Abstract
Marketed red clover products use a wide variety of labels and the isoflavone contents from the lable is ambiguous. In the present study, we analyzed the content of various isoflavone products, and determined a) the content and b) how sample matrix of red clover products affects intestinal disposition of main isoflavones within it using the human intestinal Caco-2 cell model. Analysis using high and ultra-performance liquid chromatography indicates that the isoflavone content varied significantly (p<0.05) between the chosen products. Consequently, rates of isoflavone absorption across the Caco-2 cell monolayers varied (p<0.05) greatly. Unexpectedly, permeabilities of biochanin A and formononetin (two key biomarkers) were found to be significantly affected (p<0.05) by the product matrix. As expected, biochanin A was the only isoflavone with noticeable metabolite peaks in both apical and basolateral sides. Interestingly, rates of metabolism and the polarity of the glucuronidated biochanin A excretion were also significantly altered (p<0.05) by product matrix. Studies using breast cancer resistance protein inhibitor dipyridamole showed that both the apical and basolateral excretion of biochanin A glucuronides were significantly (P<0.05) reduced (7.5 and 9.4-fold, respectively) when dipyridamole is present. This provides evidence that BCRP is the main transporter responsible for the apical efflux of isoflavone glucuronides. In conclusion, the isoflavone contents of the marketed red clover products are highly variable, and product matrix significantly affected intestinal disposition of red clover isoflavones by altering their absorption rates, permeabilities, biochanin A glucuronide excretion rates, and the polarity of biochanin A glucuronide excretion. This research provides scientific evidence to support the standardization effort so that consumers can make intelligent product choices.
INTRODUCTION
Isoflavones, a subclass of flavonoids, are phytochemicals that resemble the chemical structure of female hormone estrogens. Therefore, isoflavones are termed as phytoestrogens and their purported effects range from prostate and breast cancer prevention in preclinical models 1,2, menopausal hormone replacement therapy 3, alleviation of various aging-related and hormone-dependent disorders (e.g. osteoporosis), and improvement of cardiovascular systems (for reviews, see 1,4-7. Isoflavones have generated much interest in clinical nutrition and disease prevention 4,8-10, leading to various clinical trials for cancer prevention 11,12.
The use of soy isoflavone supplements in large-scale clinical trials for their chemopreventive effects have led to a recent trend, where the general public (especially women) is eagerly consuming natural isoflavone supplement products, even though their effects are not yet proven. This industry spawns annual sales exceeding 20 billion dollars. Among the numerous isoflavone supplement products offered on the market today, isoflavones are usually derived from soybeans that contain isoflavones such as daidzein, glycitein and genistein or from red clover (Trifolium pretense) that mainly contains biochanin A and formononetin.
This widespread use of self-administered isoflavones is somewhat unsettling since much remains to be proven including efficacy and possible side effects. In order to further the preclinical and clinical research to show if isoflavone supplements are beneficial to human health, we must overcome several barriers with the first being a lack of standardization in the marketed products.
In a previous study from our laboratory, a comparison among thirteen different retailed soy isoflavone products showed that the exact compositions of these products varied drastically from product to product 13. Only four out of 13 products contained 90% or more of the isoflavone contents claimed on the label. Some products not only did not contain the claimed isoflavones but also contained unknown impurities that exceeded 40%. Furthermore, the major isoflavone components of a product changed significantly over time making it highly variable between batches.
In the present study, we extended our investigation by analyzing the isoflavone contents (as a measure of quality) of various marketed red clover products. We hypothesized that changes in isoflavone contents will impact intestinal absorption and metabolism of the main isoflavones contained in the red clover. We also determined how inhibitor of breast cancer resistance protein (BCRP/ATP-binding cassette, subfamily G, member 2), dipyridamole will affect the excretion (via efflux) of isoflavone glucuronides. As an important reference point, the red clover standard from United States Pharmacopeia (USP) was used along with the purchased products. Developed under an important initiative termed “Dietary Supplement Verification Program (or DSVP),” this red clover standard can be used as a “Mark of Quality” for dietary supplement products.
