Abstract
Hypoplastic coronary artery disease (HCAD) is a rare condition that may lead to myocardial infarction (MI) and sudden death. We discovered HCAD in a young man who developed chest pain after heavy drinking and who was found to have suffered an MI. His ECG showed ST-segment elevation with Q waves in the anterior leads, and echocardiography revealed apical dyskinesia with moderate left ventricular (LV) dysfunction. Coronary angiography showed hypoplasia of the left anterior descending (LAD) artery. 99mTc-tetrofosmin-gated myocardial perfusion scintigraphy showed a large, fixed perfusion defect in the anteroseptal and apical segments. Sixty-four-slice cardiac CT and cardiac MR imaging demonstrated thinning of the apical wall with calcification and delayed enhancement, supporting the diagnosis of long-standing MI. The patient was discharged symptom-free on medication for ischemic heart failure two weeks after admission. Although HCAD is very uncommon, it should be considered in children and young adults who suffer MI or sudden cardiac death.
Keywords: Coronary vessel anomalies, Myocardial Infarction
Introduction
Hypoplastic coronary artery disease (HCAD) refers to congenital underdevelopment of one or more epicardial coronary arteries or their major branches with greatly decreased luminal diameter or length.1-3) HCAD has rarely been documented in living patients, and it is associated with a high rate of sudden death and poor outcome.1-7) We report a case of HCAD in a young man, which was discovered after the patient suffered a myocardial infarction (MI).
Case
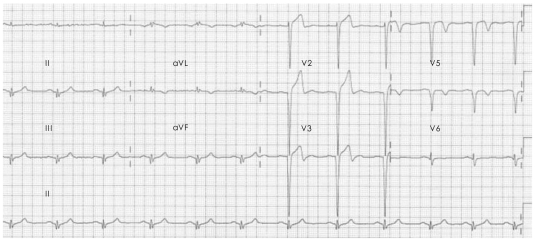
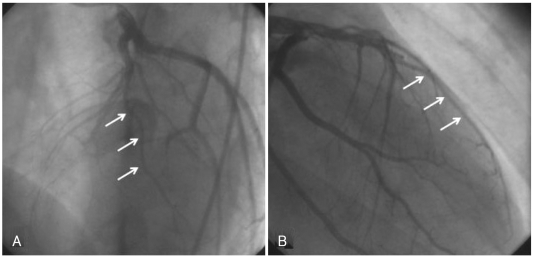
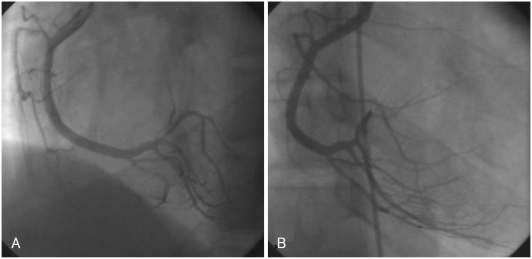
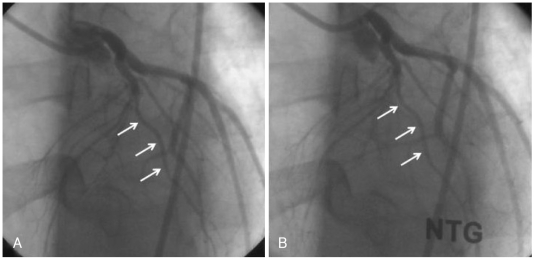
A 20-year-old man presented to the emergency department with two hours of persistent substernal chest pain, which started after heavy alcohol consumption. He had experienced dyspnea on exertion for the past two years. He was a seven pack-year smoker, but he had no other significant clinical history. Initial physical examination was normal, and ECG showed ST-segment elevation with Q waves in the anterior precordial leads (Fig. 1). Cardiac enzymes were normal, and echocardiography showed a dilated left ventricle (LV) with apical dyskinesia and moderate LV dysfunction (EF=33%). Emergent coronary angiography showed no atherosclerotic lesion, but did reveal hypoplasia of the mid-to-distal left anterior descending (LAD) artery without compensatory collateral vessels supplying the apex (Fig. 2). A myocardial bridge was noted at the mid-LAD. The other coronary arteries were unremarkable and of normal size. A posterior branch of the right coronary artery (RCA) supplied the inferior aspect of the interventricular septum (Fig. 3). Intracoronary infusion of nitroglycerin did not change the diameter or the morphology of any of the coronary arteries (Fig. 4).
Fig. 1.
ECG on admission showing ST-segment elevation and Q waves in the anterior precordial leads. ECG: electrocardiogram.
Fig. 2.
Left coronary artery angiograms showing a hypoplastic mid-to-distal left anterior descending coronary artery (arrows). A: left anterior oblique view. B: right anterior oblique view.
Fig. 3.
Right coronary artery angiograms showing right dominant coronary artery circulation, with a posterior descending artery supplying the inferior aspect of the interventricular septum. A: left anterior oblique view. B: right anterior oblique view.
