Abstract
The nervous system must provide a mechanism for very precise discrimination of differing patterns of activity, yet at the same time, there must be a mechanism for generalization to prevent all experiences from being independent and novel. Pattern separation and completion by cortical circuits contribute to these processes, respectively. Based on theoretical and computational models of the piriform cortex and experimental designs developed for hippocampal spatial memory, we provide evidence for pattern separation and completion in the olfactory system and demonstrate its predictive power for behavioral odor perception.
Keywords: piriform cortex, olfactory bulb, odor perception, hippocampus, perceptual stability, perceptual discrimination, odor, memory
Introduction
With the exception of a few narrowly tuned labeled lines, sensory perception generally involves an initial analytical process, breaking sensory experience into features (spots of light, specific frequencies of sound, submolecular moieties of volatile molecules), followed by synthetic/configural processes that result in perceptual objects (the sight of a car, the sound of a voice, the smell of a rose). Perception at the level of objects, rather than at the level of collections of independent features, has two somewhat opposing consequences. First, it can promote discrimination—a difference between two objects can be noticed even in the absence of an ability to identify the differing features. Second, subtle changes in the object can be ignored, promoting perceptual stability.
Synthetic or configural object processing generally involves two critical components at the neural circuit level. First, there must be an anatomical basis for convergence of diverse feature input onto individual target neurons. Second, in most systems displaying synthetic or configural processing, a mechanism exists for remembering previously experienced patterns of input. This latter component makes synthetic processing robust in the face of degraded or noisy inputs (the system can fill in missing features), while at the same time it allows enhanced sensory discrimination of familiar stimuli.
In olfaction, perceptual and hedonic responses to monomolecular odorants can in some cases be predicted simply from the molecular structure and specific ligand–receptor interactions.1–3 However, most naturally occurring scents are complex mixtures, which evoke unique spatiotemporal patterns of activity, which can vary both within and between stimulus exposures.4,5 Odorant mixtures composed of even a small number of components are perceived as objects,6 distinct from their components. Both theoretical and computational models7–10 predict that cortical circuits underlying processing of these mixtures support pattern completion and separation. New data from our lab demonstrate that ensembles of anterior piriform cortex can make these calculations and predict behavioral performance.
Balancing Perceptual Discrimination and Perceptual Stability
The ability of some cortical circuits to fill in features missing from a familiar input pattern is called pattern completion, and allows for perceptual stability. For example, an animal may be able to recognize its burrow visually based on the pattern of trees, shrubs, and rocks in the vicinity. Should a storm knock one of those trees down, the remaining set of features should be sufficient to allow the animal to still recognize its way home. Similarly, if your friend’s face is partially obscured by their cell phone, you may still recognize them through pattern completion. As described in more detail below, pattern completion is not simply due to generalization or a lack of sufficient information reaching the nervous system to allow a discrimination between two patterns. Rather, it is an active “filling in the blank” process based on past experience with the input patterns. Without perceptual stability, natural variation in complex sensory inputs would lead to massive confusion, with each new experience a novel one. Our ability to predict future events through classical conditioning, for example, would be severely limited if each new ring of the bell varied slightly and required a new learned association with food.
As two input patterns become more distinct, and/or as the significance of making a discrimination between two patterns increases, some cortical circuits allow for pattern separation—a decorrelation or orthogonalization of activity evoked by the two patterns. Pattern separation allows for perceptual discrimination, often of even highly overlapping patterns.
Circuit Characteristics Associated with Pattern Completion and Separation Computations
As noted above, pattern separation and completion can occur in most sensory systems; however, specific neural circuit characteristics may facilitate the computations involved.9,11–13 An auto-associative array is one such circuit. In an auto-associative array, activity of output neurons feeds back into the circuit as input.9 This feedback, combined with synaptic plasticity, wherein previously active synapses are strengthened, allows circuits to learn past input patterns.14 Both the piriform cortex and hippocampal formation share this circuitry and are considered characteristic of a content-addressable memory system.13 In both cases, inputs to the circuit are broadly dispersed and highly overlapping, representations of inputs are sparse, and an auto-associative mechanism allows storing previously experienced input patterns, against which new inputs can be compared. Thus, afferent input to either the hippocampal formation or the anterior piriform cortex is broadly dispersed, with any given target neuron receiving direct excitatory input from a relatively small percentage of the afferent fiber population, with minimal spatial topography. This results in a small, scattered population of target neurons responsive to any particular complex patterned input. The sparse encoding helps maintain separation between cell populations activated by different input patterns, reducing interference and facilitating discrimination.15
Next, a strong association fiber system, which expresses activity-dependent synaptic plasticity, allows for co-active neurons to become linked into an ensemble, despite potentially being spatially dispersed. This linkage is activity-dependent, with excitatory interconnections between the neurons within the ensemble (auto-association) strengthened by past periods of co-activation. If neurons A, B, and D are all activated in response to a particular odor, the excitatory connections between them become potentiated, while connections between A and C and E (not co-activated) remain weak. This synaptic potentiation helps form the basis of pattern completion.
