Abstract
Multiple myeloma (MM) is a progressive disease that results from dysregulated proliferation of plasma cells. Although, causative factors such as genetic events and altered expression of anti-apoptotic factors have been described in a number of patients, the mechanistic details that drive myeloma development and continued growth of malignant cells remain largely undefined. Numerous growth factors, including interleukin (IL)-6, Insulin-like growth factor-1 and IL-10 have been shown to promote growth of MM cells suggesting a significant role for cytokines in this disease. Interferon (IFN)-λ1 is a new member of the Class II cytokine family that, similar to IFN-α, has been shown to mediate viral immunity. In light of data supporting a role for cytokines in myeloma, we investigated the significance of IFN-λ1 on myeloma cell biology. Our studies show for the first time that myeloma cells bind to soluble IFN-λ1, and that IFN-λ1 induces myeloma cell growth and protects against dexamethasone-induced cell death. Our data also show that IFN-λ1 induces phosphorylation of STAT1, STAT3 and Erk. Taken together, our results suggest that IFN-λ1 may regulate myeloma cell biology and could prove to be therapeutically important.
Keywords: myeloma, IL-29, IFN-λ
Introduction
Interferon (IFN)-λ1, IFN-λ2 and IFN-λ3 (also called interleukin (IL)-29, IL-28a and IL-28b) are new members of the class II cytokine family that, similar to IFN-α, have been shown to mediate viral immunity. On account of its anti-viral activity, IFN-λ1 is thought to have biologic activity comparable to that of type I interferons. Treatment of cell lines with IFN-λ1, IFN-λ2 and IFN-λ3 results in the protection of viral infection and upregulation of major histocompatibility complex class I expression,1,2 and they were also found to have anti-proliferative and pro-apoptotic effects on a variety of tumor cell lines.3,4 The receptor for IFN-λ1 and IFN-λ2, which has parologous sequences with 81% amino acid identity, consists of the IL-10Rβ chain and the IL-28Rα chain.1,2 Stimulation of cells expressing IL-10Rβ and IFN-λR1 (IL-28Rα) results in the phosphorylation of Jak1, Tyk2, members of the STAT family and also results in the activation of the IFN–stimulated gene factor 3 transcription complex.1-3 However, the effects of IFN-λ1 on cell growth and viability were found to be more limited than IFN-α, and this is likely due to a narrower cellular distribution of the IFN-λR1 relative to the IFN-α/β receptor.2,4 In addition to their similarity to type I interferons, IFN-λ1 and IFN-λ2 also share structural properties with the IL-10-related family of cytokines, suggesting that they may have biologic properties distinct from type I interferons.
Interferons are potent immune modulatory molecules that have pleiotropic effects (anti-viral, anti-proliferative and stimulation of cytokines) on cells of the immune system.5 Although typically shown to be growth inhibitory, IFN-α has been shown to induce cell proliferation of certain cell types, including myeloma cell lines.6,7 Similar results have been seen in multiple myeloma (MM) patients, where clinical treatment with IFN-α resulted in development of aggressive plasma cell leukemia.8,9 MM is a universally fatal disease characterized by the significant accumulation of malignant plasma cells in the bone marrow. Currently, there is no curative treatment for this disease, underscoring the need to achieve a better understanding of the mechanisms underlying myeloma cell growth and survival control. The ability of IFN-α to induce growth of myeloma cells raises the question of whether or not IFN-λ1 would have the same effect. Furthermore, there is evidence suggesting a role for IL-10, whose receptor shares the IL-10Rβ subunit with IFN-λR1, in myeloma cell growth,10,11 once again suggesting a potential role for IFN-λ1 in the biology of this disease.
In light of the data supporting a role for IFN-α and IL-10 in myeloma, we investigated the significance of IFN-λ1 on myeloma cell biology. Our studies show for the first time that myeloma cells bind to soluble IFN-λ1, that IFN-λ1 is present in the malignant bone marrow environment, and that IFN-λ1 induces myeloma cell growth. Our data also show IFN-λ1 induces phosphorylation of STAT1 and STAT3 and upregulates activation or the Mitogen-activated protein kinase pathway. Taken together, our results suggest that IFN-λ1 may be an important molecule in myeloma cell biology.