MATERIALS AND METHODS
Materials
Cloned Caco-2 TC7 cells were a kind gift from Dr. Moniqué Rousset of INSERM U178 (Villejuit, France). Biochanin A, formononetin, daidzein and genistein were purchased from Indofine Chemicals (Somerville, NJ). Hanks’ balanced salt solution (HBSS, powder form) and dipyridamole were purchased from Sigma-Aldrich (St Louis, MO). Powdered red clover standard extract was purchased from USP (catalog no. 1599500). Three (3) red clover isoflavone supplement products were randomly obtained from health food/grocery outlets in Houston, Texas area and two (2) red clover isoflavone supplement products were purchased on the internet at www.drugstores.com (Table 1). All other materials (typically analytical grade or better) were used as received.
Table 1.
Amounts (nmoles) of Genistein and Daidzein at 0hr (donor) and 4hrs (receiver) in Caco-2 transport studies using six red clover supplement products
| Daidzein Amount (nmol) | Genistein Amount (nmol) | |||||
|---|---|---|---|---|---|---|
| Extract Name | AP at 0 hr | BL at 4 hr | %Transported | AP at 0 hr | BL at 4 hr | %Transported |
| Eclectic Institute | 1.63±0.01 | 0.25±0.02 | 15.3% | 0.40±0.01 | 0.35±0.02 | 87.5% |
| Solaray | 0.62±0.06 | 0.14±0.01 | 22.6% | 5.95±0.08 | 0.93±0.03 | 15.6% |
| Gaia Herbs | 6.95±0.38 | 3.87±0.58 | 55.7% | 10.4±0.58 | 1.65±0.18 | 15.8% |
| Promensil | 2.36±0.05 | 0.74±0.03 | 31.4% | 0.85±0.01 | 0.39±0.01 | 45.8% |
| Rimostil | 3.62±0.19 | 0.96±0.16 | 26.5% | 0.28±0.04 | 0.11±0.02 | 39.3% |
| USP | 0.72±0.04 | 0.18±0.01 | 25.0% | 2.49±0.04 | 0.73±0.01 | 29.3% |
Extraction Procedures
Promensil® and Rimostil® tablets were reduced to fine powder. Red clover products from Eclectic Institute and Solaray are in capsule form and thus the content of each capsule was weighed after being emptied. Red clover from Gaia Herbs was in liquid form while the USP red clover standard extract is in its original fine powder form. All of these products were extracted by adding a 6-ml mixture of acetonitrile and ethanol (50%:50%) to each 100 mg product (solid or liquid) and then sonicating the resulting suspension/solution for 30minutes in a water bath at room temperature. The mixture was next centrifuged at 3500 rpm for 15 minutes. The supernatant was further diluted (1:50) with mobile phase A, and analyzed for isoflavone content using HPLC and UPLC after treatment (as described later). The remaining samples were stored at 4°C until use, and isoflavone contents were stable during storage (not shown).
HPLC Analysis of Isoflavones
The conditions for analyzing isoflavones were as follows: system, Agilent 1090 with diode array detector running the ChemStation software; column, Aqua (Phenomenex, Gilroy, CA), 5 μm, 150×0.45 cm; mobile phase A, 100% acetonitrile, mobile phase B, water (0.04%H3PO4 and 0.06%C6H15N, pH 3.0); gradient, 0 to 3 min, 80% B, 3 to 25, 80-50% B, 25 to 26, 50-80% B; wavelength, 254 nm; and injection volume, 200μl. There was a 1-min interval between the end of the run and the next injection to allow the column to be reequilibrated with 80% mobile phase B.
UPLC Analysis of Isoflavones
Selected samples were also run on ultra-performance LC that is much more efficient than HPLC. The conditions for analyzing isoflavones using UPLC were as follows: system, Waters Acquity UPLC with photodiode array detector and Empower software; column, Acquity UPLC BEH C18 (Waters, Milford, MA), 1.7 μm, 2.1×50 mm; mobile phase A, 100% acetonitrile, mobile phase B, water (0.06%C6H15N and 0.045% CH2O2:Methanol 90:10); gradient, 0 to 0.3 min, 0% B, flow rate = 1ml/min, 0.3 to 1.80, 0-50% B, flow rate = 0.925 ml/min, 1.80 to 2.10, 50-100% B, flow rate = 0.925ml/min, 2.10 to 2.40, 100% B, flow rate = 0.925, 2.40 to 2.50, 100%-0% B, flow rate = 1ml/min; wavelength, 254 nm; and injection volume, 10μl.
