Abstract
Prostate cancer (PCa) is one of the solid tumors that metastasize to the bone. Once there, the phenotype of the bone lesions becomes depends upon the balance between osteoblastogenesis and osteoclastogenesis. We previously reported that over-expression of phosphoglycerate kinase 1 (PGK1) in PCa cell lines enhanced bone formation at the metastatic site in vivo. Here, the role of PGK1 in the bone formation was further explored. We demonstrate that PCa-derived PGK1 induces osteoblastic differentiation of bone marrow stromal cells. We also found that PGK1 secreted by PCa inhibits osteoclastogenesis. Finally, the expression levels of the bone specific markers in PCa cell themselves were higher in cells over expressing PGK1 than controls. Together, these data suggest that PGK1 secreted by PCa regulates bone formation at the metastatic site by increasing osteoblastic activity, decreasing osteoclastic function, and expressing an osteoblastic phenotype by PCa themselves.
Keywords: Prostate cancer, Phosphoglycerate kinase 1 (PGK1), Osteoblastogenesis, Osteoclastogenesis
Introduction
Prostate cancer (PCa) is a common neoplasm and the second leading cause of cancer deaths in American males. Almost all men who die from PCa, have hormone-refractory disease with skeletal metastases (1, 2). Due to progress in systemic chemotherapy and radiotherapy, the prognosis of individuals with PCa is improving (3). However, the symptoms of the skeletal metastases, such as pathological fractures, bone pain, and spinal cord compression, remain incurable (4, 5).
We recently demonstrated that SDF-1/CXCL12 and its receptors (CXCR4 and RDC1/CXCR7) play important roles in PCa bone metastases and growth in bone (6-12). One part of the mechanism activated by CXCL12 signaling is an increase in angiogenesis (9). CXCL12 signaling reduces the expression and secretion of phosphoglycerate kinase 1 (PGK1) expression (11). PGK1 is an ATP-generating glycolytic enzyme that forms part of the glycolytic pathway that is often highly expressed in PCa (13) and is regulated by hypoxia-inducible factor-1α (HIF-1α) (14). Extracellular PGK1 facilitates the cleavage of plasminogen generating the vascular inhibitor angiostatin (15-18) which is known as an important regulator of an ‘angiogenic switch’ (11). Thus, CXCL12 signaling down-regulates PGK1 secretion suggesting a mechanism for metastatic PCa to grow in tissues with high CXCL12 levels (11).
One of the most intriguing aspects of our previous study was that when PCa cells were induced to over express PGK1 in the bone microenvironment, extracortical bone formation was observed adjacent to the growing tumor (11). In most animal models of human PCa disease, an osteolytic bone phenotype predominates. Yet, in patients with skeletal metastases, a predominantly osteosclerotic bone phenotype is typically observed, osteolytic and mixed lesions are not uncommon. Mechanisms for the osteoclerotic bone phenotype is that several factors produced by PCa, such as bone morphogenetic protein (BMP), transforming growth factor-β (TGF-β), insulin-like growth factor (IGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), induce osteoblastic proliferation and differentiation (19). Other possibilities include alterations in the local levels of vascular endothelial growth factor (VEGF) secreted by PCa regulates the bone formation indirectly by supporting angiogenesis (20). At present, however, the molecular mechanisms of the osteoblastic progression at the skeletal metastatic site in PCa remain unclear (4, 21).
In this study, we explored the role of PGK1 secreted by PCa in the development of an osteosclearotic bone phenotype. We demonstrate that PCa-derived PGK1 induces osteoblastic commitment from bone marrow stromal cells (BMSCs) and inhibits osteoclast formation. Moreover, over expression of PGK1 in PCa cells themselves induces expression of several osteoblastic markers. These results suggest that PGK1 may serve as a key moderator of bone remodeling at metastatic bone sites.
Materials and Methods
Cell Cultures
PC3 was obtained from the American Type Culture Collection (ATCC; Rockville, MD). C4-2B was derived from the parental LNCaP cell lines that were serially passaged in mice to obtain a more metastatic cell line (22). RAW 264.7, murine osteoclast precursors, was also obtained from the ATCC. PC3 and C4-2B were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) and RAW 264.7 was cultured in α-MEM (Invitrogen), supplemented with 10% (v/v) fetal bovine serum (FBS; Invitrogen), 1% (v/v) penicillin-streptomycin (Invitrogen), and 1% (v/v) l-glutamine (Invitrogen). All cultures were maintained at 37°C, 5% CO2, and 100% humidity.
Bone Marrow Cell Preparations
The marrow was flushed from the femur, tibia, and humeri with Hank's buffer salt solution (Gibco, Grand Island, NY) using a 5-ml syringe fitted with a 23-gauge needle. A single-cell suspension was obtained by gentle agitation through the syringe. Debris and remaining cellular aggregates were removed by passing the cell suspension over a 40 μm-mesh nylon cell strainer (BD Biosciences, San Diego, CA). The cells were plated in α-MEM with 10% FBS and 1% penicillin and streptomycin. After 3 days, non-adherent cells were removed and fresh media were replaced.
PGK expression constructs and Small interfering RNA knockdown of PGK1
A 1.33-kb human PGK1 (hPGK1) cDNA was isolated by reverse transcription-PCR from total RNA extracted from PC3 cells (11). The forward and reverse primers were 5′-AGTACATATGTCGCTTTCTAACAAGCTG-3′ (positions, 80-100) and 5′-AGTAGGATCCCTAATGCCAAGTGGAGATGCA-3′ (positions, 1,409-1,389), respectively. For small interfering RNAs (siRNA) knockdown of PGK1, two groups of primers corresponding to nucleotide sequences in open reading frame were synthesized [position, 186-203; Si1, 5′-gatccccACAACCAGAGGATTAAGGCttcaagagaGCCTTAATCCTCTGGTTGTttttta-3′ (forward oligonucleotide) and 5′-agcttaaaaaACAACCAGAGGATTAAGGCtctcttgaaGCCTTAATCCTCTGGTTGTggg-3′ (reverse oligonucleotide); position, 192-210; Si2, 5′-gatccccAGAGGATTAAGGCTGCTGTttcaagagaACAGCAGCCTTAATCCTCTttttta-3′ (forward oligonucleotide) and 5′-agcttaaaaaAGAGGATTAAGGCTGCTCTtctcttgaaACAGCAGCCTTAATCCTCTggg-3′ (reverse oligonucleotide)]. As scrambled primers, [5′-gatccccAAAACCGACGGCTATCTCTttcaagagaAGAGATAGCCGTCGGTTTTttttta-3′ (forward oligonucleotide) and 5′-agcttaaaaaAAAACCGACGGCTATCTCTtctcttgaaAGAGATAGCCGTCGGTTTTggg-3′ (reverse oligonucleotide)] were used. PCa cells were transfected by lipofection (PC3Control, PC3PGK1, PC3SiControl, PC3SiPGK1, C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGk1) (11).
