Abstract
Background and Objectives
Kawasaki disease (KD) is an acute inflammatory process affecting the arterial walls that results in panvasculitis. Recent studies have shown that even after resolution of the disease, endothelial dysfunction persists and may progress to atherosclerosis. The pulse wave velocity (PWV) and the ankle-brachial index (ABI) are simple and non-invasive methods for evaluating the degree of atherosclerosis, and are known as the predictors of cardiovascular disease in adults. Carotid intima-media thickness (cIMT) is also known as a predictor of cardiovascular disease. We conducted this study to determine the change in arterial stiffness by measuring the PWV, ABI, and cIMT in children who have recovered from KD.
Subjects and Methods
Twenty-five patients with KD and coronary aneurysm were recruited. They all recovered from KD and were normal for more than 8 years. Fifty-five healthy children were evaluated as the control group. Their height, weight, body mass index, and blood pressure (systolic, diastolic, and the mean) were measured. The PWV, ABI, ejection time (ET), and pre-ejection period (PEP) were measured by ultrasonography. The cIMT was measured by ultrasonography.
Results
The left brachial ankle PWV was significantly higher in the KD group (1020.6±146.5 cm/sec) than the control group (984.0±96.5 cm/sec). The ABI did not differ between the two groups. There was no difference in PEP/ET and cIMT.
Conclusion
The PWV in children who recovered from KD was higher than the control group. Long-term follow up is necessary in children after recovery from KD even if there is no abnormality in echocardiography.
Keywords: Kawasaki disease, Arterial stiffness, Ankle-brachial index, Carotid arteries
Introduction
Kawasaki disease (KD) is an acute inflammatory disease that affects the systemic blood vessels, and it was first reported in Japan in 1967.1) It can occur regardless of the patient's age, but usually occurs in children <5 years of age. The prevalence varies between geographic areas, and it occurs at a higher incidence in Asian countries, such as Korea or Japan.2),3)
The signs and symptoms which are noted during the acute phase persist for 10 days and most of the symptoms gradually improve.2) In approximately 20% to 25% of untreated pediatric cases, however, coronary aneurysms may occur, which may lead to myocardial infarction or sudden death.4) Since the use of immunoglobulins to treat KD, the incidence of coronary aneurysms has greatly decreased. Nevertheless, in 10% of patients coronary aneurysms frequently occur.5)
According to recent studies, endothelial dysfunction persists even after KD and this may lead to early stage of atherosclerosis. It has also been reported that endothelial dysfunction occurs in children who have no abnormal findings in the coronary arteries during the acute phase.6) Echocardiography or coronary angiography are effective diagnostic modalities in cases in which the coronary aneurysms or stenoses form, but cannot be detected during the early stage of atherosclerosis.
Pulse wave velocity (PWV) is a simplified, non-invasive method that can measure the severity of atherosclerosis, and it is known as a prognostic factor for cardiovascular diseases in adult patients.7),8) Carotid intima-media thickness (cIMT) is also known as an indicator for atherosclerosis in both pediatric and adult patients.9) Increased cIMT is observed in pediatric patients who have obesity,10) hyperlipidemia,11) and diabetes mellitus.12) It has been reported that an increased cIMT is noted in children who recover from KD with no coronary artery lesions compared to healthy children.13),14) However, Ikemoto et al.15) reported contradictory results.
Long-term histopathologic dysfunction has been reported, even in cases in which both echocardiographic and angiographic findings were normal following recovery from the coronary aneurysm.6),13)
In the current study, we measured the PWV and cIMT in children who recovered from KD complicated by coronary aneurysms and determined the degree of arterial stiffness.
Subjects and Methods
Study population
The current study was conducted in 25 pediatric patients who were hospitalized in the Department of Pediatrics of Ewha Womans University Hospital with a diagnosis of KD accompanied by a coronary aneurysm. The patients were treated with 2 g/kg of immunoglobulin and high-dose aspirin (50 mg/kg/d). The administration of low-dose aspirin (5 mg/kg/d) was discontinued after the coronary artery lesion recovered to normal and >8 years has elapsed since the recovery. Fifty-five healthy children served as normal controls.