Materials and methods
Cells and reagents
The KAS-6/1 and KP-6 MM cell line has been described earlier.11 Bone marrow or pleural aspirate mononuclear cells were isolated as described earlier12 from normal donors or MM patients providing written informed consent. MM cells were purified using anti-CD138 microbeads (Miltenyi, Auburn, CA, USA). Zymogenetics (Seattle, WA, USA) provided recombinant PEG-IFN-λ1 and biotinylated IFN-λ1. Phycoerythrin-conjugated strepavidin was purchased from Caltag (Burlingame, CA, USA). Mouse IgG control was purchased from Pharmingen (San Diego, CA, USA). Recombinant IL-6, IL-10 and insulin-like growth factor-1 were purchased from Peprotech (Rocky Hill, NJ, USA), and INF-α from Sigma (St Louis, MO, USA). Anti-STAT1(pY701) and anti-STAT3(pY705) were purchased from Becton Dickenson (Franklin Lakes, NJ, USA). Anti-ERK2 and phospho-ERK1/2 were purchased from Cell Signaling (Beverly, MA, USA).
Flow cytometry
Cells (1 × 106) were washed in phosphate-buffered saline containing 0.5% bovine serum albumin and incubated with 0.1 μg biotinylated recombinant IFN-λ1 or MsIg-biotin control for 30 min at 4 °C. Cells were washed and incubated with strepavidin–phycoerythrin for 30 min at 4 °C, washed and analyzed using FACScalibur and FlowJo (Tree Star, Ashland, OR, USA). For determination of phospho-STAT1 and phospho-STAT3, 1 × 106 serum-starved KAS-6/1 cells or freshly isolated myeloma cells were cultured alone or with 0.1 μg/ml IFN-λ1 for 15 min at 37 °C. The cells were then fixed using Cytofix buffer for 10 min at 37 °C followed by Perm buffer III for 30 min on ice. Cells were stained using the manufacturer's (Becton Dickenson) recommended protocol and analyzed as stated above. For IFN-λ1 binding, the change in mean fluorescence intensity (ΔMFI) was calculated by dividing the MFI of IFN-λ1 staining by its isotype-matched control. For STAT activation, the ΔMFI was calculated by dividing the MFI of phospho-STAT staining in the cytokine-activated cells by the MFI in untreated cells.
PCR
RNA was isolated from eight MM patients and KAS-6/1 cells using Trizol (Invitrogen, Carlsbad, CA, USA). cDNA was generated with SuperScript III First-Strand Synthesis SuperMix (Invitrogen). PCR amplification of IL-10Rβ, IL-28Rα and β-actin was done using HotStarTaq Master Mix (Qiagen, Valencia, CA, USA) with the following primers: IL-10Rβ forward 5′-GGCTGAATTTGCAGATGAGCA-3′; IL-10Rβ reverse 5′-GAAGACCGAGGCCATGAGG-3′; IL-28Rα forward 5′-ACCTATTTTGTGGCCTATCAGAGCT-3′; IL-28Rα reverse 5′-CGGCTCCACTTCAAAAAGGTAAT-3′; β-actin forward 5′-CCAAGGCCAACCGCGAGAAGATGAC-3′; β-actin reverse 5′-AGGGTACATGGTGGTGCCGCCAGAC. PCR products were separated on a 1% agarose gel and visualized with ethidium bromide staining.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue blocks were obtained from Mayo Clinic Tissue Registry and 4 mm sections were cut from each block. Slides were deparaffinized and endogenous peroxidase activity was quenched by incubation in 50/50% solution of 3% hydrogen peroxide and absolute methanol. All slides were pre-treated for 30 min with ethylenediaminetetraacetic acid (1 mm, pH 8.0) in steamer and cooled prior to staining. The slides were placed on a DAKO autostainer with the following autostainer activities: Background Sniper (Biocare Medical, Walnut Creek, CA, USA) followed by rabbit anti-IFN-λ1 (1 μg/ml, Abcam Inc, Cambridge, MA, USA), MACH 4™ MR HRP-Polymer (Biocare Medical) and VectorÒ NovaRED™ Substrate Kit (Vector Laboratorie, Burlingame, CA, USA). Slides were counterstained with hematoxylin. All slides were observed with light microscopy (Olympus America Inc., Melville, NY, USA) with images being captured with a SPOT RT camera (Diagnostic Instruments, Burlingame, CA, USA) at ×600 magnification.
