Abstract
Objectives
This study sought to evaluate whether pulse wave velocity (PWV), a noninvasive index of arterial stiffness, is a predictor of the longitudinal changes in systolic blood pressure (SBP) and of incident hypertension.
Background
Although arterial stiffness is believed to underlie, in part, the age-associated changes in SBP, particularly at older ages, few longitudinal studies in humans have examined the relationship between arterial stiffness and blood pressure.
Methods
Pulse wave velocity was measured at baseline in 449 normotensive or untreated hypertensive volunteers (age 53 ± 17 years). Repeated measurements of blood pressure were performed during an average follow-up of 4.9 ± 2.5 years.
Results
After adjusting for covariates including age, body mass index, and mean arterial pressure, linear mixed effects regression models showed that PWV was an independent determinant of the longitudinal increase in SBP (p = 0.003 for the interaction term with time). In a subset of 306 subjects who were normotensive at baseline, hypertension developed in 105 (34%) during a median follow-up of 4.3 years (range 2 to 12 years). By stepwise Cox proportional hazards models, PWV was an independent predictor of incident hypertension (hazard ratio 1.10 per 1 m/s increase in PWV, 95% confidence interval 1.00 to 1.30, p = 0.03) in individuals with a follow-up duration greater than the median.
Conclusions
Pulse wave velocity is an independent predictor of the longitudinal increase in SBP and of incident hypertension. This suggests that PWV could help identify normotensive individuals who should be targeted for the implementation of interventions aimed at preventing or delaying the progression of subclinical arterial stiffening and the onset of hypertension.
The incidence and prevalence of hypertension increase with advancing age. Hypertension afflicts approximately 50 million individuals in the U.S. and over 1 billion individuals worldwide (1). Furthermore, hypertension is associated with increased morbidity and mortality (2). Among hypertensive subjects with at least 1 other cardiovascular risk factor, the 6-year incidence of fatal and nonfatal myocardial infarction is 11%, and the 6-year incidence of total cardiovascular events is 33% (3). These rates are likely underestimates because hypertension is under-recognized and inadequately treated in the community (4). Thus, strategies to identify normotensive subjects who are at increased risk for developing hypertension as they age and efforts to elucidate the mechanisms that underlie this increased risk are critical to the development and implementation of measures that may effectively delay or prevent hypertension.
Carotid-femoral pulse wave velocity (PWV) is a marker of central arterial stiffness that can be measured noninvasively. Pulse wave velocity is an independent predictor of coronary heart disease and stroke in healthy subjects (5), and an independent predictor of mortality in the general population (6), in hypertensive subjects (7), in older community-dwelling (8) and hospitalized (9) individuals, and in patients with end-stage renal disease (10). Both age (11) and distending blood pressure (BP) (12) are major determinants of PWV. It is believed that age-associated arterial stiffening is accelerated by chronic elevations in BP caused by structural and functional alterations in the walls of the central elastic arteries (13). In turn, arterial stiffness is believed to underlie, in part, the age-associated changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP), particularly at older ages (14). To the best of our knowledge, the relationship between PWV and the longitudinal changes in BP has not been reported. Thus, the aims of this study were to evaluate whether, in healthy volunteers, PWV is an independent predictor of the longitudinal changes in SBP and of incident hypertension.
Methods
Study population
The BLSA (Baltimore Longitudinal Study of Aging) is a prospective study of community-dwelling volunteers who undergo approximately 2½ days of medical, physiological, and psychological examinations at regular intervals (15). Pulse wave velocity was performed on a subset of 714 BLSA participants, chosen in a random fashion on days when testers were available, and who were free of overt coronary artery disease as defined by history of angina pectoris, documented myocardial infarction, or major Q waves on resting electrocardiograms (Minnesota Code 1:1 or 1:2). Of those, 92 were excluded from this analysis because they were on concurrent BP-lowering medications at the time of the PWV measurement, 17 were excluded because of missing covariates, and 156 were excluded because they did not have any follow-up visit. All subjects signed an informed consent at each study visit.
