Abstract
Background and Objectives
It is thought that patients with diabetes mellitus (DM) have a poor prognosis after an acute myocardial infarction (AMI), but the effect of diabetes on the outcomes of hypertensive patients with AMIs has not been elucidated in the Korean population. The aim of this study was to investigate the effects of diabetes on long-term clinical outcomes following AMIs in patients with hypertension.
Subjects and Methods
Using data from the Korea Acute Myocardial Infarction Registry (November 2005 to December 2006), 2,233 hypertensive patients with AMIs were grouped as follows based on the presence of DM: group I, diabetic hypertension (n=892, 544 men, mean age=66.2±10.9 years); and group II, non-diabetic hypertension (n=1341, 938 men, mean age=63.9±12.8 years). The primary study outcomes included in-hospital death and major adverse cardiac events (MACE; cardiac death, myocardial infarction (MI), repeat percutaneous coronary intervention, and coronary artery bypass surgery) at the 1 year follow-up.
Results
Hypertensive patients with DM were older and more likely to be women. The diabetic group had lower blood pressure (p<0.001), a lower left ventricular ejection fraction (p<0.001), a more severe degree of heart failure (p<0.001), a longer duration of coronary care unit admission (p<0.001), and a higher incidence of hyperlipidemia (p=0.007). The N-terminal pro-brain natriuretic peptide level (4602.5±8710.6 pg/mL vs. 2320.8±5837.9 pg/mL, p<0.001) was higher and the creatinine clearance (62.4±29.9 mL/min vs. 73.0±40.8 mL/min, p<0.001) was lower in the diabetic group than the non-diabetic group. Coronary angiographic findings revealed more frequent involvement of the left main stem (p=0.002) and multiple vessels (p<0.001) in the diabetic group. The rate of in-hospital death was higher in the diabetic group (p<0.001). During follow-up, the rates of composite MACE at 1 month, 6 months, and 12 months were higher in the diabetic group (p<0.001).
Conclusion
In hypertensive patients with AMI, DM was associated with worse clinical and angiographic features, with a higher risk of development of severe heart failure, and an increased risk of MACE on long-term clinical follow-up.
Keywords: Diabetes mellitus, Hypertension, Myocardial infarction
Introduction
Diabetes mellitus (DM) is a common disease with substantial morbidity and mortality.1),2) Hypertension is a well-known risk factor for coronary artery disease and there is considerable evidence for an increased prevalence of hypertension in diabetics, affecting up to 60%.2-5) Moreover, each pathophysiologic disease entity serves to exacerbate the other.3),6) The presence of hypertension in diabetic patients significantly increases the risk of cardiovascular disease (CVD), including acute coronary syndrome.5),7) Generally, hypertension in type 2 diabetics clusters with other CVD risk factors, such as microalbuminuria, central obesity, insulin resistance, dyslipidemia, hypercoagulation, increased inflammation, and left ventricular hypertrophy.5) This clustering of risk factors in diabetics ultimately results in the development of CVD, which is the major cause of premature mortality in patients with type 2 DM. Previous studies are in agreement that DM is an independent risk for mortality after an acute myocardial infarction (AMI).8-10) Also, other several studies have shown that hypertension is associated with a worse prognosis after an AMI.11-13) Despite this consensus, the effect of DM on the outcomes of hypertensive patients following an AMI has not been clearly demonstrated within the Oriental population.
Therefore, we conducted the present study to analyze the clinical impact of DM on patients with hypertension following an AMI who were registered in the Korea Acute Myocardial Infarction Registry (KAMIR).
Subjects and Methods
Patient population and study design
The KAMIR is a prospective, multi-center, observational registry designed to examine current epidemiology, in-hospital management, and outcome of patients with AMIs in Korea for the commemoration of the 50th anniversary of the Korean Circulation Society.14),15) Among the patients with AMIs, 2,233 hypertensive patients who were followed-up at 1 year were included in the present study. These patients were grouped as follows based on the presence of DM: group I, patients who were assigned to the diabetic hypertensive group (total 892, 544 males, mean age=66.2±10.9 years); and group II, patients who were assigned to the non-diabetic hypertensive group (total 1,341, 938 males, mean age=63.9±12.8 years). Hypertension was defined as a blood pressure ≥140/90 mmHg, or when the patient was taking antihypertensive medication based on a history of diagnosed hypertension. DM was defined as a fasting plasma glucose level ≥126 mg/dL, a random plasma glucose level ≥200 mg/dL, or when the patient was taking oral hypoglycemic agents or using subcutaneous insulin based on a history of diagnosed DM. Eligible patients for this study were required to have all three of the following: 1) symptoms of ischemia increasing or occurring at rest, 2) an elevated cardiac troponin-I level (≥2.0 ng/mL) or creatine kinase-myocardial infarction (CK-MB) (19 U/L, exceeding twice the upper limit of normal), and 3) ischemic changes assessed by electrocardiography (ST-segment elevation, depression, or T wave inversion of ≥0.2 mV in two contiguous leads). Our study included patients in cardiogenic shock state (Killip class IV).
