deficits have been shown in the upper extremities of patients with stroke,3,17 as well as the lower extremities in patients with incomplete SCI.12 Jayaraman et al.12 suggests that these temporal deficits might have great functional implications, especially in activities such as walking that require repetitive, reciprocal activations of the muscle. Like persons with incomplete SCI, children with CP may not be able to generate enough joint moment in the amount of time available to sufficiently generate forward propulsion.
Potential Mechanisms
The reduced strength of children with CP, which was exhibited in this study and in previous studies, could be due to impairments in neural activation and/or changes in muscle properties. Data from several different studies in the literature suggest that it is combination of both neural and muscular factors11,18,20,25.
The nervous system modulates muscle force by using both recruitment and rate modulation.19 Previous studies have suggested that both the number of motor units that can be voluntarily activated and the maximum firing rate may be altered in children with CP.20,25 Rose and McGill20 examined neuromuscular activation (i.e. the ratio of surface electromyogram (EMG) amplitude to maximal M-wave amplitude) in children with CP. Surface EMGs for the gastrocnemius and tibialis anterior were measured during plantarflexion and dorsiflexion MVICs, and the amplitude of the M-wave was measured during electrical stimulation of either the peroneal or tibial nerve. During voluntary contractions, children with CP had lower surface EMGs than their able-bodied peers, but their M-wave amplitudes were comparable, thereby resulting in a lower neuromuscular activation ratio. From this, the authors concluded that children with CP had an equivalent number of motor units available; however, children with CP voluntarily activated fewer motor units during MVIC testing. Moreover, the relationship between motor unit recruitment and firing rates was similar between able-bodied children and children with CP for low level contractions (<20% MVIC), but the projected maximum firing rate of children with CP was approximately half that of able-bodied children. Therefore, children with CP may not be able to increase motor unit firing rates above a certain point, thereby limiting the force output of that muscle. In this study, although lower activation ratio and lower peak firing rate may explain the differences in MVICs, it is unlikely that they account for the observation that children with CP have lower rates of moment development and relaxation. Neural control mechanisms in children with CP may differ in a manner that reduces the rate of change in recruitment or reduces the rate of increase in firing rate, both of which would contribute to the observed differences in temporal measures.
Muscle properties of children with CP have also been shown to be different from their able-bodied peers.11,18 Rose et al.18 showed that children with CP have increased variation in the size of muscle fibers, as well as altered distribution of Type-I and Type-II fibers. The altered fiber type distribution was highly variable between subjects as 3/10 had a highly disproportionate count (>70%) of Type-I fibers, while another subject had a highly disproportionate count (>70%) of Type-II fibers. More recently, Friden and Lieber11 showed that spastic muscle fibers from children with CP had shorter resting lengths, which would affect the force generating capability. Slow twitch muscle fibers have 25% of the maximum shortening velocity of fast twitch fibers2. Although shortening velocity would influence rate of moment production, at most, this could account for the rates of children with CP being 25% of the rates of able-bodied children. For plantarflexion, dorsiflexion, and hip extension values of 22, 25, and 23%, respectively of the able-bodied rates were calculated. Since all of the muscles tested in this study probably contain a mixture of fiber types, the effect of fiber type distribution likely accounts for only a portion of the observed differences in rates of moment development and relaxation. While changes in muscular architecture may account for some of the changes to the temporal characteristics of moment generation in children with CP, this strongly suggests the involvement of a neural component as well.
Future Considerations
This study measured the temporal properties of joint moment generation during isometric tasks. Functional activities require dynamic force generation using both concentric and eccentric contractions. Although a few correlations were found between the temporal characteristics and the GMFM-66, isometric tasks may not accurately convey the moment generation deficit that is experienced during ADLs. Future studies should examine these temporal moment generation properties under concentric and eccentric isokinetic tasks. Furthermore, it will be important to perform these measurements on a larger sample of children with CP that spans a broader range of neuromotor deficits than the group used in this study.
This study provides a characterization of lower extremity motor function that includes both strength and temporal measures that we believe may have clinical relevance. Exercise interventions in children with CP have resulted in increased strength.5 Therefore it is possible that properly designed therapeutic interventions could improve temporal characteristics of moment generation in a manner that improves functional ability during ADLs. It is suggested that both strength and the temporal aspects of moment generation should be monitored and trained in the clinic.
Acknowledgments
Funding for this work was provided by IGERT NSF (NSF-DGE-9987619) (A. Downing) and the National Institute of Neurological Disorders and Stroke: National Research Service Award (1 F31 NS053010) (A. Downing). The authors gratefully acknowledge Manoshi Bhowmik-Stoker, Alison Conovaloff, Elliott Downing, and Jaime Stovall for their help conducting this study, Dr. Meghan Warren for assistance with the statistical analysis, and the children who participated.
Abbreviations
- ADLs
activities of daily living
- CP
cerebral palsy
- DF
dorsiflexion
- DOF
degrees of freedom
- EMG
electromyogram
- GMFCS
Gross Motor Functional Classification System
- GMFM-66
Gross Motor Function Measure – 66
- HE
hip extension
- HF
hip flexion
- KE
knee extension
- KF
knee flexion
- MDmax
maximum rate of moment development
- MRmax
maximum rate of moment relaxation
- MVIC
maximum voluntary isometric contraction
- PF
plantarflexion
- ROM
range of motion
- RT
reaction time, time from auditory signal to 5% MVIC
- SCI
spinal cord injury
- Ton
time from 10% to 70% MVIC on ascending segment
- Toff
time from 10% to 70% MVIC on descending segment
Footnotes
Material Presented Elsewhere: Portions of this material were presented at the Society for Neuroscience Annual Meeting in November 2007, San Diego, CA.
