Abstract
Background and Objectives
Left ventricular (LV) remodeling (LVR) after an acute myocardial infarction (AMI) has important clinical implications. We have investigated the prognostic relevance of ventricular systolic dyssnchrony as an indicator of LVR after an AMI.
Subjects and Methods
We enrolled 92 patients (males, 72.8%; mean age, 61.0±13.0 years) with an AMI who underwent successful percutaneous coronary intervention. We analyzed the baseline characteristics, the laboratory and echocardiographic findings, and we performed follow-up echocardiography 6 months after the AMI. The patients were divided into two groups: 1) the presence of LVR, which was defined as an increment of LV end systolic volume (LVESV) >20% compared with the baseline examination; and 2) the absence of LVR.
Results
Twenty-seven patients (29.3%) developed LVR after a 6 month follow-up. There was no statistically significant difference in the clinical and angiographic findings between the two groups. With respect to the laboratory findings, the LVR group had a higher peak creatine kinase MB (CK-MB) (149.9±155.0 vs. 74.6±69.7 U/L, p=0.001) and troponin-I (70.2±73.3 vs. 43.2±39.5 ng/mL, p=0.024) level than the group without LVR. With respect to echocardiographic findings, the baseline LV ejection fraction (EF) and LVESV were not significantly different (LVESV, 73.0±37.3 vs. 91.3±52.0 mL, p=0.013; and EF, 58.3±13.3 vs. 55.6±11.8%, p=0.329) between the groups with and without LVR, respectively. The degree of LV dyssynchrony, which was assessed by tissue Doppler imaging, was significantly higher in the LVR group than the group without LVR (75.2±43.4 vs. 38.3±32.5 ms), and the degree of LV dyssynchrony was an independent predictor for LVR based on multivariate analysis {hazard ratio (HR)=0.097, p<0.001}. In receiver operating characteristics (ROC) curve analysis, the area under the curve (AUC) was 0.754 and a cutoff value of 45.9 predicted the development of LVR with 74.1% sensitivity and 72.3% specificity.
Conclusion
The presence of LV dyssynchroncy immediately after a myocardial infarction is an important predictive factor for development LVR.
Keywords: Ventricular remodeling, Myocardial infarction, Dyssynchrony
Introduction
Dyssynchrony of left ventricular (LV) contraction is an indicator of a temporal discrepancy in myocardial contraction among different segments of the left ventricle. The concept of dyssynchrony has been more frequently proposed since the introduction of cardiac resynchronization therapy (CRT) for intractable heart failure.1),2) Various parameters representing dyssynchrony have been evaluated for validation as predictors of response to CRT;3) however, the clinical implications of dyssynchrony after an acute myocardial infarction (AMI) have not been fully evaluated.
Left ventricular remodeling (LVR) after an AMI is considered to be an important prognosticator.4) A number of studies have shown a significant correlation between the presence of LVR and adverse clinical outcomes,5-7) thus predicting LVR is important in risk stratification and devising management plans.
An AMI has been shown to have an impact on myocardial contractile synchronicity.8) In that study, the degree of LV dyssynchrony was associated with infarction size. Accordingly, we have assumed that dyssynchronous contraction results in an undesirable influence on the myocardium, such as tethering of the myocardium, eventually leading to remodeling of the left ventricle. With that assumption in mind, in the current study we have attempted to identify the relationship between LVR and dyssynchrony immediately after an AMI.
Subjects and Methods
Patient population
We enrolled 92 patients (mean age, 61±13 years; males, 72.8%) who were admitted to Chonnam National University Hospital (CNUH) with a diagnosis of AMI. The inclusion criteria were: 1) patients presenting with typical ischemic pain and/or electrocardiogram (ECG) changes; and 2) elevated troponin I (TnI) levels, defined as >0.01 ng/mL at the time of beginning of the ischemic symptoms.
All of the patients received standard management, according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines,9) and underwent primary percutaneous coronary intervention (PCI). Thrombolysis In Myocardial Infarction (TIMI) III flow was achieved without serious complications in all patients.