Proliferation assays
Serum-starved myeloma cells were cultured in 96-well flat-bottom microtiter plates (Costar, Cambridge, MA, USA) at a density of 2.5 × 104 cells per well in the presence of 0.01–1000 ng/ml IFN-λ1, 4000 U/ml IFN-α, 0.1 μg/ml BLyS, 100 μg/ml IL-10 and 2 ng/ml IL-6 alone or in combination for 3 days at 37 °C in the presence of 5% CO2. PD98059 (Biosource, Camarilo, CA, USA) was used at a concentration of 50 μm. Cultures were pulsed with 1 μCi tritiated thymidine (3H-TdR; 5.0 Ci/mmol, Amersham, Piscataway, NJ, USA) for 18 h, harvested and 3H-TdR incorporation levels determined using a Beckman scintillation counter.
Cell viability studies
To determine the effect of IFN-λ1 on cell viability, patient's MM or KAS-6/1 cells were cultured in RPMI + 10% FCS alone or with the addition of 0.1 μg/ml. IFN-λ1 for 48 h. For the dexamethosone experiments, KAS-6/1 cells were cultured in RPMI + 10% FCS alone or with 0.1 μg/ml IFN-λ1, 1 ng/ml IL-6, or 10 nM dexamethasone (Sigma) alone or in combination for 48 h. Cell viability was determined by Annexin/propidium iodide staining. Briefly, 1 × 106 MM cells were stained with 1 μg of Annexin V-fluorescein isothiocyanate (Caltag, Burlingame, CA, USA) for 20 min at 4 °C. Cells were washed and 0.5 μg of propidium iodide was added to each sample and immediately analyzed by FACS as described above. Cells that stained negative for both Annexin and propidium iodide were considered viable.
Western blot analysis
Serum-starved KAS-6/1 cells (10 × 106) were left unstimulated or stimulated with 0.5 μg/ml IFN-λ1, 0.1 μg/ml IL-10, 0.05 μg/ml IL-6, 10 000 U/ml IFN-α or 25 ng/ml phorbol 12-myristate 13-acetate (PMA) for 10 min at 37 °C, and lysed in 150 μl of RIPA buffer. A total of 50 μl of lysate were separated by SDS-polyacrylamide gel electrophoresis, and proteins were transferred to an Immobilon P membrane (Millipore, Bedford, MA, USA). Membranes were incubated with anti-phospho-ERK1/2 (Cell Signaling, Danvers, MA, USA) followed by HRP-linked sheep anti-rabbit secondary antibody. Immunoreactive proteins were detected using enhanced chemiluminescence (Pierce, Rockford, IL, USA). The membrane was stripped with Restore Plus Stripping Buffer (Pierce) and reprobed with anti-ERK2 (Cell Signaling) as described above. Western blot films were scanned and the integrated density value was determined using AlphaImager 3400 (Alpha Innotech, San Leandro, CA, USA). The fold increases in Erk phopsphorylation was calculated by dividing the integrated density value of the phosphorylated Erk band by that of the total Erk for each stimulation condition. Those values were then compared to the nil control, which was given a value of one.
Results
IFN-λ1 binding and expresson in MM
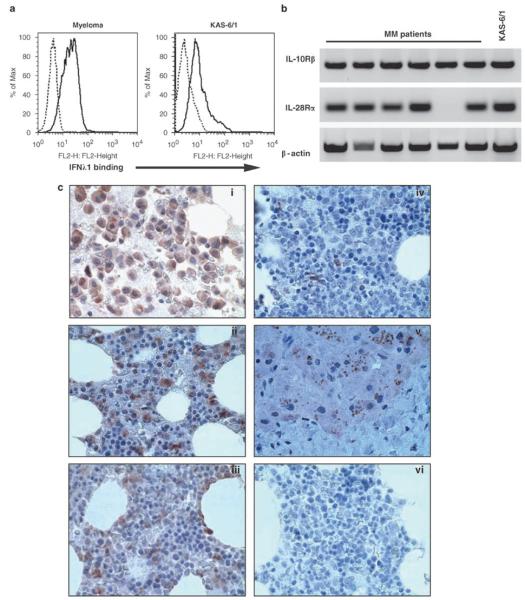
We initially began by testing the ability of CD138+ MM cells or the KAS-6/1 MM cell line to bind soluble biotinylated IFN-λ1. CD138+ MM cells bound IFN-λ1 at varying degrees with 15/20 specimens showing IFN-λ1 binding over that of the isotype control (ΔMFI = 2.64, n = 15) (Figure 1a). Similar to the MM the KAS-6/1 cell lines also bound IFN-λ1 (ΔMFI = 12.4, n = 2). Normal human peripheral blood B cells well as a number of lymphoma B cell lines did not bind significant levels of IFN-λ1, suggesting that MM cells may be unique in their ability to bind IFN-λ1 (data not shown).