References
- 1.Bohannon RW. Measurement, nature, and implications of skeletal muscle strength in patients with neurological disorders. Clin Biomech. 1995;10:283–292. doi: 10.1016/0268-0033(94)00002-o. [DOI] [PubMed] [Google Scholar]
- 2.Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. Journal of Electromyography and Kinesiology. 1999;9:87–95. doi: 10.1016/s1050-6411(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Canning CG, Ada L, O'Dwyer N. Slowness to develop force contributes to weakness after stroke. Arch Phys Med Rehabil. 1999;80:66–70. doi: 10.1016/s0003-9993(99)90309-x. [DOI] [PubMed] [Google Scholar]
- 4.Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- 5.Damiano DL, Abel MF. Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–124. doi: 10.1016/s0003-9993(98)90287-8. [DOI] [PubMed] [Google Scholar]
- 6.Damiano DL, Martellotta TL, Quinlivan JM, Abel MF. Deficits in eccentric versus concentric torque in children with spastic cerebral palsy. Med Sci Sports Exerc. 2001;33:117–122. doi: 10.1097/00005768-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Damiano DL, Martellotta TL, Sullivan DJ, Granata KP, Abel MF. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Arch Phys Med Rehabil. 2000;81:895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- 8.Damiano DL, Vaughan CL, Abel MF. Muscle response to heavy resistance exercise in children with spastic cerebral palsy. Dev Med Child Neurol. 1995;37:731–739. doi: 10.1111/j.1469-8749.1995.tb15019.x. [DOI] [PubMed] [Google Scholar]
- 9.Elder GCB, Kirk J, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–550. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- 10.Engsberg JR, Ross SA, Collins DR. Increasing ankle strength to improve gait and function in children with cerebral palsy: a pilot study. Pediatr Phys Ther. 2006;18:266–275. doi: 10.1097/01.pep.0000233023.33383.2b. [DOI] [PubMed] [Google Scholar]
- 11.Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;26:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 12.Jayaraman A, Gregory CM, Bowden M, Stevens JE, Shah P, Behrman AL, Vandenborne K. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord. 2006;44:680–687. doi: 10.1038/sj.sc.3101892. [DOI] [PubMed] [Google Scholar]
- 13.Kramer JF, MacPhail HE. Relationships among measures of walking efficiency, gross motor ability, and isokinetic strength in adolescents with cerebral palsy. Pediat Phys Ther. 1994;6:3–8. [Google Scholar]
- 14.Lee SCK, Stackhouse SK, Binder-Macleod SA, Smith BT. Contractile and fatigue characteristics of quadriceps femoris muscle in children with and without cerebral palsy. Neurology Report. 2001;25:131–132. [Google Scholar]
- 15.Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28:464–471. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 16.Lowes LP, Westcott SL, Palisano RJ, Effgen SK, Orlin MN. Muscle force and range of motion as predictors of standing balance in children with cerebral palsy. Phys Occup Ther Pediatr. 2004;24:57–77. doi: 10.1300/j006v24n01_03. [DOI] [PubMed] [Google Scholar]
- 17.McCrea PH, Eng JJ, Hodgson AJ. Time and magnitude of torque generation is impaired in both arms following stroke. Muscle Nerve. 2003;28:46–53. doi: 10.1002/mus.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose J, Haskell WL, Gamble JG, Hamilton RL, Brown DA, Rinsky L. Muscle pathology and clinical measures of disability in children with cerebral palsy. J Orthop Res. 1994;12:758–768. doi: 10.1002/jor.1100120603. [DOI] [PubMed] [Google Scholar]
- 19.Rose J, McGill KC. The motor unit in cerebral palsy. Dev Med Child Neurol. 1998;40:270–277. doi: 10.1111/j.1469-8749.1998.tb15461.x. [DOI] [PubMed] [Google Scholar]
- 20.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47:329–336. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- 21.Ross SA, Engsberg JR. Relation between spasticity and strength in individuals with spastic diplegia cerebral palsy. Dev Med Child Neurol. 2002;44:148–157. doi: 10.1017/s0012162201001852. [DOI] [PubMed] [Google Scholar]
- 22.Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther. 2000;80:873–885. [PubMed] [Google Scholar]
- 23.Schiffman JM, Luchies CW. The effects of motion on force control abilities. Clin Biomech (Bristol, Avon) 2001;16:505–513. doi: 10.1016/s0268-0033(01)00014-6. [DOI] [PubMed] [Google Scholar]
- 24.Schultz AB, Alexander NB, Ashton-Miller JA. Biomechanical analyses of rising from a chair. J Biomech. 1992;25:1383–1391. doi: 10.1016/0021-9290(92)90052-3. [DOI] [PubMed] [Google Scholar]
- 25.Stackhouse SK, Lee SCK, Smith BT, Binder-Macleod SA. Use of a topical anesthetic during the assessment of voluntary muscle activation in children with cerebral palsy. Neurology Report. 2001;25:148. [Google Scholar]
- 26.Thelen DD, Riewald SA, Asakawa DS, Sanger TD, Delp SL. Abnormal coupling of knee and hip moments during maximal exertions in persons with cerebral palsy. Muscle Nerve. 2003;27:486–493. doi: 10.1002/mus.10357. [DOI] [PubMed] [Google Scholar]
- 27.Wang HY, Yang YH. Evaluating the responsiveness of 2 versions of the gross motor function measure for children with cerebral palsy. Arch Phys Med Rehabil. 2006;87:51–56. doi: 10.1016/j.apmr.2005.08.117. [DOI] [PubMed] [Google Scholar]
- 28.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]