Variables measured
Height and weight were determined for all participants. Body mass index (BMI) was calculated as body weight (kg)/height2 (m2). Smoking status was ascertained by a questionnaire. Total and fractionated cholesterol, triglycerides, and plasma glucose were determined in the fasting state as previously described (16).
Blood pressure determinations were performed in the morning, after a light breakfast, with subjects in the seated position, after a 5-min quiet resting period. Brachial BP was measured in triplicate by auscultation in both arms with a mercury sphygmomanometer using an appropriately sized cuff. The BP values used in this study are the average of the second and third measurements on both the right and left arms. Values for SBP and DBP were defined by Korotkoff phases I and V, respectively. Pulse pressure (PP) was computed as PP = (SBP − DBP); mean arterial pressure (MAP) was computed as MAP = DBP + (1/3 × PP).
Pulse wave velocity was measured using nondirectional transcutaneous Doppler probes (model 810A, 9 to 10-Mhz probes, Parks Medical Electronics, Inc., Aloha, Oregon) as previously described (11): A minimum of 10 arterial flow waves from the right common carotid artery and the right femoral artery were simultaneously recorded, and were averaged using the QRS for synchronization. Pulse wave velocity was calculated as the distance traveled by the flow wave (measured with an external tape measure over the body surface) divided by the time differential between the feet of simultaneously recorded carotid and femoral arterial flow waves.
Statistical analyses
All analyses were performed using the SAS package (version 8.1, SAS Institute Inc., Cary, North Carolina). Data are presented as mean ± SD or proportions. Comparisons between groups were performed using chi-square tests for categorical variables and two-sample t tests for continuous variables.
The predictors of SBP at the last visit were evaluated with multiple regression analyses. The longitudinal changes in SBP (and PP) were analyzed with linear mixed effects regression models (17), which easily accommodate unbalanced, unequally spaced observations (18), and consequently are ideal tools for analyzing longitudinal BLSA data (16,19). In these models, the dependent variable (i.e., SBP or PP) was assessed on the baseline visit and during all follow-up visits, whereas the independent variables were only measured on the baseline visit. The following variables, measured or calculated on the baseline visit, were entered into all of the models as fixed effects: age, age2, gender, race, smoking, BMI, heart rate, MAP, total and high-density lipoprotein (HDL) cholesterol, triglycerides, fasting plasma glucose, and PWV.
In mixed-effects regression models, the interaction term between a fixed effect variable and time evaluates whether this variable is a predictor of the longitudinal changes in the dependent BP variable. Thus, the interaction terms between time and all of the aforementioned fixed effect variables were evaluated, but only the statistically relevant (p < 0.1) ones were included in the models.
All models also included intercept and time as random terms. Random effects allow each participant’s beginning value to vary from the population average (intercept) and the longitudinal trajectory to vary from the population average longitudinal trajectory (slope). No structure was imposed on the covariance matrix of these random effects, and the errors were assumed to be independent with constant variance.
Among the subgroup of individuals in this cohort who were normotensive (SBP <140 mm Hg and DBP <90 mm Hg) at baseline, incident hypertension (HTN) was defined as SBP ⩾140 mm Hg, or DBP ⩾90 mm Hg, or the use of antihypertensive medications on a follow-up visit. The variables that predicted the future development of HTN were evaluated with Cox proportional hazards models. In a first set of models, it was noted that the interaction term between duration of follow-up and PWV was significant (p < 0.0001), indicating that PWV’s ability to predict incident HTN differed according to the duration of follow-up. The cohort was therefore stratified according to median follow-up duration. Within each stratum, stepwise Cox models were constructed in which age and baseline SBP were forced into the models because they are well established predictors of HTN, and the following variables were added in a stepwise forward manner, whereby only the statistically significant ones were retained in the models: gender, race, smoking, BMI, DBP, PWV, heart rate, total cholesterol, HDL cholesterol, triglycerides, and glucose. The proportionality assumption was confirmed for these models.