Study protocol
Baseline clinical characteristics, including age, gender, and risk factors of coronary artery disease, such as diabetes and hypertension, were recorded at the time of admission. Among various laboratory data, the peak levels of creatine kinase MB (CK-MB), TnI, and N-terminal pro b-type natriuretic peptide (NT-pro-BNP) were evaluated for estimation of the infarction burden. Two-dimensional echocardiography was carried out within 48 hours of admission. The mean time to echocardiography was 26±15 hours. The parameters assessed during echocardiography were left ventricular ejection fraction (LVEF), left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV), left atrial dimension (LAD), the ratio of mitral inflow peak velocity (E)/mitral annular peak early velocity (E'), and the severity of mitral regurgitation. Follow-up echocardiography was performed 6 months after the AMI and all of the initial parameters were evaluated again.
The left ventricular volume was estimated by the modified Simpson's method. LVR was defined as an increment of LVESV by 20% compared with the initial value. Dyssynchrony in contraction of the left ventricle was assessed by the color-coded tissue doppler image (TDI) method.10),11) Specifically, the degree of dyssynchrony was represented by the maximal difference between the time to peak systolic velocity (Ts) measured at each 6 segments of the left ventricle. The enrolled patients were divided into two groups: 1) the presence of LVR after 6 months of follow-up; and 2) the absence of LVR after 6 months of follow-up.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Continuous variables are presented as the mean±standard deviation. The difference of continuous variables between groups was analyzed with an independent sample t-test. Categorical data were compared using a chi-square test.
The correlation between degree of dyssynchrony and change of LVESV was assessed with a bivariate correlation. In addition, multivariate logistic regression was performed to demonstrate statistical independence of the results. To determine the optimal threshold of the dyssynchrony index for prediction of LVR, receiver operating characteristic (ROC) curve analysis was applied. For all analyses, a p<0.04 was considered statistically significant.
Statistical analysis was performed using Statistical Package for Social Science (SPSS) 15.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
Among 92 patients, 67 patients (72.8%) were males. The mean age was 61±13 years. The final diagnosis was ST elevation myocardial infarction (STEMI) in 58 patients (63%) and non-ST elevation myocardial infarction (NSTEMI) in 34 patients (37%). Forty-eight patients (52.2%) had multi-vessel disease. In detail, 24 patients (26.1%) had two-vessel disease and 24 patients (26.1%) had three-vessel disease. The LAD was the infarction-related artery in 43 patients (46.7%). All of the patients received PCI and obtained TIMI III flow without complications.
On the baseline echocardiographic exam, the mean ejection fraction (EF), left ventricular end diastolic dimension (LVEDD), LVEDV, left ventricular end systolic dimension (LVESD), and LVESV were 56.4±12.2%, 50.9±6.7 mm, 176.7±52.1 mL, 35.1±8.4 mm, and 85.9±48.8 mL, respectively. Moderate-to-severe LV dysfunction was defined as an EF <40%, and was observed in 11 patients (11.2%). Eleven patients (11.2%) had diastolic dysfunction, defined as an E/E' ratio >15. Mitral regurgitation > grade II was observed in 10 patients (10.8%). The degree of LV dyssynchrony was estimated by the maximum difference of Ts in six standard myocardial segments using color-coded TDI. The mean dyssynchrony index at baseline was 49.5±39.4 ms. Thirty patients (32.6%) in whom the dyssynchrony index was >64 ms were considered to have dyssynchrony.
Six-month follow-up results
On follow-up echocardiography, the LVEF was improved >10% in 18 patients (19.6%). In contrast, the LVEF was decreased >10% in 16 patients (17.4%) and 58 patients (63%) had no significant change.
The mean LVESD at the 6 month follow-up was 85.9±48.8 mL. The LVESV was increased in 50 patients (54.3%) compared to the baseline examination. Twenty-seven patients (29.3%) had LVR.
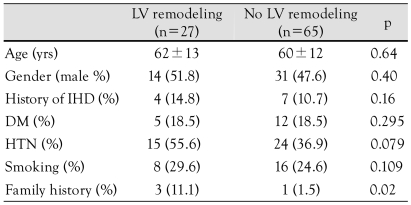
When the entire study population was divided into two groups according to the presence or absence of LVR, there was no significant difference in the baseline characteristics between the two groups, except for a family history of ischemic heart disease (Table 1).
Table 1.