Caco-2 Cell Culture
The culture conditions for growing Caco-2 cells have been described previously 14-16. The seeding density (100,000 cells/cm2), growth media (Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum), and quality control criteria were all implemented in the present study as they were described previously 14,15. Caco-2 TC7 cells were fed every other day, and the monolayers (4.2 cm2 total areas) were ready for experiments from 19 to 22 days post seeding.
Transport Experiments in the Caco-2 Cell Culture Model
Experiments in triplicate were performed in pH 7.4 HBSS 14,15. The protocol for performing cell culture experiments was the same as that described previously 16. Briefly, the cell monolayers were washed three times with 37°C, pH 7.4 HBSS. The transepithelial electrical resistance values were measured, and those with transepithelial electrical resistance values less than 420 ohms × cm2 were discarded. The monolayers were incubated with the buffer for 1 h and the incubation medium was then aspirated. Because of the intrinsic concentration differences that exist between red clover compounds, all test solutions loaded onto the apical side of the cell monolayer contained the same concentration of biochanin A (10 μM). Additionally, transport experiments were also conducted using dipyridamole (10 μM) in the test solutions of USP red clover extract. Two donor (or apical) samples were taken at the beginning and at the end of an experiment, and four receiver (or basolateral) samples (400 μl) were taken every 60 min, followed by the addition of 400 μl of fresh buffer to keep a constant volume at the receiver side. Fifty microliters (50 μl) of 94% acetonitrile/6% glacial acetic acid containing 100 μM testosterone were added to each sample as the internal standard. Afterward, the mixture was centrifuged at 13,000 rpm for 8 min, and the supernatant was analyzed by HPLC and/or UPLC.
Data Analysis
Rates of transport (Vt) were calculated based on the amounts of isoflavone transported as a function of time (Eq.1) where V is the volume of the receiver in units of ml or cm3 (typical volume is 2.5 ml) and dC/dt is the rate of concentration change in the receiver side at a unit of μM/min or μM/sec. In order to account for the concentration differences between products, experimental rates of absorption for all four isoflavones from the Caco-2 transport studies (fixed at 10 μM for biochanin A) were back-calculated to correspond to the actual concentration of each isoflavone normally found in 1mg/ml of their respective product. The Permeability (P) of isoflavones to cross a cellular membrane was calculated by dividing the rate of transport (Vt) by the surface area (A) of the monolayer and the initial concentration (Ci) of these compounds at the loading side with the assumption that concentration on the other side of the membrane is negligible (Eq. 2).
| (1) |
| (2) |
Statistical Analysis
One-way ANOVA, and Student’s t-test were used to analyze the data (NCSS 2001, Kaysville, Utah). The prior level of significance was set at 5%, or p<0.05.
RESULTS
Determination of optimal isoflavones extraction method from red clover products
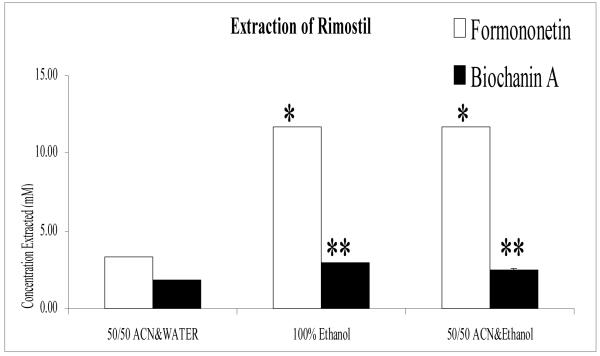
Three extraction solvents were compared using 50% acetonitrile in water, 100% pure ethanol, or an equal mix of acetonitrile and ethanol. The results showed significant (p<0.05) increase in extraction efficiency of red clover extract when ethanol is used. The most pronounced difference was in the extraction of the isoflavone formononetin from Rimostil powder (Fig. 1), where the concentration of formononetin extracted using either 100% ethanol or 50% acetonitrile and 50% pure ethanol was 3.5-fold higher than using 50% acetonitrile in water. Thus, all red clover products analyzed in this study were extracted subsequently using an equal mix of acetonitrile and ethanol. When using this solvent mixture, a second extraction of the 100 mg red clover insoluble matters (excluding Gaia) with 6 ml equal mixture of acetonitrile and ethanol did not yield significant amounts of additional isoflavones.
Fig. 1.