Figure 1.
IFN-λ1 binding and expression in multiple myeloma. (a) KAS-6/1 cells or freshly isolated CD138+ MM cells were stained with biotin-conjugated IFN-λ1 for 30 min on ice, washed and incubated with PE-streptavidin (black line). Biotinylated mouse immunoglobulin served as a negative control (dashed line). (b) Expression of IL-10Rβ and IL-28Rα mRNA was determined by PCR. β-actin served as a loading control. (c) Multiple myeloma (i–iii), normal bone marrow (iv) and liver tissue sections were stained with an IFN-λ1-specific antibody as described in Materials and methods. Isotype controls were run for all specimens and a representive example is shown in (vi). IFN, Interferon; IL, interleukin; MM, multiple myeloma.
To confirm expression of the IFN-λ1 receptors, we performed PCR for IL-10Rβ and IL-28Rα in MM patient specimens and the KAS-6/1 cell line (Figure 1b). IL-10Rβ was expressed by all MM specimens (8/8) and KAS-6/1, whereas the IL-28Rα was expressed in 7/8 MM specimens and KAS-6/1. Expression of IL-10Rβ on KAS-6/1 was confirmed by FACS (data not shown).
To our knowledge, IFN-λ1 expression levels in the bone marrow microenvironment of normal individuals or MM patients have not yet been reported. Therefore, we next examined bone marrow sections from MM patients for expression of IFN-λ1 using immunohistochemistry. MM (n = 10) or normal bone marrow (n = 2) tissue sections were stained with an IFN-λ1-specific antibody or an isotype-matched control to determine its expression. Staining for IFN-λ1 was variable, 6/10 bone marrow sections from MM patients stained positive for IFN-λ1 (Figure 1bi–iii), whereas 4/10 were negative or stained weakly. A small number of cells within the normal bone marrow stained for IFN-λ1 (Figure 1biv), although not to the same extent as the MM specimens. Liver tissue and an isotype control are shown as positive and negative controls, respectively (Figure 1bv–vi).
IFN-λ1 promotes growth of myeloma cells
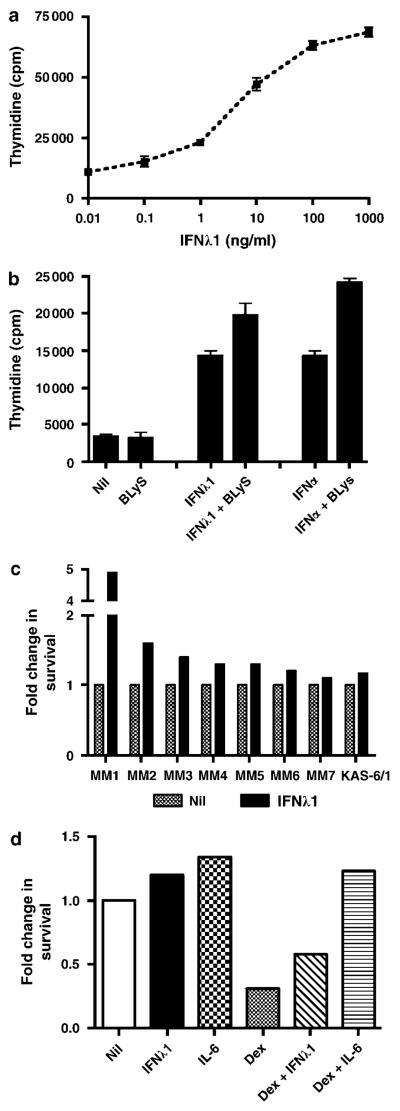
Although the anti-viral effects of IFN-λ1 have been characterized, the effect of IFN-λ1 on cell proliferation and survival has been variable and somewhat cell type dependent.4,13,14 The ability of myeloma cells to bind IFN-λ1 suggests that it may elicit biologic effects in this cell type, and we, therefore, wanted to determine the survival and proliferative consequence of IFN-λ1 on MM cells. Using the KAS-6/1 myeloma cell line as a model, we first tested the effect of IFN-λ1 on cell proliferation. When KAS-6/1 cells were cultured with 0.01–1000 ng/ml IFN-λ1, we saw a dose-dependent increase in cell proliferation suggesting that KAS-6/1 myeloma cells are growth responsive to IFN-λ1 (Figure 2a). As a positive control, IL-6 (2 ng/ml) was included in the experiment, and as expected, induced KAS-6/1 proliferation (mean c.p.m. = 140 311 ± 10 377). To determine if IFN-λ1-mediated proliferation was cell line specific, we tested its effect on the KP-6 cell MM line. We