Abstract
Background and Objectives
The purpose of this study was to investigate the relationship between chronic atrial fibrillation (AF) and reduced pulmonary function.
Subjects and Methods
Eighty-six chronic AF patients who were enrolled from annual health examination programs were studied using echocardiography and pulmonary function tests (PFT). Echocardiography and PFT matched for age, gender, and year performed were selected by the control group who had normal sinus rhythms. Patients with ejection fractions <50%, valvular heart disease, or ischemic heart disease were excluded.
Results
In the chronic AF patients, the forced expiratory volume at one second (FEV1), FEV1%, and FEV1/forced vital capacity (FVC) were significantly reduced, and the right ventricular systolic pressure was significantly increased. Episodes of heart failure were more frequently associated with the chronic AF patients than the controls. In particular, the FEV1% had the most meaningful relationship to chronic AF after an adjustment for cardiovascular risk factors {p=0.003, Exp (B)=0.978, 95% confidence interval (CI):0.963-0.993}.
Conclusion
Reduced FEV1%, which represents the severity of airway obstruction, was associated with chronic AF, and the greater the pulmonary function impairment, the greater the co-existence with AF and congestive heart failure in those with preserved left ventricular systolic function.
Keywords: Atrial fibrillation, Chronic obstructive lung disease, Pulmonary function tests, Forced expiratory volumes
Introduction
Atrial fibrillation (AF) is frequently observed among supraventricular rhythm disorders. The prevalence of AF increases with age.1-4) Pulmonary function, however, has a tendency to decrease progressively with age. The supraventricular and ventricular arrhythmias are also common in chronic obstructive lung disease (COPD).5-7) The reasons are thought to be due to hypoxia, hypercarbia, pulmonary hypertension, and myocardial ischemia, which are easily provoked by this limited ventilatory condition.8-11) Furthermore, the frequencies of various types of arrhythmias are related to the temporal state of the pulmonary function in these patients. Cardiac arrhythmias have a tendency to become aggravated when ventilatory function deteriorates, and ameliorated when it improves; however, there is still controversy regarding the relationship between AF and reduced pulmonary function.
The purpose of the current study was to determine the relationship between chronic AF and pulmonary function status among subjects with preserved left ventricular systolic function (LVSF), and the difference in pulmonary function between the chronic AF group and the controls who presented with sinus rhythm (SR).
Subjects and Methods
Subjects
Eighty-six chronic AF patients were enrolled from an annual health examination program between August 2006 and June 2007, and were studied by echocardiography and pulmonary function tests (PFT). Echocardiography and PFT matched for age, gender, and year performed were selected by the control group who had normal sinus rhythm (SR) (Table 1). The subjects with a left ventricular ejection fraction (LVEF) <50% and with valvular heart disease were excluded from the study. Also, any patients who had a history of percutaneous coronary intervention, coronary artery bypass surgery, or Q-waves on a surface electrocardiogram (ECG) were excluded. Chronic AF was considered when AF was detected consecutively >2 ECGs during a 1 month interval. Finally, the subject was allocated into either the chronic AF group or the control group, depending on whether the cardiac rhythm was AF or normal SR.
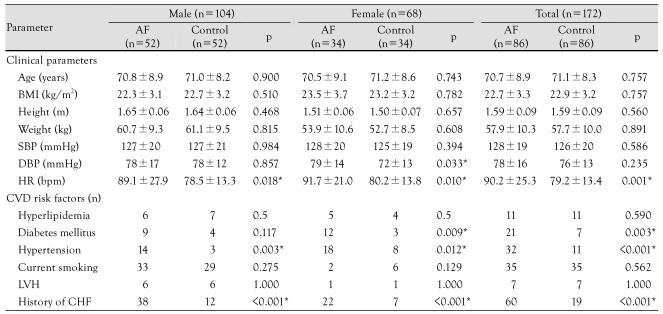
Table 1.
