Abstract
We describe here a novel Forelimb Locomotor Assessment Scale (FLAS) that assesses forelimb use during locomotion in rats injured at the cervical level. A quantitative scale was developed that measures movements of shoulder, elbow, and wrist joints, forepaw position and digit placement, forelimb-hindlimb coordination, compensatory behaviors adopted while walking, and balance. Female Sprague-Dawley rats received graded cervical contusions ranging from 200–230 (“mild”, n=11) and 250–290 kilodynes (“moderate”, n=13) between C5–8. Rats were videotaped post-injury as they walked along an alley to determine deficits and recovery of forelimb function. Recovery of shoulder and elbow joint movement occurred rapidly (within 1–7 days post-injury), whereas recovery of wrist joint movement was slower and more variable. Most rats in all groups displayed persistent deficits in forepaw and digit movement, but developed compensatory behaviors to allow functional forward locomotion within 1–2 weeks post-injury. Recovery of forelimb function as measured by the FLAS reached a plateau by 3 weeks post-injury in all groups. Rats with mild contusions displayed greater locomotor recovery than rats with moderate contusions, but exhibited persistent deficits compared to sham controls. Reliability was tested by having seven raters (3 internal, 4 external) from different laboratories, independently and blindly score videos of all rats. The multivariate correlation between all raters, all animals, and all time-points ranged from r2=0.88–0.96 (p<0.0001), indicating a high inter-rater reliability. Thus, the FLAS is a simple, inexpensive, sensitive, and reliable measure of forelimb function during locomotion following cervical SCI.
Keywords: Cervical injury, contusion, forelimb, locomotion, FLAS, behavior, rats
INTRODUCTION
In the last four years there has been a surge in the development and characterization of rodent cervical spinal cord injury (SCI) paradigms (Anderson et al., 2004, 2005, 2007; Velardo et al., 2004; Collazos-Castro et al., 2005; Pearse et al., 2005; Baussart et al., 2006; Gensel et al., 2006; Choo et al., 2007; Onifer et al., 2007; Schaal et al., 2007; de Rivero Vaccari et al., 2008; Sandrow et al., 2008). Expanding cervical SCI research is increasingly important as we learn from clinical epidemiology that the worldwide incidence of SCI ranges from 10.4–83 per million inhabitants per year (Wyndaele and Wyndaele, 2006) and that the proportion of individuals with cervical SCI ranges from 25–76%, depending on the country (Ackery et al., 2004). Regaining arm and hand function is the highest priority for people living with quadriplegia (Anderson, 2004) and the SCI population in general is receptive to the concept of incremental improvements. There is general consensus in the research field that the functional consequences of therapeutics that produce inter-segmental regeneration or plasticity will be easiest to test in cervical injury models because the distance of axonal growth that would be required to improve function is less than when assessing hindlimb function following injuries at thoracic levels.
There are many outcome measures available to assess forelimb impairments resulting from cervical SCI. Some examples include the grip strength meter (Anderson et al., 2004, 2005, 2007), food pellet reaching task (Whishaw and Pellis, 1990, Metz and Whishaw, 2000), cylinder task (Shallert and Lindner, 1990; Gharbawie et al., 2004), grooming task (Bertelli and Mira, 1993; Gensel et al., 2006), Montoya staircase (Montoya et al., 1991), horizontal ladder beam (Soblosky et al., 2001; Metz and Whishaw, 2002), and the sticker removal task (Schrimsher and Reier, 1992). Few studies, however, have assessed forelimb function during quadrupedal locomotion following cervical SCI in rodents. Part of the reason could be that there has not been a validated outcome measure to assess forelimb use during locomotion. The industry standard for evaluating deficits in hindlimb open-field locomotion in rats is the BBB scale (Basso et al., 1995), and a related scale has been developed for mice called the BMS scale (Basso et al., 2006). However, both of those were designed for and validated in animals with thoracic lesions and are inappropriate for assessing open-field locomotion in animals with forelimb impairments. One study has described a scale to evaluate deficits in forelimb function during open-field locomotion in rats (Cao et al., 2008). This scale was a modification of the BBB scale, had scoring categories ranging from 0 to 17, and was used along with a battery of other assessments to analyze the effect of administering a nogo-66 receptor antagonist peptide. This scale was only tested in animals subjected to a mild cervical lesion, which was a unilateral C3/4 lateral funiculus injury that produces very mild and transient forelimb locomotor deficits. Very recently, another scale was developed to evaluate forelimb deficits during open-field locomotion (Martinez et al., 2009). This scale was also a modification of the BBB scale, but it has a scoring range from 0–20 and it does not categorize behavioral recovery. This scale was only tested in rats subjected to partial, unilateral cervical spinal cord lesions. Thus, there have been no reports of a forelimb locomotor scale to assess deficits following bilateral injury at the cervical level.
Here, we describe a novel Forelimb Locomotor Assessment Scale (FLAS) designed to evaluate impairments resulting from a midline cervical contusion injury in rats. The FLAS was designed to be a comprehensive measure of forelimb function during quadrupedal locomotion in rats subjected to graded, midline cervical injuries that could detect levels of function ranging from complete non-use to normal use. We developed this tool in rats subjected to a clinically-relevant midline contusion injury of varying forces applied at different cervical levels, described in detail in the companion manuscript (Anderson et al., 2009). Our intent was to develop the FLAS as an inexpensive, reliable, and relatively simple tool that could be used across laboratories with a high degree of reliability.
METHODS
SCI Surgery
Experimental animals were female Sprague-Dawley rats (from Harlan, Inc., San Diego, CA) that were 200–230g at the beginning of each experiment and between 3–4 months of age. In two separate experiments, a total of 24 rats received a cervical spinal cord lesion surgery and 4 received sham surgery (n=28). All lesions were assessed via histology, as described in the companion paper (Anderson et al., 2009).
For surgery, rats were anesthetized with an intraperitoneal injection of Ketamine and Xylazine (100mg/kg and 10 mg/kg, respectively; Western Medical Supply, Inc., Arcadia, CA). Hair overlying the cervical vertebrae was removed by shaving, the skin was treated with betadine and incised, and the layers of muscle overlying the vertebral column were bluntly dissected. A dorsal laminectomy was then performed on the fifth, sixth, or seventh/eighth cervical vertebra (C5, C6, or C7/8), depending upon the study group. The impactor probe was centered over the exposed spinal cord and lesions aimed at the midline were created using an Infinite Horizons (IH) Impactor, (Precision Systems & Instrumentation, Lexington, KY). The vertebral column was stabilized by clamping the vertebrae immediately rostral and caudal to the exposed spinal cord with stabilizing forceps. Two types of lesions were created, termed “mild” and “moderate”, each with the dura mater left intact and with zero dwell time. The mild lesion was one in which the force of the impactor was preset to 200 kilodynes. The moderate lesion was one in which the force was preset to 250 kilodynes. The diameter of the head of the impactor probe was 3.5 mm, which was larger than the standard 2.5 mm probe. We used a larger diameter custom made impactor tip because the 2.5mm tip that comes with the IH device was designed for thoracic contusions. The cervical enlargement is larger than the thoracic spinal cord and so a larger diameter probe is required to avoid narrow injuries at the midline. Sham-operated controls received a C5 dorsal laminectomy only.
After creating the lesions, the muscle was sutured in layers, and the skin was closed with wound clips. Post-operatively, rats received 5 ml per 100 kg of 0.9% saline, 2.5 mg/kg Baytril, and 0.01 mg/kg Buprenorphine subcutaneously and were placed on a water jacketed warming pad at 37°C overnight. For the first week post-injury, intensive animal care was administered each day. Saline (5ml/100kg), Baytril (2.5mg/kg), and Buprenorphine (0.01mg/kg) were administered subcutaneously each morning. Bladders were manually expressed every day for the first week and residual urine was collected and weighed each morning prior to the administration of fluids. There was no noticeable impairment in voiding ability (i.e., bladders were empty or contained minimal urine when expressed). Body weight was measured every morning (also prior to the administration of fluids) for the first 8 days post-injury and once per week for the remainder of the experiment. Diet supplements (Fruit Loop cereal) and regular food pellets were placed on the floor of each cage to provide easy access for the rats. Nutri-cal (2ml, Henry Schein, Melville, NY) was administered orally for the first week post-injury.