Comparison of the concentration (mM) of Formononetin and Biochanin A extracted from Rimsotil using 3 different extraction solvents. Use of ethanol significantly (p<0.05) increased the concentration of formononetin and BCA extracted from Rimostil as well as other red clover products. Each bar is the average of three determinations and error bars are the standard deviations of the mean.
HPLC and UPLC profiles of various red clover products
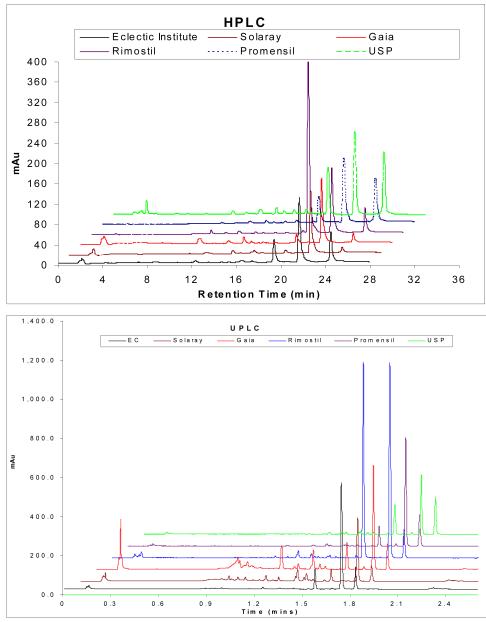
Analysis of five chosen red clover products and the USP red clover standard extract showed substantial differences in their HPLC and UPLC chromatographic profiles (Fig. 2A & B). Even though chromatographs from both HPLC and UPLC both depicted accurate chromatographic profiles of red clover isoflavones, UPLC is more efficient in separating compounds with run time decreased from 25 min to 3min per sample. As evident from the chromatographs, red clover consists of mainly formononetin and biochanin A, whereas daidzein and genistein are also present in minute quantities. Therefore, concentrations of formononetin and biochanin A should be the main determinants used to compare the isoflavone content differences between products. In our previous study of soy isoflavone supplement products, we noticed a lot of peaks other than soy isoflavone’s peaks in 13 products that were analyzed and classified them as impurities 13. In our analysis of 5 red clover products and the USP red clover standard extract, peaks resulting from these impurities (i.e., peaks other than biochanin A, formononetin, genistein and daidzein) are more prevalent in the chromatograph profiles of red clover products than from the standard USP red clover extract (Fig. 2A & B).
Fig. 2.
HPLC (2A) and UPLC (2B) chromatographs that show both the quantitative and the compositional differences among 5 red clover natural isoflavone supplement products as compared to the USP red clover standard. Each bar is the average of three determinations and error bars are the standard deviations of the mean.
Isoflavones contents of red clover products
The concentration of formononetin found in Gaia, Solaray, Eclectic Institute, Rimostil and Promensil red clover products in 1/60 (W/V) solvent (an equal mix of acetonitrile and ethanol) extracts were 69.8μM, 30.27μM, 180.93μM, 11.65mM and 1.20mM, respectively. The same 1/60 (W/V) solvent extract of the USP red clover standard yielded a formononetin concentration of 2.26mM. Among all the tested products, significant differences (p<0.05) were found between their formononetin concentrations with a 385-fold difference between the lowest (Solaray) and the highest (Rimostil) concentration detected. Significant (p<0.05) concentration differences were also noticed for the biochanin A found in Gaia, Solaray, Eclectic Institute, Rimostil and Promensil, which had biochanin A concentrations of 112.7μM, 80.52μM, 384.7μM, 2.48mM and 3.62mM, respectively. The USP red clover standard extract had a biochanin A concentration of 7.15mM. From this data, the biochanin A concentration difference between the least (Solaray) and the most (Rimostil) concentrated product was 45 folds.