Baseline characteristics of the study population
*statistically significant. AF: atrial fibrillation, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, CVD: cardiovascular disease, LVH: left ventricular hypertrophy, CHF: congestive heart failure
Assessment of risk factors
The clinical data on risk factors for cardiovascular disease that were related to AF were obtained from a comprehensive review of each patient's medical record. Therefore, each patient's age, gender, and history of systemic hypertension, diabetes mellitus, hyperlipidemia, and current smoking were investigated (Table 1). Systemic hypertension was defined by documenting the clinical diagnosis, or with evidence of an elevated systolic blood pressure of 140 mmHg and/or diastolic blood pressure of 90 mmHg, in the absence of any acute medical illnesses. Diabetes mellitus was based on the clinical documentation of the condition, and whether the treatment consisted of dietary therapy, oral hypoglycemic agents, and/or insulin. Hyperlipidemia was defined by a fasting total cholesterol level of 240 mg/dL and/or a low-density lipoprotein cholesterol level of 160 mg/dL, or by the use of cholesterol-lowering drugs. Current smoking referred to the active use of tobacco products at the time of enrollment in the study. Furthermore, despite all the subjects presenting with preserved LVSF, a previous history of heart failure (HF) was also evaluated by the Framingham heart failure diagnostic criteria12) because diastolic heart failure is not uncommonly associated with elderly patients and AF with a rapid ventricular response is a well-known cause of reversible cardiomyopathy.
Echocardiographic examination
In the echocardiographic study, the conventional two-dimensional grayscale imaging, the pulsed or continuous wave Doppler study, and color Doppler imaging were performed according to previously validated recommendations.13) The semi-quantitative visual estimation and the modified Simpson's method were used to assess LVSF, and the patients with a LVEF <50% by either of the two methods were excluded. The wall-motion score index was also applied to rule out cases with regional wall motion abnormalities, which were calculated by dividing the sum of the scores by the number of visualized segments. The left atrial (LA) dimension was measured at the point of end-systole just before the frame that preceded the mitral valve opening from the parasternal long-axis view. The peak systolic pressure of the right ventricle (pRVSPr) was carefully estimated using a previously validated method13) when tricuspid regurgitation was present. Subjects with insufficient aortic or mitral valves (greater than a mild degree), stenosis of the valves, or structural abnormalities were excluded from the study.
Evaluation of pulmonary function
The forced expiratory volume at one second (FEV1), forced vital capacity (FVC), mean forced expiratory flow during the middle half of the FVC (FEF25-75%), and the ratio of the FEV1-to-FVC (FEV1/FVC) were measured by an electronic spirometer (Vmax 229 pulmonary function test/cardiopulmonary exercise testing instrument; Sensomedics, Yorba Linda, CA, USA). Also, the peak expiratory flow (PEF) and the expiratory flow at 25%, 50%, and 75% FVC (FEF25%, FEF50%, and FEF75%, respectively) were obtained from the flow-volume curve. Body plethysmography was applied to the measurement of the residual volume (RV), total lung capacity (TLC), airway resistance (Raw), and lung compliance (Gaw). The single breath technique with carbon monoxide was adopted for the measurement of the diffusion capacity of the lungs (DLCO). Specially-trained technicians performed all of these measurements. Moreover, all of the values, except for the FEV1/FVC, which was measured with the spirometer, were automatically recalculated as percentile predicted values of the age- and gender-matched normal values using a computer software program. For example, the FEV1% was obtained from the following formulae, in which H is the height (cm) and A is the age (year): FEV1%=0.092 H/2.54-0.032 A-1.26 (males); and FEV1%=0.089 H/2.54-0.025 A-1.932 (females).
Statistical analysis
The data are presented as the mean values ±standard deviations (SDs), unless otherwise stated. Continuous data on the two groups were compared with an independent sample t-test, and categorical data were compared with a chi-square test. To reveal the relationship of the dichotomous data with the continuous data, logistic regression analysis was applied (SPSS, version 13.0; SPSS Inc., Chicago, IL, USA). The statistical differences were considered significant at a p<0.05.
Results
Baseline characteristics
The mean age, body mass index (BMI), and systolic and diastolic blood pressures (BP) of the AF group were similar to the controls. These findings, except for the diastolic BP in females, were also noted when comparing those parameters between the gender-specific subgroups. Regardless of gender, the resting heart rate (HR) of the AF group was significantly higher than the controls (p=0.001). The incidence of hyperlipidemia and current smoking did not differ between the AF and control groups. The AF group, however, had a significantly higher incidence of hypertension for both genders, and diabetes in females. The incidence of previous HF was much higher in the AF than in the control groups, as expected (60% vs. 19%, p<0.001).
Characteristics of echocardiography and pulmonary function tests of the atrial fibrillation and control groups
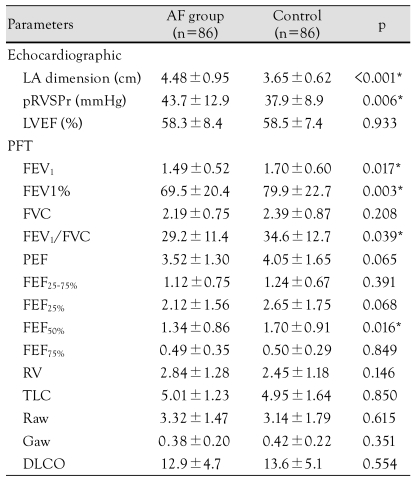
The LA dimension and pRVSPr of the AF group were significantly greater than the controls (4.48±0.95 cm vs. 3.65±0.62 cm, p<0.001, and 43.7±12.9 mmHg vs. 37.9±8.9 mmHg, p=0.006, respectively), even though the LVEF of the two groups was similar (Table 2).