Methods
Anthropometric data
In our patients, height was measured using a height measurement system and the weight was measured up to the first decimal point after the zero point was adjusted. The body mass index (BMI) (kg/m2) was defined as the value calculated by the division of weight (kg) by the square value of height (m2), and it was expressed up to the first decimal point.
Blood pressure measurement
Blood pressure was measured with the use of an oscillometric blood pressure monitor after stabilization was attempted for >10 minutes with a blood pressure cuff with a diameter of 2/3 of the arm thickness. After 5 minutes, the blood pressure was measured twice and the measurements were then averaged. This was the mean value of blood pressure of the subject patients.
Pulse wave velocity, ankle brachial index, ejection time, and pre-ejection period
PWV and ABI were measured using a VP-1000 (Colin Co. Ltd, Komaki, Japan). PWV, ankle brachial index (ABI), the blood pressure of the extremities, EKG, and heart sounds were synchronously measured and then automatically recorded. An electrode was contacted on both wrists and a microphone was attached to the left margin of the sternum. Then, the extremities were wrapped by a cuff which was connected to a pulse monitor. The volume wave and time difference emitted from the pulse monitor were recorded. The pulse wave was defined as the value obtained by dividing the distance between the two points by the time spent in transferring the pulse. In the current study, the pulse wave was measured in the brachial artery and ankle (baPWV). The ABI was defined as the ratio between the systolic pressure measured in the ankle and that measured in the brachial artery.
Ejection time (ET) was defined as the time elapsed until the semilunar valve was closed since it was first opened. The pre-ejection period (PEP) was defined as the time elapsed until the semilunar valve was opened since the initial time point for the QRS complex. To minimize the errors due to the difference in heart rate depending on age, the PEP/ET was calculated.
Carotid intima-media thickness
cIMT was measured with a B ultrasonogram to which a 12.5 MHz linear transducer was installed (iU22, Intelligent Ultrasound System; Philips, Amsterdam, The Netherlands). cIMT measurement was performed in all patients by one experienced technician unaware of the group to which the subject patients were assigned.
The subject patients were given the test in a lying position while they slightly rounded their head. Within 1 cm of the junction of the right common carotid artery, the cIMT and vascular diameter of the vessel during the systolic and diastolic periods were measured. cIMT was defined as the distance between the two points which were observed to be bright on echocardiography. The compliance and distensibility of the carotid artery were calculated based on the following formulae:
-
Lumen cross-sectional area (mm2)=πdD2/4
Wall cross-sectional area (mm2)=π(dD/2+ IMT)2-π (dD/2)2
Cross-sectional compliance (mm2·mmHg-1)=[π (sD2-dD2)]/4ΔP
Cross-sectional distensibility (mmHg-1·10-2)=(sD2-dD2) /(dD2·ΔP)
IMT: intima-media thickness (mm)
sD: systolic diameter (mm)
dD: diastolic diameter (mm)
ΔP: pulse pressure (mmHg)
Echocardiography
In children who were diagnosed with KD, two-dimensional echocardiography was performed during the acute phase prior to the administration of immunoglobulins, after 2 months, during the recovery phase, and every year thereafter. On the parasternal short axis view, the proximal area of the left and right coronary arteries was examined. In accordance with the diagnostic criteria recommended by the Ministry of Health and Welfare in Japan, the coronary artery was defined as abnormal in children aged <5 years of age in whom the diameter of the coronary artery was >3 mm, those ≧5 years of age in whom the diameter of the coronary artery was >4 mm, cases in which the lumen of the segment was >1.5 times the adjacent segment, and those in whom the lumen of the coronary artery was clearly irregular.