Behavioral Testing
The Supplementary Materials contains step-by-step instructions for each of the components described below for acclimation, recording, administering, and analyzing the FLAS.
A. Acclimation
A simple procedure was used to acclimate rats to the testing environment (Supplementary Materials part A). This was performed one week prior to injury. On the 1st day, a clear plexiglass alley was placed in the center of an open, shallow enclosure (a kiddie pool as used when assessing animals with the BBB scale). The alley was 36.5 inches long, 4.5 inches wide, with sides that were 7 inches tall (see Supplementary Material). Rats were placed in the enclosure in groups and were allowed to explore the alley for 30 min. A similar procedure was performed on the 2nd through 4th days, with the addition of a white back-board being placed behind the alley leaning against one side. This was done to emulate the environment during the videotaping. Subsets of animals were placed in the enclosure (cage-mates, animals housed in groups of 4 or 5). Animals from each subset were placed individually at one end of the alley and allowed to run/walk to the other end for a total of 15 minutes. On the 5th day (i.e. the day prior to SCI surgery), the alley was placed on a counter (outside the enclosure) and each animal was videotaped while traversing the alley according to the videotaping instructions described below.
Techniques that were used to encourage animals to traverse the alley included putting a black box at the end of the alley toward which the animal was walking and placing sugary cereal and a buddy rat (from the same cage) in the black box. It was also helpful for the observer to stand at the end of the alley with the animal walking toward him/her.
Following injury, familiarity of the environment was reinforced by repeating the acclimation described above two days per week (non-testing days) for 15 minutes per day.
B. Video recording of behavior (Supplementary Materials part B)
On each testing day, rats were placed individually into the start end of the alley and videotaped as they moved to the darkened compartment at the opposite end of the alley. Video recording was performed on the following schedule:
1 day prior to SCI surgery
Weeks 1–2 post-injury: 3 times/week
Weeks 3–4 post-injury: 2 times/week
Weeks 5 & beyond: 1 time/week
To enhance uniformity and consistency throughout the study, a set of criteria were developed for technical staff to follow while videotaping the rats as they traversed the alley (described in detail in the Supplementary Materials). These criteria included always placing the alley on a countertop of the same height, always putting the video camera on a tripod at the same height and distance from the alley, and always using the same amount of zoom (adjusted so that the animal’s entire body, from nose to tail, could be seen as the rat traversed the alley while still allowing the forepaws and digits to be clearly observed). Additional criteria were established to control for the length of time spent recording each animal. If the animal was not capable of walking, it was placed in the alley so that both forelimbs could be observed simultaneously and was videotaped for 1 minute. If an animal was capable of walking, then passes were recorded for a maximum of 7 minutes. A PASS was defined as a minimum of 4 consecutive forward steps of the hindlimb without stopping or turning. A STEP was defined as forward then backward movement of the hindlimb that resulted in forward movement of the body and re-establishment of weight support on the hindlimb. The forelimb was not used to count steps because spinal cord injured animals often performed small compensatory movements with the forelimb (“patting” or “sliding”) while walking, described below. Ten passes were recorded for each animal at each time point; 5 of those passes were of the animal walking one direction and 5 passes were of the animal walking the opposite direction. This was to ensure that both forelimbs of the animal could be evaluated individually and that multiple passes were available for scoring. If the rat stopped walking while in the alley, it was “reset” by picking it up and putting it back at the beginning of the alley.
Videotapes were converted to digital files using the Windows Movie Maker software program, burned onto CDs for long-term storage, and behavior was scored while viewing with the Windows Media Player program. CDs were sent to external laboratories for scoring and inter-rater reliability testing. Copies of these CDs will be made available to any investigator seeking to learn the FLAS to allow direct comparison with the scores reported here.
C. Scoring of the recorded behavior (Supplementary Materials part C)
Each behavioral feature of the FLAS that was rated contributed one point to the overall score. The final score was the sum of all the observations made. The assessment scale was divided into three sections (Appendix) depending on whether the rat was able to make full passes, operationally defined as taking four consecutive forward steps with the hindlimbs. The scoring range of the FLAS was from 0–64, with 64 representing “normal” walking behavior of female Sprague-Dawley rats that were 3–6 months old and 0 representing a rat that was unable to move anything other than its’ head.
A hierarchy of scoring rules was established in order to document the progression of recovery of locomotor function immediately following injury through 8 weeks post-injury. As described above, a full pass was defined as 4 consecutive forward steps of the hindlimbs. Based upon those criteria, the 1st scoring rule was that if a rat was not capable of making any full passes, then it was observed for 1 full minute and Sections 1 & 2 of the score sheet were completed. During that 1 minute time period, the predominant movements of the joints (shoulder, elbow, wrist), predominant position of the digits (non-functional, partially functional, normal) and paws (non-plantar, plantar), and predominant activities of the forelimbs (sliding, patting, stepping) were recorded.
Any change in joint angle was scored as a movement. Regarding wrist joint movement, it was important to ensure that any movement observed was not due to passive bending of the wrist as a result of weight bearing. No wrist joint movement was a common observation. Regarding forepaw digit position, non-functional referred to digits being flaccid or clenched in a fist, partially functional referred to digits being partially extended or flexed, abnormally spread apart, or having any other altered appearance, and normal meant that all the digits were flat on the surface as was consistently observed in non-injured animals. Plantar placement of the forepaw was restricted to observations during which the paw was actively placed in the plantar position. All other positions were rated as non-plantar, including walking on a fist or passively dragging the digits along the alley surface.
Several abnormal behaviors were observed. One common behavior was locomotion with or without shoulder joint movement, with the elbow locked in extension or in flexion and with the forepaw remaining in contact with the floor rather than being lifted. This was referred to as “sliding”, which would be scored as no movement for the elbow joint and sliding with regard to forelimb activity. Another common behavior observed was “patting”, where the animal extended the forelimb forward and rapidly raised and lowered the paw; patting was seen with or without stepping movements. When rats took 1, 2, or 3 normal steps, but did not complete a pass (4 steps), this was rated as stepping without a full pass under forelimb activity.
The 2nd scoring rule was that if a rat performed at least 1 full pass, then Sections 1 and 2 of the score sheet were skipped and only Section 3 was completed. During our study, we rated passes while observing each side of the rat separately, as described above in the videorecording section. The first three passes observed on each side were scored. During each pass, the presence or absence of forelimb joint movement (shoulder, elbow, wrist) and the predominant position of the digits (non-functional, partially functional, normal) and paws (non-plantar, plantar) were recorded. Additionally, forelimb activity during forward locomotion was recorded. This included the presence or absence of sliding or patting behaviors and stepping with plantar or non-plantar forepaw placement during each pass. Forelimb-hindlimb coordination during each pass was rated. Coordination was defined as one forelimb step for every hindlimb step taken, with the hindlimbs alternating. If there was patting or sliding during any part of a 4-step pass, then, by definition, the pass was not coordinated. Coordination was scored from both sides of the animals (i.e. scored 3 passes while looking at the left side of the body and 3 passes while looking at the right side of the body). Finally, body posture and trunk stability were observed during all passes and an overall rating was recorded. For body posture, if any part of the body deviated from the body posture of a non-SCI rat during locomotion, it was rated as abnormal. For trunk stability, if it was grossly or obviously impaired, it was rated as unstable.
For all rating (Sections 1, 2, and 3), if there was uncertainty about whether particular movements or positions were seen, the score reflected the deficit.
D. Assigning numbers to the scored behavior (Supplementary Materials part D)
For each category on the score sheet, a number in parentheses represented the value of each behavior. After the behaviors were recorded on the score sheet, the values were tallied to generate a score for each side of the body. This design feature was incorporated into the scoring system so that if the spinal cord lesion induced asymmetric damage/impairment, a score could be reported for each side individually. If the spinal cord lesion induced bilaterally symmetric damage, then scores for the two sides could be averaged.