We analyzed baseline demographic and clinical characteristics, relevant laboratory results and pharmacotherapy. Echocardiography was performed in all patients before discharge. Major adverse cardiac events (MACE) at the 1 month, 6 months, and 1 year clinical follow-up were evaluated and were defined as the composite of 1) cardiac death, 2) non-cardiac death, 3) non-fatal myocardial infarction (MI), and 4) re-percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG). Re-infarction was defined as the recurrence of symptoms or electrocardiographic changes in association with a rise in cardiac enzymes above the upper limit of normal. All data were recorded on a standardized, electronic, web-based registry at http://www.kamir.or.kr.
Statistical analysis
We compared the in-hospital mortality, the duration of admission to the coronary care unit, and the incidence of MACE during 1 month, 6 months, and 1 year of clinical follow-up between the 2 groups. The predictive factors for MACE at the 1 year clinical follow-up were calculated by multiple logistic regression analysis.
The Statistical Package for Social Sciences (SPSS) for Windows, version 15.0 (SPSS, Inc., Chicago, IL, USA) was used for all analysis. Continuous variables with normal distributions were expressed as the mean±standard deviation (SD) and they were compared with the use of an unpaired Student's t-test. Categorical variables were compared with the use of the chi-square test, where appropriate. A p<0.05 was deemed to be statistically significant.
Results
Baseline clinical characteristics of the study population
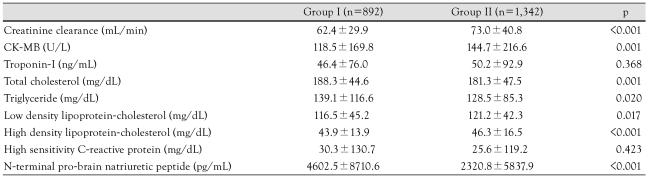
Among the 2,233 hypertensive patients with AMI who were followed-up for 1 year, there were 892 diabetic patients and 1,342 non-diabetic patients. The baseline clinical characteristics and laboratory findings of the study population are described in Table 1 and 2. Hypertensive patients with DM were older (p<0.001) and more likely to be women (p<0.001). Also, patients with DM had a higher proportion of smokers (p<0.001) and hyperlipidemia (p=0.007). The diabetic group had lower blood pressure (p<0.001), a lower left ventricular ejection fraction (p<0.001), more severe heart failure (p<0.001), and a higher proportion of ST segment elevation myocardial infarction (STEMI; p=0.005). N-terminal pro-brain natriuretic peptide level (p<0.001) was higher and creatinine clearance (p<0.001) was lower in the diabetic group than the non-diabetic group.
Table 1.
Baseline characteristics according to the presence of diabetes
Table 2.
Laboratory findings according to the presence of diabetes
CK-MB: creatine kinase-myocardial band isoenzyme
Coronary angiographic findings
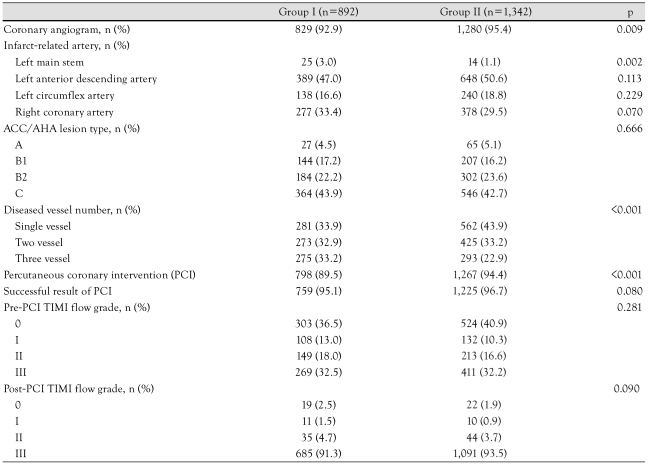
In the diabetic hypertension group, coronary angiography was performed in 92.9% of the patients during hospitalization, compared with 95.4% of the patients in the non-diabetic hypertension group. Also, PCI was done in 89.5% of patients in the diabetic group relative to 94.4% in the non-diabetic group, but the success rate of PCI showed no significant difference between the 2 groups. The baseline coronary angiographic characteristics are shown in Table 3. The most common infarct-related artery was the left anterior descending artery in both groups. The types of lesions according to the American College of Cardiology/American Heart Association (ACC/AHA) classification and the Thrombolysis In Myocardial Infarction (TIMI) flow grade before and after PCI showed no significant differences between the two groups. Left main stem and multivessel involvement were more prevalent in the diabetic group than the non-diabetic group (p=0.002 and p<0.001 respectively).
