Abstract
Background and Objectives
Hypertension develops as a result of cardiac hypertrophy and fibrosis or as a result of exchange of the extracellular matrix. In particular, matrix metalloproteinase (MMP)-3 is a major enzyme involved in the reconstruction of the arterial intima through activation of other MMPs. We analyzed MMP-3 genotypes in hypertensive and normotensive adolescents and sought to determine if a particular genotype is a predictor of cardiovascular complications.
Subjects and Methods
Forty-four hypertensive adolescents and 59 healthy adolescents were included in this study. Serum aldosterone, renin, insulin, angiotensin converting enzyme (ACE), insulin, homocysteine, vitamin B12, folate, MMP-1, MMP-2, MMP-3, MMP-9, tissue inhibitors of matrix metalloproteinases (TIMP)-1, and TIMP-2 were measured. MMP-3 genotypes were analyzed using a polymerase chain reaction (PCR) primer. The carotid intima media thickness (IMT), diameter, and brachial ankle pulse wave velocity (baPWV) were evaluated using ultrasound.
Results
In hypertensive adolescents, blood pressure, anthropometric data, carotid IMT, baPWV, serum pro-MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 were no different between the 6A/6A group and the 5A/6A group. Serum MMP-9 was higher in the 5A/6A group than in the control group. Aldosterone, insulin, and homocysteine were higher in the 6A/6A group than in the control group, and vitamin B12 and folate were lower in the 6A/6A group than in the control group.
Conclusion
In conclusion, serum MMP-3 levels were not significantly different in different MMP-3 genotypes in hypertensive adolescents. However, few patients were included in this study. Further investigation is necessary to clarify the relationship between MMP-3 genotype and cardiovascular risk.
Keywords: Matrix metalloproteinases, Hypertension, Genes, Adolescent
Introduction
Hypertension is influenced by the interaction of various environmental and genetic factors. The genetic influence is speculated to be responsible for 30-40% of the blood pressure variation.1) Although much progress has been made during the past 20 years with regard to our knowledge of the genetic background of essential hypertension, studies concerning the candidate genes in various populations have produced inconsistent and often unclear results.2),3)
Hypertension is associated with cardiovascular remodeling, including myocardial hypertrophy and fibrosis, which have been shown to be characterized by an increase in extracellular collagen matrix.4-6) Hypertension increases stiffness in the large arteries by inducing hypertrophy and causing changes in the extracellular matrix (ECM).7),8) The structural and functional manifestations of hypertensive left ventricle (LV) remodeling are associated with significant changes in the ECM composition.9) Matrix metalloproteinases (MMPs) are proteases that play an important role in the protein synthetic-lytic equilibrium of connective tissue.10-12) Most MMPs are secreted into the extracellular milieu as inactive proteins. Their activity is modulated by tissue inhibitors of metalloproteinases (TIMPs).12)
There have been several studies exploring the relationship among MMPs, their inhibitors, and LV structure and function in patients with hypertension.13-18) MMPs are associated with cardiovascular disease and atherosclerosis; in particular, MMP-2 and MMP-9 play important roles in the progression of atherosclerosis.18) In a community-based study, atherosclerosis was found to be related to plasma MMP-9 levels not only in patients with systolic hypertension, but also in younger, apparently healthy individuals.19)
MMP-3 may be particularly significant in arterial wall remodeling. This is due to its broad substrate spectrum, which includes most major constituents of the arterial wall: fibronectin, collagen types IV, V, IX, and X, gelastin, laminin, elastin, and proteoglycan proteins. In addition, MMP-3 activates other MMPs, such as MMP-1 and MMP-9.20)
However, the specific molecular and biochemical determinants that contribute to this ECM remodeling process in patients with hypertensive heart disease have not been fully elucidated. Most previous genetic studies have noted an association between MMPs and cardiovascular disease, though this has not been totally consistent. However, there has been no study addressing MMP-3 genotype in hypertensive adolescents.