Table 2.
Characteristics of echocardiographic examination and pulmonary function tests
AF: atrial fibrillation, LA: left atrium, pRVSPr: peak right ventricular systolic pressure, LVEF: left ventricular ejection fraction, PFT: pulmonary function test, FEV1: forced expiratory volume at on second, FVC: forced vital capacity, FEV1/FVC: ratio of the FEV1 to FVC, PEF: peak expiratory flow, FEF25-75%: mean forced expiratory flow during the middle half of the FVC, FEF25%: expiratory flow at the 25%, FEF50%: expiratory flow at the 50%, FEF75%: expiratory flow at the 75%, RV: residual volume, TLC: total lung capacity, Raw: airway resistance, Gaw: lung compliance, DLCO: diffusion capacity of the lung
Among the parameters of the PFT, the FEV1, FEV1%, and FEV1/FVC were significantly lower in the AF group than the control group. The other parameters were not statistically different between the two groups (Table 2). Therefore, ventilatory dysfunction, rather than SR, appeared to be more associated with the AF group. When logistic regression was performed with the FEV1, FEV1%, and FEV1/FVC as a function of the cardiac rhythm and with the adjustment of risk factors {hypertension, diabetes mellitus, and congestive heart failure (CHF)}, FEV1% had the most meaningful relationship with AF {p=0.003, Exp (B)=0.978, 95% confidence interval (CI): 0.963-0.993}; FEV1 also exhibited a meaningful relationship {p=0.019, Exp (B)=0.520, 95% CI: 0.301-0.897}. An inverse correlation between FEV1% and the pRVSPr was observed in this study, which was in agreement with the secondary pulmonary hypertension results of the reduced ventilatory function. Furthermore, an increase in the pRVSPr represented a close relationship with AF {p=0.009, Exp (B)=1.052, 95% CI: 1.013-1.092}.
Categorization of the subjects according to the forced expiratory volume at one second range
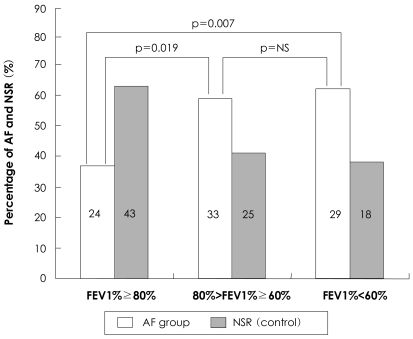
The FEV1% values were categorized into three subgroups by applying cutoff levels of 80% and 60% of the predictive value in both groups, which were the levels adopted in many previous studies. In the cases in which the FEV1% was >80% of the predictive value, SR was most frequently observed. AF was most commonly observed in cases in which the FEV1% was <60% (Fig. 1). Using the Mantel-Haenszel estimation, the prevalence of AF in the cases with a FEV1% between 60% and 80%, and <60% exhibited odds ratios (ORs) of 2.265 (p=0.019) and 2.887 (p=0.007), respectively, when compared to cases with a FEV1% >80%. The prevalence of AF in the cases with a FEV1% <60% did not differ from the prevalence of AF in the cases with a FEV1% between 60% and 80% (OR=1.221, p=0.619).
Fig. 1.
Histogram showing percentage of atrial fibrillation and normal sinus rhythm groups, stratified into FEV1% categories. FEV1%: forced expiratory volume at one second, AF: atrial fibrillation, NSR: normal sinus rhythm, NS: not significant.