Echocardiography was performed using a Hewlett Packard 5500 (Philips, Andover, MA, USA) and the transducer with a frequency of 3.5 and 5 MHz. The ventricular function was assessed by measuring the myocardial performance index (MPI) and the EF of the left ventricle. The MPI was obtained by dividing the isovolumetric contraction time (ICT) and the isovolumetric relaxation time (IRT) by the ET. The ejection fraction was measured with the use of the modified Simpson method, for which the endsystolic volume (ESV) was subtracted from the enddiastolic volume (EDV), and the result was divided by the EDV on a four-chamber view.
Tissue Doppler imaging (TDI) was performed based on the following methods. Sample volume (3-5 mm) and Nyquest limit (15-20 cm/sec) were set at a low value. The wall filter was also set at the lowest value possible. The peak wall velocity was expressed as the positive value when the transducer was approached and a negative value when it became remote from the transducer.
On apical four-chamber view, at the base of the interventricular septum, the peak early and late diastolic myocardial velocities (E, myocardial velocity; and A, myocardial velocity), and the peak systolic myocardial velocity (S, myocardial velocity) were measured.
Statistical analysis
Data which were examined and all the measurements were expressed as the mean±standard deviation (SD), for which statistical analysis was performed using a Student t-test. A p<0.05 was considered statistically significant. All the statistical analyses were performed using SPSS, version 11.0.
Results
Clinical characteristics
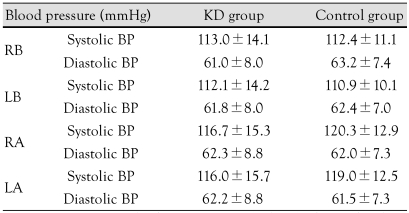
The mean age of the KD group (12.6±2.0 years) was significantly lower than the control group (14.5±0.7 years; p<0.05). However, there were no significant differences in height, weight, and BMI between the two groups (Table 1). In addition, there were no significant differences in blood pressure, which was measured in the right upper arm, left upper arm, right ankle, and left ankle between the two groups (Table 2).
Table 1.
Anthropometric data of the study group
*p<0.05: significantly different from the control group. KD: Kawasaki disease, BMI: body mass index
Table 2.
Comparison of blood pressure in the Kawasaki disease group and the control group
p>0.05. KD: Kawasaki disease, RB: right brachial, LB: left brachial, RA: right ankle, LA: left ankle
An assessment of cardiac function by echocardiography
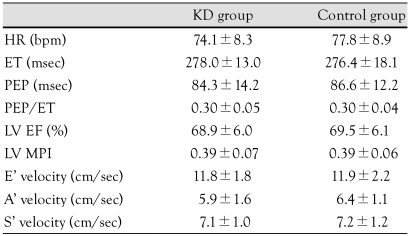
The heart rate was 74.1±8.3 times/min in the KD group and 77.8±8.9 times/min in the control group. There was no significant difference between the two groups. The ejection time was 278.0±13.0 msec in the KD group and 276.4±18.1 msec in the control group, with no significant difference between the two groups. The PEP was 84.3±14.2 msec in the KD group and 86.6±12.2 msec in the control group (p>0.05) (Table 3).
Table 3.
Comparison of cardiac function by echocardiography in the Kawasaki disease and control groups
p>0.05. KD: Kawasaki disease, HR: heart rate, ET: ejection time, PEP: pre-ejection period, LV EF: left ventricle ejection fraction, LV MPI: left ventricle myocardial performance index, E' velocity: peak early diastolic myocardial velocity, A' velocity: peak late diastolic myocardial velocity, S' velocity: peak systolic myocardial velocity
The EF measured by echocardiography was 68.9±6.0% in the KD group and 69.5±6.1% in the control group and showed no significant difference between the two groups. The MPI was 0.39±0.07 in the KD group and 0.39±0.06 in the control group (p>0.05) (Table 3).
The peak early diastolic myocardial velocity which was measured using TDI was 11.8±1.8 cm/sec in the KD group and 11.9±2.2 cm/sec in the control group (p>0.05); the peak late diastolic myocardial velocities were 5.1±1.7 cm/sec and 6.4±1.1 cm/sec, respectively. There were no significant differences in these parameters between the two groups. The peak systolic myocardial velocity was 7.1±1.1 cm/sec in the KD group and 7.2±1.2 cm/sec in the control group; there were no significant differences in these parameters between the two groups (Table 3).