The maximum score possible for Section 1 and 2 was 17. For the shoulder, elbow, and wrist categories, a 0 was assigned for no movement, a 1 for predominantly no movement, and a 2 for predominant movement during the 1 minute observation period. For the predominant digit position category, a 1 was assigned for non-functional, a 2 for partially functional, and a 3 for normal position. For the predominant paw placement category, non-plantar was assigned a 1 and plantar was assigned a 2. For forelimb activity (sliding, patting, and stepping-without-a-full-pass categories), no movement was assigned a 0, predominantly no movement received a 1, and predominant movement received a 2. The sum of all movements was tallied for each side of the animal, then averaged.
The maximum score possible for Section 3 was 64. Based on the hierarchy rules described above, if an animal was able to complete at least 1 full pass, then only Section 3 was scored. A base score of 17 was assigned to any animal scored on Section 3 which is the score they would have received for functions in Sections 1 and 2. Right and left sides of the animal were scored separately. For the shoulder, elbow, and wrist categories, a 0 was assigned for no movement during a pass. If a movement was observed in only 1 pass, then a score of 1 was assigned; if a movement was observed in 2 passes, a 2 was assigned and if a movement was observed in 3 passes a 3 was assigned. For the predominant digit position category and the predominant paw placement category, each pass was assigned a value and, as described above, non-functional received a 1, partially functional received a 2, normal received a 3, non-plantar received a 1, and plantar received a 2. Then, for each category, the value of the passes was summed. The sliding, patting, stepping with non-planter placement, and stepping with plantar placement categories were used collectively to assign a value of 1 to 4 for each pass. If there was no sliding, patting, or non-plantar placement and there was plantar placement, then this was considered normal behavior and a value of 4 was assigned. If there was sliding or patting with plantar placement, then a value of 3 was assigned. If there was no sliding or patting but there was non-plantar placement, then a value of 2 was assigned. If there was sliding or patting along with non-plantar placement, then a value of 1 was assigned.
For the forelimb-hindlimb coordination category, each side was scored separately. If coordination was not observed in any of the three passes for a given side, then a 0 was assigned. If coordination was observed in only 1 pass, then a 1 was assigned. If coordination was observed in 2 passes, then a 4 was assigned and if coordination was observed in all 3 passes for a given side, then a 9 was assigned. Finally, abnormal body posture was assigned a 0 and normal posture a 1. Similarly, trunk instability received a 0 while normal stability received a 1.
Lesion Quantification
The companion manuscript (Anderson et al, 2009) describes the methods of tissue preparation and histological processing. All of the sets of stained horizontal sections from each animal were analyzed, from dorsal to ventral, using an Olympus IM80 microscope and the freely available ImageJ software. In every other section of the series (so every 4th section overall) the diameter of the spinal cord immediately rostral and caudal to the lesion was measured, the minimum width of spared lateral tissue at the center of the lesion was measured, and the rostro-caudal length of the lesion was measured.
The percentage of lateral spared tissue at the lesion epicenter was determined by utilizing the measurements from the section containing the epicenter plus two sections immediately dorsal and two sections immediately ventral to the epicenter (n = 5 sections). For each section, the spared lateral tissue on each side of the lesion cavity was summed and then divided by the average diameter of the spinal cord rostral and caudal to the lesion and multiplied by 100, then the average of the 5 sections was calculated (see Figure 4 in the companion manuscript Anderson et al., 2009). That value was identified for each spinal cord and used in subsequent analyses.
Figure 4.
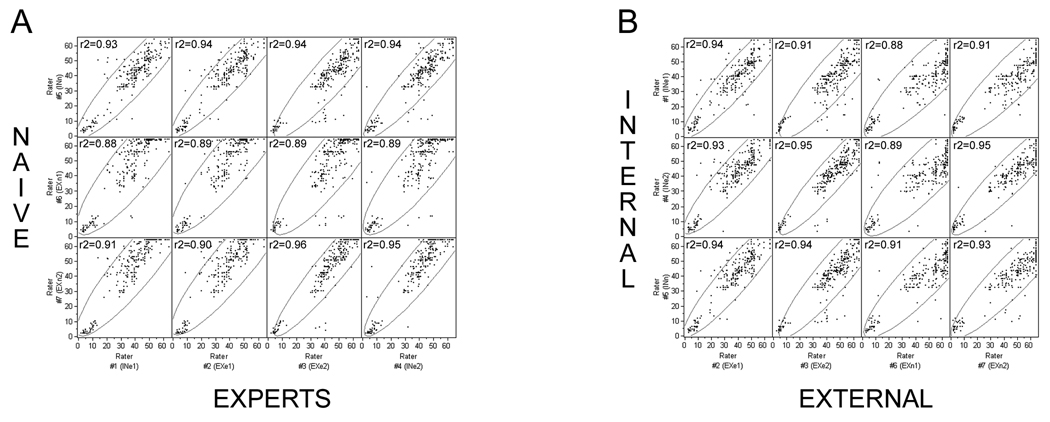
Inter-rater reliability is high regardless of experience or laboratory location. A, naïve versus expert raters. B, internal versus external raters (n = naïve; e = expert; IN = internal; EX = external). The encircled points represent the 95% (α level) bivariate normal density ellipses depicting the degree of correlation between a dependent variable and independent variable.
The percentage of total spared ventral gray matter, from the central canal through the ventral spinal cord, at the epicenter of the lesion was determined by first identifying the section containing the central canal, then measuring the width of spared gray matter, if any, on each side of the center of the lesion. This was done in every other section (i.e. every 80µm) from the central canal through the entire ventral half of the spinal cord. The measurements were summed across all sections and both sides of the lesion to yield a single value for spared ventral gray matter. This value was divided by the sum across all sections measured of the average diameter of the spinal cord (measured as described above) and multiplied by 100. Additionally, the completeness of the lesion (dorsally, laterally, and ventrally) was visually assessed, as well as the extent of damage or sparing of the labeled CST.
Statistical Analysis
Rats were grouped post hoc based upon the severity of the contusion (mild or moderate) or the spinal level of the injury epicenter (identified by counting dorsal roots during dissection). A mean FLAS score was obtained for each rat at each time point by averaging the corresponding scores of the seven raters. The Kruskal-Wallis test (a non-parametric analogue of the one-way ANOVA) was performed to identify differences between groups (sham vs mild vs moderate; C5 mild vs C6 mild vs C7/8 mild; C6 moderate vs C7/8 moderate) and across time post-injury. The Dunn’s test was used for post-hoc analysis to correct for multiple comparisons.
Inter-rater reliability was analyzed with the JMP®7.0.1 Statistical Discovery Software program. Multivariate correlations were performed to compare internal raters to external raters, naïve raters to expert raters, and all raters. All rats and time points were used in the analyses. Correlation coefficients are reported in the figures (i.e. scatterplot matrices), as well at the 95% bivariate normal density ellipses. The JMP®7.0.1 program was also used to analyze the bivariate relationship between contusion force and FLAS outcome.
RESULTS
Rationale for characteristics comprising the FLAS
When we began developing the FLAS, we intended to base it on a rational prediction of the expected deficits and progression of locomotor recovery. For example, when considering a single step cycle, the forelimb must flex at the elbow, dorsiflex the paw, rotate the shoulder to extend the forelimb rostrally, extend the elbow and dorsiflex the paw, plant the paw (extension) and maintain extension to allow weight bearing. The muscle groups involved are in the shoulder (ex. acromiotrapezius, levitor claviculae, and spinodeltoideus), upper forearm (ex. biceps and triceps), and lower forearm (ex. extensor/flexor carpi radialis, extensor/flexor carpi ulnaris, extensor digiti quarti, extensor pollicus longus, palmaris longus, and digitorum profundus). The motoneuron pools innervating the shoulder reside between C2–C4, the motoneuron pools innervating the biceps and extensor carpi radialis reside primarily at C5, the motoneuron pools innervating the flexor carpi radialis reside primarily at C6, the motoneuron pools innervating the triceps reside primarily at C7, and the motoneuron pools innervating the remaining lower forearm muscles reside between C7-T1 (McKenna et al., 2000). One would expect that damage to particular motoneuron pools would result in the loss of function to the corresponding muscles and that without function in those muscles, forelimb use during locomotion would be disrupted in predictable ways. One would also predict a hierarchical order of muscles necessary to complete a step cycle, i.e. shoulder muscles first to allow for rostral-caudal movement of the forelimb, biceps for elbow flexion, triceps for elbow extension, and multiple lower forearm muscles for paw placement.
