Abstract
Background and Objectives
The prognosis and natural history of bradycardia related to drugs such as beta-blockers and non-dihydropyridine calcium channel blockers are not well known.
Subjects and Methods
We retrospectively analyzed 38 consecutive patients (age 69±11, 21 women) with drug-related bradycardia (DRB) between March 2005 and September 2007. A drug-associated etiology for the bradycardia was established based on the medical history and patient response to drug discontinuation. The mean follow-up duration was 18±8 months.
Results
The initial electrocardiogram (ECG) showed sinus bradycardia (heart rate ≤40/min) in 13 patients, sinus bradycardia with junctional escape beats in 18 patients, and third-degree atrioventricular (AV) block in seven patients. Drug discontinuation was followed by resolution of bradycardia in 60% of patients (n=23). Among them, five (17.8%) patients resumed taking the culprit medication after discharge and none developed bradycardia again. Bradycardia persisted in 10 (26.3%) patients despite drug withdrawal, and a permanent pacemaker was implanted in seven of them. Third-degree AV block, QRS width, and bradycardia requiring temporary transvenous pacing were significantly associated with the bradycardia caused by drugs.
Conclusion
Beta-blockers were the most common drugs associated with DRB. However, in one quarter of the cases the DRB was not associated with drugs; in these patients permanent pacemaker implantation should be considered.
Keywords: Bradycardia, Atrioventricular block, Arrhythmia, Drugs
Introduction
According to the current guidelines of the American College of Cardiology/American Heart Association for pacemaker implantation, clinically significant symptomatic bradycardia is one of the major indications for pacemaker implantation.1) However, if symptomatic bradycardia is drug-induced and the condition is expected to resolve after withdrawal of the culprit medication, pacemaker implantation is generally considered unnecessary.1) Clinically significant bradycardia can be induced by beta-blockers and non-dihydropyridine (DHP) calcium-channel antagonists such as verapamil and diltiazem. Although drug-related bradycardia is frequently observed in clinical practice, it is a poorly defined clinical problem. It is unclear whether drug-related bradycardia (DRB) represents the presence of serious underlying sinus and/or atrioventricular (AV) node dysfunction and conduction disturbance. Therefore, the aim of this study was to assess the prognosis and natural history of DRB.
Subjects and Methods
Study subjects
In this retrospective study, we reviewed all cases that visited the emergency departments of three university hospitals (Kyungpook National University Hospital, Keimyung University Dongsan Medical Center, and Daegu Catholic University Medical Center) in Daegu, Korea, between March 2005 and September 2007 with a diagnosis of DRB. Thirty-eight consecutive patients that were regularly taking beta-blockers and/or non-DHP calcium channels antagonists were included.
Patients were included in this study if they presented with any of the following four electrocardiogram (ECG)-predefined cardiac rhythm abnormalities: 1) sinus bradycardia (≤40 beats/min), 2) sinus bradycardia with junctional escape beats, 3) second-degree AV block, and 4) third-degree AV block. Patients were excluded if their bradycardia was attributed to an acute myocardial infarction, other anti-arrhythmic drugs, digitalis toxicity, electrolyte imbalances, or failure of a previously implanted pacemaker device.
Cause-and-effect relationship between drugs and bradycardia
The cause-and-effect relationship between medication use and bradycardia was defined according to the response to drug discontinuation. Patients were classified into one of the three following groups: 1) DRB caused by drugs: DRB that resolved within 48 hours after drug withdrawal and never recurred during the follow-up period; 2) DRB not caused by drugs: DRB that persisted after drug withdrawal or recurred in the absence of drug therapy during the follow-up period; 3) DRB undetermined correlation: bradycardia that resolved within 48 hours after drug withdrawal, but the cause-and-effect relationship could not be determined because the bradycardia did not recur despite restarting drug therapy during the follow-up period.
Follow-up and outcome
The mean follow-up duration was 18±8 months. Therapies with beta-blockers and/or non-DHP calcium channel blockers were discontinued soon after admission. The recurrence of clinically significant and symptomatic bradycardia during the follow-up period was assessed. All patients were asked to report their symptoms at their out-patient clinic visits or during phone interviews.