For construction of full-length PGK1 expressing lentiviral vector, infectious viral particles were prepared by co-transfection of 293T cells with the hPGK1 construct, a packing vector (ABM, Richmond, BC, Canada), and a lentiviral vector (ABM), according to the manufacturer's protocol. BMSC were transfected with hPGK1 expressing vector (BMSCPGK1) or scrambled control vector (BMSCControl).
Generation of conditioned medium (CM)
PCa cells were seeded on 100-mm tissue culture dishes at a concentration of 2 x106 cells per well in RPMI 1640 supplemented with 10% (v/v) FBS. After 48 h, supernatant was collected, cleared from any free-floating cells by centrifugation for 5 min at 1000g, filtered through a 0.2-mm sterilizing filter, and then stored at -80°C. The concentrations of conditioned medium were normalized to total protein.
Animals
All experimental procedures were approved by the University of Michigan Committee for the Use and Care of Animals (UCUCA). C57BL/6 mice (4- to 6-week-old) and male SCID mice (4- to 6-week-old) were obtained from Charles River Laboratories (Wilmington, MA). Male athymic (nude) mice (4- to 5-week-old) were purchased from Harlan Bioscience (Indianapolis, IN). Animals were caged under standard conditions and fed a laboratory diet and tap water ad libitum.
RNA Extraction and Real-Time RT-PCR
Total RNA was isolated using RNeasy Mini Kit (QIAGEN, Valencia, CA), and first-strand cDNA was synthesized in a 20 μL reaction volume using 0.4 μg of total RNA. RT products were analyzed by real-time PCR in TaqMan® Gene Expression Assays of several target genes: mouse BMP2, BMP6, Runx2, OCN, and β-Actin (Applied Biosystems, Foster City, CA). Real-time PCR analysis was performed using 15.0 μl of TaqMan® Universal PCR Master Mix (Applied Biosystems), 1.5 μl of TaqMan® Gene Expression Assay (forward and reverse primers at 18 μM and Taqman probe at 5 μM), 1 μl of the cDNA, and 12.5 μl of RNAse/DNAse-free water in a total volume of 30 μl. Reactions without template and/or enzyme were used as negative controls. The 2nd step PCR reaction was run for 40 cycles (95°C for 15 min) after an initial single cycle of 95°C for 30 sec and 60°C for 5 min to activate the Taq polymerase. The PCR product was detected as an increase in fluorescence using an ABI PRISM 7700 instrument (Applied Biosystems). RNA quantity (CR) was normalized to the housekeeping gene β-Actin control by using the formula CR=2(40-Ct of sample)-(40-Ct of control). The threshold cycle (Ct) is the cycle at which a significant increase in fluorescence occurs.
Immunohistochemistry
The PCa cells-injected tibias were fixed in 10% formalin at 4°C and decalcified in 10% EDTA (pH 7.4) for paraffin embedding. Seven μm sections of the decalcified long bones were then cut with a microtome (Leica RM2125 RT; Leica Microsystems, Bannockburn, IL). Mason's trichrome staining (Sigma-Aldrich, St. Louis, MO) and TRAP staining (Sigma-Aldrich) were performed and data expressed as numbers of osteoblasts and osteoclasts per millimeter of bone surface. Sections were also stained with anti-Runx2 antibody (1:250, rabbit polyclonal; Abcam, Cambridge, MA), anti-von Willebrand factor (factor VIII-related antigen) antibody (1:250, rabbit polyclonal; Dako North America, Carpinteria, CA), or an IgG isotype-matched control (Sigma-Aldrich).
PCa cells (PC3Control, PC3PGK1, PC3SiControl, PC3SiPGK1, C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGk1) were cultured in Lab-Tek II 4-chamber slides (Nalge Nunc International, Naperville, IL) at 5 × 104 cells / chamber. After 24 hours, the cells were fixed in 4% paraformaldehyde for 25 minutes at room temperature, and endogenous peroxidase activity quenched with 75 mM NH4Cl and 20 mM Glycine in PBS at room temperature for 10 minutes. Thereafter, the cells were incubated with anti-Runx2 antibody or an IgG isotype-matched control. Antibody detection was performed by using a rabbit HRP-AEC staining kit (R&D Systems, Minneapolis, MN) for 1 h at room temperature. Images were acquired on a Zeiss LSM510 microscope. The staining intensities of the slides were quantified with Image J software, and data were normalized to the total cell numbers.
Enzyme-Linked Immunosorbent Assays (ELISA)
Alkaline phosphatase activity (ALP) (Sigma-Aldrich) and osteocalcin (OCN) levels (Biomedical Technologies, Stoughton, MA) of the cell lysates and animal serum were determined by double-antibody sandwich method assembled with commercially available components, according to the directions of the manufacturer. PGK1 levels were determined by direct ELISA using 100 μL of 5 μg/mL murine anti-PGK monoclonal IgM against a rhPGK1 (11). Data were normalized to total protein.