The purposes of this study were to compare the incidence of the MMP-3 genotype in hypertensive and normotensive adolescents and to assess the effect of MMP-3 gene polymorphisms on carotid artery IMT and pulse wave velocity (PWV), which are markers of early atherosclerosis and vessel remodeling.
Subjects and Methods
Patients
Forty-four hypertensive patients and 59 healthy adolescents of similar age participated in this study (all patients greater than 16 but less than 17 years of age). Systolic blood pressures were greater than 140 mmHg or diastolic blood pressures were greater than 90 mmHg in all patients. All participants provided written informed consent.
Anthropometric measurements
All hypertensive students were referred to our pediatric clinic. Their elevated blood pressures were ascertained by averaging three blood pressure measurements made after 10 minutes of rest with an oscillometric monitor. No patients had ever been diagnosed or treated for hypertension before. Height and body weight were measured, and body mass index (BMI) and obesity index (OI) were calculated from these figures. BMI was defined as weight (kg) divided by height squared (m2). OI was calculated by the following equation using the standard weight as the value corresponding to the 50th percentile weight of Korean adolescents.
Obesity index (%)=(weight measured-standard weight)/standard weight×100
Obesity was defined as OI above 120 percent. Fat mass and fat distribution were measured by bioelectrical impedance analysis (Inbody 3.0, Biospace, Seoul, Korea).
Intima-media thickness of the common carotid artery
Carotid artery measurements were made using a real-time B-mode ultrasound imager (iU22, intelligent Ultrasound System; Philips, Amsterdam, Netherlands) and a 12.5 MHz probe. For all subjects, the IMT and lumen diameter were measured in the same carotid arterial segment by the same radiologist. Patients were directed to lie down in a supine position for 30 minutes before measurements were made. The following equations were used to calculate carotid artery compliance and elasticity.
Lumen cross-sectional area=πdD2/4
Wall cross-sectional area=π (dD/2+IMT)2-π (dD/2)2
Cross-sectional compliance={π (sD2-dD2)}/4ΔP (mm2·mmHg-1)
Cross-sectional distensibility=(sD2-dD2)/(dD2ΔP) (mmHg-1·10-2)
IMT (mm)
Systolic diameter (sD, mm)
Diastolic diameter (dD, mm)
ΔP: pulse pressure
Pulse wave velocity and ankle brachial index
Brachial-ankle PWV (baPWV) and ankle brachial index (ABI) were measured by VP-1000 (Colin Co., Komaki, Japan). PWV, ABI (the ratio of systolic blood pressure in the ankle to that in the brachial artery), blood pressures in both extremities, electrocardiography, and heart sounds were obtained simultaneously using volume plethysmographic technique. Cuffs were wrapped on both the arms and ankles, and electrocardiogram electrodes were placed on the left sternal border. As the pulse wave contours in the four extremities were recorded, the cuffs inflated and deflated automatically. Cuffs were attached to the plethysmographic sensor, which determined the volume pulse form. Blood pressures were measured from the oscillometric pressure sensor. BaPWV was determined based on the pulse transit time and the distance between these two segments. The distance of each segment was calculated automatically, based on the height of each subject. All measurements were made during regular sinus rhythm.
Serum aldosterone, renin, insulin, angiotensin converting enzyme, homocysteine, vitamin B12, and folate levels
Venous blood was drawn from all patients after overnight fasting. Samples were kept at -70℃ for subsequent assay. Serum concentrations of aldosterone, renin, insulin, vitamin B12, and folate were evaluated by radioimmunoassay (RIA) with a COBRA-II Gamma Counter (Packard, Meriden Coonecticut, CA, USA). Angiotensin converting enzyme (ACE) was measured with enzyme linked immuno sorbent assay (ELISA) using COBAS MIRA (Roche, Switzerland). Homocysteine was measured by chemiluminoimmunoassay (CLIA) with an ADVIA Centaur HCY (Bayer, Tarrytown, New York, NY, USA).