found that IFN-λ1 could also induce cell proliferation of KP-6 cells, in a dose-dependent manner, although to a lesser extent than seen KAS-6/1 (data not shown). We next compared the proliferative capacity of IFN-λ1 to IFN-α (Figure 2b). These experiments were also performed in the presence of BLyS, a TNF family member, which has been shown earlier to augment cytokine-mediated growth of MM cells.15 IFN-λ1 alone stimulated KAS-6/1 cells to proliferate at levels similar to that seen with IFN-α. As seen with IL-6 or Insulin-like growth factor-1,15 the addition of BLyS to IFN-λ1 or IFN-α consistently (n = 5) enhanced KAS-6/1 cell proliferation.
Figure 2.
IFN-λ1 mediated cell growth of multiple myeloma cells. (a) KAS-6/1 cells were cultured in the presence of 0.01–1000 ng/ml IFN-λ1 for 3 days. Values represent the mean of triplicate values of a representative experiment (n = 3). (b) KAS-6/1 cells were cultured in the presence of 0.1 μg/ml IFN-λ1, 4000 U/ml IFN-α or 0.1 μg/ml BLyS, alone or in combination, for 3 days. Values represent the mean of triplicate values of a representative experiment (n = 5). (c) KAS-6/1 cells or freshly isolated CD138+ MM cells were cultured in RPMI + 10% FCS with the addition of 0.1 μg/ml IFN-λ1 and cell viability was determined after 3 days as described in Materials and methods. (d) KAS-6/1 cells were cultured in RPMI + 10% FCS with the addition of 0.1 μg/ml IFN-λ1, 1 ng/ml IL-6 or 10 nM dexamethasone alone or in combination, and cell viability was determined after 3 days. A representative experiment is shown (n = 3). IFN, Interferon; IL, interleukin; MM, multiple myeloma.
We next determined the effect of IFN-λ1 on spontaneous MM cell death in the presence of serum (Figure 2c). When we cultured CD138+ MM or KAS-6/1 cells with IFN-λ1 for 48hrs we saw a modest, but consistent, pro-survival effect (1.3-fold increase, n = 7 for MM and a 1.1-fold increase for KAS-6/1). When feasible, survival experiments were performed for longer time points and similar trend was seen (data not shown). These data suggest that, unlike other cell types where IFN-λ1 has been shown to be pro-apoptotic, IFN-λ1 has little effect on spontaneous MM cell viability.
Because cytokines, such as IL-6, have been shown to protect MM cells from drug-induced cell death, we wanted to determine if IFN-λ1 would have a similar effect (Figure 2d). When KAS-6/1 cells were cultured in the presence of 10 nM dexamethasone (dex) alone 69% of the cells died (n = 3, mean 73%). When IFN-λ1 was combined with dex, we saw a 1.9-fold increase in cell viability (n = 3, mean fold increase = 2.6) compared with dex alone. As expected, IL-6 protected against dex-mediated cell death and almost completely abolished any effect of dex on cell viability. IFN-λ1 and IL-6 alone induced a slight increase in cell viability over the nil control. These data suggest that IFN-λ1 can protect MM cells from dex-induced cell death, although not to the same degree as IL-6.