Baseline clinical characteristics
LV: left ventricle, IHD: ischemic heart disease, DM: diabetes mellitus, HTN: hypertension
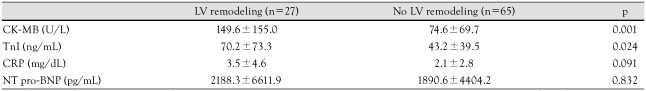
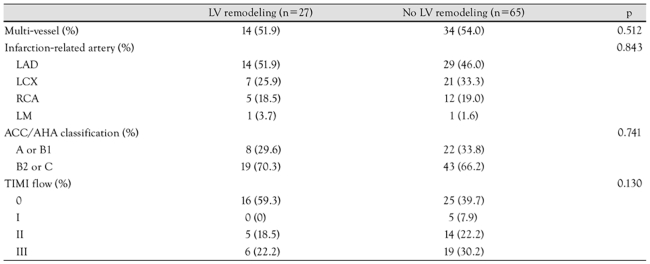
The laboratory data pertaining to the presence or absence of LVR are shown in Table 2. The peak CK-MB and TnI levels were significantly higher in the LVR group than the group without LVR. The peak CK-MB level in the LVR group was 149.6±155.0 U/L compared to 74.6±69.7 U/L in the group without LVR (p=0.001). The TnI level was 70.2±73.3 ng/mL in the LVR group and 43.2±39.5 ng/mL in the group without LVR (p=0.024). In contrast, the C-reactive protein (CRP) and NT pro-BNP levels did not differ significantly between the two groups. The incidence of left anterior descending artery involvement, multi-vessel disease, and TIMI flow before PCI was determined by coronary angiography; there was no significant difference between the two groups in any of the three parameters (Table 3).
Table 2.
Laboratory findings
LV: left ventricle, CK-MB: creatine kinase MB, TnI: troponin I, CRP: C-reactive protein, NT pro-BNP: N-terminal pro b-type natriuretic peptide
Table 3.
Coronary angiographic findings
LV: left ventricle, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, LM: left main coronary artery, ACC/AHA: American College of Cardiology/American Heart Association, TIMI: thrombolysis in myocardial infarction
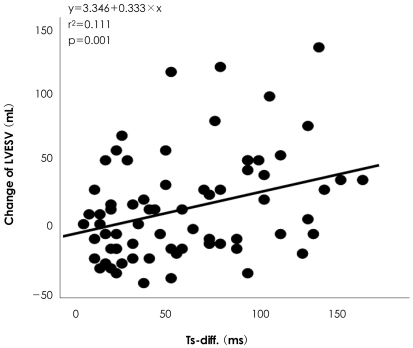
On baseline echocardiographic data, there were no significant differences in LVESV, LVEDV, EF, left atrium (LA) diameter, mitral regurgitation (MR) grade, and incidence of diastolic dysfunction between the two groups, as shown in Table 4. The only difference in baseline echocardiography was the dyssynchrony index, as assessed by color-coded TDI. The mean dyssynchrony index in the LVR group was 75.2±43.3 ms, which was significantly greater than the group without LVR (38.8±32.5 ms; p<0.001). The difference in the dyssynchrony index is depicted in Fig. 1. We then verified the correlation be tween the dyssynchrony index and the change in LVESV. As shown in Fig. 2, the change in LVESV had a positive correlation with the dyssynchrony index (r2=0.111, p=0.001).
Table 4.
Baseline echocardiographic data
LVEDV: left ventricular end diastolic volume, LVESV: left ventricular end systolic volume, EF: ejection fraction, LAD: left atrium dimension, MR: mitral regurgitation, Ts-diff.: maximum difference between time to peak systolic velocity
Fig. 1.
Individual value of LV dyssynchrony index according to LV remodeling. Plotting of maximum difference in time to peak systolic time (Ts-diff.) showed a significantly higher mean value in the LV remodeling group. LV: left ventride, Ts-diff.: maximum difference between time to peak systolic velocity.
Fig. 2.
Correlation between LV dyssynchrony index and change in LVESV. This figure showed a positive correlation between LVESV and the dyssynchrony index (r2=0.111, p=0.001). LVESV: left ventricular end systolic volume, Ts-diff.: maximum difference between time to peak systolic velocity.