Fig. 4.
Left anterior oblique view of the left coronary artery showing hypoplasia of the left anterior descending artery (arrows). Intracoronary infusion of nitroglycerin did not change the diameter, excluding the presence of vasospasm. A: before infusion of nitroglycerin. B: after infusion of nitroglycerin.
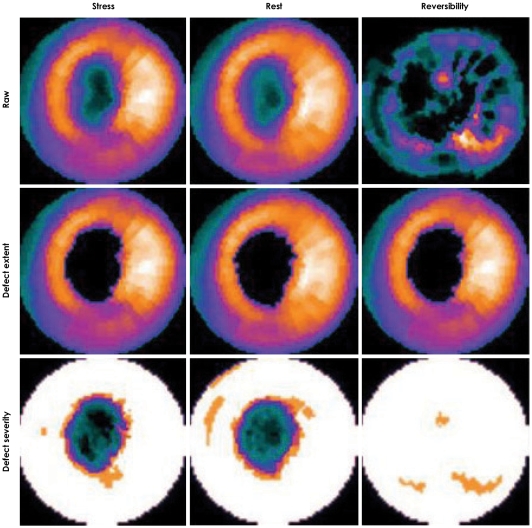
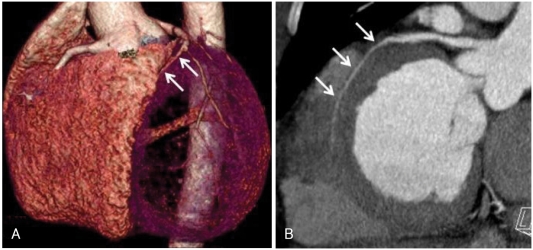
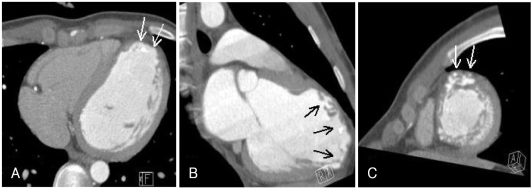
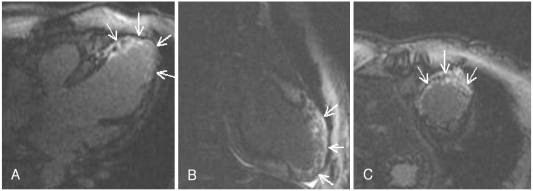
A stress test with 99mTc-tetrofosmin-gated myocardial perfusion scintigraphy showed a large, fixed perfusion defect involving the anteroseptal and apical segments (Fig. 5). Sixty-four-slice cardiac CT demonstrated hypoplasia of the LAD in its mid-to-distal portion (Fig. 6) and showed focal thinning of the LV wall at the apex, with calcifications suggestive of long-standing MI (Fig. 7). No associated anomalies, such as a single coronary ostium, an atretic coronary ostium, or a high takeoff position, were found. Similar findings were observed on cardiac MR imaging with delayed enhancement (Fig. 8). The final diagnosis was anterior MI due to HCAD. The patient was discharged on medication for ischemic heart failure (aspirin, clopidogrel, carvedilol, ACE inhibitor, aldosterone antagonist, and a nitrate) 14 days after admission.
Fig. 5.
99mTc-tetrofosmin-gated myocardial perfusion scintigraphy showing a fixed apical and anterior defect. Three perfusion polar maps (from left to right: stress, rest, and reversibility) show extent and severity of perfusion defect.
Fig. 6.
Cardiac CT angiography demonstrating hypoplasia of the left anterior descending artery in its mid-to-distal portion (arrows). A: three-dimensional volume rendering image, left anterior view. B: curved multiplanar reformatted image, left posterior view.
Fig. 7.
Contrast-enhanced cardiac CT scans showing thinning of the apical wall of the left ventricle with calcifications (arrows), suggestive of long-standing myocardial infarction. A: four-chamber view. B: two-chamber view. C: short-axis view.
Fig. 8.
Contrast-enhanced magnetic resonance images showing diffuse thinning and aneurysmal dilatation of the apical wall of the left ventricle with delayed enhancement (arrows), suggesting old myocardial infarction. A: four-chamber view. B: two-chamber view. C: short-axis view
Discussion
The diagnosis of HCAD is usually made if there is an obvious difference in the size of the lumina of the main branches of the coronary arteries or if the length is markedly decreased.4),5) There are two groups of hypoplastic coronary arteries: those that occur in association with other abnormalities and those that occur in isolation. The majority of the reported cases are isolated and are often diagnosed at necropsy.1),2) In living patients, the diagnosis is made using coronary angiography.6),7)
HCAD often manifests as sudden death, especially in young adults and athletes.1-3) It is unusual to see a patient with MI and isolated HCAD diagnosed on coronary angiography, as was seen in this case. His MI and presenting symptoms may be attributable to coronary artery spasm caused by autonomic and/or endothelial dysfunction at the hypoplastic site.8) A milking effect at the myocardial bridge might also have occurred in our patient. In order to document the presence of spasm, an ergonovine or acetylcholine challenge might be necessary. Intravascular ultrasonography during the evaluation of coronary spasm could be of use in excluding atherosclerotic coronary obstructive disease. These tests could have been helpful in our patient, with respect to elucidating the causal relationship between HCAD and MI. However, unlike the effort-related ischemia typical of fixed obstructive lesions, ischemia associated with coronary anomalies is not always reproducible. It usually occurs under inconsistent or exceptional clinical conditions, such as extreme exertion.9-14) Furthermore, it remains controversial whether coronary anomalies may predispose to obstructive atherosclerotic disease independent of the presence of atherosclerosis risk factors.15) Thus, difficult though it is to uncover the exact mechanisms involved, HCAD could be reasonably understood to have induced MI complicated by heart failure in this otherwise healthy young man.