Matrix metalloproteinase-1, matrix metalloproteinase-2, matrix metalloproteinase-3, matrix metalloproteinase-9, tissue inhibitors of matrix metalloproteinases-1, and tissue inhibitors of matrix metalloproteinases-2
Commercially available sandwich ELISA assays (Molecular Devices, Palmcity, FL, USA) were used to determine MMP-1, MMP-2, MMP-3, MMP-9, TIMP-1, and TIMP-2 levels. We placed samples in a plate coated with specific total MMP-1 polyclonal antibodies and made specific antibodies. We then washed off the detached materials and added pro-MMP-1-specific enzymelinked polyclonal antibodies to each well. After substrate solution was added, total MMP-1 conjugates radiated. The absorbance was read spectrophotometrically with a microtiter plate reader. MMP-2, MMP-3, MMP-9, TIMP-1, and TIMP-2 levels were measured using the same method.
Determination of the matrix metalloproteinase-3 genotype
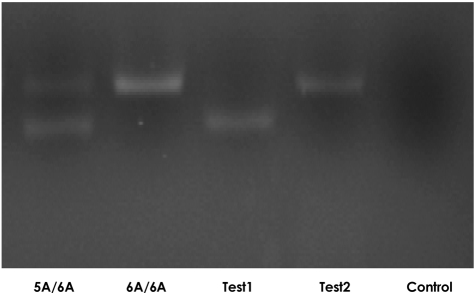
The sequences of polymerase chain reaction (PCR) primers were 5'-GATTACAGACATGGGTCACA-3' (forward primer) and 5'-TTTCAATCAGGACAAGACGAAGTTT-3' (reverse primer). PCR was carried out in a total volume of 25 mL: 15 ng of genomic DNA, 5 pmol of each primer, 200 mM each of dATP, dCTP, dGTP, and dTTP, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 5 mM MgCl2, and Taq polymerase (TaKAaRa). The solution was overlaid with 25 mL of liquid paraffin and incubated for 2 minutes at 95℃, 30 seconds at 53℃, and 30 seconds at 72℃, with an additional 2 minutes extension at 70℃ at the end of 35 cycles. The 120 bp PCR product was cleaved with 10 U of Xnm I (New England Biolab, Bererly, MA, USA) and electrophoresed on 4% agarose; the allele produced one band of 120 bp (Fig. 1).
Fig. 1.
MMP-3 gene expression in hypertensive adolescents and controls. The allele produced one band of 120 bp size. Test1: 5A/5A mutant, Test2: 6A/6A normal, Control: saline.
Statistical analysis
We performed all statistical analyses using the SPSS/PC software package program (SPSS version 11.0). Descriptive statistics were presented as means and standard deviations.
The correlations among continuous variables were determined using the Student t-test and one-way analysis of variance (ANOVA). P less than 0.05 were considered statistically significant.
Results
Matrix metalloproteinase-3 genotype frequencies in hypertensive and normotensive adolescents
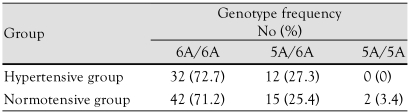
MMP-3 genotype frequencies were 6A/6A 72.7% (n=32) and 5A/6A 27.3% (n=12) in hypertensive patients and 6A/6A 71.2% (n=42), 5A/6A 24.4% (n=15), and 5A/5A 3.4% (n=2) in control patients (Table 1). The genotype frequencies did not significantly differ between hypertensive and normotensive adolescents.
Table 1.
MMP-3 genotype frequencies in the hypertensive and normotensive groups
p>0.05 vs. control. MMP: matrix metalloproteinase
Blood pressure
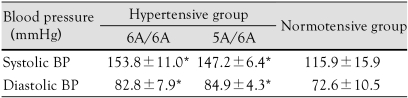
Blood pressure was significantly higher in the hypertensive group compared to the control group, but neither systolic nor diastolic blood pressure showed a statistically significant difference according to genotype (Table 2).
Table 2.