Results
The baseline characteristics of the study cohort stratified by gender are shown in Table 1. These normotensive and untreated hypertensive subjects were followed up for an average of 4.9 ± 2.5 years (range 1 to 12 years), representing an average of 3.1 visits per person. Specifically, 171 participants had 2 visits, 150 had 3 visits, 71 had 4 visits, 41 had 5 visits, and 16 had 6 or more visits. For those who were started on antihypertensive medications during the follow-up period, their BP data were censored at the last visit on which they were not receiving antihypertensive medications (i.e., the visit immediately preceding the one with medications).
Table 1.
Baseline Characteristics of the Study Cohort
| Variable | Overall | Men | Women |
|---|---|---|---|
| n | 449 | 201 | 248 |
| Age (yrs) | 53 ± 17 | 54 ± 17 | 51 ± 16 |
| Race (white) | 348 (77%) | 157 (78%) | 191 (77%) |
| Height (cm) | 170 ± 10 | 178 ± 7 | 163 ± 7* |
| Weight (kg) | 74 ± 16 | 83 ± 14 | 67 ± 13* |
| BMI (kg/m2) | 25 ± 4 | 26 ± 4 | 25 ± 4† |
| Smoking (ever) | 232 (52%) | 115 (57%) | 117 (47%)‡ |
| Heart rate (beats/min) | 69 ± 12 | 68 ± 13 | 69 ± 11 |
| SBP (mm Hg) | 125 ± 17 | 128 ± 17 | 123 ± 18‡ |
| DBP (mm Hg) | 80 ± 10 | 82 ± 9 | 78 ± 10* |
| PP (mm Hg) | 46 ± 13 | 46 ± 13 | 46 ± 14 |
| MAP (mm Hg) | 95 ± 11 | 97 ± 11 | 92 ± 11* |
| PWV (m/s) | 6.9 ± 2.5 | 7.2 ± 2.7 | 6.7 ± 2.3‡ |
| Cholesterol (mg/dl) | 183 ± 38 | 181 ± 33 | 184 ± 42 |
| LDL (mg/dl) | 112 ± 33 | 113 ± 30 | 111 ± 36 |
| HDL (mg/dl) | 49 ± 13 | 43 ± 11 | 54 ± 13* |
| Triglycerides (mg/dl) | 101 ± 72 | 117 ± 87 | 87 ± 52* |
| Glucose (mg/dl) | 97 ± 14 | 100 ± 16 | 95 ± 12† |
p < 0.0001.
p < 0.001.
p < 0.01 compared with men.
BMI = body mass index; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MAP = mean arterial pressure; PP = pulse pressure; PWV = pulse wave velocity; SBP = systolic blood pressure.
PWV and longitudinal SBP
We first evaluated the association between variables measured at baseline and SBP measured on the last visit, with multiple regression analyses. As expected, age, BMI, and MAP were independently associated with higher SBP on the last visit (Table 2); in addition, PWV was also independently associated with higher SBP on the last visit, and explained 4% of its variance. Next, we took advantage of the repeated measurements of BP at each follow-up visit to characterize the longitudinal changes in SBP over time in our cohort. Using linear mixed-effects regression models, we evaluated the predictors of the longitudinal changes in SBP. In these models, a statistically significant interaction term between time and a predictor variable indicates that the longitudinal changes in SBP are influenced by this variable. As shown in Table 3, age, BMI, and MAP (p = 0.09, p = 0.009, p < 0.0001 respectively for the interaction terms with time) were predictors of the longitudinal changes in SBP. In addition, PWV was also an independent predictor of the longitudinal increase in SBP (p = 0.003 for the interaction term with time). The contribution of PWV to the mixed effects models was evaluated by comparing models that included and excluded PWV: after adjusting for the other covariates in the model, PWV accounted for 16% of the variance in the longitudinal changes in SBP (20). Of note, the main effect of PWV was statistically significant (p = 0.006) when the model was run without the interaction term between PWV and time.
Table 2.