In practice, however, recovery of forelimb movements did not occur in the way that would be predicted. Shoulder and elbow movement usually occurred first, followed by varying degrees of recovery of wrist movement. Some animals did not move their shoulder(s) and/or elbow(s) but were able to bear weight on their forelimbs and locomote forward with their hindlimbs. Animals often showed persistent deficits in paw placement and digit position, particularly non-plantar placement when the forepaw was in a tight fist, which would be rated as non-functional and non-plantar, or partially flexed/extended digits with plantar placement, which would be rated as partially-functional and plantar. Despite these persistent deficits, which were sometimes quite profound, animals showed recovery of the ability to use their forelimbs for locomotion in a highly functional manner.
Animals also displayed abnormal behaviors that would not be predicted, (sliding and/or patting of the forelimb while walking) (Supplementary Materials part C). These behaviors are not seen in un-injured rats. While walking, an un-injured rat performs plantar placement of its forepaws and its digits are relatively flat. Passes with this behavior were rated as “normal” and given a score of 4. The presence of sliding, patting, and/or non-plantar placement was rated as “abnormal” behavior during walking, and was thus given a score of 3, 2, or 1 depending on the combination presented. Finally, most rats showed persistent deficits in coordination and balance as well, mostly likely due to the presence of compensatory forelimb behaviors. Because the nature of the deficits and recovery were not entirely what would be predicted, we developed the FLAS based on observation of the deficits over time.
Histology
Lesion extent was assessed both qualitatively and quantitatively by examining horizontal, serial sections and is described in detail in the companion paper Anderson et al. Overall, in mild and moderate contusions the dorsal portion of the cord and the central gray matter were more severely damaged than the ventral portion of the cord. Moderate contusions tended to produce more lateral white matter damage. The extent of damage was variable across animals, but was clearly related to the severity of the contusion. The blindly scored FLAS data were grouped for analyses based on post-hoc anatomical verification of injury level and contusion parameters (Table 1). Table 2 lists the contusion force on impact, the amount of displacement, and the final mean FLAS score for each animal in the mild and moderate groups. Detailed analyses of the lesions revealed a relationship between the degree of tissue sparing and the force of the contusion. Quantification of the percentage of spared lateral tissue at the epicenter of the lesion is presented in the companion paper, along with quantification of the lesion length at the epicenter. Examples of tissue damage induced by mild or moderate midline contusions are shown in Figure 4 of the companion paper (Anderson et al., 2009).
Table 1.
Number of animals in each lesion group
| C5 | C6 | C7/8 | TOTAL | |
|---|---|---|---|---|
| MILD | 4 | 4 | 3 | 11 |
| MODERATE | 0 | 2 | 11 | 13 |
| SHAM | 4 | 0 | 0 | 4 |
| TOTAL | 8 | 6 | 14 | 28 |
Table 2.
Contusion force, displacement, and consequent final FLAS score.
| MILD | Force (kDynes) |
Displacement (mm) |
FLAS 54dpi mean score |
|---|---|---|---|
| exp 2 #7 C5 | 200 | 1.22 | 55 |
| exp 2 #6 C5 | 202 | 1.02 | 56 |
| exp 2 #5 C5 | 205 | 1.38 | 62.5 |
| exp 2 #3 C5 | 200 | ? | 54 |
| exp 2 #11 C6 | 208 | 0.97 | 52.5 |
| exp 2 #9 C6 | 219 | 1.09 | 54 |
| exp 2 #4 C6 | 207 | 1.34 | 60 |
| exp 3 #17 C6 | 231 | 1.29 | 51 |
| exp 3 #16 C7/8* | 208 | 1.18 | 45.5 |
| exp 3 #18 C7/8* | 211 | 1.25 | 33 |
| exp 2 #12 C7/8 | 205 | 1.32 | 55 |
| MEAN ± SD | 209±9 | 1.21±0.14 | 53±9 |
| MODERATE | Force (kdynes) |
Displacement (mm) |
FLAS 54dpi mean score |
| exp 2 #19 C6 | 259 | 1.20 | 40.5 |
| exp 2 #18 C6 | 275 | 1.18 | 32 |
| exp 3 #6 C7/8 | 252 | 1.27 | 43 |
| exp 3 #14 C7/8* | 276 | 1.73 | 56 |
| exp 3 #10 C7/8 | 267 | 1.11 | 39 |
| exp 2 #15 C7/8 | 272 | 1.06 | 39 |
| exp 2 #13 C8 | 255 | 0.78 | 49 |
| exp 3 #9 C7 | 287 | 1.27 | 31 |
| exp 3 #7 C7/8* | 250 | 1.27 | 51 |
| exp 3 #8 C7 | 267 | 1.22 | 40 |
| exp 3 #13 C7 | 264 | 1.13 | 47 |
| exp 3 #2 C7 | 283 | 1.20 | 47 |
| exp 2 #14 C7 | 255 | 1.00 | 41 |
| MEAN ± SD | 266±12 | 1.19±0.21 | 43±7 |
outlier = excluded from bivariate analyses due to large inconsistency between force and FLAS.
Time course of recovery
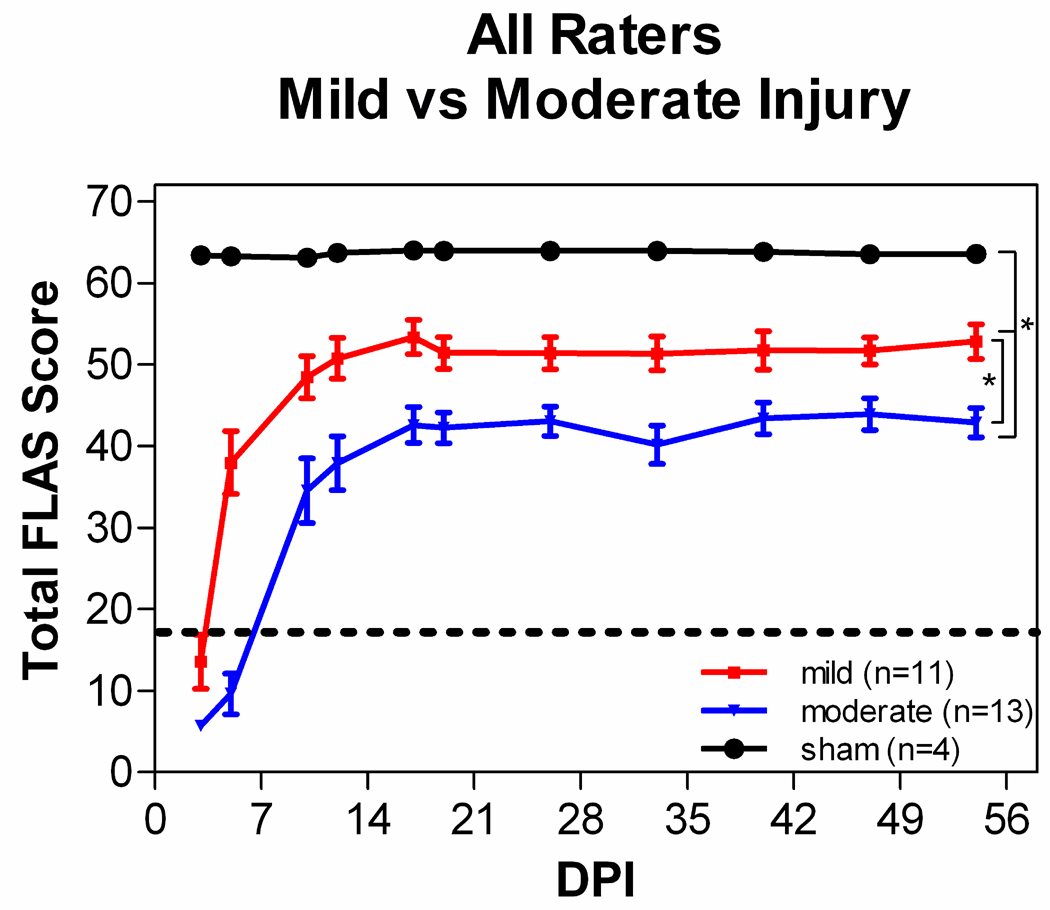
All rats were significantly impaired immediately following either a mild or moderate contusion. They had difficulty moving around the cage, but they were able to lie on their ventral surfaces and move their head and neck. Within 1 or 2 days, animals with mild contusions began moving their upper body significantly and then used a variety of compensatory mechanisms to move their entire body around the cage (e.g. crawling, scooting, hopping, etc). Animals with moderate contusions were significantly impaired for 3 to 7 days. During the first week post-injury a significant amount of attention and care were administered to all rats (see Anderson et al., 2009). Despite the initial impairment, rats were able to recover a significant amount of forelimb locomotor ability following either a mild or moderate contusion centered at or below the fifth cervical level. The degree of recovery, however, was dependent upon the force of the contusion (Fig. 1, 2A).