Comparison of BPs according to MMP-3 genotype in the hypertensive and normotensive groups
*p<0.05 vs. control. BP: blood pressure, MMP: matrix metalloproteinase
Anthropometric data
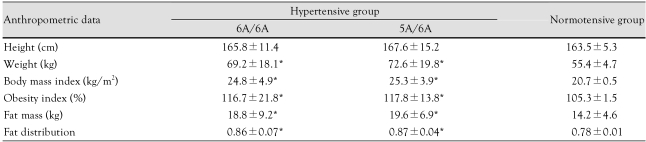
There were no significant differences in anthropometric data such as height, weight, BMI, OI, fat mass, or fat distribution between patients with the 6A/6A genotype and those with the 5A/6A genotype (Table 3).
Table 3.
Anthropometric data according to MMP-3 genotype in the hypertensive and normotensive groups
*p<0.05 vs. control. MMP: matrix metalloproteinase
Carotid intima media thickness and pulse wave velocity
Carotid IMT and PWV were significantly higher in the hypertensive group than in the control group, but there was no significant difference between genotypes (Table 4).
Table 4.
Comparison of carotid IMT PWV and ABI according to MMP-3 genotype in the hypertensive and normotensive groups
*p<0.05 vs. control. IMT: intima media thickness, MMP: matrix metalloproteinase, PWV: pulse wave velocity, ABI: ankle brachial index, RbaPWV: right brachial-ankle pulse wave velocity, LbaPWV: left brachial-ankle pulse wave velocity, R-ABI: right ankle-brachial index, L-ABI: left ankle-brachial index
Serum aldosterone, renin, insulin, angiotensin converting enzyme, vitamin B12, and folate levels
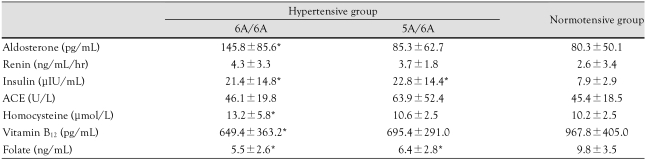
Serum aldosterone (145.8±85.6 pg/mL vs. 80.3±50.1 pg/mL) and homocysteine (13.2±5.8 µIU/mL vs. 10.2±2.5 µIU/mL) levels were significantly higher in the 6A/6A genotype group than in the control group.
Insulin levels were significantly higher in the hypertensive group than in the control group. Vitamin B12 levels were lower in the 6A/6A genotype group than they were in the control group (649.4±363.2 pg/mL vs. 967.8±405.0 pg/mL). Folate levels were significantly lower in the hypertensive group than in the control group (Table 5).
Table 5.
Comparison of aldosterone, renin, insulin, angiotensin converting enzyme (ACE), homocysteine, vitamin B12, and folate levels according to MMP-3 genotype in the hypertensive and normotensive groups
*p<0.05 vs. control. MMP: matrix metalloproteinase, ACE: angiotensin converting enzyme
Serum matrix metalloproteinase and tissue inhibitors of matrix metalloproteinases levels according to matrix metalloproteinase-3 genotype
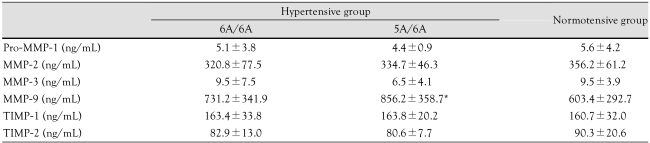
Serum MMP-3 levels were higher in the 6A/6A genotype group than in the 5A/6A genotype group, but not to a statistically significant degree. Serum MMP-9 levels were higher in the 5A/6A genotype group than in the control group (856.2±358.7 ng/mL vs. 603.4±292.7 ng/mL). There were no significant differences in pro-MMP-1, MMP-2, MMP-9, TIMP-1, or TIMP-2 levels according to MMP-3 genotype (Table 6).
Table 6.
Comparison of serum MMP and TIMP levels according to MMP-3 genotypes between hypertensive group and normotensive group
*p<0.05 vs. control. MMP: matrix metalloproteinase, TIMP: tissue inhibitor of metalloproteinase
Discussion
In our study, the MMP-3 genotype frequencies were 6A/6A 72.7% and 5A/6A 27.3% in hypertensive adolescents. The incidence was not significantly different between hypertensive and normotensive adolescents.