Transport rates of isoflavones in extracts of different red clover products
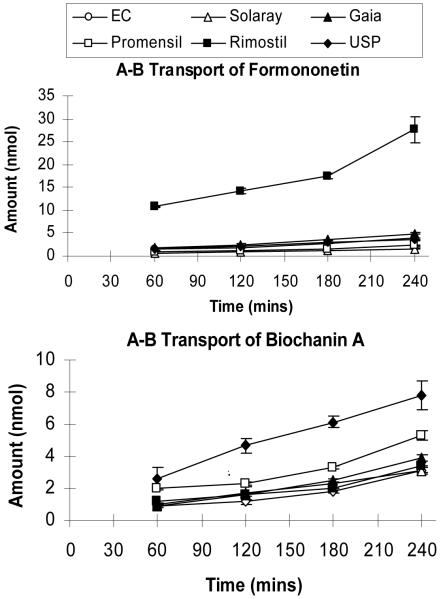
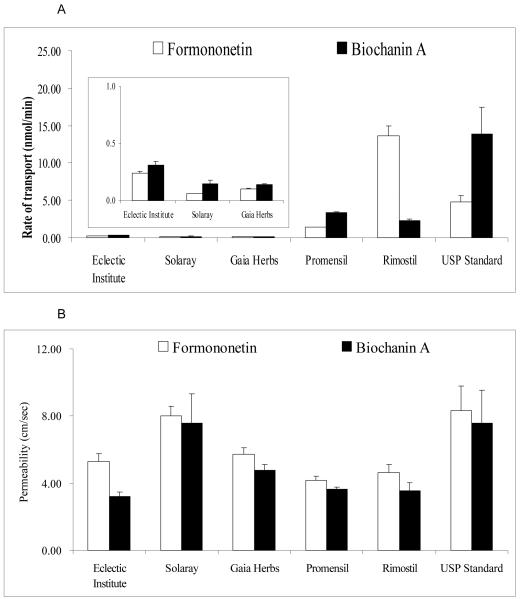
The amount of formononetin and biochanin A transported across the Caco-2 cell monolayer was measured by loading a diluted product extract that contains 10 μM biochanin A (Fig.3). The measured rates of transport of formononetin and biochanin A were normalized to a (donor) loading concentration of 1 mg of the product per ml of optimized solvent. The results indicated that intestinal transport rates (nmol/min) of formononetin and biochanin A in red clover compounds were dependent on isoflavone content in that a lower content would translate into a slower rate of absorption across the Caco-2 cells (Fig. 4A). Surprisingly, permeabilities of biochanin A and formononetin were dependent on (extracted) product matrix, with the highest permeability (Solaray and USP standard) being twice as much as the lowest permeability (Eclectic Institute and Promensil) (Fig.4B). Interestingly, in each product matrix, the permeability of formononetin was similar to that of biochanin A. For the other two isoflavones (genistein and daidzein) that were present in lower abundance, we also observed significant impact of product matrix on amounts of isoflavone transported at 4 hr, in that higher initial donor (apical) concentrations lead to higher 4 hr receiver (basolateral) concentration (Table 1). More interestingly, the largest difference in % transport (4hr) for genistein and daidzein was not observed with Solaray or USP standard (Table 1). Here, we did not calculate permeability because concentrations of genistein and daidzein at receiver side was often very low at earlier time points, which often make it impossible to quantify. Therefore, this precluded them for being used as part of the multiple time points needed for calculating the transport rates and permeability via equation 1.
Fig. 3.
Time course of absorption for formononetin and biochanin A in six red clover extracts. Experiments, in triplicates, were run using the Caco-2 cell culture model. Amounts of isoflavone absorption from the apical to the basolateral side were measured as a function of time. The incubation time points for all isoflavones were 60, 120, 180 and 240 min. The concentration of isoflavones was normalized to a biochanin A concentration of 10 μM in the starting donor solution.
Fig. 4.
Calculated rates of transport (nmol/min) and permeability (cm/secx10-6) of formononetin and biochanin A in 6 red clover extracts. Concentrations of both isoflavones in each red clover compound were back-calculated based on experimentally determined concentration from 100 mg extraction of product. The rate of transport represents the speed at which 1mg/ml of either pure formononetin or biochanin A is transported. Permeabilities (x10-6 cm/sec) were calculated based on experimental value where 10 μM of biochanin A-equivalent concentration of each extract was used.
Metabolism and excretion of biochanin A in extracts of different red clover products
The sample matrix was different for all six red clover products tested as represented by different UPLC and HPLC profiles. We hypothesized that this composition difference may influence the metabolism of isoflavones in the Caco-2 transport studies. The results from these studies show that biochanin A was the only isoflavone metabolized with noticeable metabolite peaks in both the apical and basolateral sides (Table 2). The biggest difference in the total (apical+basolateral) amounts of metabolite excreted was more than 3 times (USP > Rimostil). For all product extract, the glucuronidated biochanin A was preferentially excreted to the apical side (p<0.05 for all products). The biggest % difference (140%) in polarity is displayed by Eclectic Institute whereas the smallest was by Gaia and Solaray (27%). In the studies involving dipyridamole, results showed that the efflux of BCA glucuronides were significantly (P<0.05) reduced when dipyridamole is introduced than without (Table 2). Apical and basolateral excretion of BCA glucuronides when treated with dipyridamole was reduced 7.5 and 9.4-fold, respectively (Table 2).