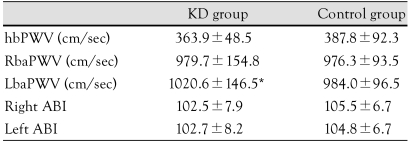
Pulse wave velocity, and ankle-brachial index
The right brachial-ankle pulse wave velocity (Rba-PWV) was 979.7±154.5 cm/sec in the KD group and 976.3±93.5 cm/sec in the control group; there were no significant differences in the RbaPWV between the two groups. However, the LbaPWV was significantly higher in the KD group than the control group (1020.6±146.5 cm/sec vs. 984.0±96.5 cm/sec; p<0.05) (Table 4). There were no significant differences in the LbaPWV between the two groups.
Table 4.
Comparison of pulse wave velocities and ankle brachial index in the Kawasaki disease and control groups
*p<0.05: significantly different from the control group. KD: Kawasaki disease, hbPWV: heart-brachial pulse wave velocity, RbaPWV: right brachial-ankle pulse wave velocity, LbaPWV: left brachial-ankle pulse wave velocity, ABI: ankle brachial index
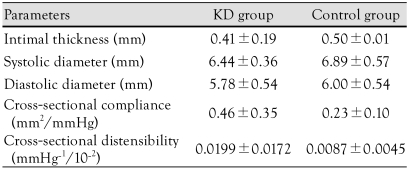
Carotid intima-media thickness
The cIMT was 0.41±0.19 mm in the KD group and 0.50±0.01 mm in the control group. The systolic diameter was 6.44±0.36 mm in the KD group and 6.89±0.57 mm in the control group. The diastolic diameter was 5.78±0.54 mm in the KD group and 6.00±0.54 mm in the control group. There were no significant differences in these parameters. The compliance was 0.46±0.35 mm2/mmHg in the KD group and 0.23±0.10 mm2/mmHg in the control group (p>0.05). The distensibility was 0.0199±0.0172 mmHg-1·10-2 in the KD group and 0.0087±0.0045 mmHg-1·10-2 in the control group (p>0.05) (Table 5).
Table 5.
Comparison of carotid intimal medial thickness in the Kawasaki disease and control groups
p>0.05: significantly different from control group. KD: Kawasaki disease
Discussion
According to the current study, the PWV was increased, even in cases in which the echocardiographic findings were normal following the recovery of KD during the acute phase. KD is an acute febrile disease that causes an idiopathic systemic vasculitis, and it usually develops in children <5 years of age. The prognosis varies depending on the severity of complications of coronary artery diseases. Coronary aneurysm that occurs during the acute phase is a severe complication, and may lead to myocardial infarction or sudden death.4) The damage to the coronary artery complicated by KD was first introduced by Kato et al.16) It has been reported that coronary aneurysms occur in approximately 25% of untreated patients.5) It has also been reported that 50% of all the cases of coronary aneurysms recover within several years. In some patients, however, it may lead to coronary artery stenosis or occlusion, and this may be followed by myocardial infarction or sudden death.17)
According to recent studies, endothelial dysfunction may persist, even after several years have passed since KD was treated, and this might lead to an early stage of atherosclerosis.18) This poses the necessity for long-term follow-up in patients with KD. It has been reported that systemic endothelial dysfunction is observed in adult patients with a past history of KD, and it could be one of the risk factors for the early stage onset of atherosclerosis.15) A histopathologic study has indicated that a coronary aneurysm might be one of the risk factors for atherosclerosis in patients with a history of KD.19) The histopathologic findings which exist in patients with acute KD have also been observed in pediatric patients who had normal findings in the absence of an aneurysm on echocardiography, which includes extensive vasculitis, such as edema, necrosis, and leukocytic infiltration of the endothelium of the coronary arteries.6)
There is no definite evidence demonstrating an association between endothelial dysfunction following KD and atherosclerosis. Several studies have shown, however, that an endothelial injury is a key factor in the development of atherosclerosis.20-22) According to Ross et al.22) atherosclerosis is an inflammatory disease and endothelial dysfunction is the first step in the progression to inflammatory disease. In cases of KD, endothelial dysfunction can be a factor for inducing atherosclerosis due to the inflammatory responses during the acute phase. Systemic endothelial dysfunction is present for several years after recovery from acute KD, even in patients who had no invasion of the coronary artery in the early stage. Since KD can be the major factor for end-stage vascular complications, long-term follow-up is mandatory in all patients who had a past history of KD.