Blood pressure, anthropometric data, carotid IMT, and baPWV were no different between the MMP-3 6A/6A and 5A/6A groups in hypertensive adolescents. Serum aldosterone, insulin, and homocysteine levels were higher in the 6A/6A group than in the control group, while vitamin B12 and folate levels were lower in the 6A/6A group. Serum MMP-3 levels were slightly higher in the 6A/6A group than in the 5A/6A group, but not to a statistically significant degree. Serum MMP-9 levels were higher in the hypertensive group with the 5A/6A genotype than in the control group. However, there was no statistically significant difference between serum pro-MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 levels.
Most previous genetic studies have noted an association between MMPs and cardiovascular disease, though this finding has not been completely consistent. Genetic variation in a number of MMP and TIMP genes has been implicated as a risk factor for atherosclerosis. However, the studies indicating these relationships have been generally small and have produced conflicting results.21)
Medley et al.22) reported that the MMP-9 genotype influences large artery stiffness through effects on aortic gene and protein expression. MMP-9 genotype (C-1562T promoter polymorphism) T-allele carriers (C/T and T/T) had stiffer large arteries and higher carotid pulse and systolic blood pressure than C/C homozygotes did. The higher expression of MMP-9 associated with the T allele may influence large arteries to become stiffer through vascular remodeling, which disrupts their mechanical integrity. This relationship may be due to increased degradation of important elastic components of the ECM secondary to elevated MMP-9 gene expression and protein levels in the aorta.22)
An association was found between MMP-9 R279Q and internal carotid artery bulb IMT, but there was no linear trend between allele number and IMT and no association with common carotid artery or bulb IMT. Associations were noted between three polymorphisms (MMP-1 A-519G, MMP-3 5A/6A, and TIMP-3 T-1296C) and hypertension.21) These findings provide little support for genetic variants of MMP as direct risk factors for IMT. However, the interaction between MMP variants and hypertension suggest that hypertensive carriers of these alleles may be at greater risk for increased IMT and future cardiovascular disease.21)
Altered MMP activity has been noted to be involved as a mechanism in early vascular remodeling secondary to hypertension. ECM is hypothesized to impede left ventricular diastolic filling, thus contributing to symptomatic diastolic dysfunction in diabetic and hypertensive patients.13) Several studies have been dedicated to determining the relationship among MMPs, their inhibitors, and LV structure and function.14-17)
Plasma MMPs play a role in the development of diastolic dysfunction in hypertensive patients. Saglam et al.14) found that plasma MMP-3 and MMP-9 levels were significantly higher in patients with left ventricular hypertrophy (LVH).
In an adult study, aortic and brachial PWV, as well as serum MMP-9, MMP-2, and elastase levels, were increased in older subjects with isolated systolic hypertension (ISH), compared with older controls.19) In our study, MMP-9 levels were significantly higher in 5A/6A genotype patients compared with normal adolescents. Carotid IMT and PWV were significantly higher in hypertensive adolescents compared with normal controls. However, IMT and PWV were no different according to MMP-3 genotype. Although the exact duration of hypertension was unknown in the patients in our study, it is probably too short to cause a significant difference in MMP levels according to genotype. IMT thickness appears to represent not only early atherosclerosis, but also adaptive remodeling, particularly in hypertension.23) The effects of MMP genetic variants may not be pronounced enough to be detected by increases in IMT in normotensive subjects at this early stage. However, as hypertension and the degree of vascular remodeling increase, the effect of these genetic variants will be manifested.
In adult studies, MMP-9 levels have been found to correlate significantly with aortic and brachial PWV at an early time point.19),22) Aortic stiffness is related to MMP-9 and elastase levels, not only in ISH, but also in younger, apparently healthy individuals. This suggests that elastases including MMP-9 may be involved in arterial stiffening and the development of ISH.19) Hypertensive patients showed higher levels of MMP-9 and TIMP-1. Spearman's correlation analysis showed that serum levels of MMP-9 and TIMP-1 were significantly and positively correlated with PWV in hypertensive patients.