Western Blot Analyses
The cells were lysed by freeze-thawing in ice-cold lysis buffer (50 mM Tris-HCl, 1% NP-40, 120 mM NaCl, 1 mM EDTA, 25 mM NaF, 40 mM beta-glycerol phosphate, 0.1 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride and 1.0% mammalian protease inhibitor cocktail (Sigma-Aldrich). The nuclei and cellular debris were removed by centrifugation at 16000 × g for 15 minutes at 4°C. SDS-PAGE was performed in 10% polyacrylamide gels. Each lane contained 30 μg of cell (lysate) protein. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). Residual protein binding sites on the membrane were blocked with Tris-buffered saline with Tween 20 buffer containing 5% nonfat dry milk. The membranes were then incubated with the primary antibody (a monoclonal anti-PGK1 IgM antibody (23) and an anti-Runx2 antibody) overnight. After washing with Tris-buffered saline with Tween 20, the secondary antibody (anti-species specific horseradish peroxidase) was added. Finally the proteins were visualized by autoradiography using an enhanced chemiluminescence detection system (Amersham Pharmacia, Piscataway, NJ). The densities of the bands were quantified with Image J software (version 1.40; National Institutes of Health (NIH), Bethesda, MD).
In Vitro Osteoblastogenesis
BMSCs (1 × 105 cells / well) were plated onto a 24-well culture plate with osteogenic media containing 50μg/ml ascorbic acid (Sigma-Aldrich) and 10 mM β-glycerophosphate (Sigma-Aldrich). BMSCs were treated with either vehicle, rhBMP2 (200 ng/ml) (R&D Systems), rhBMP6 (200 ng/ml) (R&D Systems), or rhPGK1 (50 ng/ml). In some case, 10% (v/v) CM derived from PCa cells (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1) were added to the culture. At 21 days, osteoblastogenesis from BMSCs were evaluated by real-time RT-PCR and Alizarin Red staining (Sigma-Aldrich).
In Vitro Osteoclastogenesis
Marrow mononuclear cells (MMCs) (1x105 cells / well) or RAW 264.7 cells (3x104 cells / well) were plated onto 96-well culture plates. Cells were treated with RANKL (50 ng/ml) (R&D Systems) and/or rhPGK1 (10-50 ng/ml) every other day for 7 days. In some case, 10% (v/v) CM derived from PCa cells (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1) were added to the culture. Thereafter, osteoclastogenesis were evaluated by TRAP staining (Sigma-Aldrich).
Intratibial Injections
PC3Control and PC3PGK1 cells were inoculated intratibially to measure the effect of PGK1 on bone formation. The animals were anesthetized, and both legs were cleaned with betadine and 70% ethanol. Thereafter, the cells (1 × 105 cells / 10 μl) were injected through the cortex of the anterior tuberosity of the tibia with a drill-like motion to prevent cortical fracture using a 25-μl syringe fitted with a 25-gauge needle. After 4 weeks, animals were euthanized and tibias were fixed in 10% formalin at 4°C. Tibias were further decalcified in 10% EDTA (pH 7.4) for 10 days and embedded in paraffin.
Vertebral Body Transplants
Vertebral Body Transplants were performed, as previously described (24). Lumbar vertebrae were isolated from mice 4 to 7 days after birth. The vertebrae were sectioned into single vertebral bodies. SCID mice were used as transplant recipients. Four vertebral bodies per mouse were implanted into subcutaneous pouches. Before implantations, PCa cells (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1) were introduced into vertebral bodies (10000 cells/10 μl of PBS). Vertebral bodies were collected at 4 weeks.
Bony Ossicles Transplants
BMSCControl and BMSCPGK1 were assessed for their potential to form bony ossicles in vivo. Cells (2 × 106 cells) were incorporated into a gelatin sponge (Gel-foam; Pharmacia & Upjohn, Kalamazoo, MI). These cell/scaffold constructs were transplanted subcutaneously into 4- to 5-week-old male C57BL/6 mice. At 5 weeks, the bony ossicles were harvested and fixed in aqueous buffered zinc formalin for 24 hours at 4°C.
In Vivo Assessment of Bone Formation
For micro-computed tomography (micro-CT) analysis, specimens were scanned at 8.93 μm voxel resolution on a micro-CT scanner (EVS Corporation, London, ON, Canada), with a total of 667 slices per scan. GEMS MicroView software (GE Healthcare Bio-sciences, Piscataway, NJ) was used to make a three-dimensional reconstruction from the set of scans. A fixed threshold (1,500) was used to extract the mineralized bone phase and actual bone volume fracture (BVF) and bone mineral density (BMD) were calculated. For histomorphometry, specimens were paraffin embedded, sectioned, stained for hematoxylin and eosin (H&E).
Statistical Analysis
Numerical data are expressed as mean ± standard deviation. Statistical analysis was performed by ANOVA or unpaired two-tailed Student's t test using the GraphPad Instat statistical program (GraphPad Software, San Diego, CA) with significance at p < 0.05.
Results
Local Expression of PGK1 by PCa Induce Bone Formarion In Vivo
Previously, we demonstrated that the secretion of the glycolytic enzyme PGK1 by PCa cells enhanced the formation of extracortical woven bone in vivo (11). To determine whether PGK1 secreted by PCa regulates bone formation, PCa cell lines over-expressing PGK1 (PC3PGK1) or control vector (PC3Control) were injected intratibially into immune deficient mice. After 4 weeks, the animals were euthanized and the skeletal lesions were evaluated. Significantly more osteoblastic bone formation (Figure 1A&C) and less osteoclastic bone resorption (Figure 1B&C) were found in the PC3PGK1 cells-bearing animals than the PC3Control cells-injected animals. When the bones of the PC3PGK1 cells-injected animals were evaluated for the expression of the osteoblast-specific transcription factor Runx2, higher levels of expression were noted compared with animals injected with PC3Control cells (Figure 1D&E). Moreover, the levels of bone-specific alkaline phosphatase and osteocalcin in the serum recovered from animals injected with the PC3PGK1 cells were increased compared with animals bearing PC3Control cells (Figure 1F). These data suggest that PGK1 is secreted by PCa induces bone formation by increasing osteoblastic activities and/or decreasing osteoclastic functions.
Figure 1. PGK1-derived from PCa enhances the bone formation in vivo.