Activation of STAT1 and STAT3 by IFN-λ1
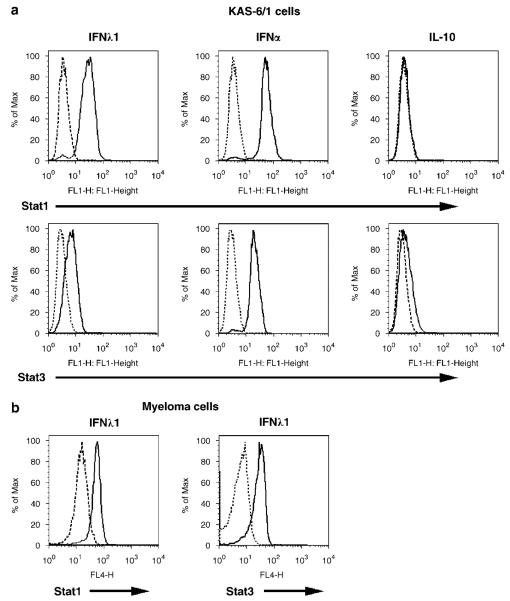
Similar to other class II cytokine family members, IFN-λ1 has been shown to activate the Jak–STAT pathway. Overexpression of either full-length IL-28R or a chimereic IL-10R1/IL-28R results in the phosphorylation of STAT1, 3 and 5,1,3 and treatment of intestinal epithelial cells with IFN-λ1 results in the phosphorylation of Akt and ERK-1/2.13 To determine if a similar signaling cascade was activated in MM cells, we first studied the effect of IFN-λ1 on STAT activation (Figure 3a). As IFN-λ1 shares biologic and structural properties with IFN-α and IL-10, we included these cytokines in the study for comparison. Stimulation of KAS-6/1 cells with IFN-λ1 results in the phosphorylation of both STAT1 and STAT3 (ΔMFI = 6.71 and 2.26, respectively; n = 3). IFN-α also induced phosphorylation of STAT1 and STAT3, whereas IL-10 had no effect on STAT1 phosphorlyation and induced a slight upregulation of phosphorylated STAT3. When we examine the ability of IFN-λ1 to activate STATs in 15 MM patient specimens (Figure 3b), we found that IFN-λ1 induced phosphorylation of both STAT1 (ΔMFI = 1.76, n = 13) and STAT3 (ΔMFI = 1.79, n = 9); however, no detectable STAT activation was seen in a subset of patients (n = 2 for STAT1 and n = 6 for STAT3).
Figure 3.
Activation of the STAT pathway by IFN-λ1. (a) KAS-6/1 or (b) CD138+ patient MM cells were stimulated with 0.1 mg/ml IFN-l1, 10 000 U/ml IFN-α or 0.1 mg/ml IL-10 for 10 min, and STAT1 and −3 phosphorylation was determined by FACS as described in Materials and methods. IFN, Interferon; IL, interleukin; MM, multiple myeloma.
IFN-λ1-mediated myeloma cell growth is dependent of activation of the Erk pathway
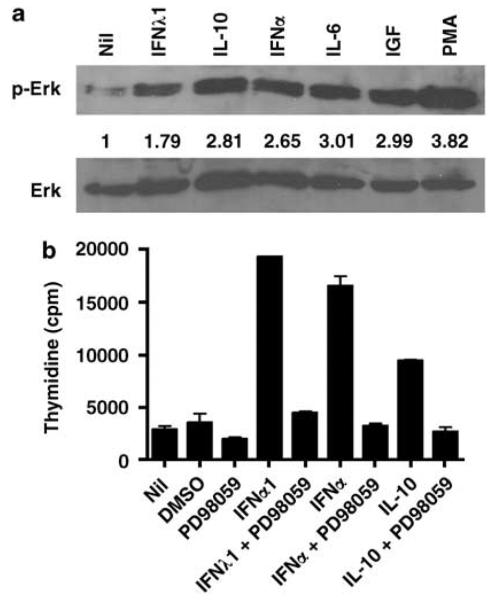
In addition to activation of STATs, we wanted to determine the effect of IFN-λ1 on activation of the Erk pathway (Figure 4a). KAS-6/1 cells were stimulated with IFN-λ1, IL-10 and IFN-α, and we found that Erk was phosphorylated in response to IFN-λ1 treatment (Figure 4b, top panel). The degree of phosphorylation seen in response to IFN-λ1 (1.79-fold) was less than IL-10 (2.81) and IFN-α (2.65), but was consistently detected (n = 3). IL-6, Insulin-like growth factor-1 and PMA served as positive controls for Erk activation. Expression of total Erk protein levels are shown in the lower panel of Figure 4b and were used for normalization of phosphorylated Erk.