The MMP-9/TIMP-1 system may play an important role in the determination of arterial function, and these findings may have implications for the involvement of the MMP-9/TIMP-1 system in the pathophysiology of cardiovascular disease.24)
The concomitant increases in both MMPs and TIMPs may indicate abnormal ECM turnover at the post-translational level.25) MMP-1 is likely to be relevant in the clinical setting. Ishikawa et al.26) demonstrated that plasma MMP-1 levels significantly correlate with both the pulse pressure and the mean blood pressure in hypertensive human patients with LVH. However, in our study, plasma pro-MMP-1 levels were not significantly different between hypertensive and nomotensive adolescents.
Early and late chronic pressure overload induces distinct changes in ECM phenotypes in particular MMPs and TIMPs. MMPs are predominantly attenuated during the initial pressure overload, with increases in MMPs noted during the late phase of chronic pressure overload.25)
MMPs and TIMPs play an important role in collagen degradation.25) However, whether the determinants of ECM composition, such as the balance between MMPs and TIMPs, are altered in hypertensive heart disease is unknown.9) With respect to hypertensive heart disease, increased TIMPs and decreased MMPs favor decreased collagen degradation and increased collagen accumulation. TIMPs tightly regulate the activities of MMPs.18) Patients with hypertension, but normal LV structure and function, have been shown to have normal MMP/TIMP profiles.9) Changes in MMP profiles that favor decreased ECM degradation have been associated with LVH and diastolic dysfunction, and increased TIMP-1 levels have been shown to predict the presence of congestive heart failure.9) Although these findings should be confirmed in a larger prospective study, these data do suggest that changes in the MMP/TIMP balance may play an important role in the structural, functional, and clinical manifestations of hypertensive heart disease.9) It seems likely, therefore, that ongoing changes in MMPs and TIMPs will contribute to the phenotypic and structural changes present in hypertensive heart disease.9)
We noted no significant difference in serum MMP-3 levels according to MMP-3 genotype in hypertensive adolescents. Plasma levels do not necessarily reflect the net ECM proteolytic activity that occurs within the myocardium. The myocardium is not the only source of MMPs and TIMPs in LVH patients. Therefore, plasma MMP and TIMP levels represent the sum of MMPs and TIMPs released from both cardiac and non-cardiac sources.
Our study is limited in that it was small; we studied only 44 adolescents. It is also possible we failed to show the relationship between MMP-3 genotype and cardiovascular risk because our subjects were adolescents who had high blood pressure of short duration. Large sample size increases statistical power and reduces the problem of incomplete penetrance in individuals that may have subclinical hypertension, which will lead to clinical events in subsequent years. Carotid IMT has been successfully used to demonstrate robust, replicable associations with other cardiovascular candidate genes.27)
Our findings provide some support for genetic variants of MMP as direct risk factors for IMT, at least in the early stages of hypertension, through vessel remodeling and thickening.