PC3Control or PC3PGK1 cells were injected intratibially in order to determine the role of PGK1-derived from PCa in the bone formation. At 4 weeks, the histologic analyses of lesions resulting from intratibia injection of PC3PGK1 versus PC3Control cells were performed. (A) Mason's tetracrome stain and (B) TRAP stain of the bone/tumor interface. (C) The numbers of the osteoblasts and osteoclasts on the bone surface in (A) and (B). (D) Expression of Runx2 protein in murine tibia. Immunolocalization of Runx2 protein was visualized using a monoclonal antibody (mAb) to Runx2 or an IgG isotype matched control antibody. Original magnification: 40x. (E) Runx2 positive area from (D). (F) Alkaline phosphatase activities and osteocalcin levels in the serum from the tumor-bearing animals were evaluated by ELISA. Data are presented as mean ± standard deviation. Significant difference is from PC3Control. These data demonstrated that PCa-derived PGK1 enhanced osteoblastogenesis in vivo. Arrows: Positive areas. Bar = 100 microns.
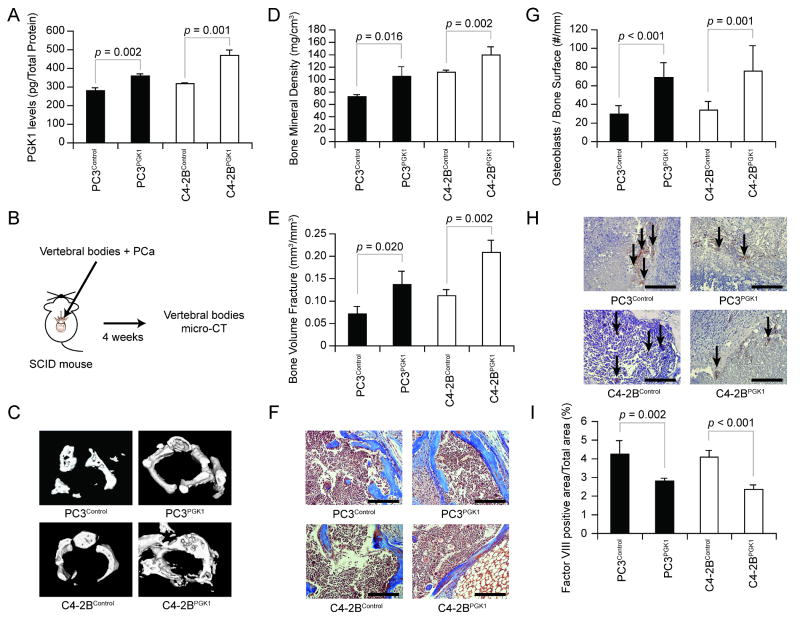
To further explore the bone formation effects of PCa cells that secrete different levels of PGK1, and to verify that the aforementioned results were not cell line specific, additional cell lines over expressing PGK1 were established and used in conjunction with an in vivo model of bone formation that was recently developed by our group which uses transplantation of vertebral bodies (24). First, to evaluate the efficiency of transfections, ELISA for PGK1 that is secreted by PCa cell lines (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1) were performed to demonstrate that enhanced secreted PGK1 levels were achieved (Figure 2A). Next, PCa cells were injected directly into the vertebral bodies derived from 4-to7-day-old animals, and transplanted into immunodeficient hosts (Figure 2B). Micro-CT was performed at 4 weeks to evaluate the bone growth of vertebral bodies (Figure 2C-E). As expected, the bone growth was greater in the vertebral bodies transplanted with PGK1-overespressed PCa, compared to controls (Figure 2C-E). Interestingly, significantly more osteoblastic bone formation occurred (Figure 2F&G), while less vasculalization was found in the vertebral bodies that were implanted with PGK1-overespressed PCa than controls (Figure 2H&I). These data further suggest that PGK1 that is secreted by PCa regulates the bone formation.
Figure 2. PGK1-derived from PCa is involved in the bone formation in vivo.
(A) PGK1 levels in CM from PCa cells (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1) were evaluated by ELISA. Data are presented as mean ± standard deviation. Significant difference is from controls. (B) Experimental schema. PCa cells (1 × 104 cells; n = 4) were injected into vertebral bodies and then vertebral bodies were implanted in into SCID mice. At 4 weeks, (C) 3-dimensional micro-computed tomography measurements of the vertebral bodies were performed. Bone parameters including (D) bone mineral density (BMD) and (E) bone volume fractions (BVF) were calculated. Data are presented as mean ± standard deviation. Significant difference is from controls. (F) Mason's tetracrome stain and of the bone/tumor interface. Original magnification: 40x. Bar = 100 microns. (G) The numbers of the osteoblasts on the bone surface in (F). Data are presented as mean ± standard deviation. Significant difference is from controls. (H) The histologic evaluation of microvessel growth (factor VIII) in tumors with different levels of PGK1. Original magnification: 40x. Arrows: microvessels. Bar = 100 microns. (I) Factor VIII positive area from (D). Data are presented as mean ± standard deviation. Significant difference is from controls. These data demonstrated that PCa-derived PGK1 is involved in the bone formation in vivo.