Heart transplantation may be indicated in patients who develop ischemic cardiomyopathy with end stage heart failure. Implantable cardioverter-defibrillator (ICD) therapy can also be considered for patients who have post-MI heart failure, because sudden death is a frequent issue in individuals with this coronary anomaly.16) Given that our patient had chronic stable heart failure and there was no evidence of persistent myocardial ischemia on the myocardial perfusion scan, periodic ECG and Holter monitoring were planned in lieu of prophylactic ICD therapy.17),18)
In conclusion, HCAD is a rare congenital anomaly predisposing to MI and sudden death. A high index of suspicion should be exercised for the possibility of HCAD in children and young adults who present with MI and/or sudden cardiac death.
References
- 1.Roberts WC, Glick BN. Congenital hypoplasia of both right and left circumflex coronary arteries. Am J Cardiol. 1992;70:121–123. doi: 10.1016/0002-9149(92)91407-u. [DOI] [PubMed] [Google Scholar]
- 2.Zugibe FT, Zugibe FT, Jr, Costello JT, Breithaupt MK. Hypoplastic coronary artery disease within the spectrum of sudden unexpected death in young and middle age adults. Am J Forensic Med Pathol. 1993;14:276–283. doi: 10.1097/00000433-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kim MS, Han JK, Lee SE, et al. Cases of right ventricular myocardial infarction in patients with an absent or hypoplastic right coronary artery. Korean Circ J. 2007;37:84–86. [Google Scholar]
- 4.Amabile N, Fraisse A, Quilici J. Hypoplastic coronary artery disease: report of one case. Heart. 2005;91:e12. doi: 10.1136/hrt.2004.047621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipsett J, Cohle SD, Berry PJ, Russell G, Byard RW. Anomalous coronary arteries: a multicenter pediatric autopsy study. Pediatr Pathol. 1994;14:287–300. doi: 10.3109/15513819409024261. [DOI] [PubMed] [Google Scholar]
- 6.Göl MK, Ozatik MA, Kunt A, et al. Coronary anomalies in adult patients. Med Sci Monit. 2002;8:CR636–CR641. [PubMed] [Google Scholar]
- 7.Casta A. Hypoplasia of the left coronary artery complicated by reversible myocardial ischemia in a newborn. Am Heart J. 1987;114:1238–1241. doi: 10.1016/0002-8703(87)90204-3. [DOI] [PubMed] [Google Scholar]
- 8.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology and clinical relevance. Circulation. 2002;105:2449–2454. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 9.Maron B, Roberts WC. Causes and implications of sudden cardiac death in athletes. In: Akhtar M, Myerburg RJ, Ruskin JN, editors. Sudden Cardiac Death. Philadelphia: Williams & Wilkins; 1994. pp. 238–255. [Google Scholar]
- 10.Virmani R, Burke AP, Farb A. The pathology of sudden cardiac death in athletes. In: Williams RA, editor. The Athlete and Heart Disease. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 249–272. [Google Scholar]
- 11.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 12.Waller BF. Exercise-related sudden death in young (age ≤30 years) and old (age ≥30 years) conditioned subjects. In: Wenger NK, editor. Exercise and the Heart. 2nd ed. Philadelphia: FA Davis; 1985. pp. 9–73. [PubMed] [Google Scholar]
- 13.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 14.Cheitlin MD . Coronary anomalies as a cause of sudden death in athletes. In: Estes NA, Salem DN, Wang PJ, editors. Sudden Cardiac Death in the Athlete. Armonk, NY: Futura Publishing Company; 1998. pp. 379–391. [Google Scholar]
- 15.Blake HA, Mahion WC, Mattingly TW, et al. Coronary artery anomalies. Circulation. 1964;30:927–934. doi: 10.1161/01.cir.30.6.927. [DOI] [PubMed] [Google Scholar]
- 16.Gradaus R, Wollman C, Kobe J, et al. Potential benefit from implantable cardioverter-defibrillator therapy in children and young adolescents. Heart. 2004;90:328–329. doi: 10.1136/hrt.2003.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buxton AE, Lee KL, Fisher JD, Josephson M, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 18.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]