Figure 1.
The mean FLAS recovery pattern is influenced by contusion force. All animals with mild contusions compared to moderate contusions compared to sham injury. Scores for each group represent the mean scores and the 95% confidence interval from all of the raters for all animals within that group (*p<0.001). In each case, results from mild injury are less than those from moderate injury.
Figure 2.
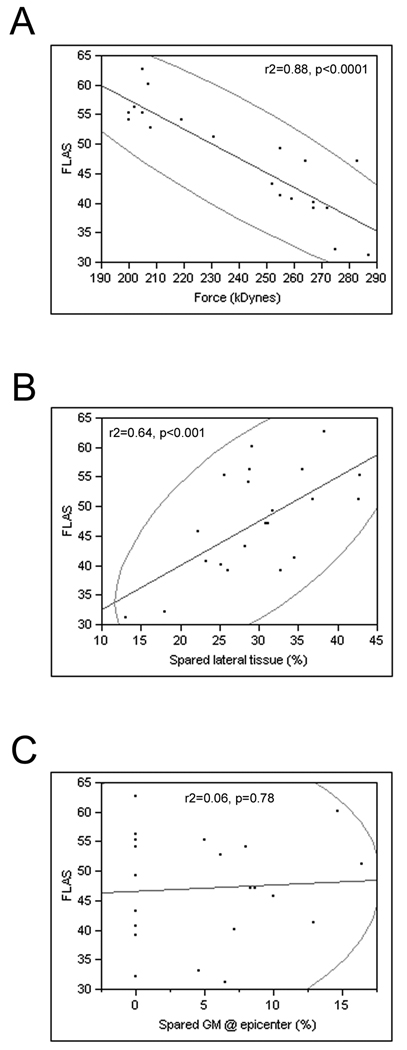
FLAS recovery is correlated to contusion force and spared lateral tissue. A, The scatterplot shows the negative correlation between contusion force and mean FLAS score at the end of the experiment, B, the positive correlation between the percentage of spared lateral tissue and mean FLAS scores, and C, no correlation between the percentage of spared ventral gray matter at the epicenter of the lesion and mean FLAS scores. The encircled points represent the 95% (α level) bivariate normal density ellipses depicting the degree of correlation between a dependent variable and independent variable.
Persistent deficits were observed in digit position, and to a lesser degree in paw placement. The deficits in digit position consisted of three categories, 1) the digits being clenched together in a fist, 2) the digits being completely flaccid, or 3) the digits being overly abducted or abnormally flexed. Persistent deficits were also observed in forelimb-hindlimb locomotion and balance in all groups. Sliding or patting of the forelimb(s) while walking prohibits forelimb-hindlimb coordination. Digit position contributes a maximum of 9 points per paw to the total FLAS score and forelimb-hindlimb coordination contributes a maximum of 9 points per side to the total score. Those deficits are reflected in Figure 1 by the final scores averaging around 50 and 40 for mild and moderate contusions, respectively.
Scale development
The FLAS was conceptually developed to be similar to the BBB, but redesigned to address the locomotor impairments and unique compensatory behaviors exhibited following cervical midline contusion injuries. The components of the FLAS were developed in a similar manner as the BBB, in that “scoring categories were established for the early, intermediate, and late phases of recovery by observational analysis of open field locomotion at various time points … postoperative” (Basso et al., 1995). The internal raters created draft versions of the FLAS and modified it several times to improve its sensitivity, comprehensiveness, and utility. A preliminary study was carried out with a separate group of animals with cervical injuries to develop the FLAS (rats from 1st experiment in Anderson et al., 2009). After the instructions for acclimating the rats and videotaping the sessions were finalized, the score sheet was developed and refined and then two large experiments were conducted (rats from 2nd and 3rd experiment in Anderson et al., 2009), for which the data are reported here. All of the videos from those 2 experiments were blindly and independently rated by the internal and external raters. The final component of the study was to develop the system for assigning numerical values to the observed behavior.
Video recordings of rats walking in the plexiglass alley at various times post-injury were evaluated blindly by 7 raters. Three were individuals at the home institution (“internal”), of which 2 were individuals with significant experience performing behavioral assessments in spinal cord injured rodents (“experts”) and 1 individual who had no experience evaluating rodent behavior associated with SCI (“naïve”). The four other individuals were from 2 outside institutions (“external”). Each external institution provided 1 expert and 1 naïve rater. Thus, there were a total of 3 internal and 4 external raters and a total of 4 expert and 3 naïve raters. All external raters learned the scale from the supplementary detailed instructions available on-line with this article.
Scale validation
Validity of the BBB was demonstrated based on 2 factors, 1) the ability of the scale to predict injury severity and 2) the relationship of scores to anatomical lesions (Basso et al., 1995). We used those same 2 factors to test the validity of the FLAS.
The FLAS Can Predict Injury Severity
For all injury groups, the recovery of shoulder and elbow joint movement for locomotion occurred rapidly (within 1–7 days post-injury), whereas recovery of wrist joint movement was slower and more variable. The range of 1–17 on the FLAS represented the early phase of recovery, which included varying degrees of movement of the shoulder, elbow, wrist, paw, and digits during the immediate post-injury time period when animals were not yet able to perform a complete pass (i.e. behavior was recorded on Sections 1 and/or 2 of the score sheet). The upper end of this range included compensatory forelimb movements (sliding and patting) and stepping during incomplete passes. During this phase of recovery, compensatory behaviors were rated positively in order to represent increasing levels of activity that rat’s exhibit prior to recovering the ability to perform a complete pass. As described in more detail below, compensatory behaviors were rated negatively when rats were able to perform a complete pass because they represented incomplete recovery of “normal” locomotor behavior.
The recovery of locomotor ability occurred quickly in rats with mild contusions, within 1 week post-injury as mentioned above. The transition from not being able to complete a full pass to rapidly traversing the alley occurred suddenly in all of the rats with mild lesions, regardless of the level of the injury; i.e., animals were unable to traverse the alley on one day and were able to accomplish the task the next day, rather than occurring in a successive, graded manner between the two stages of recovery. That transition is demarcated by the dotted line in Figure 1. It is possible that collecting data on locomotor ability everyday during the first 2 weeks could reveal a successive, graded pattern of recovery. However, the recovery of the ability to traverse the alley did not require recovery of “normal” locomotor behavior. Animals used multiple compensatory behaviors while traversing the alley, which included sliding and/or patting of one or both forelimbs. Those compensatory behaviors of the forelimb impacted other aspects of locomotion, including forelimb-hindlimb coordination, body posture, and trunk stability. Rats with moderate contusions progressed more slowly, but still not in a graded manner, and by 2 weeks post-injury all were able to traverse the alley. The range of 17–30 on the FLAS represented the intermediate phase of recovery, which included the transition from not being able to complete a full pass to being able to complete a full pass. The range of 30–50 on the FLAS represented the late phase of recovery, which included significant improvements in locomotor ability over time. The transition between the intermediate and late stages of recovery did occur in a graded manner as well as further improvements during the late stage of recovery. Behavioral improvements in locomotion reached a plateau by 3 weeks post-injury in all groups (Fig. 1), which was a similar phenomenon observed when using the BBB to measure locomotor recovery following a thoracic contusion (Basso et al., 1995).