PGK1 by PCa Regulates Osteoblastic Differentiation of BMSCs
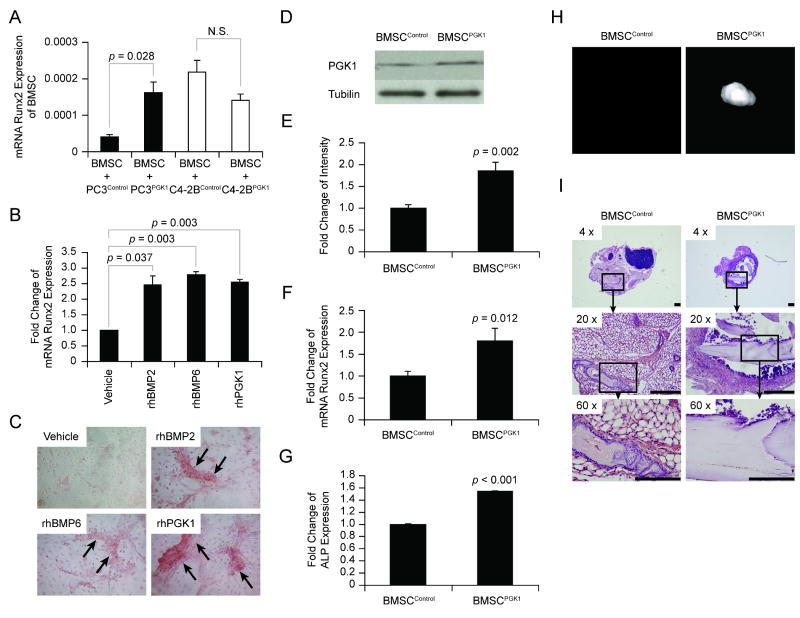
To determine if PGK1 secreted by PCa is able to induce osteoblastic induction of mixed bone marrow stromal cells (BMSCs), BMSCs were treated with conditioned medium (CM) derived from various PCa cell lines. BMSCs treated with PC3Control CM expressed less Runx2 mRNA compared to BMSCs treated with PC3PGK1 CM, whereas CM from C4-2BPGK1 did not have an osteoblastic effect on BMSCs (Figure 3A). To determine if PGK1 is directly able to induce BMSCs towards an osteoblastic lineage, BMSCs were treated in vitro with rhPGK1. As positive controls for factors that induce a bone phenotype, BMSCs were also treated with BMP2 and BMP6 (Figure 3B&C). At 21 days were examined for Runx-2 expression and mineralization of their extracellular matrix by staining for alizarin red. The data demonstrate that PGK1-treated BMSCs express more Runx2 mRNA (Figure 3B) and mineralized their extracellular matrix to a greater extent than did vehicle-treated BMSCs (Figure 3C). To further determine the role of PGK1 in bone formation, PGK1 was over-expressed in BMSCs (BMSCPGK1) (Figure 3D&E). The expression of Runx2 mRNA (Figure 3F), and alkaline phosphatase levels (Figure 3G) of the BMSCPGK1 were considerably higher than the levels expressed by BMSCControl cells. To validate the in vitro findings, 1 × 106 BMSCControl and BMSCPGK1 cells were implanted into immune competent mice. At 5 weeks, the resulting bony ossicles were evaluated by micro-CT and histological analyses. A round dense bony ossicle was visualized by micro-CT in the implants generated with BMSCPGK1 cells, while little or no signal was found in the implants derived from BMSCControl cells (Figure 3H). Subsequent histologic analyses demonstrated less bone formed in the implants established with BMSCControl cells compared to implants containing BMSCPGK1 cells (Figure 3I). These data suggest that PGK1 secreted by PCa regulates osteoblastic commitment of BMSCs, and further suggest that PGK1 plays an important role in the bone formation in vivo.
Figure 3. PGK1 induces osteoblastic Differentiation.
To determine if PGK1 induces mesenchymal osteoblastic differentiation, the BMSCs (2 × 105 cells / well) were plated onto a 24-well plate with osteogenic media. (A) The BMSCs were treated with 10% (v/v) CM derived from PCa cells (PC3Control, PC3PGK1, C4-2BControland C4-2BPGK1). At 21 days, the expression of Runx2 mRNA in BMSCs was determined by real-time RT-PCR. Significant is from control. N.S.: No significant. (B) BMSCs were treated with rhBMP2, rhBMP6, and rhPGK1. At 21 days, the expressions of Runx2 mRNA in BMSCs were determined by real-time RT-PCR. Significant difference is from vehicle treatment. (C) Representative Alizarin Red staining from (B). Next, to determine the role of PGK1 in bone formation, BMSCs were over-expressed PGK1 or control vectors. (D) Western blott analysis of PGK1 expression in BMSCControl and BMSCPGK1 cells. (E) The quantitative data of (D). (F) The expression of Runx2 mRNA was compared by real-time RT-PCR and normalized to β-actin as a loading control. (G) Alkaline phosphatase activity in the cell lysates were evaluated by ELISA. Significant difference is from BMSCControl. In (H) and (I), BMSCControl or BMSCPGK1 (2 × 106 cells; n = 3) were implanted in gelatin sponges into C57BL/6 mice. At 5 weeks, (H) 3-dimensional micro-computed tomography measurements of the bony ossicles were performed. (I) Representative H&E staining of the bony ossicles. All data are presented as mean ± standard deviation. These data demonstrated that PGK1 secreted by PCa cells induces osteoblastic commitment from BMSCs. Arrows: Positive areas. Bar = 100 microns.
PGK1 by PCa Inhibits Osteoclatogenesis
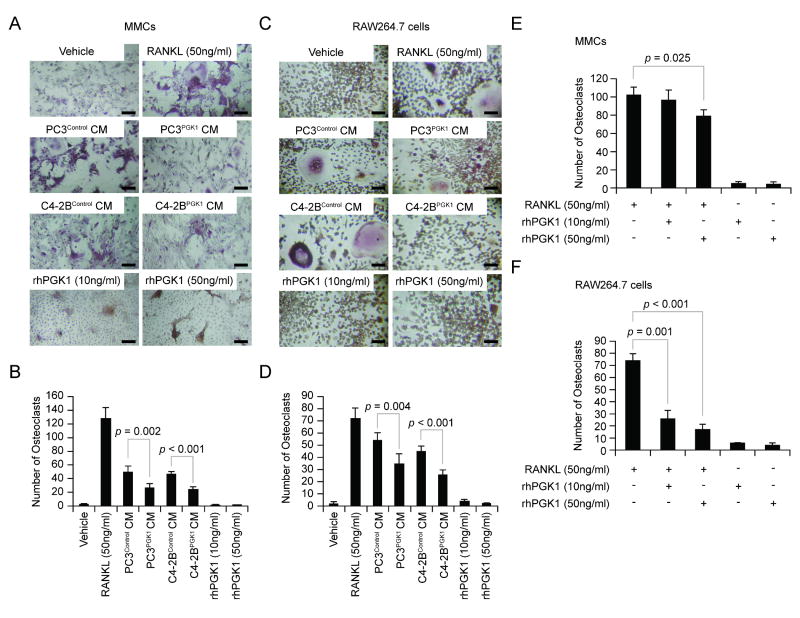
Next, we explored if PGK1 directly regulates osteoclastogenesis. The rationale for these studies was that the histologic analyses suggested that reductions in osteoclast numbers were associated with increased bone formation (Figure 1A-E). For these studies, mixed marrow mononuclear cells (MMCs) and osteoclast precursor cell line RAW264.7 cells were treated with RANKL, rhPGK1, and CM derived from PCa cells (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1) for 7 days. Thereafter, TRAP staining was performed and multinucleated TRAP-positive cells in the cultures were counted. As expected, the RANKL enhanced osteoclastogenesis in both MMCs and RAW264.7 cells (Figure 4A-D). Interestingly, compared to the control cells, CM from PC3PGK1 and C4-2BPGK1 prevented osteoclast formation by both MMCs and RAW264.7 cells (Figure 4A-D). Moreover, rhPGK1 itself had no direct effects on the generation of osteoclasts (Figure 4A-D). To address whether PGK1 itself affects osteoclastgenesis, MMCs and RAW264.7 cells were treated with RANKL and/or rhPGK1. Intriguingly, rhPGK1 inhibited RANKL-derived osteoclast formation (Figure 4E&F). These data suggest that PGK1-derived from PCa inhibits osteoclast formation in the marrow.