Table 2.
Concentration (μM) and amount (nmoles) of formononetin and biochanin A glucuronide at 4hrs in Caco-2 transport studies using extracts obtained from USP red clover standard with and without the addition of dipyridamole (10μM). Concentration of biochanin A from extraction was targeted to be at approximately 10 μM with actual dosed concentration (back-calculated from experimental data) to be slightly higher
| USP | USP + Dipyridamole | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Caco-2 Sides | Form Dosed | Formo-Gluc | BCA Dosed | BCA-Gluc | Form Dosed | Formo-Gluc | BCA Dosed | BCA-Gluc | |
| Conc (μM) | Apical | 5.71±0.17 | 0.53±0.18 | 14.46±0.52 | 9.58±1.51* | 12.11±0.11 | 0.82±0.05 | 19.60±0.83 | 1.27±0.17* |
| Basolateral | 6.09±0.54 | 0.43±0.03 | 16.28±1.02 | 6.92±0.77* | 12.17±0.20 | 0.32±0.04 | 18.95±1.43 | 0.74±0.07* | |
| Amount (nmoles) | Apical | 14.30±0.43 | 1.32±0.45 | 36.21±1.30 | 23.91±3.78* | 30.28±0.28 | 2.05±0.13 | 49.00±2.08 | 3.18±0.43* |
| Basolateral | 15.23±1.35 | 1.08±0.08 | 40.73±2.04 | 17.30±1.93* | 30.43±0.50 | 0.80±0.10 | 47.38±3.58 | 1.85±0.18* | |
The symbol indicates that there is a statistically significant (P<0.05) difference between the concentration (and subsequent amount) of isoflavone glucuronides in either apical and/or basolateral side when Caco-2 cells were treated either with or without dipyridamole.
Lack of correlation between actual isoflavone content and product label values
We examined if the labels of the red clover products accurately report their isoflavone content since each product claimed they contained a certain amount of isoflavones. We also listed or calculated recommended daily product dose, amount per dose, the actual isoflavone amounts present in each dose and the actual amount required to reach the USP red clover standard (50mg of USP red clover standard was extracted to yield 6.6mg of isoflavones). As expected, the actual isoflavone contents found in each product varied tremendously and are not accurately reported in their respective labels (Table 3).
Table 3.
Source profiles, daily dose, perceived and actual values of various red clover products as compared to the USP standard. All red clover isoflavones were extracted using the optimized method and quantified using HPLC
| Brand Name | Lot# | Red Clover Source | Daily Dose (mg) | Number of Doses per Container | Amount of Isoflavones Per Day (mg) | Dosage (mg) required reach 6.6mg of USP Standard Isoflavonesa |
|---|---|---|---|---|---|---|
| Eclectic Institute | 6997/4 | Fresh-Freeze Dried Blossoms | 600 | 45 caps | 5.67 | 698 |
| Solaray Red Clover Blossoms | 090109 | Blossoms | 1125 | 100 caps | 4.14 | 1793 |
| Gaia Herbs | 59305-2004 | Organic tops (Liquid herbal extract) | 2666 | 22.5serving | 6.08 | 2894 |
| Promensil by Novogen | 2L0449-R3106 | Plant extract | 40 | 30 tablets | 3.24 | 81.5 |
| Rimostil by Novogen | 28587-3113 | Leaf extract | 57 | 30 tablets | 13.11 | 28.7 |
| USP Standard | FOC188 | N/A | 50 mg | N/A | 6.60 | 50 mg |
Spending calculated according to a 50mg dose of the USP red clover standard, which contains 6.60mg isoflavones.