Statistical analysis
The data are expressed as the mean±SD or percentages. Patients were categorized into the three previously mentioned groups. All comparisons between the baseline variables were assessed with the Chi-square test or the analysis of variance (ANOVA) test for categorical or continuous variables, respectively. The Least Significant Difference (LSD) post hoc test was performed to investigate significant differences among the three groups. For all analyses, a two-sided p<0.05 was considered statistically significant. Statistical analysis was performed using Statistical Package for Social Science (SPSS) version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patients, their symptoms, and presenting rhythms
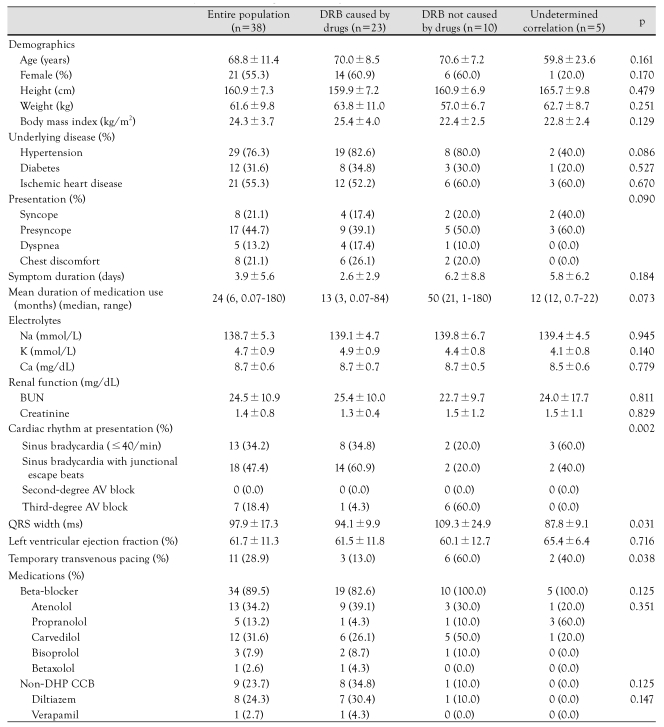
Baseline characteristics of the study population are shown in Table 1. The mean age was 68±11 years, and 21 (55.3%) patients were women. Sinus bradycardia with junctional escape beats was the most common ECG finding and was identified in 18 (47.4%) patients. Sinus bradycardia was noted in 13 (34.2%) patients, and third-degree AV block in seven (18.4%) patients. There was no patient with a second-degree AV block. Thirty-four (89.5%) patients were on beta-blockers and nine (23.7%) were on non-DHP calcium channel blockers. Five (13.2%) patients were on both beta-blockers and non-DHP calcium channel blockers.
Table 1.
Baseline characteristics of 38 patients with drug-related bradycardia
DRB: drug-related bradycardia, BUN: blood urea nitrogen, AV: atrioventricular, Non-DHP CCB: Non-dihydropyridine calcium channel blocker
The indications for treatment with these drugs were hypertension (n=29) and ischemic heart disease (n=21). The most common symptom was presyncope (n=17), followed by syncope (n=8), chest discomfort (n=8), and dyspnea (n=5). There were no significant differences in symptoms according to the type of ECGs (p=0.09). The mean time from initiation of the first symptom suggestive of bradycardia to admission was 3.9 days. Two-dimensional echocardiography was performed in all patients and it showed a mean left ventricular ejection fraction (LVEF) of 61.7±11.3%. The mean QRS duration was 97.9±17.3 msec. Temporary pacing was provided in 11 (28.9%) patients.
Patients with "DRB caused by drugs" were similar to those with "DRB not caused by drugs" or "undetermined correlation" with regard to the demographic features, underlying diseases, presenting symptoms, symptom duration, laboratory findings, LVEF, and medication history (Table 1). There were no significant differences in the mean duration of medication among the three groups (p=0.073). The mean duration of medication tended to be longer among the patients with "DRB not caused by drugs" compared to the patients with "DRB caused by drugs" (p=0.140). The median duration of medication was three and 21 months in the "DRB caused by drugs" and in the "DRB not caused by drugs", respectively. There were significant differences among the three groups in the ECG characteristics: patients with "DRB caused by drugs" more commonly had sinus bradycardia with junctional escape beats and less commonly third-degree AV block; patients with "DRB not caused by drugs" more commonly had third-degree AV block, whereas patients with "bradycardia of undetermined correlation" more commonly had sinus bradycardia (p=0.002). The QRS width in the patients with "DRB not caused by drugs" was significantly longer than in the patients with "DRB caused by drugs" or "bradycardia of undetermined correlation" (p=0.031). Compromising bradycardia requiring temporary transvenous pacing was more common in the patients with "DRB not caused by drugs" (p=0.038).