Figure 4. PGK1 Inhibits Osteoclatogenesis.
To evaluate if PGK1 effects on osteoclastogenesis, the MMCs (1x105 cells / well) or RAW264.7 cells (3x104 cells / well) were plated into 96-well plates. The cells were treated with either RANKL (50 ng/ml), rhPGK1 (10-50 ng/ml), or 10% (v/v) CM derived from PCa cells (PC3Control, PC3PGK1, C4-2BControl, and C4-2BPGK1). At 7 days, TRAP staining was performed. (A) Representative TRAP-positive cells of the MMC cultures. Original magnification: 20x. Bar = 50 microns. (B) The numbers of TRAP-positive cells from (A). (C) Representative TRAP-positive cells of the RAW264.7 cells. Original magnification: 20x. Bar = 50 microns. (D) The numbers of TRAP-positive cells from (C). Data are presented as mean ± standard deviation. Significant difference is from control. To evaluate if PGK1 inhibits osteoclastogenesis, (E) MMCs (1x105 cells / well) or (F) RAW264.7 cells (3x104 cells / well) were plated onto a 96-well plate. The cells were treated with RANKL (50 ng/ml) and/or rhPGK1 (10-50 ng/ml). At 7 days, TRAP staining was performed. Data are presented as mean ± standard deviation. Significant difference is from RANKL treatment. These data demonstrated that PGK1 inhibits the osteoclast formation.
Expression of PGK1 by PCa induces an Osteoblastic Phenotype
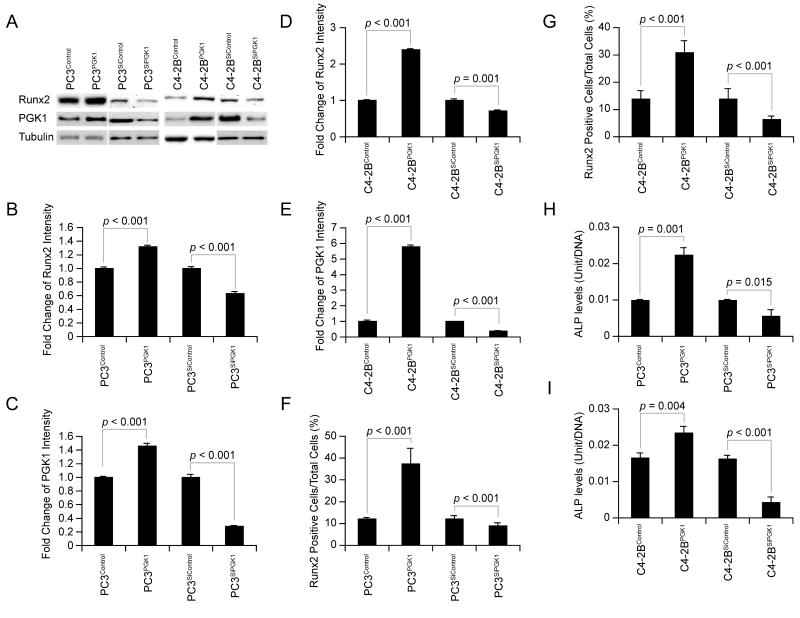
Another explanation of the bone formation in the tumor metastatic sites could be that PCa cells themselves express an osteoblastic phenotype. To address this possibility, we compared the expression of the osteoblastic markers between PGK1-overexpressed PCa, PGK1-knocked down PCa, and controls. Significantly more Runx2 expression in PC3PGK1 and C4-2BPGK1 was detected by western blot (Figure 5A&B&D) and by immunohistochemistry (Figure 5F&G and Supplemental Figure 1A&B), whereas significantly less Runx2 expression in PC3SiPGK1 and C4-2BSiPGK1 was detected by western blot (Figure 5A&B&D) and by immunohistochemistry (Figure 5F&G and Supplemental Figure 1A&B), As shown in Figure 5H&I, alkaline phosphatase activities in the cell lysates of the PC3PGK1 and C4-2BPGK1 cells were also elevated, compared to the controls. In comparison, alkaline phosphatase activities in the cell lysates of the PC3SiPGK1 and C4-2BSiPGK1 cells declined, compared to the controls (Figure 5H&I). These data suggest that tumor-derived PGK1 regulates the expression of bone related phenotypes.
Figure 5. PGK1 expression in PCa cells regulates expression of an osteoblastic phenotype.
To determine if PGK1 induces an osteoblastic phenotype in PCa cells, PGK1 was over-expressed and knocked-down in PC3 and C4-2B cells. (A) Western blot analysis for Runx2 and PGK1 expression in the PCa cells (PC3Control, PC3PGK1, PC3SiControl, PC3SiPGK1, C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGK1). Quantitative assessment of data from (B&C) PC3Control, PC3PGK1, PC3SiControl, and PC3SiPGK1 cells and (D&E) C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGK1 cells. Runx2 positive cells from immunohistochemistry for Runx2 expressed by (F) PC3Control, PC3PGK1, PC3SiControl, and PC3SiPGK1 cells and (G) C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGK1 cells using monoclonal antibodies. (H&I) Alkaline phosphatase activity in the cell lysates of the PCa cells was elevated by ELISA. Data are presented as mean ± standard deviation. Significant difference is from controls. These data demonstrated that PCa-derived PGK1 regulates their own expression of osteoblastic phenotypes.