Table 3.
Coronary angiographic findings according to the presence of diabetes
ACC/AHA: American College of Cardiology/American Heart Association, TIMI: Thrombolysis In Myocardial Infarction
In-hospital outcomes and major adverse cardiac events
The clinical outcomes during the 1 year follow-up are shown in Table 4. The estimated in-hospital mortality was 9.8% in the diabetic group and 3.8% in the non-diabetic group (p<0.001). The duration of admission to the coronary care unit was significantly longer in the diabetic group (4.5 vs. 3.2 days, p<0.001). The rates of cardiac death were higher in the diabetic group at the 1 month, 6 months, and 12 months clinical follow-ups (p=0.002, p<0.001, and p<0.001 respectively). The rates of CABG at 1 month and non-cardiac death at 12 months were higher in the diabetic group (p=0.028 and p=0.025 respectively). The incidence of composite MACE was higher in the diabetic group at the 1 month, 6 months, and 12 months clinical follow-ups (p<0.001, p<0.001, and p<0.001 respectively).
Table 4.
Clinical outcomes and MACE during follow-up and 12 months after discharge
MACE: major adverse cardiac event
Survival curve and multivariate analysis of predictors of 1 year major adverse cardiac events
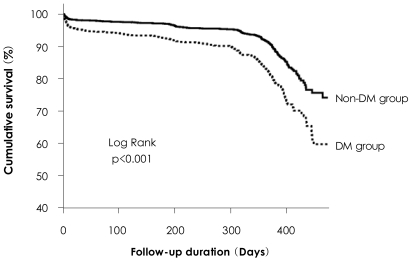
The Kaplan-Meier survival curve for mortality showed a significantly higher mortality in diabetic hypertensive patients (Fig. 1). Multivariate analysis was conducted to identify the independent predictors of the 1 year MACE using the meaningful factors in univariate analysis (Table 5 and 6).
Fig. 1.
Kaplan-Meier survival curves for the 2 groups. Survival curve analysis reveals significantly higher mortality rate in diabetic group than non-diabetic group.
Table 5.
Univariate analysis for the predictors of 1 year major adverse cardiac events
HMG Co-A: 3-hydroxy-3-methylglutaryl coenzyme A
Table 6.
Laboratory findings according to the MACE
MACE: major adverse cardiac event, CK-MB: creatine kindase-myocardial band isoenzyme
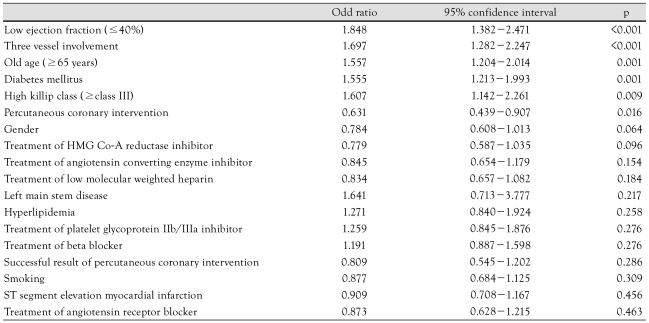
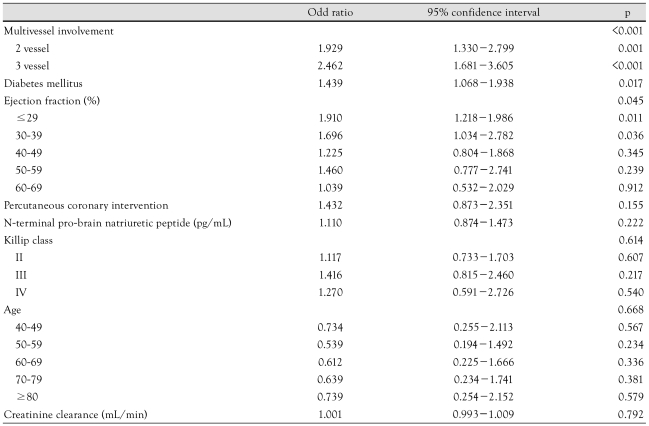
The independent predictors for 1 year MACE were 3-vessel involvement (p=0.001), presence of DM (p=0.017), and a lower ejection fraction (p=0.045) (Table 7).