Multiple Regression Analysis Evaluating the Predictors of Last Visit SBP
| Variable | Parameter Estimate | Standard Error | p Value |
|---|---|---|---|
| Age (yrs) | 0.32 | 0.06 | <0.0001 |
| Gender (men) | 0.65 | 1.78 | 0.71 |
| Race (white) | −1.22 | 2.00 | 0.54 |
| Smoking (ever) | 2.48 | 1.61 | 0.12 |
| BMI (kg/m2) | 0.61 | 0.22 | 0.006 |
| MAP (mm Hg) | 0.60 | 0.08 | <0.0001 |
| PWV (m/s) | 1.56 | 0.38 | <0.0001 |
| Heart rate (beats/min) | 0.08 | 0.06 | 0.20 |
| Total cholesterol (mg/dl) | −0.005 | 0.02 | 0.83 |
| Triglycerides (mg/dl) | −0.009 | 0.01 | 0.50 |
| HDL cholesterol (mg/dl) | −0.001 | 0.07 | 0.98 |
| Glucose (mg/dl) | −0.02 | 0.06 | 0.75 |
The predictor variables were all measured at the baseline visit. Model R2 = 0.42.
Abbreviations as in Table 1.
Table 3.
Predictors of Longitudinal SBP Derived From a Linear Mixed-Effects Regression Model
| Variable | Coefficient | Standardized Coefficient | 95% Confidence Interval | p Value |
|---|---|---|---|---|
| Time (yrs) | 3.14 | 0.14 | 0.61 to 5.66 | 0.02 |
| Age (yrs) | −0.37 | 0.25 | −0.68 to −0.06 | 0.02 |
| Age2 (yrs2) | 0.006 | 0.08 | 0.002 to 0.008 | <0.0001 |
| Gender (men) | 0.61 | 0.03 | −1.26 to 2.47 | 0.52 |
| BMI (kg/m2) | 0.25 | 0.11 | −0.01 to 0.50 | 0.06 |
| MAP (mm Hg) | 1.03 | 0.47 | 0.93 to 1.12 | <0.0001 |
| PWV (m/s) | 0.29 | 0.12 | −0.16 to 0.74 | 0.21 |
| Time × age | 0.02 | 0.04 | −0.002 to 0.038 | 0.09 |
| Time × BMI | 0.10 | 0.06 | 0.02 to 0.183 | 0.009 |
| Time × MAP | −0.08 | −0.12 | −0.11 to −0.05 | <0.0001 |
| Time × PWV | 0.22 | 0.08 | 0.07 to 0.36 | 0.003 |
The model also included race, smoking, heart rate, cholesterol, HDL, triglycerides, and glucose, which were all nonsignificant.
Abbreviations as in Table 1.
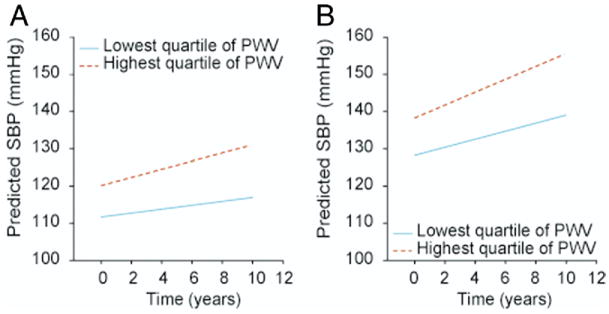
Figure 1 provides an example that illustrates the effects of PWV on the predicted longitudinal increase in SBP. When stratified according to age-decade-specific quartiles of PWV, a 40-year-old man whose PWV is in the highest quartile has, on average, a higher SBP at baseline than a 40-year-old man whose PWV is in the lowest quartile (120 mm Hg vs. 111 mm Hg), and an accelerated increase in SBP over the ensuing 10 years (11 mm Hg vs. 5 mm Hg) (Fig. 1A). Similarly, a 70-year-old man whose PWV is in the highest quartile has, on average, a higher SBP at baseline than a 70-year-old man whose PWV is in the lowest quartile (138 mm Hg vs. 128 mm Hg), and an accelerated increase in SBP over the ensuing 10 years (17 mm Hg vs. 11 mm Hg) (Fig. 1B).
Figure 1. Baseline and Predicted 10-Year Longitudinal Changes in SBP.
Baseline and predicted 10-year longitudinal changes in systolic blood pressure (SBP) for men with starting ages 40 years (A) and 70 years (B) whose pulse wave velocity (PWV) at baseline was in the highest versus lowest quartile. For both starting ages, men whose baseline PWV was in the highest quartile showed an accelerated increase in SBP compared with men whose PWV was in the lowest quartile. Quartiles of PWV were separately defined for each age-decade.