At the 6 month follow-up, there were no significant differences in the incidence of patients in the groups with and without LVR taking an angiotensin converting enzyme inhibitor, an angiotensin receptor blocker, and a beta-blocker.
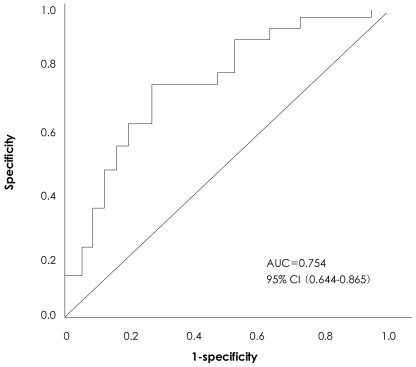
To identify the independency of the LV dyssynchrony index as a predictor of LV remodeling, we performed multivariate logistic regression for variables which showed statistical significance on univariate testing (a family history of ischemic heart disease, and the peak TnI and CK-MB levels). The results are shown in Table 5. The dyssynchrony index remained an independent predictor of LVR after adjustment for other variables (HR=0.097; 95% CI, 0.028-0.343, p<0.001). To determine the optimal cut-off value of the dyssynchrony index for LVR, receiver operating characteristics analysis (ROC) was performed. The area under the curve (AUC) was 0.754, which indicates good predictability. The peak systolic time difference >45.9 ms predicted the development of LVR with a sensitivity of 74.1% and a specificity of 72.3%. The results of ROC analysis are shown in Fig. 3.
Table 5.
Multivariate analysis for predictor of LV remodeling
Ts-diff.: maximum difference between time to peak systolic velocity, TnI: troponin I, CK-MB: creatine kinase MB, LAD-IRA: infarct related artery was left anterior descending artery, TIMI: thrombolysis in myocardial infarction
Fig. 3.
Receiver operating characteristics (ROC) curve of LV dyssynchrony index. Area under curve (AUC) was 0.754, which indicates good predictability. The value, 45.9 ms, predicted development of LV remodeling with a sensitivity of 74.1% and a specificity of 72.3%. LV: left ventricle.
Discussion
We demonstrated herein that LV dyssynchrony is an independent predictor of LVR compared to other parameters. The severity of LVR was positively correlated with the degree of LV dyssynchrony.
Unfavorable remodeling of the left ventricle has been one of the important sequelae after an AMI which causes progressive left ventricular dysfunction.6),12) In the current study, 16 patients (17.4%) had worsening of LV systolic function, as defined by a decrease in LVEF >10%.13)
The definition of LVR has varied between studies because the term, remodeling, implies multiple meanings, including morphology and volume. We adopted the increment of LVESV >20% as the definition of LVR. The majority of previous studies regarding LVR after an AMI chose a change in LVESV as the definition of remodeling due to good reproducibility.14-16)
In the current study, 29.3% of patients had evidence of LVR at the 6 month follow-up. The incidence of LVR was higher compared with the results of previous studies which showed a 16% to 20% incidence.17),18) This result may be due to the large infarction burden in the study population that was presumed by a high peak cardiac enzyme level.
The various predictors for LVR after an AMI have been tested for validation.19-21) The current established predictors are infarction size, involvement of the left anterior descending artery, and the presence of hypertension.22-24) Among the known predictors for LVR, infarction size, which is estimated by the peak level of cardiac enzymes, was in agreement with the findings in the current study. Even though the incidence of LAD involvement and hypertension had a higher tendency in the LVR group than the group without LVR, there were no significant differences between the two groups. The lack of differences in the incidence of LAD involvement and hypertension may reflect the small population number; enrollment of a larger number of patients in a future study may clarify the discrepancy.
In the current study, we evaluated the possibility of LV systolic dyssynchrony as a predictor of LVR. The previous studies involving LV systolic dyssynchrony mainly focused on identifying responders after CRT. The clinical implication in AMI has not been fully evaluated yet. Some studies have demonstrated the impact of LV dyssynchrony on AMI. In those studies, the presence of LV dyssynchrony reflected a larger infarction burden, which increased the change in unfavorable remodeling, and chronic dyssynergy was sufficient alone to induce ischemic LVR in patients with AMIs.25),26) Thus, we assumed that myocardial dyssynchrony after an AMI may be a primary indicator of remodeling.