Discussion
Skeletal metastases resulting from PCa typically result in osteosclerotic bone lesions. Yet, in most animal models of human disease osteolytic lesions are most commonly observed (19). The molecular mechanisms that account for the predominantly osteoblastic skeletal phenotype in humans of PCa disease remain unclear (4, 21). Recently, we observed CXCL12 signaling plays a major role in localizing PCa to the marrow (6-12). One of the major down stream targets of CXCL12 signaling is the inhibition of PGK1 expression (11). Secreted PGK1 acts as a disulfide reductase that cleaves plasminogen generating the vascular inhibitor angiostatin (15-18). Limiting PGK1 secretion provides a mechanism for metastatic PCa to grow in tissues with high CXCL12 levels by promoting angiogensis (11). However, during the course of these studies we observed that that over expression of PGK1 in PC3 cells resulted in excessive formation of woven bone reminiscent of the osteoblastic lesions typically observed in PCa disease. Here, we determined that PCa cell lines that over express PGK1 have enhanced expression of several osteoblastic markers. In addition PGK1 expression in PCa induced osteoblastic commitment from mixed bone marrow progenitor cells. Moreover, over-expression of PGK1 by PCa inhibited osteoclast bone formation. These results suggest that PGK1 plays a central role in regulating the induction of expression of osteoblast-specific phenotypes by three potential mechanisms that are inhibiting osteoclastic activity, enhancing osteoblast formation, and possibly altering the phenotype of the cancer cell itself into osteomemetic cells.
One mechanism whereby PCa expression of PGK1 is likely to play a role in the expression of an osteoblastic bone phenotype is to regulate osteoblastic commitment of mesenchymal stem or progenitor cells. In this study that we observed that addition of PGK1 directly to bone marrow stromal cells, or conditioned medium derived from the PCa cells themselves, had a stimulating effect on the formation of mineralized bone nodules. However, how PGK1 regulates mineralization is not clear. One possible scenario is that PGK1 may play a role in the selective survival of MSCs during hypoxic events. Recent work by Balduino have demonstrated that MSC populations appear to localize to the subendosteal region (25) which in other studies have been demonstrated to represent areas of hypoxia in the marrow (26, 27). Alternatively, PGK1 may be directly involved in promoting the expansion of osteoblastic progenitor cells or the commitment of preosteoblasts to a mature phenotype. It is also conceivable that PGK1 functions by an alternative mechanism in that several reports have demonstrated that mineralization in vivo during development occurs most efficiently following vascular invasion of the bone analogue. In fact, it seems just as likely that PGK1 would have to function to promote osteogenesis independent of its effects on the vasculature (28-30). However, the expression of PGK1 was previously noted to be exceptionally strong in odontogenic epithelial cells and the surrounding mesenchymal cells of the tooth germ from embryonic day (E) 10.5 to E18.0, suggesting that PGK1 expression may in fact be a feature of a mineralizing phenotype (31).
Normal bone remodeling is a dynamic balance of bone forming and bone degrading activity. One mechanism which may lead to an overall increase in bone formation when PGK1 is over expressed in the context of PCa is to either reduce osteoclast numbers or activities. Our data suggests that PCa derived PGK1 inhibits osteoclast formation which would serve to uncouple bone formation from resorption. How this occurs is also not clear. Hypoxia and HIF-1α develop the osteolytic bone metastases by suppressing osteoblast differentiation and promoting osteoclastogenesis (32). One clue as to the mechanism comes from our previous work which demonstrates that over expression of PGK1 in PCa cells results in the reduction of IL-8 and VEGF synthesis (11). While VEGF is involved in the vascular formation, VEGF also plays an important role in the osteoclast activity (33-35). In osteopetrotic (op/op) mice that have lack of the functional macrophage colony-stimulating factor (M-CSF)-known to activate the osteoclast formation, a single injection of VEGF rescued their osteoclast recruitment (33, 34). The osteoclast differentiation is also regulated by receptor activator of NF-κB ligand (RANKL) (33). However, we recently found that the changing PGK1 expression in PCa did not alter the RANKL levels in PCa (data not shown). Thus, PGK1 may prevent the osteoclastogenesis by suppressing VEGF secretion.
That PGK1 could regulate the expression of an osteomiminic phenotype by PCa cells themselves is a real possibility in that we observed that PGK1 induces the expression of Runx2. Runx2 is a transcriptional factor known to induce osteoblastic differentiation (36, 37). We detected more Runx2 mRNA and protein when PGK1 was expressed in PCa cells themselves or in lesions generated by PGK1 over expressing PCa cells. In addition, over expression of PGK1 also resulted in enhanced expression of osteocalcin and alkaline phosphatase by PCa cells. Each of which are critical components of the osteoblast phenotype. In other studies, osteoblastic PCa cells (MDA PCa 2a and MDA PCa 2b) themselves have been shown to participate in the generation of osseous tissues through Runx2 signaling pathway, whereas little osteoblastic reaction are typically found in the osteolytic PCa cells (PC3) (36). Previous work by our group has demonstrated that Wnt/BMPs signaling pathway plays a crucial role in determining the phenotype of the bone metastatic lesion in PCa (38, 39). Although the detailed molecular mechanisms remain unclear, PGK1 may be involved in the mineralization by PCa themselves. However, a large screen of Wnt/BMP signaling cascades were unable to identify which if any members of the pathway were direct PGK1 targets (data not presented).
Previously, we presented data that demonstrates that high levels of PGK1 are essential for tumor growth, but limit angiogenesis when secreted extracellularly. However, at sites of high CXCL12 production such as bone, lymph node, liver, however, PGK1 secretion is likely to be inhibited. Thus, CXCL12 signaling through CXCR4 generates an ‘angiogenic switch’ that may be necessary for metastatic growth (11). Together, these data demonstrate that the chemokine axis and PGK1 represent at least one of the critical determinants for metastasis of PCa as well as a mechanism for a pro-angogenic switch that promotes tumor growth. Yet, it is difficult to reconcile our previous report with our current knowledge of the role of PGK1 in PCa bone disease. From a therapeutic stand point, stimulating PGK1 expression would appear to represent a reasonable strategy if indeed its major effect would be to limit tumor growth at metastatic sites such as the marrow. On the other hand, enhanced PGK1 expression would appear to promote the osteosclerotic phenotype of PCa disease associated with bone metastases. For PGK1 to become an effective therapeutic target, further knowledge as to how to shift the balance from tumor growth without negatively impacting bone health will be required.