For example, assume that a complex input pattern activates a specific population of interconnected target neurons. That pattern is represented or encoded by the activity of that ensemble of neurons (ABD). In the event of a partial or degraded familiar input, which activates only a subset of the original target neurons (AB), the missing neuron activity (D) can be filled in by the activity of strengthened association fibers, essentially bypassing the missing afferent input features to complete the representation (ABD).
Pattern Separation and Completion in the Hippocampal Formation
The processes and consequences of pattern separation and completion have perhaps most thoroughly been examined in the hippocampal formation.13,16 The hippocampal formation receives its primary afferent input from the multisensory entorhinal cortex. The hippocampal formation is composed of several major subregions: the dentate gyrus, which receives direct input from the entorhinal cortex; CA3, which receives a strong input from the dentate gyrus as well as a smaller direct input from the entorhinal; and CA1, which receives input from CA3 and the entorhinal cortex.17 The hippocampal formation is critically involved in memory for a variety of complex patterns such as spatial mapping of the animal’s environment and the context and detail of episodic events in the animal’s life. Pattern separation in this system allows memories to be stored as distinct maps or episodes, different from similar environments or events. Pattern completion allows recognition of a modified though familiar environment, or a partial input to evoke a complete memory.
In the hippocampal formation, pattern separation and completion appear to be anatomically segregated to some degree. The dentate gyrus, with its very sparse representations of entorhinal cortical input and minimal auto-association connections, is specialized to perform pattern separation.18 Thus, for example, even very slight differences in a spatial environment are encoded differently (decorrelated) by ensembles of dentate gyrus neurons.19
In contrast, CA3 and CA1 neurons can perform pattern completion if there are small changes in the input pattens.19–22 Thus, CA3 and CA1 ensembles can ignore slight changes in a spatial environment and allow stability in the animal’s spatial map by filling in missing components through potentiated association connections. It must be emphasized that the dentate gyrus, which is afferent to CA3 and thus to CA1, can decorrelate these small pattern changes. That is, there is sufficient information within the nervous system to allow discrimination to occur. However, as described above, it is not adaptive to have all new experiences novel; some completion of degraded inputs can be very useful. Ensembles within the hippocampal CA3 and CA1, presumably through plasticity within auto-associative networks, promote this pattern completion. As patterns diverge further, however, CA3 neurons nonlinearly shift toward pattern separation, essentially allowing an abrupt transition between encoding patterns as similar/same and as different.19,20,22
Pattern Separation and Completion in Olfaction
Similar to other sensory systems and hippocampal-based memory, olfactory perception includes a highly synthetic process wherein complex mixtures of scores or hundreds of odorant molecules can result in a unitary perceptual odor.23 Odorants and odorant mixtures induce complex spatiotemporal patterns of activity in the olfactory bulb,24–29 providing information about molecular features present in the stimulus,4 as well as some interactions between those features.30 It has been hypothesized that the piriform cortex may be a site critical for olfactory pattern separation and completion.9
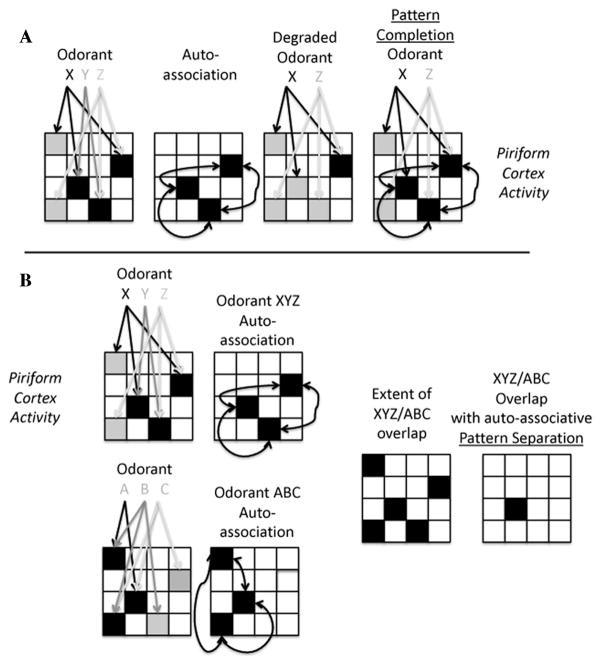
Like the hippocampus, the piriform cortex is a trilaminar cortex with patchily distributed afferent input and an extensive, highly plastic association fiber system. The piriform neural architecture conforms to an auto-associative array as described above.9 Odorant stimulation evokes activity in a specific set of mitral cells projecting to the piriform cortex, each conveying information about specific olfactory sensory neuron activity, and thus the presence of specific molecular features. Piriform cortical target neurons receive convergent input from multiple input neurons,31,32 but in addition receive excitatory input from other piriform cortical neurons,33 similar to the CA3/CA1 circuit described above. The association fiber synapses express activity-dependent plasticity,34 and thus can be strengthened during co-activation of cells responding to different features of the same odor. Thus, as described above for hippocampus, piriform cortical ensembles could ignore slight changes in an odor mixture’s features (e.g., missing a subset of the mitral cells normally activated by a familiar odor) and allow stability in the animal’s odor perception by filling in missing components through potentiated association connections (Fig. 1).