PWV and longitudinal PP
Using mixed-effects linear regression models, we also evaluated the determinants of the longitudinal changes in PP. In the overall cohort, only age was an independent predictor of the longitudinal increase in PP (p < 0.0001 for the interaction term with time), whereas PWV was only a determinant of higher PP (p = 0.03), but not of longitudinal changes in PP (Online Table 1). This is likely related to accelerated increases in DBP among younger individuals with higher PWV (data not shown), which balance the longitudinal increases in SBP. Indeed, when the analyses were repeated after stratifying the cohort into tertiles of age, PWV was an independent predictor of the longitudinal increase in PP (p = 0.04 for the interaction term with time) among individuals over the age of 60 years (highest tertile) (Online Table 2).
PWV as a predictor of incident hypertension
Because PWV was an independent determinant of the longitudinal increase in SBP, we next evaluated whether PWV could also predict the future development of HTN among the 306 individuals from our cohort who were normotensive at the baseline visit, during which PWV was assessed. Over a median follow-up of 4.3 years (mean 4.7 ± 2.5 years, range 2 to 12 years), HTN developed in 105 (34%). Of note, unlike the preceding set of analyses, censoring was not performed on the visit preceding the one with medication.
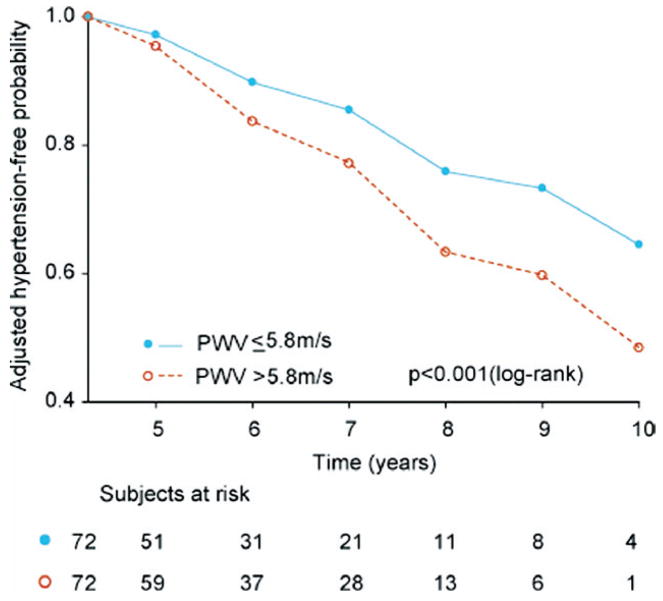
The baseline characteristics of the subjects who remained normotensive during follow-up versus those in whom hypertension developed, stratified according to median follow-up duration, are shown in Table 4. The predictors of incident HTN were evaluated with stepwise Cox models, in which age and baseline SBP were forced into the models. In the group with shorter follow-up duration, no other variable was significant when added to the model. In contrast, in the group with follow-up duration greater than the median (in which all subjects remained normotensive for the first 4.3 years), beyond age (hazard ratio [HR] 1.02 per 1 year, 95% confidence interval [CI] 0.99 to 1.04, p = 0.2) and SBP (HR 1.05 per 1 mm Hg, 95% CI 1.01 to 1.09, p = 0.006), both HDL (HR 0.96 per 1 mg/dl, 95% CI 0.93 to 0.99, p = 0.02) and PWV (HR 1.10 per 1 m/s, 95% CI 1.00 to 1.30, p = 0.03) (Fig. 2) were independent predictors of incident HTN.
Table 4.