DISCUSSION
Analysis of isoflavone content and profiles using the optimized extraction method clearly showed that they varied greatly between products (Fig.2). This analysis was enabled by an optimization of the solvent used in extraction method that minimizes the possibility that isoflavones were not detected as the result of poor extraction. This difference between isoflavone contents affects their absorption rates across the Caco-2 cell monolayer (Fig.3A and Table 1). It also affects the permeability of biochanin A and formononetin (Fig.3B, Table 1), and the metabolism of biochanin A (Table 2). These results suggest that isoflavone content in each product will likely impact their bioavailabilities, which could potentially affect their biological activity or efficacy.
Our studies indicate that ethanol is a critical reagent for optimal extraction of red clover isoflavones, where formononetin and biochanin A are the major components. This is somewhat different from the result of an earlier investigation, where acetonitrile or a solvent mixture of 50%acetonitrile in water was shown to be the best solvent for the extraction of soy isoflavones, which contain mostly glucoside and also a smaller amounts of aglycone forms of genistein and daidzein 17. This suggests the importance of optimizing the extraction procedure for each isoflavone in each different sample matrixes.
HPLC and UPLC chromatographic profiles of extracted from different red clover products showed drastic differences in isoflavone contents (Fig.2). As expected, formononetin and biochanin A were the main isoflavones present in red clover products. Daidzein and genistein, two isoflavones abundant in soybeans, were often present but only in minute quantities. In addition to differences in known isoflavones, other peaks in the chromatographic profiles that represent impurities are also very different between products and between the products and the USP standard.
We hypothesized that differences in isoflavone content would lead to different absorption rates. The results showed that normalized transport rates of biochanin A and formononetin were directly influenced by isoflavone content (Fig.3, Fig.4A). A correlating relationship could be observed since higher content usually results in higher rate of transport (not shown). Similar relationships were found for genistein and daidzein (Table 1).
We also hypothesized that permeability of different isoflavones would stay the same in a different product matrix, since these isoflavones are presumed to be absorbed via passive diffusion. However, this was not the case as permeabilities of formononetin and biochanin A and the % transport of genistein and daidzein were all affected by the sample matrix (Table 1, Fig.4B)
In addition to differences in permeability (% absorption) and absorption rates, the extent of metabolism (as represented by biochanin A glucuronide formation and polarity of biochanin A glucuronide excretion) were also affected by product matrix using the Caco-2 cell culture model. This was unexpected since we had used the same loading concentration of biochanin A at the donor side. This difference was probably not due to differences in the concentrations of formononetin present in the various products since higher formononetin concentrations are not correlated with faster or slower metabolism. Formononetin was rarely found to have metabolized at all in red clover matrix as was observed earlier 22, which was very different from when pure formononetin or biochanin A was used in rat intestinal microsomes 23: in this case they were metabolized at a comparable rate 23.
To further understand the mechanisms of above observed matrix effects, BCRP inhibitor dipyridamole was used in attempt to significantly reduce both the apical and basolateral excretion of biochanin A metabolites (Table 2). This result is consistent with the work of Morris and co-workers, who have shown that isoflavones themselves can inhibit BCRP, MDR1 and MRP1 18-20. Therefore, our data suggest that the BCRP might be a predominant efflux transporters involved in the apical efflux of isoflavone metabolites. However, our data from previous research show that apical to basolateral transport of genistein was shown to be similar to its basolateral to apical transport 21. Nevertheless, these data clearly warrant further research into the role and capacity of BCRP in the efflux of isoflavone metabolites.
The ultimate cause of observed discrepancies in isoflavone contents is likely due to lack of standardization among many red clover sources used by the manufacturers of these products. Based on product labels, red clover sources varied from red clover leaf extracts (Rimostil) to red clover tops (Gaia) to fresh-freeze dried blossoms (Eclectic Institute). The variations in materials will certainly cause differences in isoflavone concentrations. Furthermore, because of the fact that botanicals can also vary in composition due to geographical location (harvesting) as well as growing conditions, future studies will and should also investigate the comparison of content on intestinal absorption for individual products; perhaps utilizing models such as the in situ intestinal perfusion model. Moreover, possible differences in extract production processes and inadequate quality control could further attribute to content differences. We already described the biological implication for the observed difference in isoflavone contents, and we will next describe the consequence for average consumers.
Our studies showed that actual amount of isoflavones present in each tablet/dose differed greatly from the respective labels of the commercial red clover products we tested. This translates to larger differences in dosage amount required to reach a set USP red clover standard product (Table 3). Therefore, the product label is not a good predictor of quality and content in that higher reported dosing strength does not guarantee larger amounts of isoflavones available for absorption or better quality. Taken together, these inaccuracies are highly detrimental to consumers and the market place because there is no scientific basis behind the product labeling.