Rats with a mild contusion experienced slightly less severe locomotor deficits immediately post-injury compared to rats with a moderate contusion. Rats with mild contusions also exhibited faster locomotor recovery and reached a higher functional level, although they still exhibited persistent deficits compared to sham operated rats (Fig. 1). A Kruskal-Wallis test revealed significant differences in FLAS scores between groups over time (H=25.22, df=2, p<0.0001) and post-hoc analyses demonstrated that rats with a mild contusion displayed a statistically greater degree of locomotor recovery than rats with a moderate contusion (p<0.05) at all times post-injury. Thus, the FLAS is sensitive to lesion severity. There was a significant negative correlation between the final FLAS score and contusion force (Fig. 2A, r2=0.88, p<0.0001). This relationship was similar to that observed between contusion force and recovery of forepaw gripping ability (Anderson et al., 2009).
Relationship Between FLAS Scores and Anatomical Lesions
There was a relationship between FLAS scores and percent of lateral spared tissue at the epicenter (Fig. 2B,). Scatterplot comparisons demonstrated that there was a significant positive correlation between the percent of lateral spared tissue at the epicenter and final FLAS score (r2=0.64, p<0.001). There was no relationship, however, between FLAS scores and the percent of spared ventral gray matter at the epicenter (Fig. 2C, r2=0.06, p=0.77).
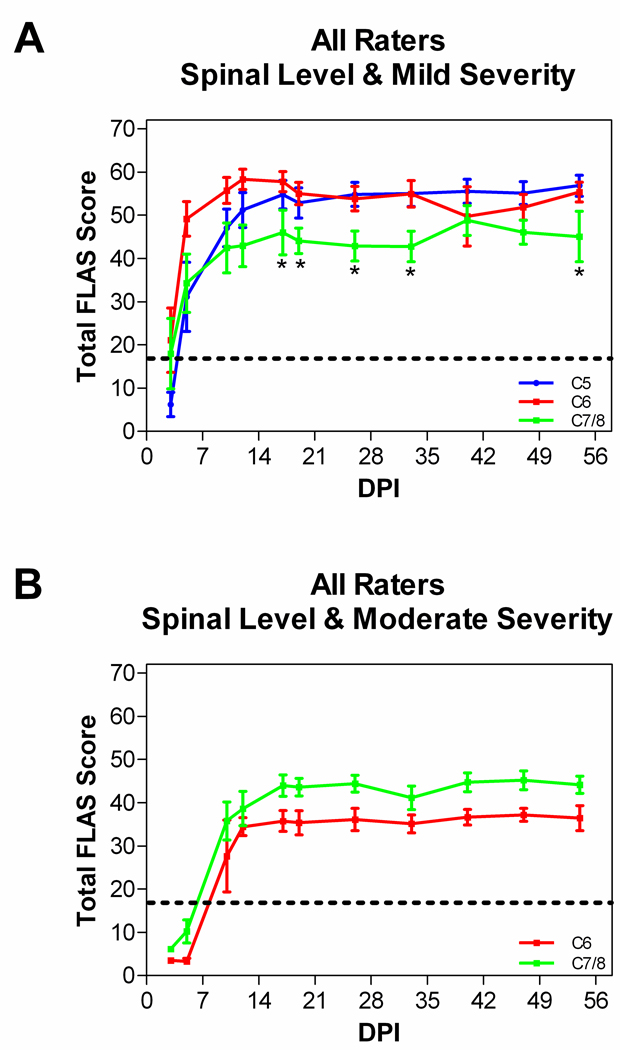
The location of the injury in the spinal cord had only a minor impact on forelimb locomotor ability. Figure 3A shows the FLAS recovery curve for all animals that received a mild contusion at the spinal level of C5, C6, or C7/8. Rats with a C5 or a C6 mild contusion exhibited very similar recovery patterns and recovered the best walking ability of all groups, reaching a plateau around 55 points. Rats with a C7/8 mild contusion displayed more impairments in the recovery of walking, reaching a plateau around 45 points. A Kruskal-Wallis test revealed differences in FLAS scores between the groups and over time (H=12.12, df=2, p=0.0023) and post-hoc analyses demonstrated that rats with a C5 or a C6 mild contusion displayed a statistically greater degree of locomotor recovery than rats with a C7/8 mild contusion (p<0.05). There were no significant differences between rats with a C5 or C6 mild contusion. Figure 3B demonstrates the FLAS recovery curve for all animals that received a moderate contusion at the spinal level of C6 or C7/8. No rats at C5 received a moderate contusion. Rats with a C6 moderate contusion reached a plateau around 35 points and rats with a C7/8 moderate contusion reached a plateau around 40 points (not significantly different).
Figure 3.
Lesion level has minor influence on FLAS recovery pattern. A, animals with a mild contusion at spinal level C5, C6, or C7/8. Animals with a C5 or C6 mild injury exhibit greater recovery of forelimb locomotion compared to animals with a C7/8 mild injury (*p<0.05). B, animals with a moderate contusion at C6 or C7/8 (no significant differences). Scores for each animal represent the mean and the 95% confidence interval from all 7 raters.
Inter-rater reliability
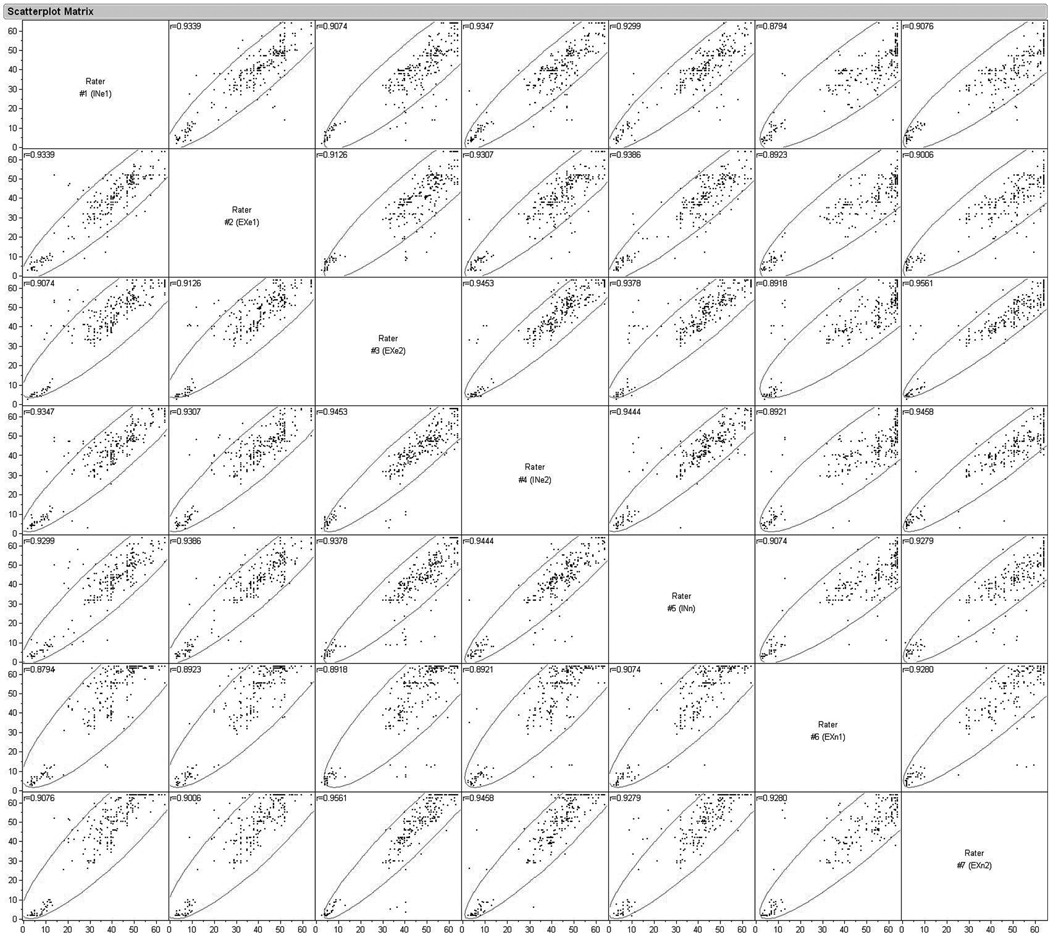
To test for reliability, seven raters (3 internal, 4 external), from different laboratories, independently and blindly scored videotape records of all animals, at all times post-injury. Raters from external labs learned the scoring system from the instructions provided herein, and had no other explicit training. Multivariate comparisons were made between naïve versus expert raters (Fig. 4A) as well as internal versus external raters (Fig. 4B). The multivariate correlation between all raters, all animals, and all time-points ranged from r2=0.88 to r2=0.96 (p<0.0001), indicating a very high inter-rater reliability regardless of whether the raters were naïve or experienced or learned the FLAS solely from the written instructions provided (Fig. 5).