The distensibility of the carotid artery represents a relative change of arterial volume in response to that of pressure, and the compliance represents the absolute change in arterial volume in response to that of pressure. Senzaki et al.23) reported that there is an a characteristic increase in impedence, a decrease in total peripheral arterial compliance, and an increase arterial wave reflexion, regardless of the presence of coronary artery lesions in patients with KD. It has also been reported that there is stiffness of the aorta, carotid artery, and brachio-radial artery in the late stage in children who have a history of KD.24-26) These findings suggest that the degree of stiffness of central and peripheral arteries is greater following the onset of KD. In the current study, however, despite a lack of statistical significance, the distensibility and compliance were approximately two times higher on average in patients with KD compared to healthy controls. Presumably, this might be not only because there was a difference in the age between the two groups, but also because a small number of patients were enrolled in the current study. Henceforth, further long-term follow-up studies are warranted with a larger group of patients.
Suzuki et al.27) reported that cIMT was increased on intravascular ultrasound in the areas where coronary aneurysm was persistently present or lost. Cheung et al.13) also reported similar results. Noto et al.25) also noted that an increased cIMT was associated with systemic arterial stiffness following recovery form KD with no major changes in the lipid profile. The vascular wall of the carotid artery, which was dilated to a lesser extent, represents the secondary change of the arterial wall following the onset of extensive vasculitis. These findings may be precursors to atherosclerosis in patients with KD who have coronary lesions.
However, Ikemoto et al.15) reported contradictory results. cIMT was not significantly different between the two groups. In the current study, the results were the same.
Nato et al.25) reported that the coronary artery was less dilated and more thickened in patients with KD than healthy controls. This was not associated with the changes in lipid profile. Since there was a significant correlation between the coronary artery and atherosclerosis of the carotid artery, an extracranial examination of carotid artery can be used to predict the occurrence of invasion of the coronary artery by atherosclerosis. Cheung et al.26) analyzed such cardiovascular risk factors as the concentration of serum lipids and PWV in patients who achieved a recovery from KD, and these authors noted that high density lipoprotein-cholesterol and apolipoprotein A-1 (apoA-I) were lower, and apolipoprotein B (apo B) and PWV were higher in patients who had coronary aneurysms compared to healthy controls. These authors also noted that apoB and PWV were higher, even in patients who had no coronary aneurysms compared to healthy controls. These reports indicate that the cardiovascular risk factors are increased, with no respect to the presence of coronary aneurysms, even after treatment in children with a past history of KD. Also, in the current study, LbaPWV was significantly higher in patients with KD compared to healthy controls. But there was no significant difference in RbaPWV between the two groups. Presumably, this might be because a very small number of patients were assigned to the KD group. Further long-term follow-up studies are therefore warranted in a larger group of patients.
The cIMT is a useful indicator that predicts the future occurrence of cardiovascular diseases or early stage atherosclerosis. Of the prognostic indicators for atherosclerosis, baPWV is relatively advantageous over other tests in a primary care setting. The cIMT requires special technical expertise and the results are dependent on the examiner.
The pathophysiology of KD cannot be assured, although it is involved in the consecutive recovery process due to the structural changes during the acute phase.28) Even in patients who have no coronary lesions, such findings as a pre-atherosclerotic lipid profile,26) endothelial function,23) systemic arterial stiffness24) and increased cIMT are observed.