Table 7.
Multivariate analysis for the predictors of one year major adverse cardiac events
Discussion
The aim of this study was to examine the relationship between DM and clinical outcomes in 2,233 hypertensive patients with AMIs in the KAMIR during 12 months of follow-up. Our results demonstrated that diabetic hypertensive patients with AMIs have a poorer outcome in clinical and angiographic features, and a higher risk of death on short- and long-term follow-up relative to non-diabetic hypertensive patients.
DM is associated with a high risk of CVD and is the leading cause of end-stage renal disease, blindness, and non-traumatic amputations. Hypertension by itself is a powerful risk factor for cardiovascular morbidity and mortality. Although the effects of DM and hypertension on the cardiovascular system are often distinct, we can easily presume that their combined presence in the same patient is devastating to the cardiovascular system. Epidemiologic studies have provided evidence for the coexistence of hypertension and DM and possibly point toward a common genetic and environmental factor promoting both DM and hypertension. Similarly, clustering of hypertension, insulin resistance, frank type 2 DM, hyperlipidemia, and central obesity has been documented in several populations.16) Insulin resistance, increased tissue inflammation, and reactive oxygen species production, resulting in endothelial dysfunction, increased tissue renin-angiotensin-aldosterone activity, and increased sympathetic nervous system activity have all been implicated in this complex pathophysiology of DM and hypertension. It is estimated that about 25% to 47% of persons with hypertension have insulin resistance or impaired glucose tolerance.17) With insulin resistance there is impaired biologic and physiologic tissue responses to insulin. The relationship between insulin resistance, DM, and hypertension is complex and interrelated. Untreated patients with essential hypertension have higher fasting and postprandial insulin levels than age- and gender-matched normotensive persons, regardless of body mass; a direct correlation between plasma insulin levels and blood pressure exists.5),18) Interestingly, the relationship between hyperinsulinemia and hypertension is not observed in secondary hypertension.18) This indicates that insulin resistance and hyperinsulinemia are not consequences of hypertension, but rather a genetic predisposition that exists for both diseases. This notion is supported by the observation that there is abnormal glucose metabolism in the offspring of hypertensive patients.19) Thus, there is a strong association between hypertension, DM, and insulin resistance.
Both hypertension and DM are well-identified risk factors for atherogenesis. Coronary artery disease is much more common in patients with both DM and hypertension than in patients with hypertension alone, and the development of atherosclerosis was found to be accelerated, with more plaque fissure and a lower coronary perfusion reserve index when DM and hypertension coexist.20),21) Our study revealed that diabetic hypertensive patients have a higher prevalence of multivessel involvement than the non-diabetic population following an AMI and multivessel disease is related to worse long-term clinical outcome. Patients with combined DM and hypertension also tend to have impaired systolic and diastolic ventricular function with more severe left ventricular hypertrophy and congestive heart failure than counterparts with hypertension alone.20),22) The extensive degenerative changes in the diabetic hypertensive heart may be related to abnormalities in the microcirculation. The most striking microscopic findings of the hypertensive diabetic heart seem to be the distribution of dense interstitial connective tissue throughout the myocardium. As expected, we found that in diabetic hypertensive patients left ventricular ejection fraction was significantly lower. In our analysis of patients, the combination of DM and hypertension was more common in females, but after univariate analysis, the risk of death was not significantly different among male and female patients as in a previous study.23)
The most important result of this study was that DM is a strong risk factor for cardiovascular events; hypertensive patients with DM were more likely to have a cardiovascular event than non-diabetic hypertensive patients. Our study showed that the in-hospital mortality rate for diabetic hypertensive patients with an AMI was higher than for non-diabetic hypertensive patients. In many previous studies, hospital case-fatality rates for diabetic patients with acute coronary syndrome were almost twice as high as those for non-diabetic patients.24-27) Also, these results agree with a previous study which reported DM to be one of the most important risk factors for new cardiovascular events following an AMI.28) Factors, such as diabetic cardiomyopathy, small vessel disease, increased platelet activity, decreased fibrinolysis, and autonomic neuropathy leading to ventricular arrhythmia may account for the poor prognostic outlook of diabetic hypertensive patients.29),30) In our analysis, long-term clinical outcomes of diabetic hypertensive patients following an AMI showed worse progression relative to the non-diabetic hypertensive population.