Relationship between congestive heart failure and atrial fibrillation and forced expiratory volume at one second in patients with preserved LVSF
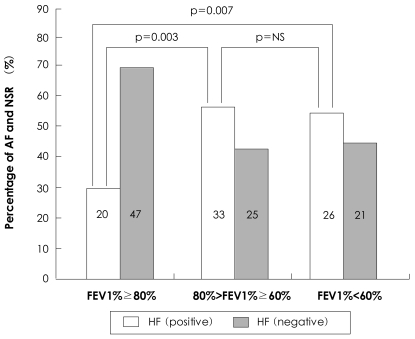
A normal ejection fraction can exist in patients with symptoms of heart failure, and is regarded as an indication of diastolic heart failure. Moreover, AF can induce an enhanced ventricular response, which will result in the aggravation of heart failure. In this study, a history of CHF was observed more frequently in the AF group than in the control group. The FEV1% had a meaningful relationship with CHF {p=0.008, Exp (B)=0.980, 95% CI: 0.965-0.995}. The mean value of the FEV1% also differed significantly according to whether or not the patient had a history of heart failure (69.6±19.7% vs. 79.0±23.2%, p=0.006). The prevalence in the patients with a FEV1% between 60% and 80% and <60%, had an OR of 3.102 (p=0.003) and 2.910 (p=0.007), respectively, compared to a FEV1% >80% (Fig. 2). The prevalence of CHF in the patients with a FEV1% <60% did not differ from the prevalence of CHF in the patients with a FEV1% between 60% and 80% (OR=0.938, p=0.871).
Fig. 2.
Histogram showing relationship of heart failure with atrial fibrillation, stratified into FEV1% categories. FEV1%: forced expiratory volume at one second, AF: atrial fibrillation, NSR: normal sinus rhythm, NS: not significant, HF: heart failure.
Discussion
In several studies that used multivariate analyses with correction for age, the major risk factors for AF were hypertension, heart failure, diabetes mellitus, and valvular heart disease. Bundle branch block and left ventricular hypertrophy were regarded as potential predictors of AF.14) However, the results regarding the relationship between AF and pulmonary function have been discordant. The FEV1% was reported to be an important predictive factor for AF in the Copenhagen City Heart Study,15) which was comprised of a healthy age-stratified cohort (n=13,430) with a prospective design. Psaty et al.16) also reported that the occurrence of AF was related to reduced pulmonary function in the Cardiovascular Health Study (n=5,201). In contrast, the Renfrew/Paisley study14) (n=15,406) did not demonstrate a significant correlation between the FEV1% and AF, whereas it reported the traditional major risk factors to be significant. Similarly, the Framingham study1) (n=4,731) found no relationship between the FEV1 and AF.
To address these discordant results on the relationship between AF and reduced pulmonary function, the current study was conducted as a case-control study with consecutive enrollment of patients. In this study, a reduced FEV1% was significantly associated with chronic AF, and this finding was supported by the results of the preceding two studies. The Renfrew/Paisley study,14) which reported the opposite results, observed a small number of new-onset AF cases (n=19) after a short follow-up and reported cardiomegaly as the most powerful predictor for AF (OR=14.0) after a long-term follow-up, which means that subjects with a decreased LVSF were enrolled. In the Framingham study,1) there was a high rate of heart disease. For example, the prevalence of valvular heart disease was 7% in males and 9% in females; patients with valvular heart disease were excluded from our study. Therefore, the inclusion of these strong risk factors may have obscured the relationship between the FEV1% and AF in the two aforementioned studies.
The pathophysiologic mechanisms that connect reduced pulmonary function to chronic AF have not been clearly determined, but there are several suggestive explanations. With respect to the first explanation, recent observations have indicated that ectopic beats that initiate AF often originate in the pulmonary veins.17),18) Reduced ventilatory function could easily trigger ectopic beats by deterioration of the blood gas composition, such as occurs with hypoxia, and pulmonary hypertension results in stress on the right atrium and connecting vessels, thus perpetuating AF. In this study, the pulmonary artery pressure (pPAPr) in the AF group was significantly higher than the control group. The second explanation can be assumed to be the chronic inflammatory processes that involve the cardiopulmonary system. Anatomically, the pulmonary circulation directly drains into the left atrium. Also, obstructive airway disease is the result of chronic inflammation of the airways of the lungs that consequently manifests in reduced FEV1 or FEV1%. Recent studies have reported that these processes are associated with an increase in several inflammation-sensitive proteins (ISP), such as high sensitivity-C-reactive protein (CRP), fibrinogen, and cytokines, including interleukin-6 (IL-6), IL-1, tumor necrosis factor (TNF)-α, and the complement system.19-22) For example, the level of hs-CRP is known to increase in patients with AF; hsCRP is synthesized from the liver by stimulation of IL-6, -11, and -12 and is frequently associated with fibrosis of the atrial tissue and myocarditis on histologic examination. Fibrosis of the atrial tissue is now accepted as a perpetuating factor of AF. Therefore, it can be speculated that chronic inflammation is a plausible mechanism that connects reduced pulmonary function with chronic AF, which was clinically manifested as the relationship between chronic AF and decreased FEV1% in this study. The third explanation involves the hemodynamic consequences of AF. It is well-recognized that the loss of the atrial contraction decreases cardiac output and causes an increase in backward pressure and congestion of the lungs, especially the small airways. As a consequence, those hemodynamic alterations not only result in atrial dilation, wall stretching, and electrical remodeling of the atrial tissue, but also hinders the ventilatory function of the lungs.23),24) In this study, when comparing the parameters for estimating small airway function, FEF25-75%, FEF25%, and FEF75%, but not FEF50%, did not differ between the groups. Also, the DLCO exhibited no manifestations compatible with that assumption. Therefore, the results of this study do not sufficiently support the aforementioned assumption. However, the possibility that these parameters may present meaningful results by an exaggeration of the hemodynamic effects, such as an exercise challenge, cannot be abandoned entirely because these parameters were measured only during the resting state.