Clinical course after medication withdrawal
The culprit medications were discontinued in all patients soon after hospitalization (Fig. 1). Drug discontinuation was followed by spontaneous resolution of bradycardia within 48 hours in 28 (73.7%) out of 38 patients. Five among them resumed taking the culprit medication after discharge, and none of these patients developed bradycardia again during the follow-up period; their bradycardia was classified as "undetermined correlation". The bradycardia in the remaining 23 (60.5% out of 38 patients was considered "DRB caused by drugs". The bradycardia persisted in 10 (26.3%) patients despite drug withdrawal. Therefore, they were considered "DRB not caused by drugs". Permanent pacemaker implantation was performed in seven of the 10 patients with "DRB not caused by drugs". The cardiac rhythms in the seven patients requiring permanent pacing were sinus bradycardia in one; sinus bradycardia with junctional escape beats in one; and third-degree AV block in five.
Fig. 1.
Flow chart describing the clinical course of 38 patients with drug-related bradycardia. PM: pacemaker.
Discussion
We present data on 38 patients with clinically significant and symptomatic DRB responsible for the development of syncope, presyncope, dyspnea, and chest discomfort. All patients were on beta-blockers and/or non-DHP calcium channels antagonists at the time of presentation. The main findings of this retrospective analysis were that about one quarter of the DRB was not caused by, but revealed by drugs, and that beta-blockers were the most common 'offending' drug involved in the development of bradycardia.
Does drug-related bradycardia suggest a benign prognosis?
When symptomatic bradycardia is related to extrinsic causes, removal of potentially reversible causes of the bradycardia is always the first approach to management.2-4) Severe symptomatic bradycardia has been observed during therapy with beta-blockers and non-DHP calcium channels antagonists.3-5) In patients with clinically significant bradycardia secondary to drug treatment, one must decide whether to discontinue, reduce the dosage, or continue medication use if there is no acceptable alternative; in the latter case pacing therapy should be considered.1) Such decisions are presently being made on the basis of clinical judgment rather than published guidelines because little is known about the prognosis and natural history of patients with drug-related bradycardia.6) A recent study reported that druginduced AV block, in patients treated with beta-blockers and non-DHP calcium channels antagonists, the AV block 'truly caused by drugs' was found in only 15% of the patients, and in the majority of cases, the 'culprit' drugs were found to be 'innocent bystanders'.7)
It is possible that clinically significant and symptomatic 'drug-induced' bradycardia in other patients might also be primarily due to underlying sinus and/or AV node disease.6),8-10) It is often reported in the literature that therapeutic doses of drugs, generally, do not cause clinically significant bradycardia in patients with structurally normal hearts9-12); however, in the heart with underlying latent disease of the sinus and/or AV node, bradycardia may occur due to a triggering effect caused by drug-related bradycardia. Bradycardia usually develops after prolonged medication use in these cases,10-12) and may have different underlying mechanisms than in cases associated with true pro-arrhythmic events. The true proarrhythmic events commonly occur within several days from the start of anti-arrhythmic drug therapy at initial relatively low doses. When significant bradycardia is observed in patients taking drugs such as beta-blockers and/or non-DHP calcium channel antagonists, the possibility of significant underlying sinus or conduction system disease or both must be considered.
Prediction of drug-related bradycardia from the medication history and electrocardiogram findings
Combined drug therapy, advanced age, a history of prior myocardial infarction, decreased systolic performance, and ventricular arrhythmia increase the risk of bradyarrhythmic complications associated with drug therapy.2),8),12) In a study of patients with high-degree AV block, a QRS width ≥120 ms was an independent predictor for the progression of a high-degree AV block after drug withdrawal.13) In this study, third-degree AV block, QRS width, and bradycardia requiring temporary transvenous pacing were significantly associated with bradycardia in cases truly associated with drugs.
Permanent pacing in drug-related bradycardia
There is a paucity of data regarding the incidence of drug-related bradycardia in specific cardiac conditions.9),14),15-17) In a recent report, the incidence of drug-related bradycardia requiring permanent pacing was estimated to be 1-15% among patients on a variety of anti-arrhythmic agents used for different indications.6) In another report, resolution of an AV block with drug discontinuation was more likely to be transient, and the reported 56% rate of "AV block relapse" was likely an underestimation; additional cases might have been detected with a longer follow-up.7) Although these patients could be discharged without permanent pacing, according to current guidelines,1) it was suggested that pacemaker implantation should be considered in such patients.7)
The limitations of this study included the following. First, the retrospective nature of the analysis and the small sample size are major limitations and increase the likelihood of a selection bias. Second, the relative short follow-up period in some patients might have underestimated the frequency of bradycardia truly caused by drugs. Third, we did not perform a sinus node function test. Sinus node function testing is not indicated in patients with DRB according to the Korean National Health Insurance System guidelines. Fourth, we did not perform routine 24 hour ECG monitoring. Patients might have asymptomatic sinus bradycardia or AV block that would be identified during 24 hour ECG monitoring. We only evaluated symptomatic bradycardia as a possible indication for pacemaker implantation in this study. Fifth, we could not perform a multivariate analysis because of the small sample size, and independent predictors of bradycardia truly associated with drugs could not be established in this study.