Lastly, we would like to state that this paper aims to determine the differences found in red clover products found in the market compared to the USP red clover standard. This paper did not attempt to endorse or demote a specific brand of red clover product since we only sample the marketed product once. The quality of these products could vary over time. The product that was shown here to be the “best” here could be the “worst” one in another sampling. To protect the consumers, FDA has just proposed new rules for dietary supplement manufacturers. If the manufacturers endorse and implement the upcoming new FDA regulation governing currect good manufacturing practices, standardization efforts could produce better and more consistent products for consumers.
In conclusion, large differences in isoflavone content between the red clover products significantly impacted absorption rates, permeability, and metabolism of various isoflavones within these products. Therefore, our results support that standardization processes such as those provided by the DSVP program from USP. Because there is tremendous ambiguity in the labeling among the products, the labels are not reflective of the actual amount of pure red clover isoflavones. Consumers will be better served by new FDA regulation that emphasizes standardization and current good manufacturing practice. Finally, this study further supports the necessity of an independent assay to precede any efficacy trials of dietary or herbal supplement in humans.
Acknowledgments
This study was supported by The National Institutes of Health (NIH) Grant CA 87779 and GM70737 to MH. S.W.J.W. was funded by USP fellowship 2005-2006 from United States Pharmacopeia.
LITERATURE CITED
- 1.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 2.Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr. 2000;71(6 Suppl):1705S–1707S. doi: 10.1093/ajcn/71.6.1705S. discussion 1708S-1709S. [DOI] [PubMed] [Google Scholar]
- 3.Muthyala RS, Sheng S, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA. Bridged bicyclic cores containing a 1,1-diarylethylene motif are high-affinity subtype-selective ligands for the estrogen receptor. J Med Chem. 2003;46(9):1589–1602. doi: 10.1021/jm0204800. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129(3):758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 5.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90(23):157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 7.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 8.Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83(7):2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 9.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3 Suppl):439S–450S. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes Control. 1998;9(6):553–557. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- 11.Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, Llorens V, Lieberman R, Crowell JA, Poisson BA, Bergan RC. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(11):1213–1221. [PubMed] [Google Scholar]
- 12.Crowell JA. The chemopreventive agent development research program in the Division of Cancer Prevention of the US National Cancer Institute: an overview. Eur J Cancer. 2005;41(13):1889–1910. doi: 10.1016/j.ejca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Chua R, Anderson K, Chen J, Hu M. Quality, labeling accuracy, and cost comparison of purified soy isoflavonoid products. J Altern Complement Med. 2004;10(6):1053–1060. doi: 10.1089/acm.2004.10.1053. [DOI] [PubMed] [Google Scholar]
- 14.Hu M, Chen J, Tran D, Zhu Y, Leonardo G. The Caco-2 cell monolayers as an intestinal metabolism model: metabolism of dipeptide Phe-Pro. J Drug Target. 1994;2(1):79–89. doi: 10.3109/10611869409015895. [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Chen J, Zhu Y, Dantzig AH, Stratford RE, Jr., Kuhfeld MT. Mechanism and kinetics of transcellular transport of a new beta-lactam antibiotic loracarbef across an intestinal epithelial membrane model system (Caco-2) Pharm Res. 1994;11(10):1405–1413. doi: 10.1023/a:1018935704693. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 17.Griffith AP, Collison MW. Improved methods for the extraction and analysis of isoflavones from soy-containing foods and nutritional supplements by reversed-phase high-performance liquid chromatography and liquid chromatography-mass spectrometry. J Chromatogr A. 2001;913(12):397–413. doi: 10.1016/s0021-9673(00)01077-3. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen H, Zhang S, Morris ME. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci. 2003;92(2):250–257. doi: 10.1002/jps.10283. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther. 2003;304(3):1258–1267. doi: 10.1124/jpet.102.044412. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65(5):1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304(3):1228–1235. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 22.Jia X, Chen J, Lin H, Hu M. Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J Pharmacol Exp Ther. 2004;310(3):1103–1113. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Wang S, Jia X, Bajimaya S, Tam V, Hu M. Disposition of Flavonoids via Recycling: Comparison of Intestinal versus Hepatic Disposition. Drug Metab Dispos. 2005 doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]