In addition, a close relationship existed between the FEV1% and symptomatic episodes of heart failure in the AF group {p=0.011, Exp (B)=0.965, 95% CI: 0.939-0.002} after adjustment for the risk factors (hypertension and diabetes mellitus), whereas no relationship was observed in the control group. AF can cause heart failure through a rapid ventricular response, which results in an elevated end diastolic pressure and backward congestion, even in patients with a normal ejection fraction. Hence, these findings imply that reduced ventilatory function as also attributed to heart failure from a rapid ventricular rate during AF.
Clinical implications and limitations
In clinical practice, β-blockers, which can adversely affect ventilation, are commonly administered to alleviate the ventricular rate in AF with preserved LVSF, as are β-agonists, which can aggravate the cardiac rhythm to manage reduced lung function. Prudent use of those drugs is likely to be needed because chronic AF co-existing with reduced ventilatory function was not rare in this study.
Similar to other case control studies, the cause-and-effect relationship and the underlying mechanisms were not clearly determined in this study, but the FEV1% was revealed to be a co-morbid factor for chronic AF. Thus, a further study on these issues should be pursued.
In conclusion, this study showed that reduced pulmonary function was related to AF as a co-morbid factor, and was clearly attributed to hemodynamic alterations, such as congestion during chronic AF, even when a normal ejection fraction was present.
References
- 1.Benjamin EJ, Levy D, Vaziri SM, Dágostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Tomita F, Kohya T, Sakurai M, et al. Prevalence and clinical characteristics of patients with atrial fibrillation: analysis of 20,000 cases in Japan. Jpn Circ J. 2000;64:653–658. doi: 10.1253/jcj.64.653. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implication. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 5.Sideris DA, Katsadoros DP, Valianos G, Assioura A. Type of cardiac dysrhythmias in respiratory failure. Am Heart J. 1975;89:32–35. doi: 10.1016/0002-8703(75)90006-x. [DOI] [PubMed] [Google Scholar]
- 6.McCord J, Borzak S. Multifocal atrial tachycardia. Chest. 1998;113:203–209. doi: 10.1378/chest.113.1.203. [DOI] [PubMed] [Google Scholar]
- 7.Kothari SA, Apiyasawat S, Asad N, Spodick DH. Evidence supporting a new rate threshold for multifocal atrial tachycardia. Clin Cardiol. 2005;28:561–563. doi: 10.1002/clc.4960281205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khokhar N. Cardiac arrhythmias associated with acute respiratory failure in chronic obstructive pulmonary disease. Mil Med. 1981;146:856–858. [PubMed] [Google Scholar]
- 9.Levine PA, Klein MD. Mechanisms of arrhythmias in chronic obstructive lung disease. Geriatrics. 1976;31:47–56. [PubMed] [Google Scholar]
- 10.Thomas AJ, Valabhji P. Arrhythmia and tachycardia in pulmonary heart disease. Br Heart J. 1969;31:491–495. doi: 10.1136/hrt.31.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holford FD, Mithoefer JC. Cardiac arrhythmias in hospitalized patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973;108:879–885. doi: 10.1164/arrd.1973.108.4.879. [DOI] [PubMed] [Google Scholar]
- 12.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 13.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–1016. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 17.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 18.Olsson SB. Atrial fibrillation: where do we stand today? J Intern Med. 2001;250:19–28. doi: 10.1046/j.1365-2796.2001.00853.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SJ, Sung KC, Lee YS, et al. Serum C-reactive protein level and its association with atrial fibrillation in Korean adults. Korean Circ J. 2005;35:309–314. [Google Scholar]
- 21.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. doi: 10.1093/eurheartj/ehi645. [DOI] [PubMed] [Google Scholar]
- 22.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764–767. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 24.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation: time course and mechanisms. Circulation. 1996;94:2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]