In summary, our work suggests that there are three possible mechanisms whereby PGK1 secreted by PCa cells may regulate mineral deposition. PGK1 expression may drive mesenchymal stem or progenitor cells down the osteoblastic lineage providing more mature osteoblasts relative to osteoclasts, while at the same time suppressing osteoclast formation. PGK1 may also alter the ability of PCa cells to themselves participate in the mineralization process. Although further investigation is needed, these results suggest that PGK1 serves as a key moderator of the bone remodeling at the tumor metastatic site.
Supplementary Material
Immunohistochemistry for Runx2 expressed by (A) PC3Control, PC3PGK1, PC3SiControl, and PC3SiPGK1 cells and (B) C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGK1 using monoclonal antibodies or immunoglobin controls. Original magnification: 20x. Arrows: Positive cells. Bar = 50 microns.
Acknowledgments
We thank Drs. Laurie K. McCauley and Kenneth J. Pienta for scientific discussions. The authors are also grateful to Dr. Tae-Geon Kwon in the Laboratory of Dr. Renny T. Franceschi (University of Michigan) for assistance with alkaline phosphatase assays. This work is directly supported by the Pediatric Oncology Research Fellowship (Y.S.), the National Cancer Institute (E.T.K. and R.S.T.), and the Department of Defense (R.S.T.).
References
- 1.Jacobs SC. Spread of prostatic cancer to bone. Urology. 1983;21(4):337–44. doi: 10.1016/0090-4295(83)90147-4. [DOI] [PubMed] [Google Scholar]
- 2.Koutsilieris M, Rabbani SA, Bennett HP, Goltzman D. Characteristics of prostate-derived growth factors for cells of the osteoblast phenotype. J Clin Invest. 1987;80(4):941–6. doi: 10.1172/JCI113186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RE, Guise TA, Lipton A, et al. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin Cancer Res. 2008;14(20):6387–95. doi: 10.1158/1078-0432.CCR-08-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 6.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7. [PubMed] [Google Scholar]
- 7.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 8.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20(2):318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17(12):1578–92. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67(1):61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Dai J, Jung Y, et al. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2007;67(1):149–59. doi: 10.1158/0008-5472.CAN-06-2971. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Shiozawa Y, Wang Y, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 13.Migita T, Oda Y, Naito S, Morikawa W, Kuwano M, Tsuneyoshi M. The accumulation of angiostatin-like fragments in human prostate carcinoma. Clin Cancer Res. 2001;7(9):2750–6. [PubMed] [Google Scholar]
- 14.Daly EB, Wind T, Jiang XM, Sun L, Hogg PJ. Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim Biophys Acta. 2004;1691(1):17–22. doi: 10.1016/j.bbamcr.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 16.Detterbeck FC, Falen S, Rivera MP, Halle JS, Socinski MA. Seeking a home for a PET, part 2: Defining the appropriate place for positron emission tomography imaging in the staging of patients with suspected lung cancer. Chest. 2004;125(6):2300–8. doi: 10.1378/chest.125.6.2300. [DOI] [PubMed] [Google Scholar]
- 17.Peyruchaud O, Serre CM, NicAmhlaoibh R, Fournier P, Clezardin P. Angiostatin inhibits bone metastasis formation in nude mice through a direct anti-osteoclastic activity. J Biol Chem. 2003;278(46):45826–32. doi: 10.1074/jbc.M309024200. [DOI] [PubMed] [Google Scholar]
- 18.Peyruchaud O, Serre CM, NicAmhlaoibh R, Sveigaard C, Clezardin P. Does tumor angiogenesis play a role in bone metastatic process? Rev Med Suisse Romande. 2004;124(2):83–4. [PubMed] [Google Scholar]
- 19.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5(1):21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 20.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 21.Goltzman D, Bolivar I, Rabbani SA. Studies on the pathogenesis of osteoblastic metastases by prostate cancer. Adv Exp Med Biol. 1992;324:165–71. doi: 10.1007/978-1-4615-3398-6_17. [DOI] [PubMed] [Google Scholar]
- 22.Wu TT, Sikes RA, Cui Q, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77(6):887–94. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Lay AJ, Jiang XM, Kisker O, et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408(6814):869–73. doi: 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- 24.Shiozawa Y, Havens AM, Jung Y, et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balduino A, Hurtado SP, Frazao P, et al. Bone marrow subendosteal microenvironment harbours functionally distinct haemosupportive stromal cell populations. Cell Tissue Res. 2005;319(2):255–66. doi: 10.1007/s00441-004-1006-3. [DOI] [PubMed] [Google Scholar]
- 26.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031–7. [PubMed] [Google Scholar]
- 27.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116(5):1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8(21):980–9. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 29.Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 30.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda JY, Kobayashi I, Kiyoshima T, et al. Glycolytic enzyme Pgk1 is strongly expressed in the developing tooth germ of the mouse lower first molar. Histol Histopathol. 2008;23(4):423–32. doi: 10.14670/HH-23.423. [DOI] [PubMed] [Google Scholar]
- 32.Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T. Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 2007;67(9):4157–63. doi: 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- 33.Niida S, Kaku M, Amano H, et al. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190(2):293–8. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engsig MT, Chen QJ, Vu TH, et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151(4):879–89. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa M, Kaneda T, Arakawa T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000;473(2):161–4. doi: 10.1016/s0014-5793(00)01520-9. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Fizazi K, Peleg S, et al. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res. 2001;61(14):5652–9. [PubMed] [Google Scholar]
- 37.Brubaker KD, Vessella RL, Brown LG, Corey E. Prostate cancer expression of runt-domain transcription factor Runx2, a key regulator of osteoblast differentiation and function. Prostate. 2003;56(1):13–22. doi: 10.1002/pros.10233. [DOI] [PubMed] [Google Scholar]
- 38.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554–60. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 39.Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68(14):5785–94. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry for Runx2 expressed by (A) PC3Control, PC3PGK1, PC3SiControl, and PC3SiPGK1 cells and (B) C4-2BControl, C4-2BPGK1, C4-2BSiControl, and C4-2BSiPGK1 using monoclonal antibodies or immunoglobin controls. Original magnification: 20x. Arrows: Positive cells. Bar = 50 microns.