Figure 1.
Schematic representation of how auto-associative networks, as found in hippocampal formation and piriform cortex, contribute to pattern completion.9,14 (A) Pattern completion. In this example, an odorant composed of features X, Y, and Z activates a dispersed population of piriform cortical neurons (individual squares within grid). Afferent fiber convergence of multiple features enhances activation of particular cells (filled squares). Co-active cortical neurons strengthen their association fiber connections via activity-dependent synaptic potentiation, in an auto-associative manner. Upon stimulation with a degraded input pattern (XZ), the previously strengthened association fiber synapses reconstruct (complete) the degraded pattern. (B) Pattern separation. Odorants XYZ and ABC activate overlapping sets of piriform cortical neurons. Through auto-associative enhancement of familiar patterns, the extent of overlap between these two patterns is reduced, enhancing discriminability.
In indirect support of this model, manipulations that disrupt normal synaptic potentiation of association fibers within piriform cortex, such as via acetylcholine muscarinic receptors,35 disrupts normal odor decorrelation by piriform cortical neurons,36 behavioral odor discrimination,37 and olfactory perceptual learning.38
Our lab has recently found more direct support for pattern separation and completion in the olfactory system, and found that cortical ensemble activity can predict odor discrimination and generalization.39 Complex odorant mixtures, composed of 10 equal-concentration components, were used as stimuli. Single units were recorded in both the mitral cell layer of the olfactory bulb and Layer II/III of the anterior piriform cortex. Responses to the 10-component mixture were compared with morphs of this mixture that were produced by removing components (leaving nine components, or eight, or seven, etc.) or by replacing components with an equal-concentration novel component. For example, we replaced isoamyl acetate, which was a component of the standard 10-component mix, with 3-methyl-2-buten-1-ol, which was not in the original mix. We examined how ensembles of mitral cell or piriform cortical neurons differentially responded to these highly overlapping mixtures by looking at cross-correlations between the evoked spike activity of a neural ensemble (>15 units) responding to one mixture compared with another overlapping mixture.
We found that on average, piriform cortical ensembles were significantly better than mitral cell ensembles at decorrelating overlapping odor mixtures. However, there were dramatic differences between these two regions depending on the degree of overlap between the mixtures. Thus, even a single missing component induced a significant decorrelation in the activity of mitral cell ensembles compared wiht the response to the full 10-component mix. Mitral cell ensembles readily separated these overlapping patterns. As the difference between mixtures increased, however, mitral cell ensemble decorrelation remained stable.
In contrast, piriform cortex ensembles showed no significant de-correlation if only a single component was missing (90% overlap). Thus, the cortical ensemble filled in the missing component and responded as if the full mixture were present—consistent with pattern completion. As more components were removed, decorrelation became more pronounced, and in fact exceeded that shown by mitral cell ensembles. That is, piriform cortical ensembles performed pattern completion when a single component was missing, but showed enhanced pattern separation compared with mitral cells as more components were removed.
Interestingly, replacement of a single component (90% overlap with the original mixture), in contrast to single-component removal (90% overlap), produced dramatic cortical ensemble pattern separation. These ensemble results suggest that introduction of a novel component into a complex mixture should be relatively easy to detect behaviorally, while removal of a single component should be difficult. Human psychophysical data of discrimination of tainted food odors support this prediction.40 In fact, in a two-alternative, forced-choice odor discrimination task using the same 10-component mixtures as in the physiological studies, we found that animals easily behaviorally discriminated replacement of a single component (regardless of molecular similarity), while they had difficulty discriminating mixtures with a single component removed. These results suggest that cortical ensembles can predict behavioral odor discriminability. When cortical ensembles perform pattern completion, animals perceive two overlapping mixtures to be the same. When the ensembles perform pattern separation, the animals perceive the two overlapping mixtures to be different. In this paradigm, mitral cell ensembles do not allow a direct prediction of behavioral performance.