The assessment of LV dyssynchrony has been performed using various echocardiographic techniques. Currently, tissue Doppler imaging is the major modality for estimation of myocardial contractile performance. The maximum difference in the peak systolic time, as measured by a color-coded tissue Doppler imaging technique, and the standard deviation of the time to peak radial strain in speckled tracking radial strain analysis are two mainstays for the assessment of dyssynchrony.27) We used a color-coded TDI technique for the study because it is easy for post-examination analysis and has relatively good reproducibility.
In the study results, the incidence of LV dyssynchrony was significantly higher in the LVR group. There was a positive correlation between the change in LVESV and the LV dyssynchrony index. Also, this relationship was tested for independency. We concluded that the presence of LV dyssynchrony was a primary factor in predicting the development of LVR. Indeed, the degree of LV dyssynchrony can predict the extent of LVR.
The clinical implications of this study primarily involve the prognostic value. Recognition of dyssynchrony may enable physicians to predict a higher probability of LVR early after patient presentation, in which greater caution and aggressive treatment will be afforded the patient. In fact, LV dyssynchrony may be more than a prognosticator. Some studies have demonstrated that stem cell transfusion following an AMI can prevent LVR,28),29) and induce restoration of LV dyssynchrony.30) The implication of such results is that LV dyssynchrony should be one of the treatment goals in patients with AMIs.
Conclusion
In patients with AMIs, the presence of LV systolic dyssynchrony is an independent predictor for the development of LVR after long-term follow-up. More clinical caution and aggressive treatment should be made available in the management of such patients.
References
- 1.Abdelhadi R, Adelstein E, Voigt A, Gorcsan J, Saba S. Measures of left ventricular dyssynchrony and the correlation to clinical and echocardiographic response after cardiac resynchronization therapy. Am J Cardiol. 2008;102:598–601. doi: 10.1016/j.amjcard.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J, Freemantle N, Ghio S, et al. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. J Am Coll Cardiol. 2008;52:438–445. doi: 10.1016/j.jacc.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Cleland J, Freemantle N, Ghio S, et al. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. J Am Coll Cardiol. 2008;52:438–445. doi: 10.1016/j.jacc.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Nicolosi GL. Echocardiography to understand remodeling and to assess prognosis after acute myocardial infarction. Int J Cardiol. 1998;65(Suppl 1):S75–S78. doi: 10.1016/s0167-5273(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 5.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 6.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 7.Udelson JE, Konstam MA. Relation between left ventricular remodeling and clinical outcomes in heart failure patients with left ventricular systolic dysfunction. J Card Fail. 2002;8(Suppl):S465–S471. doi: 10.1054/jcaf.2002.129289. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Chan AK, Yu CM, et al. Left ventricular systolic asynchrony after acute myocardial infarction in patients with narrow QRS complexes. Am Heart J. 2005;149:497–503. doi: 10.1016/j.ahj.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 9.Antman EM, Hand M, Armstrong PW, et al. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 writing committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 10.Bleeker GB, Bax JJ, Schalij MJ, van der Wall EE. Tissue Doppler imaging to assess left ventricular dyssynchrony and resynchronization therapy. Eur J Echocardiogr. 2005;6:382–384. doi: 10.1016/j.euje.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Kang SJ, Song JK, Song JM, et al. Usefulness of Doppler myocardial imaging for the quantitative assessment of ventricular asynchrony in patients with heart failure. Korean Circ J. 2004;34:492–499. [Google Scholar]