Baseline Characteristics of the 306 Subjects Who Were Normotensive on the Baseline Visit, Stratified According to Median Follow-Up Duration (4.3 Years) and Whether They Remained Normotensive (NTf) or Developed Hypertension (HTNf) During Follow-Up
| Follow-Up Duration ≤4.3 Years (Average 2.7 ± 0.8) |
Follow-Up Duration >4.3 Years (Average 6.9 ± 1.7) |
|||||
|---|---|---|---|---|---|---|
| NTf | HTNf | p Value | NTf | HTNf* | p Value | |
| N | 93 | 69 | 108 | 36 | ||
| Age (yrs) | 44 ± 14 | 62 ± 15 | <0.0001 | 44 ± 14 | 53 ± 14 | 0.0006 |
| Gender (men) | 30 (32%) | 34 (49%) | 0.02 | 39 (36%) | 20 (55%) | 0.03 |
| Race (white) | 66 (76%) | 49 (71%) | 0.72† | 90 (83%) | 27 (77%) | 0.4† |
| Height (cm) | 170 ± 7.1 | 169 ± 8.0 | 0.73† | 170 ± 6.5 | 172 ± 6.9 | 0.15† |
| Weight (kg) | 72 ± 7.3 | 73 ± 8.1 | 0.35† | 70 ± 8.0 | 73 ± 8.4 | 0.05† |
| BMI (kg/m2) | 24 ± 0.55 | 26 ± 0.54 | <0.0001† | 24 ± 0.93 | 25 ± 0.96 | 0.006† |
| Smoking history (%) | 45 (48%) | 36 (52%) | 0.48† | 56 (52%) | 15 (43%) | 0.35† |
| Heart rate (beats/min) | 69 ± 0.52 | 69 ± 0.55 | 0.60† | 68 ± 1.3 | 67 ± 1.4 | <0.001† |
| SBP (mm Hg) | 116 ± 3.8 | 121 ± 3.9 | <0.0001† | 114 ± 10 | 116 ± 14 | <0.0001† |
| DBP (mm Hg) | 75 ± 1.4 | 76 ± 1.5 | 0.0023† | 75 ± 2.1 | 76 ± 2.2 | 0.10† |
| PP (mm Hg) | 41 ± 3.4 | 45±3.7 | <0.0001† | 39±2.1 | 40±2.3 | 0.07† |
| MAP (mm Hg) | 89 ± 1.9 | 91 ± 1.8 | <0.0001† | 88 ± 1.8 | 89 ± 1.8 | 0.006† |
| PWV (m/s) | 5.8 ± 1.1 | 7.3 ± 1.2 | <0.0001† | 6.0 ± 0.9 | 6.7 ± 1.0 | 0.0005† |
| Cholesterol (mg/dl) | 170 ± 11 | 184 ± 12 | <0.0001† | 173 ± 7.3 | 178 ± 7.3 | 0.001† |
| LDL (mg/dl) | 102 ± 8.2 | 113 ± 8.3 | <0.0001† | 104 ± 6.6 | 109 ± 6.9 | 0.13† |
| HDL (mg/dl) | 49 ± 5.5 | 50 ± 6.2 | 0.52† | 49 ± 3.9 | 48 ± 4.1 | 0.11† |
| Triglycerides (mg/dl) | 85 ± 17.3 | 100 ± 17.1 | <0.0001† | 86 ± 10.1 | 90 ± 10.5 | 0.02† |
| Glucose (mg/dl) | 95 ± 3.0 | 96 ± 3.3 | 0.02† | 96 ± 1.9 | 97 ± 2.0 | <0.0001† |
These subjects remained normotensive for the first 4.3 years.
Adjusted for age and gender.
Abbreviations as in Table 1.
Figure 2. Adjusted Hypertension-Free Survival Curves for Participants Who Were Followed Up for >4.3 Years.
All participants were normotensive at 4.3 years and were stratified according to median PWV (5.8 m/s). The curves are adjusted for age, SBP, and high-density lipoprotein (HDL). Abbreviations as in Figure 1.
Discussion
The main findings of this study are that PWV, a marker of central arterial stiffening, is an independent determinant of longitudinal SBP increase in healthy BLSA volunteers, and an independent risk factor for incident hypertension among normotensive subjects followed up for longer than 4 years.