This study had several limitations. First, our study was a multi-center prospective registry, and it was not a randomized, controlled study. Thus, there was probably a selection bias when enrolling patients into both study groups.
The proportion of STEMI patients was higher in the group I patients than in the group II patients. Second, a value of blood glucose at follow-up was not recorded in the registry, therefore the relationship between adequate control of blood glucose and the prognosis of the patients was not estimated exactly. Third, whether long-term and more aggressive treatment of high blood glucose in patients with AMI can reduce adverse outcomes remains unknown. Finally, the period of our study was relatively short because our study was a comparison of the MACE at the 1 year clinical follow-up.
In conclusion, in hypertensive Korean patients with AMIs, DM was associated with worse clinical and angiographic features, with a higher risk of development of severe heart failure and increased risk of MACE on long-term clinical follow-up.
Acknowledgments
This study was performed with the support of The Korean Society of Circulation in celebration of the 50th Anniversary of The Korean Society of Circulation.
Footnotes
Korea Acute Myocardial Infarction Registry (KAMIR) Study Group of Korean Society of Cardiology: Myung Ho Jeong, MD, Youngkeun Ahn, MD, Shung Chull Chae, MD, Jong Hyun Kim, MD, Seung Ho Hur, MD, Young Jo Kim, MD, In Whan Seong, MD, Dong Hoon Choi, MD, Jei Keon Chae, MD, Taek Jong Hong, MD, Jae Young Rhew, MD, Doo Il Kim, MD, In Ho Chae, MD, Jung Han Yoon, MD, Bon Kwon Koo, MD, Byung Ok Kim, MD, Myoung Yong Lee, MD, Kee Sik Kim, MD, Jin Young Hwang, MD, Myeong Chan Cho, MD, Seok Kyu Oh, MD, Nae Hee Lee, MD, Kyoung Tae Jeong, MD, Seung Jea Tahk, MD, Jang Ho Bae, MD, Seung Woon Rha, MD, Keum Soo Park, MD, Chong Jin Kim, MD, Kyoo Rok Han, MD, Tae Hoon Ahn, MD, Moo Hyun Kim, MD, Ki Bae Seung, MD, Wook Sung Chung, MD, Ju Young Yang, MD, Chong Yun Rhim, MD, Hyeon Cheol Gwon, MD, Seong Wook Park, MD, Young Youp Koh, MD, Seung Jae Joo, MD, Soo Joong Kim, MD, Dong Kyu Jin, MD, Jin Man Cho, MD, Byung Ok Kim, MD, Sang-Wook Kim, MD, Jeong Kyung Kim, MD, Tae Ik Kim, MD, Deug Young Nah, MD, Si Hoon Park, MD, Sang Hyun Lee, MD, Seung Uk Lee, MD, Hang-Jae Chung, MD, Jang Hyun Cho, MD, Seung Won Jin, MD, Yang Soo Jang, MD, Jeong Gwan Cho, MD and Seung Jung Park, MD
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Bonora E, Kiechl S, Willeit J, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals. Diabetes. 2004;53:1782–1789. doi: 10.2337/diabetes.53.7.1782. [DOI] [PubMed] [Google Scholar]
- 3.National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Program Working Group report on hypertension in diabetes. Hypertension. 1994;23:145–158. [PubMed] [Google Scholar]
- 4.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. N Engl J Med. 2000;342:905–912. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 5.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 6.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 7.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmberg K, Ryden L. Myocardial infarction in patients with diabetes mellitus. Eur Heart J. 1988;9:259–264. doi: 10.1093/oxfordjournals.eurheartj.a062494. [DOI] [PubMed] [Google Scholar]
- 9.Behar S, Boyko V, Reicher-Reiss H, Goldbourt U. Ten-year survival after acute myocardial infarction: comparison of patients with and without diabetes. Am Heart J. 1997;133:290–296. doi: 10.1016/s0002-8703(97)70222-9. [DOI] [PubMed] [Google Scholar]
- 10.Mak KH, Moliterno DJ, Granger CB, et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. J Am Coll Cardiol. 1997;30:171–179. doi: 10.1016/s0735-1097(97)00118-6. [DOI] [PubMed] [Google Scholar]