The American Society of Cardiology recommends a regular assessment at a 3-5 year interval in patients who had cardiovascular risk factors.29) According to a study using positron emission tomography, the cardiac vascular reservoir and endothelial function were significantly impaired, even following recovery.30)
According to Cheung et al.26) there was no significant difference in blood pressure between patients and healthy controls. By contrast, Silva et al.31) reported that systolic pressure and diastolic pressure were both higher 11 years after patients had KD. In the current study, there were no significant differences in systolic and diastolic pressures between the two groups.
In spite of the normalized coronary artery by coronary angiography after recovery of KD, the intima was thickened on intravascular ultrasound, the vasoconstriction was more significantly sensitive to acetylcholine, and the vasodilation was not responsive to isosorbide dinitrate.32) This is the evidence demonstrating that the vascular wall was abnormally changed and the vascular dysfunction was present in the areas were coronary aneurysm was recovered in patients with a history of KD. These patients must be consulted for the possible prevention of risk factors for causing atherosclerosis and they must also receive a long-term follow-up study until they reach adulthood.
It remains unclear whether coronary artery lesions can recover or may lead to stenosis. Anti-coagulative treatments have contributed to diminishing the end-stage effects of KD. Regulatory factors that restrict the progression of KD are still unknown. At present, the long-term effects of immunoglobulin administration remain unclear. In cases in which KD has not progressed to stenosis, such hemodynamic factors as shear stress may be involved. Histopathologic studies have shown that the infiltration of inflammatory cells can be present approximately 6 months after the acute phase of KD elapses.32) Even the aneurysm which was recovered may have risk factors for coronary arteries during a long-term period. Atherosclerotic lesions may develop during the adult period. These patients are in need of a meticulous follow-up and they must also be advised to avoid other risk factors of causing atherosclerosis, such as smoking, obesity, hypertension, and hyperlipidemia. To prevent the progression to atherosclerotic coronary artery diseases, the use of anti-platelet agents, including low-dose aspirin would be favorable.32)
The limitations of the current study are that the control group had a slightly higher age than the KD group. Therefore, there may be slight differences in the cIMT, the vascular diameter during the systolic and diastolic periods, and the compliance and distensibility of the carotid artery between the two groups. This study was conducted during a short-term period, therefore further long-term studies are warranted in a larger group of patients. PWV was significantly higher in children with KD who recovered normally following the treatment compared to healthy controls. This emphasizes the importance of PWV for a follow-up study in patients with KD.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 3.Park YW, Han JW, Park IS, et al. Kawasaki disease in Korea, 2003-2005. Pediatr Infect Dis J. 2007;26:821–823. doi: 10.1097/INF.0b013e318124aa1a. [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Ichinose E, Yoshioko F, et al. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am J Cardiol. 1982;49:1758–1766. doi: 10.1016/0002-9149(82)90256-9. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon R, Clarkson P, Conald A, et al. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94:2103–2106. doi: 10.1161/01.cir.94.9.2103. [DOI] [PubMed] [Google Scholar]
- 7.Lee YS, Kim KS, Nam CW, et al. Clinical implication of carotidradial pulse wave velocity for patients with coronary artery disease. Korean Circ J. 2006;36:565–572. [Google Scholar]
- 8.Han SH, Park CG, Park SW, et al. High aortic stiffness assessed by pulse wave velocity is an independent predictor of coronary artery calcification and stenosis in suspected coronary artery disease patients. Korean Circ J. 2004;34:468–476. [Google Scholar]
- 9.Jeong IB, Bae JH, Kim KY, et al. The carotid intima-media thickness as a screening test for coronary artery disease. Korean Circ J. 2005;35:460–466. [Google Scholar]