Arterial stiffness as a predictor of HTN
At least 2 prior studies have identified indices of central arterial stiffness that predicted future HTN in normotensive community-based samples. Liao et al. (21) previously showed that in normotensive middle-aged (45 to 64 years) participants in the Atherosclerosis Risk in Communities (ARIC) Study, indexes of carotid arterial elasticity were independent predictors of future HTN over a 6-year mean follow-up. Similarly, Dernellis and Panaretou (22) showed that echocardio-graphic indexes of aortic stiffness were independent predictors of incident HTN in normotensive subjects across a broad age span (35 to 94 years) who were followed up for 4 years.
Our findings are in agreement with these 2 previous studies and extend them insofar as we show, for the first time, that PWV, one of the most widely used indexes of central arterial stiffness, is also an independent predictor of future HTN. Furthermore, our results show for the first time that PWV is an independent determinant of the longitudinal increase in SBP as a continuous variable, that is, beyond the arbitrary threshold values that are used to define HTN. It is worth noting that unlike the ultrasono-graphically derived indexes of carotid and aortic distensibility that were used in the 2 prior studies (21,22), PWV has been shown to be an independent risk factor for cardiovascular morbidity and mortality in diverse populations (5–10).
The finding that PWV was a predictor of incident HTN only in subjects with a follow-up duration >4 years but not in subjects with a shorter follow-up duration is not surprising because the increase in BP that is attributable to the higher arterial stiffness is expected to be a gradual process. We cannot exclude the possibility that our sample size was underpowered to discern the independent predictive effects of PWV in the group with the shorter follow-up duration, particularly because our models were adjusted for age and baseline SBP.
SBP as a predictor of arterial stiffness
It is traditionally believed that arterial stiffening is accelerated by higher SBP because of the structural and functional alterations in the walls of the central elastic arteries in response to the chronically elevated distending pressures (13). In the Framingham Heart Study (23), the longitudinal increase in pulse pressure, a surrogate measure of arterial stiffness, was greater in subjects with higher baseline SBP. In the Bogalusa Heart Study (24), the cumulative burden of SBP measured since childhood and over an average follow-up of 26.5 years was an independent predictor of brachial-ankle PWV measured in young adulthood. However, this analysis was not adjusted for baseline arterial stiffness because the latter was not assessed during childhood.
Importantly, 2 longitudinal studies of carotid–femoral PWV that did adjust for baseline arterial stiffness did not find SBP to be an independent predictor of the longitudinal changes in PWV. Benetos et al. (25) found that after a 6-year follow-up, SBP was not an independent predictor of PWV increase among normotensive or hypertensive subjects, although the increase of PWV in poorly controlled hypertensive subjects was significantly greater than that in well-controlled hypertensive subjects and in normotensive subjects. Wildman et al. (26) found that after a 2 year follow-up, SBP was not associated with the annual change in PWV, even by univariate analyses. In contrast, our present study and those by Liao et al. (21) and Dernellis and Panaretou (22) collectively provide robust evidence of the converse, namely that arterial stiffening precedes and predisposes to accelerated longitudinal increases in SBP and to future HTN. In other words, arterial stiffening is not simply an adaptive response of blood vessels to distending pressures; rather, when it is accelerated, arterial stiffening is an underlying pathophysiological cause of the increase in pressure.
Implications
Although studies attempting to characterize normotensive populations at risk for the development of HTN have largely focused on measurements of BP (27), our results suggest that PWV could potentially serve as a valuable additional tool to help in risk stratifying these individuals. Furthermore, because PWV is a risk factor for incident HTN efforts to find cost-effective and efficacious de-stiffening interventions should be intensified. Traditionally, the age-associated increase in arterial stiffness has been attributed to cumulative wear-and-tear induced fragmentation and depletion of elastin, as well as the deposition of collagen (13). Recent findings suggest that central arterial stiffness is also regulated by other processes (28), including alterations in the signaling cascades of nitric oxide, endothelin-1, and other putative cell-signaling molecules. Future studies are needed to determine whether interventions that target these pathophysiological processes can succeed in preventing or delaying the onset of HTN.