Figure 4.
IFN-λ1 mediated cell growth of multiple myeloma cells is Erk dependent. (a) Phosphorylated and total Erk levels were determined by western blot after stimulation of KAS-6/1 cells with 0.1 μg/ml IFN-λ1, 10 000 U/ml IFN-α, 0.1 μg/ml IL-10, 0.05 μg/ml IL-6, 0.1 μg/ml IGF-1 or 25 ng/ml PMA. The fold increase in phosphorylated Erk was calculated as described in Materials and methods and it is shown below the upper panel. (b) KAS-6/1 cells were cultured in the presence of 0.5 μg/ml IFN-λ1, 4000 U/ml IFN-α or 0.1 μg/ml IL-10 alone or with 50 μM PD98059 for 3 days. Values represent the mean of triplicate values of a representative experiment (n = 5). IGF-1, Insulin-like growth factor-1; IFN, Interferon; IL, interleukin.
As Erk activation is associated with cell proliferation, we next wanted to determine if IFN-λ1-mediated cell proliferation was Erk dependent. KAS-6/1 cells were stimulated with IFN-λ1, IL-10 and IFN-α alone or in the presence of the MEK inhibitor PD98059 (Figure 4b). DMSO and PD98059 alone served as controls. All three cytokines induced cell proliferation, and in the presence of the MEK inhibitor, proliferation was completely abolished (representitive experiment shown, n = 5). These data suggest that IFN-λ1, as well as IL-10 and IFN-α, mediated proliferation of KAS-6/1 cells is Erk dependent.
Discussion
To date, IFN-λ1 has been shown to have biologic activity comparable to that of type I interferons in that it mediates viral immunity and inhibits cell growth. In contrast to these initial findings, our data suggest that IFN-λ1 may have a much more pleitropic effect on cells, which may, in part, be cell type specific. Our studies clearly suggest that MM cells can bind IFN-λ1, express the IFN-λ1 receptors IL-10Rβ and IL-28Rα, and that IFN-λ1 is present in the malignant microenvironment (Figure 1). Although, these findings do not specifically address which cell in the bone marrow produces IFN-λ1, they do provide evidence that IFN-λ1 can be present at higher levels compared with normal marrows.
Our results define IFN-λ1 as a novel growth factor of MM cells, however, it was found to have little effect on spontaneous cell viability (Figure 2). The ability of IFN-λ1 to induce cell death and inhibit cell proliferation in one cell type while promoting cell growth in another is not unique and has also been seen with IFN-α.11 Although the precise mechanism of how IFN-λ1 induces variable growth responses remains to be defined, the dynamics of receptor expression and heterodimerization, as well as activation of cell type-specific signaling cascades may contribute to the unique IFN-λ1 response seen in MM cells. While seeing little effect on spontaneous MM cell death, IFN-λ1 did protect against dex-induced cell death (Figure 2d). These data suggest that expression of IFN-λ1 in the tumor microenvironment may promote MM cell viability and may, therefore, have clinical implications.
To better understand the initial signaling events that are induced by IFN-λ1 in MM cells, we characterized the effect of IFN-λ1 on activation of the STAT and Erk pathways. Taken together, the signaling studies show that IFN-λ1, like IFN-α, activates phosphorylation of STAT1, STAT3 (Figures 3a and b) and Erk (Figure 4a) in MM cells. IFN-λ1 signaling differs somewhat from IL-10 in that we detected no IL-10 mediated phopsphorylation of STAT1, however, IL-10 did induce phosphorylation of Erk. Additionally, our data showed that IFN-λ1, as well as IFN-α and IL-10, mediated cell proliferation of MM cells is Erk dependent, as the MEK inhibitor PD98059 completely inhibited cytokine-mediated proliferation (Figure 4b). PD98059 did not effect IFN-λ1-mediated STAT activation (data not shown), suggesting that MEK activation is downstream of STAT phopsphorylation. These results suggest that the upstream signaling cascades activated by IFN-λ1 in MM cells compares to that seen in other cell types, therefore, does not initially explain why IFN-λ1 has a proliferative effect in MM cells versus the anti-proliferative effect seen in other cell types. However, it is quite possible that other unique downstream targets are activated by IFN-λ1 in MM cells that drive them to respond in a unique manner.