- 12.Verma A, Meris A, Skali H, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction. The VALIANT (VALsartan In Acute myocardial iNfarcTion) echocardiographic study. JACC Cardiovasc Imaging. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Parodi G, Memisha G, et al. Prevalence, predictors, time course, and long-term clinical implications of left ventricular functional recovery after mechanical reperfusion for acute myocardial infarction. Am J Cardiol. 2007;100:1718–1722. doi: 10.1016/j.amjcard.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 14.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Yu CM, Fung JW, Chan CK, et al. Comparison of efficacy of reverse remodeling and clinical improvement for relatively narrow and wide QRS complexes after cardiac resynchronization therapy for heart failure. J Cardiovasc Electrophysiol. 2004;15:1058–1065. doi: 10.1046/j.1540-8167.2004.03648.x. [DOI] [PubMed] [Google Scholar]
- 16.Giannuzzi P, Temporelli PL, Nicolosi GL, et al. Doppler-derived mitral deceleration time as a strong prognostic marker of left ventricular remodeling and survival after acute myocardial infarction: results of the GISSI-3 echo substudy. J Am Coll Cardiol. 2004;43:1646–1653. doi: 10.1016/j.jacc.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Giannuzzi P, Temporelli PL, Bosimini E, et al. Heterogeneity of left ventricular remodeling after acute myocardial infarction: results of the Gruppo Italiano per lo Studio della Sopravvivenza-nell'Infarto Miocardico-3 Echo Substudy. Am Heart J. 2001;141:131–138. doi: 10.1067/mhj.2001.111260. [DOI] [PubMed] [Google Scholar]
- 18.Savoye C, Equine O, Tricot O, et al. Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] Study Group) Am J Cardiol. 2006;98:1144–1149. doi: 10.1016/j.amjcard.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, Aonuma K, Imanaka-Yoshida K, et al. Serum tenascin-C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2006;47:2319–2325. doi: 10.1016/j.jacc.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Nagashima M, Itoh A, Otsuka M, Kasanuki H, Haze K. Reperfusion phenomenon is a strong predictor of left ventricular remodeling after acute myocardial infarction. Circ J. 2005;69:884–889. doi: 10.1253/circj.69.884. [DOI] [PubMed] [Google Scholar]
- 21.Choi SY, Tahk SJ, Yoon MH, et al. Comparison of TIMI myocardial perfusion grade with coronary flow reserve for prediction of recovery of LV function and LV remodeling in acute myocardial infarction. Korean Circ J. 2004;34:247–257. [Google Scholar]
- 22.McKay RG, Pfeffer MA, Pasternak RC, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 23.Pirolo JS, Hutchins GM, Moore GW. Infarct expansion: pathologic analysis of 204 patients with a single myocardial infarct. J Am Coll Cardiol. 1986;7:349–354. doi: 10.1016/s0735-1097(86)80504-6. [DOI] [PubMed] [Google Scholar]
- 24.Popovic AD, Neskovic AN, Marinkovic J, Thomas JD. Acute and long-term effects of thrombolysis after anterior wall acute myocardial infarction with serial assessment of infarct expansion and late ventricular remodeling. Am J Cardiol. 1996;77:446–450. doi: 10.1016/s0002-9149(97)89335-6. [DOI] [PubMed] [Google Scholar]
- 25.Mollema SA, Bleeker GB, Liem SS, et al. Does left ventricular dyssynchrony immediately after acute myocardial infarction result in left ventricular dilatation? Heart Rhythm. 2007;4:1144–1148. doi: 10.1016/j.hrthm.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Carluccio E, Biagioli P, Alunni G, et al. Patients with hibernating myocardium show altered left ventricular volumes and shape, which revert after revascularization: evidence that dyssynergy might directly induce cardiac remodeling. J Am Coll Cardiol. 2006;47:969–977. doi: 10.1016/j.jacc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 27.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 28.Yao M, Dieterle T, Hale SL, et al. Long-term outcome of fetal cell transplantation on postinfarction ventricular remodeling and function. J Mol Cell Cardiol. 2003;35:661–670. doi: 10.1016/s0022-2828(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 29.Kang HJ, Kim HS, Na SH, et al. Six months follow up results of "granulocytes-colony stimulating factor" based stem cell therapy in patients with myocardial infarction: MAGIC cell randomized controlled trial. Korean Circ J. 2006;36:99–107. [Google Scholar]
- 30.Chang SA, Kim HK, Lee HY, et al. Restoration of left ventricular synchronous contraction after acute myocardial infarction by stem cell therapy: new insights into the therapeutic implication of stem cell therapy for acute myocardial infarction. Heart. 2008;94:995–1001. doi: 10.1136/hrt.2007.124701. [DOI] [PubMed] [Google Scholar]