Figure 5.
Multivariate correlation between all raters (n=7), all animals (n=28), and all time points (n=11) shows high reliability. (IN = internal; EX = external; n = naïve; e = expert). The encircled points represent the 95% (α level) bivariate normal density ellipses depicting the degree of correlation between a dependent variable and independent variable.
DISCUSSION
Our goal in the present study was to develop a simple scale that could be used across laboratories to evaluate gross forelimb locomotor dysfunctions induced by cervical spinal cord injury in rats. The FLAS fulfills that goal. It is sensitive to graded lesions at C6, each forelimb can be evaluated individually, the behavior can be recorded once and scored by multiple individuals in different locations at different times, the FLAS can be learned from written instructions provided herein, and the scoring reliability between raters (whether expert or novice) is very high.
Originally, our plan was to develop a categorical point system for the FLAS that would be similar to the BBB and BMS scales, in which the recovery of certain behaviors in a certain order moved animals up the scale. A modification of the BBB was created by Cao et al. (2008) for rats with a unilateral C3/4 lateral funiculus lesion. That scale consists of scores from 0 to 17, each representing a category of behavioral recovery. In our mild and moderately contused rats, the portion of the scale from 0–5 is very applicable to the recovery we saw. That range focuses on the recovery of shoulder, elbow, and wrist joint movement, specifically focusing on the number of joints being moved and the frequency of joint movement while locomoting in an open-field. The 6–11 point range addresses extensive movement of all 3 joints in addition to dorsal versus plantar stepping and the frequency at which those occur. In our study, rats with midline contusion injuries did not always exhibit recovery of all 3 joints, yet were capable of either dorsal (non-plantar) or plantar stepping with or without persistent digit deficits. Under those criteria, many of the contused animals would not score above a 5, yet would actually be locomoting quite rapidly and functionally. The 12–17 point range addressed paw position and toe clearance, which was not part of the recovery process of the contused animals. Because of the inconsistency in the order in which different components of forelimb locomotor recovery occurred in mildly and moderately contused rats, we did not create a categorical point system for the FLAS. Rather, the FLAS score represents how far away from “normal” an animal’s forelimbs usage is while walking. The highest score, 64, encompasses all of the “normal” movements a rat makes while performing quadrupedal locomotion. Similarly, the scale described by Martinez and colleagues represents how far away from normal an animal’s walking is following cervical SCI. However, it does not register new compensatory behaviors that animals may utilize in response to injury and it averages forelimb and hindlimb scores rather than focusing on forelimb use. The scale created by Cao and colleagues may be better suited to evaluate locomotor deficits induced by discreet lesions, whereas the FLAS is more appropriate for the deficits and recovery seen with contusion lesions. The scale created by Martinez and colleagues may be better suited to evaluate combined forelimb-hindlimb dysfunction induced by unilateral lesions.
Potential versatility of the FLAS
The FLAS was developed using a midline cervical contusion model. It is capable of detecting impairments resulting from lesions induced by different contusive forces. This is an important characteristic to incorporate into a tool designed to be used by multiple laboratories because different laboratories use different injury models and devices, dependent upon available resources, preferences, and scientific questions being addressed. The FLAS was designed to evaluate and score each forelimb individually. This feature provides the possibility for the FLAS to be used to evaluate animals with unilateral lesions, such as a lateral hemisection or unilateral contusion, where the forelimb without neurologic impairment can serve as an internal control. This has not been validated as part of the current study, however.
Locomotor deficits induced by midline contusion
A necessary feature of cervical injury models is that they must be incomplete so as to preserve a sufficient degree of function to allow survival of the experimental animals. The contusions we administered resulted in mild and moderate degrees of tissue damage (for detailed information see the companion manuscript Anderson et al., 2009). Both lesion severities produced significant initial deficits in gross locomotor ability, but then yielded graded functional improvements in walking, reaching a plateau by 3 weeks post-injury. The rate of recovery was related to the severity of injury. There are advantages and disadvantages to this result. The advantages are that the animals do indeed survive the contusions and do exhibit graded degrees of locomotor recovery directly related to the force of the contusion. The disadvantages are that the locomotor recovery rate is quite rapid with regard to the time at which an animal will transition from being active, but not able to make a full pass, to being able to make at least 1 full pass using modified behaviors. This is the transition from the early to intermediate stages of recovery. This transition is extremely fast and, in our opinion, will be difficult to capture in a graded manner under any scoring system. This is because the transition from not being able to complete a 4-step pass to being able to complete a pass is simply not graded, the rats can either do it or not. In fact, an animal can be unable to walk one day and be capable of making a full pass the next. Moderately injured animals took longer to reach this transition than mildly injured animals, but when the transition occurred it was just as rapid. Thus the scale is not very sensitive in the range of 15–30 (i.e. the point range representing the transition) and may require testing at more frequent intervals (daily) during the time at which transitions may be expected. The transition between the intermediate and late stages of recovery did occur in a graded manner, however, as well as additional improvements during the late stage of recovery. Hence, the FLAS is sensitive to successive changes in levels of activity immediately following injury (i.e. prior to being able to complete a full pass) and once able to complete a full pass.
Variability of recovery: Compensatory behaviors
Despite well controlled contusive forces and analytical grouping based on lesion extent via histological assessment, there was variability with regard to locomotor recovery across animals with similar lesions. However, there were strong correlations among all 7 raters with regard to individual animals. This suggests that the variability was across animals rather than across raters. It is possible that the development of compensatory behaviors could, in part, contribute to the variability in locomotor behavior observed across animals. The sliding and patting behaviors observed in our study occurred across the spectrum of injury severities and injury levels. Other studies have also observed compensatory behaviors during locomotion in rats with cervical injuries. Following a C3 unilateral hemisection, rats do not use their impaired forelimb for braking or propulsion and tend to bear more weight on their hindlimbs (Webb and Muir, 2002). Hemisected rats also develop altered kinetics of locomotion to compensate for body instability (Webb and Muir, 2002). Bilateral lesions to the dorsolateral funiculi at C2/3 followed by secondary lesions to the dorsolateral funiculus produce compensatory changes in forelimb and hindlimb ground reaction forces and timing of stepping (Kanagal and Muir, 2008). Another group has shown that rats with a mild midline contusion at C7 maintained chronic deficits in elbow extension and wrist flexion and extension, but were able to locomote in the open field (Collazos-Castro et al., 2005). The rats did exhibit compensatory behaviors while locomoting, including prolonged time in the stance phase, slower velocity, and shifted body weight support to the hindlimbs (Collazos-Castro et al., 2005). The CatWalk has recently been used to evaluate forelimb function during overground locomotion in rats with a C4/5 overhemisection (Dai et al., 2009). Several compensatory changes in locomotion were detected, including an increased base of support for the forelimbs, reduced stridelength, increased standtime, and decreased swingtime. A great degree of variability was observed between animals, despite similar lesions. Interesting, exposure to an enriched environment with daily training on a skilled task had no effect on forelimb locomotion (Dai et al., 2009).