Study limitations
The BLSA participants tend to be predominantly white, well-educated, and health-conscious individuals, which limits the generalizability of our findings to other populations. Our study only included measurements of brachial BP, which are overestimates of central SBP and PP caused by pressure wave amplification across the arterial tree. However, central BP is seldom available in clinical practice, and is rarely assessed in epidemiological and longitudinal studies of BP. Because the diagnosis of hypertension was made by the presence of antihypertensive medications in 44% of subjects in whom hypertension developed during follow-up, we were not able to evaluate whether PWV’s ability to predict hypertension varied according to the type of hypertension (i.e., systolic vs. diastolic vs. mixed). Because of the important effect of distending pressures (MAP) on arterial stiffness, adjusting the mixed-effects models for baseline MAP is necessary (and is one of the strengths of this analysis); however, this could introduce a bias in favor of the estimated coefficient of PWV because MAP is closely related to the outcome of interest (29).
Conclusions
We found that arterial stiffness, as indexed by PWV, is an independent predictor of the longitudinal increase in SBP and of incident HTN. Because higher SBP and established HTN are associated with significant cardiovascular morbidity and mortality (2,3), our findings suggest that PWV could potentially serve as a screening tool to identify normotensive individuals at higher risk, who could be targeted for pharmacological and nonpharmacological interventions aimed at preventing or delaying the progression of subclinical arterial stiffening and the onset of HTN.
Supplementary Material
Acknowledgments
The authors thank Peter V. Vaitkevicius, MD, Amit Nussbacher, MD, and Tomasz M. Rywik, MD, for their efforts in the acquisition and measurement of PWV, and Christopher H. Morrell, PhD, for statistical guidance.
Abbreviations and Acronyms
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- HTN
hypertension
- HR
hazard ratio
- MAP
mean arterial pressure
- PP
pulse pressure
- PWV
pulse wave velocity
- SBP
systolic blood pressure
APPENDIX
For supplementary tables, please see the online version of this article.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women. Life Course Analysis Hypertension. 2005;46:280–6. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 3.ALLHAT Officers and Coordinators. ALLHAT Collaborative Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 4.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991 Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 5.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 6.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 8.Sutton-Tyrrell K, Najjar SS, Kupelian V, et al. for the Health ABC Study. Aortic pulse wave velocity predicts mortality in a general population of well-functioning older adults. Circulation. 2005;111:2284–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 9.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 10.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 11.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–62. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 12.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–66. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 14.Franklin SS. Arterial stiffness and hypertension: a two-way street? Hypertension. 2005;45:349–51. doi: 10.1161/01.HYP.0000157819.31611.87. [DOI] [PubMed] [Google Scholar]
- 15.Shock NW, Greulich RC, Andres RA, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Government Printing Office; 1984. p. 45. NIH publication no. 84–2450. [Google Scholar]
- 16.Scuteri A, Najjar SS, Muller D, et al. ApoE4 allele and the natural history of cardiovascular risk factors. Am J Physiol Endocrinol Metab. 2005;289:E322–7. doi: 10.1152/ajpendo.00408.2004. [DOI] [PubMed] [Google Scholar]
- 17.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 18.Gueorguieva R, Krystal JH. More over ANOVA. Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–7. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 19.Scuteri A, Bos AJG, Brant LJ, Talbot L, Lakatta EG, Fleg JL. Hormone replacement therapy and longitudinal changes in blood pressure in postmenopausal women. Ann Intern Med. 2001;135:229–38. doi: 10.7326/0003-4819-135-4-200108210-00007. [DOI] [PubMed] [Google Scholar]
- 20.Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models and individual growth models. J Educ Behav Stat. 1998;24:323–55. [Google Scholar]
- 21.Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC study Hypertension. 1999;34:201–6. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 22.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–31. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 23.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure as a predictor of arterial stiffness in young adults: the Bogalusa heart study. Hypertension. 2004;43:541–6. doi: 10.1161/01.HYP.0000115922.98155.23. [DOI] [PubMed] [Google Scholar]
- 25.Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–7. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 26.Wildman RP, Farhat GN, Patel AS, et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–92. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 27.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–6. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 28.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–62. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 29.Yanez ND, Kronmal RA, Shemanski LR. The effects of measurement error in response variables and tests of association of explanatory variables in change models. Stat Med. 1998;17:2597–606. doi: 10.1002/(sici)1097-0258(19981130)17:22<2597::aid-sim940>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.