- 10.Tounian P, Aggoun Y, Dobem B, et al. Presence of increased stiffness of the commom carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 11.Pauciullo P, Iannuzzi A, Sartorio R, et al. Increased intima-media thickness of the common carotid artery in hypercholesterolemic children. Arterioscler Thromb. 1994;14:1075–1079. doi: 10.1161/01.atv.14.7.1075. [DOI] [PubMed] [Google Scholar]
- 12.Jarvisalo MJ, Putto-Laurila A, Jartti L, et al. Carotid artery intima-media thickness in children with type I diabetes. Diabetes. 2002;51:493–498. doi: 10.2337/diabetes.51.2.493. [DOI] [PubMed] [Google Scholar]
- 13.Cheung YF, Wong SJ, Ho MH. Relationship between carotid intima media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child. 2007;92:43–47. doi: 10.1136/adc.2006.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nato N, Okada T, Yamasuge M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107:1095–1099. doi: 10.1542/peds.107.5.1095. [DOI] [PubMed] [Google Scholar]
- 15.Ikemoto Y, Ogino H, Teraguchi M, Kobayashi Y. Evaluation of preclinical atherosclerosis by flow-mediated dilatation of the brachial artery and carotid artery analysis in patient with a history of Kawasaki disease. Pediatr Cardiol. 2005;26:782–786. doi: 10.1007/s00246-005-0921-8. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Koike S, Yamamoto M, Ito Y, Yano E. Coronary aneurysms in infants and young children with acute febrile mucocutaneous lymph node syndrome. J Pediatr. 1975;86:892–898. doi: 10.1016/s0022-3476(75)80220-4. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Mason W, Lewis AB. Regression of coronary aneurysms in patients with Kawasaki syndrome. Circulation. 1987;75:387–394. doi: 10.1161/01.cir.75.2.387. [DOI] [PubMed] [Google Scholar]
- 18.Niboshi A, Hamaoka K, Sakata K, Yamaguchi N. Endothelial dysfunction in adult patients with a history of Kawasaki disease. Eur J Pediatr. 2008;167:189–196. doi: 10.1007/s00431-007-0452-9. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Oharaseki T, Naoe OS. Pathological study of post-coronary arteritis in adolescents and young adults: with reference to the relationship between sequelae of Kawasaki disease and atherosclerisos. Pediatr Cardiol. 2001;22:138–142. doi: 10.1007/s002460010180. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 22.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 23.Senzaki H, Chen CH, Ishido H, et al. Arterial haemodynamics in patients after Kawasaki disease. Circulation. 2005;111:2119–2125. doi: 10.1161/01.CIR.0000162483.51132.25. [DOI] [PubMed] [Google Scholar]
- 24.Ooyanagi R, Fuse S, Tomita H, et al. Pulse wave velocity and ankle brachial index in patients with Kawasaki disease. Pediatr Int. 2004;46:398–402. doi: 10.1111/j.1442-200x.2004.01929.x. [DOI] [PubMed] [Google Scholar]
- 25.Nato N, Okada T, Yamasuge M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107:1095–1099. doi: 10.1542/peds.107.5.1095. [DOI] [PubMed] [Google Scholar]
- 26.Cheung YF, Yung TC, Tam SC, Ho MH, Chau AK. Novel and traditional cardiovascular risk factors in children after Kawasaki disease. J Am Coll Cardiol. 2004;43:120–124. doi: 10.1016/j.jacc.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A, Yamagishi M, Kimura K, et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol. 1996;27:291–296. doi: 10.1016/0735-1097(95)00447-5. [DOI] [PubMed] [Google Scholar]
- 28.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: I. pathology and morphogenesis of the vascular changes. Jpn Cir J. 1979;43:633–643. doi: 10.1253/jcj.43.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic fever, endocarditis, and Kawasaki disease, Council on cardiovascular diseases in the young. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 30.Furuyama H, Odagawa Y, Katoh C, et al. Altered myocardial flow reserve and endocardial function late after Kawasaki disease. J Pediatr. 2003;142:149–154. doi: 10.1067/mpd.2003.46. [DOI] [PubMed] [Google Scholar]
- 31.Saliva AA, Maeno Y, Hashimi A, Smallborn JF, Siverman ED, McCrindle BW. Cardiovascular risk factors after Kawasaki disease: a case-control study. J Pediatr. 2001;138:400–405. doi: 10.1067/mpd.2001.111430. [DOI] [PubMed] [Google Scholar]
- 32.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysm after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]