Additionally, it is also feasible that the IFN-λ1 receptors may not signal independently in MM and may be involved in receptor cross-talk. Evidence for receptor cross-talk has been characterized in MM cells, where it was found that stimulation of KAS-6/1 cells with IFN-α resulted in phosphorylation of the signaling subunit of the IL-6 receptor, gp130, and using a chimeric receptor approach it was found that IFN-α mediated phosphorylation of gp130 was Jak dependent.16 The ability of IFN-α to modulate IL-6-mediated signal transduction has importance in MM biology as IL-6 is a key growth factor for these cells. Whether or not IFN-λ1 can cross-talk with IL-6 or other growth factor receptors is yet to be defined, and further characterization of IFN-λ1 mediated cell signaling in MM cells, as well as other cell types, will hopefully lend insight into how this occurs.
In summary, our studies show for the first time that myeloma cells bind to soluble IFN-λ1, express IFN-λ1 receptors and that IFN-λ1 induces myeloma cell growth. IFN-λ1 was found to have a modest effect on spontaneous myeloma cell viability, but did protect against dex-induced cell death. Our data also show that IFN-λ1 induces phosphorylation of STAT1 and STAT3 and upregulates activation or the MAPK pathway. Taken together, our results suggest that IFN-λ1 may be an important molecule in myeloma cell biology and could prove to be therapeutically relevant.
Acknowledgements
We would like to thank Diane Jelinek for the use of the KAS-6/1 and KP-6 cell line. This study supported in part by the National Institutes of Health Grants CA062242 and ZymoGenetics Inc. and Merck Serono International SA, an affiliate of Merck KGaA, Darmstadt, Germany.
References
- 1.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 3.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 4.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 6.Jelinek DF, Aagaard-Tillery KM, Arendt BK, Arora T, Tschumper RC, Westendorf JJ. Differential human multiple myeloma cell line responsiveness to interferon-alpha. Analysis of transcription factor activation and interleukin 6 receptor expression. J Clin Invest. 1997;99:447–456. doi: 10.1172/JCI119179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelinek DF, Arora T. Effects of interferon alpha on myeloma cells: mechanisms of differential responsiveness. Curr Top Microbiol Immunol. 1997;224:261–268. doi: 10.1007/978-3-642-60801-8_27. [DOI] [PubMed] [Google Scholar]
- 8.Blade J, Lopez-Guillermo A, Tassies D, Montserrat E, Rozman C. Development of aggressive plasma cell leukaemia under interferon-alpha therapy. Br J Haematol. 1991;79:523–525. doi: 10.1111/j.1365-2141.1991.tb08068.x. [DOI] [PubMed] [Google Scholar]
- 9.Sawamura M, Murayama K, Ui G, Matsushima T, Tamura J, Murakami H, et al. Plasma cell leukaemia with alpha-interferon therapy in myeloma. Br J Haematol. 1992;82:631. doi: 10.1111/j.1365-2141.1992.tb06484.x. [DOI] [PubMed] [Google Scholar]
- 10.Lu ZY, Zhang XG, Rodriguez C, Wijdenes J, Gu ZJ, Morel-Fournier B, et al. Interleukin-10 is a proliferation factor but not a differentiation factor for human myeloma cells. Blood. 1995;85:2521–2527. [PubMed] [Google Scholar]
- 11.Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia. 1996;10:866–876. [PubMed] [Google Scholar]
- 12.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973–2979. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 13.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, et al. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G960–G968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- 14.Zitzmann K, Brand S, Baehs S, Goke B, Meinecke J, Spottl G, et al. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem Biophys Res Commun. 2006;344:1334–1341. doi: 10.1016/j.bbrc.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 16.French JD, Walters DK, Jelinek DF. Transactivation of gp130 in myeloma cells. J Immunol. 2003;170:3717–3723. doi: 10.4049/jimmunol.170.7.3717. [DOI] [PubMed] [Google Scholar]