Summary
The nervous system must provide a mechanism for very precise discrimination of differing patterns of activity, evoked either by sensory input or by memorized representations. At the same time, there must be a mechanism for generalization to prevent all experiences from being novel and thus lacking associations and predictive value. Pattern separation and completion by cortical circuits contribute to these processes, respectively. Based on theoretical and computational models of the piriform cortex and experimental designs developed for hippocampal spatial memory, we provide evidence for pattern separation and completion in the olfactory system and demonstrate its predictive power for behavioral odor perception.
Acknowledgments
Supported by grant DC 003906 to D.A.W and grant DC008982 to Rob Rennaker and D.A.W.
Footnotes
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Cleland TA, et al. Relational representation in the olfactory system. Proc Natl Acad Sci USA. 2007;104:1953–1958. doi: 10.1073/pnas.0608564104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngentob SL, et al. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan RM, et al. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Tabor R, et al. Processing of odor mixtures in the zebrafish olfactory bulb. J Neurosci. 2004;24:6611–6620. doi: 10.1523/JNEUROSCI.1834-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinks A, Laing DG. The analysis of odor mixtures by humans: evidence for a configurational process. Physiol Behav. 2001;72:51–63. doi: 10.1016/s0031-9384(00)00407-8. [DOI] [PubMed] [Google Scholar]
- 7.Granger R, Lynch G. Higher olfactory processes: perceptual learning and memory. Curr Opin Neurobiol. 1991;1:209–214. doi: 10.1016/0959-4388(91)90080-q. [DOI] [PubMed] [Google Scholar]
- 8.Barkai E, et al. Modulation of associative memory function in a biophysical simulation of rat piriform cortex. J Neurophysiol. 1994;72:659–677. doi: 10.1152/jn.1994.72.2.659. [DOI] [PubMed] [Google Scholar]
- 9.Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 10.Hopfield JJ. Odor space and olfactory processing: collective algorithms and neural implementation. Proc Natl Acad Sci USA. 1999;96:12506–12511. doi: 10.1073/pnas.96.22.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granger R, et al. Unsupervised perceptual learning: a paleocortical model. In: Hanson SJ, Olson CR, editors. Connectionist modeling and brain function: the developing interface. MIT Press; Cambridge, MA: 1990. pp. 106–131. [Google Scholar]
- 12.Hasselmo ME, et al. Associative memory function in piriform (olfactory) cortex: computational modeling and neuropharmacology. Cold Spring Harb Symp Quant Biol. 1990;55:599–610. doi: 10.1101/sqb.1990.055.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 14.Hopfield JJ. Olfactory computation and object perception. Proc Natl Acad Sci USA. 1991;88:6462–6466. doi: 10.1073/pnas.88.15.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 16.McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford University Press; New York: 2004. pp. 455–498. [Google Scholar]
- 18.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 19.Leutgeb JK, et al. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 20.Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Bakker A, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leutgeb S, et al. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DA, Stevenson RJ. Learning to smell: olfactory perception from neurobiology to behavior. Johns Hopkins University Press; Baltimore: 2006. [Google Scholar]
- 24.Spors H, et al. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 28.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie KM, et al. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci USA. 1993;90:3329–3333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joerges J, et al. Representation of odours and odour mixtures visualized in the honeybee brain. Nature. 1997:387. [Google Scholar]
- 31.Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Neville KR, Haberly L. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford University Press; New York: 2004. pp. 415–454. [Google Scholar]
- 33.Johnson DM, et al. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanter ED, Haberly LB. NMDA-dependent induction of long-term potentiation in afferent and association fiber systems of piriform cortex in vitro. Brain Res. 1990;525:175–179. doi: 10.1016/0006-8993(90)91337-g. [DOI] [PubMed] [Google Scholar]
- 35.Linster C, Hasselmo ME. Neuromodulation and the functional dynamics of piriform cortex. Chem Senses. 2001;26:585–594. doi: 10.1093/chemse/26.5.585. [DOI] [PubMed] [Google Scholar]
- 36.Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8:279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Rosa E, Hasselmo ME. Muscarinic cholinergic neuromodulation reduces proactive interference between stored odor memories during associative learning in rats. Behav Neurosci. 2000;114:32–41. [PubMed] [Google Scholar]
- 38.Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes DC, et al. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1368–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva Ferreira AC, Hogg T, Guedes de Pinho P. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J Agric Food Chem. 2003;51:1377–1381. doi: 10.1021/jf025847o. [DOI] [PubMed] [Google Scholar]