- 11.Fresco C, Avanzini F, Bosi S, et al. Prognostic value of a history of hypertension in 11,483 patients with acute myocardial infarction treated with thrombolysis. J Hypertens. 1996;14:743–750. doi: 10.1097/00004872-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction: results from international trial of 41,021 patients. Circulation. 1995;91:1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 13.Herlitz J, Bang A, Karlson BW. Five year prognosis after acute myocardial infarction in relation to a history of hypertension. Am J Hypertens. 1996;9:70–76. doi: 10.1016/0895-7061(95)00302-9. [DOI] [PubMed] [Google Scholar]
- 14.Lee KH, Jeong MH, Ahn YK, et al. Gender differences of success rate of percutaneous coronary intervention and short term cardiac events in Korea Acute Myocardial Infarction Registry. Int J Cardiol. 2008;130:227–234. doi: 10.1016/j.ijcard.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 15.Kwon TG, Bae JH, Jeong MH, et al. N-terminal Pro-B-type natriuretic peptide is associated with adverse short-term clinical outcomes in patients with acute ST-elevation myocardial infarction underwent primary percutaneous coronary intervention. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2007.12.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Cha BS, Kim HJ. Metabolic syndrome and cardiovascular disease. Korean Circ J. 2003;33:645–652. [Google Scholar]
- 17.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 18.Cho HK, Goh CW, Kim SS, et al. Insulin resistance as an associated factor of essential hypertension in Korean. Korean Circ J. 1996;26:1020–1029. [Google Scholar]
- 19.Sechi LA, Melis A, Tedde R. Insulin hypersecretion: a distinctive feature between essential and secondary hypertension. Metabolism. 1992;41:1261–1266. doi: 10.1016/0026-0495(92)90019-7. [DOI] [PubMed] [Google Scholar]
- 20.Grossman E, Messereli FH. Diabetic and hypertensive heart disease. Ann Intern Med. 1996;125:304–310. doi: 10.7326/0003-4819-125-4-199608150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Arora GD, Reeves WC, Movahed A. Alteration of coronary perfusion reserve in hypertensive patients with diabetes. J Hum Hypertens. 1994;8:51–57. [PubMed] [Google Scholar]
- 22.Cho KI, Park JH, Lee CK, et al. Isolated and combined influences of diabetes and hypertension on the myocardial function and geometry. Korean Circ J. 2006;36:411–417. [Google Scholar]
- 23.Jonas M, Reicher-Reiss H, Boyko V, Behar S, Grossman E. Hospital and 1-year outcome after acute myocardial infarction in patients with diabetes mellitus and hypertension. J Hum Hypertens. 2003;17:665–670. doi: 10.1038/sj.jhh.1001597. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson L, Hildebrandt P, Seibaek M, et al. Long-term prognosis of diabetic patients with myocardial infarction: relation to antidiabetic treatment regimen. Eur Heart J. 2000;21:1937–1943. doi: 10.1053/euhj.2000.2244. [DOI] [PubMed] [Google Scholar]
- 25.Eagle KA, Goodman SG, Avezurn A, Budaj A, Sullivan CM, Lopez-Sendon J. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE) Lancet. 2002;359:373–377. doi: 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- 26.Hsu LF, Mak KH, Lau KW, et al. Clinical outcomes of patients with diabetes mellitus and acute myocardial infarction treated with primary angioplasty or fibrinolysis. Heart. 2002;88:260–265. doi: 10.1136/heart.88.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin K, Goldberg RJ, Spencer F, et al. Implications of diabetes in patients with acute coronary syndromes. Arch Intern Med. 2004;164:1457–1463. doi: 10.1001/archinte.164.13.1457. [DOI] [PubMed] [Google Scholar]
- 28.Levantesi G, Macchia A, Marfisi R, et al. Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2005;46:277–283. doi: 10.1016/j.jacc.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 29.Calcera-Tomas, Melgarejo-Moreno A, Garcia-Alberola A, et al. Prognostic significance of diabetes in acute myocardial infarction: are the differences linked to female gender? Int J Cardiol. 1999;69:289–298. doi: 10.1016/s0167-5273(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 30.Aronson D, Rayfield EJ, Chesebro JH. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann Intern Med. 1997;126:296–306. doi: 10.7326/0003-4819-126-4-199702150-00006. [DOI] [PubMed] [Google Scholar]