In conclusion, beta-blockers were the most common drugs associated with the development of bradycardia. About one quarter of the cases with DRB were not caused by drugs, but were revealed by drugs. Close follow-up of these patients is needed because implantation of a permanent pacemaker may be required in some of them.
Acknowledgments
We thank Dr. Roberto Patarca and Dr. Sung Hee Kim for their assistance with the preparation of this manuscript.
Part of this study was presented as an abstract at the 51st Annual Scientific Meeting of the Korean Circulation Society in 2007.
References
- 1.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practical Guidelines (ACC/AHA/NASPE Committee to Update the 1988 Pacemaker Guidelines) Circulation. 2002;106:2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Maheshwari A, Tresch DD, Thakur RK. Cardiac arrhythmias in the elderly. Card Electrophysiol Rev. 2002;6:120–128. doi: 10.1023/a:1017963928016. [DOI] [PubMed] [Google Scholar]
- 3.Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med. 2000;342:703–709. doi: 10.1056/NEJM200003093421006. [DOI] [PubMed] [Google Scholar]
- 4.Shohat-Zabarski R, Iakobishvili Z, Kusniec J, Mazur A, Strasberg B. Paroxysmal atrioventricular block: clinical experience with 20 patients. Int J Cardiol. 2004;97:399–405. doi: 10.1016/j.ijcard.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 5.López-sedón J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25:1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ovysyshcher IE, Barold SS. Drug-induced bradycardia: to pace or not to pace? Pacing Clin Electrophysiol. 2004;27:1144–1147. doi: 10.1111/j.1540-8159.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 7.Zeltser D, Justo D, Halkin A, et al. Drug-induced atrioventricular block: prognosis after discontinuation of the culprit drug. J Am Coll Cardiol. 2004;44:105–108. doi: 10.1016/j.jacc.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 8.Edoute Y, Nagachandran P, Svirski B, Ben-Ami H. Cardiovascular adverse drug reaction associated with combined beta-adrenergic and calcium entry-blocking agents. J Cardiovasc Pharmacol. 2000;35:556–559. doi: 10.1097/00005344-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hauser TH, Pinto DS, Josephson ME, Zimetbaum P. Safety and feasibility of a clinical pathway for the outpatient initiation of antiarrhythmic medications in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2003;91:1437–1441. doi: 10.1016/s0002-9149(03)00395-3. [DOI] [PubMed] [Google Scholar]
- 10.Essebag V, Hadjis T, Platt RW, Pilote L. Amiodarone and the risk of bradyarrhythmia requiring permanent pacemaker in elderly patients with atrial fibrillation and prior myocardial infarction. J Am Coll Cardiol. 2003;41:249–254. doi: 10.1016/s0735-1097(02)02709-2. [DOI] [PubMed] [Google Scholar]
- 11.Maisel WH, Kuntz K, Reimond S, et al. Risk of initiating antiarrhythmic drug therapy for atrial fibrillation in patients admitted to a university hospital. Ann Intern Med. 1997;127:281–284. doi: 10.7326/0003-4819-127-4-199708150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Friedman PL, Stevenson WG. Proarrhythmia. Am J Cardiol. 1998;82:50N–58N. doi: 10.1016/s0002-9149(98)00586-4. [DOI] [PubMed] [Google Scholar]
- 13.Kenneback G, Tabrizi F, Lindell P, Nordlander R. High-degree atrioventricular block during anti-arrhythmic drug treatment: use of a pacemaker with a bradycardia-detection algorithm to study the time course after drug withdrawal. Europace. 2007;9:186–191. doi: 10.1093/europace/eul185. [DOI] [PubMed] [Google Scholar]
- 14.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 15.Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:712–718. doi: 10.1001/jama.289.6.712. [DOI] [PubMed] [Google Scholar]
- 16.Cho YJ, Lee MM, Choe SJ, et al. A randomized, double-blind clinical trial to determine the efficacy of carvedilol vs. atenolol in patients with stage 1 to 2 essential hypertension. Korean Circ J. 1998;28:359–365. [Google Scholar]
- 17.Cha DH, Cha YS, Kook JH, et al. Clinical efficacy of carvedilol in patients with moderate to severe congestive heart failure. Korean Circ J. 1998;26:523–531. [Google Scholar]