Limitations
The FLAS was developed for rats. It would likely be difficult to adapt the FLAS to mice because of their speed of locomotion, however, that has not been evaluated in the current study. Also, in our experience, mice with cervical injuries retain the ability to locomote quite well despite forelimb impairments (Anderson et al., 2004 – lateral hemisection model; Blanco et al., 2007 – lateral hemisection model and motor cortex lesion model; R. Aguilar, personal communication, cervical contusion model).
More extensive studies need to be conducted to further validate the FLAS across additional injury levels. In the current study, we did not induce moderate contusions at C5 and we did not go above C5. Additionally, the relationship between spared motoneuron pools at different distances from the lesion epicenter and final FLAS scores needs to be analyzed. These and other analyses are the subject of future investigations.
Functional locomotor ability despite persistent gripping deficits
The most predominant persistent deficits captured by the FLAS are the poor recovery of normal digit position and the presence of compensatory behaviors. In the companion paper (Anderson et al., 2009) we report impairments in grip strength in the same animals for which walking ability is reported here. There is no correlation between final FLAS score and final GSM value. Interestingly, only 4 of the 24 contused animals, for which FLAS scores are reported here, showed recovery of grip strength as tested by the grip strength meter. Yet all 24 of those animals recovered the ability to functionally locomote within 3 weeks post-injury. Those animals never recovered “normal” walking, however, as indicated by FLAS scores lower than 64. This highlights two important issues. First, rats do not need fine forelimb motor function in order to walk in a functional manner. Second, even though a rat can recover the use of its forelimbs to walk following a cervical injury, it cannot be assumed that the rat has recovered full or “normal” use of its forepaw digits that is necessary to perform gripping or other fine/skilled movements. This stresses the importance of utilizing more than 1 behavioral outcome measure in SCI studies. The FLAS would be useful for evaluations of gross forelimb function during locomotion whereas the food pellet reaching task would be useful for evaluations of highly skilled movements of the forelimb and the grip strength meter task would be useful for evaluations of fine digit flexion and extension.
Conclusion
Despite its limitations, the FLAS is sensitive to graded patterns of gross locomotor recovery. The pattern of recovery when injured at C6 is dependent upon the severity of the injury. The FLAS is simple to learn and requires very little expense (a standard grade video camera). Interrater reliability is very high regardless of whether the rater has had a significant amount of experience evaluating SCI animal behavior or is a novice. Moreover, the FLAS can be learned easily using the instructions provided in the Supplementary Materials without any additional training. The FLAS can be added to the growing number of behavioral assessment tools appropriate for evaluating impairments induced by cervical lesions, which will hopefully encourage more investigators to pursue studies of cervical SCI.
Supplementary Material
Acknowledgements
Thanks to Ardi Gunawan and Kelly Yee for technical assistance, Katina Hanford and Lauren Santi from the laboratory of Marion Murray, and Johnathan Ly from the laboratory of Jacqueline Bresnahan. This work was supported by the NIH-NO1-NS-3-2354 contract (Facilities of Research Excellence in Spinal Cord Injury), and the Roman Reed Spinal Cord Injury Research Fund of the State of California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord epidemiology. J. Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the SCI population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Abdul M, Steward O. Quantitative assessment of deficits and recovery of forelimb motor function after cervical spinal cord injury in mice. Exp. Neurol. 2004;190:184–191. doi: 10.1016/j.expneurol.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Quantitative behavioral analysis of forepaw function after cervical spinal cord injury in rats: Relationship to the corticospinal tract. Exp. Neurol. 2005;194:161–174. doi: 10.1016/j.expneurol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp. Neurol. 2007;206:318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.06.012. Advance Online Publication DOI: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadié M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol. Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Mira JC. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial-plexus reconstruction in the rat. J. Neurosci. Methods. 1993;46:203–208. doi: 10.1016/0165-0270(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Blanco JE, Anderson KD, Steward O. Recovery of forepaw gripping ability and reorganization of cortical motor control following cervical spinal cord injuries in mice. Exp. Neurol. 2007;203:333–348. doi: 10.1016/j.expneurol.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Cao Y, Shumsky JS, Sabol MA, Kushner RA, Strittmatter S, Hamers FP, Lee DH, Rabacchi SA, Murray M. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil. Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J. Neurosurg. Spine. 2007;6:255–266. doi: 10.3171/spi.2007.6.3.255. [DOI] [PubMed] [Google Scholar]
- Collazos-Castro JE, Soto VM, Gutiérrez-Dávila M, Nieto-Sampedro M. Motoneuron loss associated with chronic locomotion impairements after spinal cord contusion in rats. J. Neurotrauma. 2005;22:544–558. doi: 10.1089/neu.2005.22.544. [DOI] [PubMed] [Google Scholar]
- Dai H, MacArthur L, McAtee M, Hockenbury N, Tidwwll JL, McHugh B, Mansfield K, Finn T, Hamers FPT, Bregman BS. Activity based therapy to promote forelimb use after a cervical spinal cord injury. J. Neurotrauma. 2009 doi: 10.1089/neu.2008-0592. Advanced Online Publication, DOI: 10.1089/neu.2008.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FPT, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion in rats. J. Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Whishaw PA, Whishaw IQ. The topography of three-dimensional exploration: a new quantification of vertical and horizontal exploration, postural support, and exploratory bouts in the cylinder test. Behav. Brain Res. 2004;151:125–135. doi: 10.1016/j.bbr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kanagal SG, Muir GD. Effects of combined dorsolateral and dorsal funicular lesions on sensorimotor behaviour in rats. Exp. Neurol. 2008;214:229–239. doi: 10.1016/j.expneurol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Marinez M, Brezun JM, Bonnier L, Xerri C. A new rating scale for open-field evaluation of behavioral recovery after cervical spinal cord injury in rats. J. Neurotrrauma. 2009;26:1043–1053. doi: 10.1089/neu.2008.0717. [DOI] [PubMed] [Google Scholar]
- Metz GAS, Whishaw IQ. Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav. Brain Res. 2000;116:111–122. doi: 10.1016/s0166-4328(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The "staircase test": a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci. Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG, Wells MJ, West RM, Calloway CC, Schnell JT, Whitaker CM, Burke DA, Hubscher CH. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp. Neurol. 2007;207:238–247. doi: 10.1016/j.expneurol.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, Lo TP, Jr, Cho KS, Lynch MP, Garg MS, Marcillo AE, Sanchez AR, Cruz Y, Dietrich WD. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J. Neurotrauma. 2005;22:680–702. doi: 10.1089/neu.2005.22.680. [DOI] [PubMed] [Google Scholar]
- Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp. Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal SM, Kitay BM, Cho KS, Lo TP, Jr, Barakat DJ, Marcillo AE, Sanchez AR, Andrade CM, Pearse DD. Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant. 2007;16:207–228. doi: 10.3727/000000007783464768. [DOI] [PubMed] [Google Scholar]
- Schallert T, Lindner MD. Rescuing neurons from trans-synaptic degeneration after brain damage: helpful, harmful or neutral in recovery of function? Can. J. Psychol. 1990;44:276–292. doi: 10.1037/h0084244. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp. Neurol. 1992;117:287–298. doi: 10.1016/0014-4886(92)90138-g. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav. Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Velardo MJ, Burger C, Williams PR, Baker HV, López MC, Mareci TH, White TE, Muzyczka N, Reier PJ. Patterns of gene expression reveal a temporally orchestrated wound healing response in the injured spinal cord. J. Neurosci. 2004;24:8562–8576. doi: 10.1523/JNEUROSCI.3316-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav. Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- Wyndaele M, Wyndaele JJ. Incidence, prevalence, and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.