Further studies investigating the associations between MMP genotypes and symptomatic disease will be required to determine if these variants play a role in hypertension. IMT thickness appears to represent not only early atherosclerosis, but also adaptive remodeling, particularly to hypertension. Vascular remodeling is limited during early atherosclerotic changes, which IMT changes represent. As such, the effects of MMP genetic variants may not be pronounced enough to be detected by increases in IMT in normotensive subjects at this early stage. However, as hypertension increases the degree of vascular remodeling, the effect of these genetic variants becomes manifest.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Shin DJ, Bae YJ, et al. Association between I/D, G14480C, A22982G polymorphisms of angiotension I-converting enzyme gene and essential hypertension in the Korean population. Korean Circ J. 2004;34:1137–1147. [Google Scholar]
- 3.Lee JA, Sohn JA, Hong YM. Polymorphism of angiotensin II type 1 receptor A1166C in Korean hypertensive adolescents. Korean Circ J. 2008;38:405–410. [Google Scholar]
- 4.Jalil JE, Doering CW, Janicki JS, Pick RZ, Shroff SG, Weber KT. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res. 1989;64:1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- 5.Bashey RI, Cox R, McCann J, Jimenez SA. Changes in collagen biosynthesis, types, and mechanics of aorta in hypertensive rats. J Lab Clin Med. 1989;113:604–611. [PubMed] [Google Scholar]
- 6.Laviades C, Vain N, Fernandez J, et al. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98:535–540. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 7.Safar ME. Therapeutic trials and large arteries in hypertension. Am Heart J. 1988;115:702–710. doi: 10.1016/0002-8703(88)90830-7. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Safar ME. Large conduit arteries in hypertension: role of the renin-angiotensin system. Circulation. 1988;77:947–954. doi: 10.1161/01.cir.77.5.947. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SH, Clark LL, Pennington WR, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 10.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 11.Parks WE. A confederancy of proteinases. J Clin Invest. 2002;110:613–614. doi: 10.1172/JCI16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu TH, Werbz Z. Matrix metalloprotienases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 13.Karthikeyan VJ, Lip GY. Matrix metalloproteinases and hypertension: a link between left ventricular hypertrophy and diastolic function? Tohoku J Exp Med. 2006;208:93–97. doi: 10.1620/tjem.208.93. [DOI] [PubMed] [Google Scholar]
- 14.Saglam M, Karakaya O, Esen AM, et al. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med. 2006;208:117–122. doi: 10.1620/tjem.208.117. [DOI] [PubMed] [Google Scholar]
- 15.Tayebjee MH, Nedar SK, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9 levels in patients with hypertension: relationship to tissue Doppler indicies of diastolic relaxation. Am J Hypertens. 2004;17:770–774. doi: 10.1016/j.amjhyper.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Tayebjee MH, Lim HS, Nadar S, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinases-I is a marker of diastolic dysfunction using tissue Doppler in patients with type 2 diabetes and hypertension. Eur J Clin Invest. 2005;35:8–12. doi: 10.1111/j.1365-2362.2005.01438.x. [DOI] [PubMed] [Google Scholar]
- 17.Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17:119–124. doi: 10.1038/sj.jhh.1001518. [DOI] [PubMed] [Google Scholar]
- 18.Asano Y, Iwai S, Okazaki M, et al. Matrix metalloproteinase-9 in spontaneously hypertensive hyperlipidemic rats. Pathophysiology. 2008;15:157–166. doi: 10.1016/j.pathophys.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Yasmin, McEniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 20.Murphy G, Ward R, Gavrilovic J, Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224–230. [PubMed] [Google Scholar]
- 21.Seccia TM, Bettini E, Vukous V, et al. Extracellular matrix gene expression in the left ventricular tissue of spontaneously hypertensive rats. Blood Press. 1999;8:57–64. doi: 10.1080/080370599438400. [DOI] [PubMed] [Google Scholar]
- 22.Medley TL, Cole TJ, Dart AM, Gatzka CD, Kingwell BA. Matrix metalloproteinase-9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vasc Biol. 2004;24:1479–1484. doi: 10.1161/01.ATV.0000135656.49158.95. [DOI] [PubMed] [Google Scholar]
- 23.Rhee MY, Lee HY, Park JB. Measurements of arterial stiffness: methological aspects. Korean Circ J. 2008;38:343–350. [Google Scholar]
- 24.Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30:959–963. doi: 10.1291/hypres.30.959. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Davis HB, Dai Q, et al. Effects of early and late chronic pressure overload on extracellular matrix remodeling. Hypertens Res. 2008;31:1225–1231. doi: 10.1291/hypres.31.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa J, Kario K, Matsui Y, et al. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertens Res. 2005;28:995–1001. doi: 10.1291/hypres.28.995. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong C, Abilleira S, Sitzer M, Markus H, Bevan S. Polymorphsims in MMP family and TIMP genes and carotid artery intima-media thickness. Stroke. 2007;38:2895–2899. doi: 10.1161/STROKEAHA.107.491696. [DOI] [PubMed] [Google Scholar]