Abstract
Infection with Trypanosoma cruzi, the etiologic agent of Chagas disease is accompanied by an intense inflammatory reaction. Our laboratory group has identified adipose tissue as one of the major sites of inflammation during disease progression. Because adipose tissue is composed of many cell types, we were interested in investigating whether the adipocyte per se was a source of inflammatory mediators in this infection. Cultured adipocytes were infected with the Tulahuen strain of T. cruzi for 48–96 h. Immunoblot and quantitative PCR (qPCR) analyses demonstrated an increase in the expression of proinflammatory cytokines and chemokines, including interleukin (IL)-1β, interferon-γ, tumor necrosis factor-α, CCL2, CCL5, and CXCL10 as well as an increase in the expression of Toll-like receptors-2 and 9 and activation of the notch pathway. Interestingly, caveolin-1 expression was reduced while cyclin D1 and extracellular signal-regulated kinase (ERK) expression was increased. The expression of PI3kinase and the activation of AKT (phosphorylated AKT) were increased suggesting that infection may induce components of the insulin/IGF-1 receptor cascade. There was an infection-associated decrease in adiponectin and peroxisome proliferator–activated receptor-γ (PPAR-γ). These data provide a mechanism for the increase in the inflammatory phenotype that occurs in T. cruzi–infected adipocytes. Overall, these data implicate the adipocyte as an important target of T. cruzi, and one which contributes significantly to the inflammatory response observed in Chagas disease.
INTRODUCTION
Infection with Trypanosoma cruzi, a kinetoplastid protozoan, causes Chagas disease, an important cause of cardiac disease in endemic areas of Latin America. Recently, Chagas disease has been recognized among immigrants to the nonendemic areas of North America and Europe (1). This infection is also an emerging opportunistic infection in individuals with HIV/AIDS (2). T. cruzi invades many cell types but primarily those of the cardiovascular, reticuloendothelial, and autonomic nervous systems. Infection with this parasite is associated with an intense inflammatory response (3–5). Recently, adipose tissue has been observed to be an important target of T. cruzi infection in animal models (6). The interaction of microbial organisms with adipocytes has only recently been explored (7). For example, it has been demonstrated that CD4, CXR4, and CCR5 which are regarded as receptors for HIV are expressed on the surface of adipocytes (8). Furthermore, Dhurander’s group has suggested a possible viral etiology for certain forms of obesity (9).
Adipose tissue is not only a storage site for triglycerides, but also acts as an endocrine organ contributing to energy homeostasis, immune responses, and infection (7,10). Adipose tissue and adipocytes exert their influence through the synthesis and release of adipocyte-specific and adipocyte-enriched hormonal factors, cytokines, and extracellular matrix component collectively known as “adipokines” (7). Although little attention has been paid to the role of adipose tissue in infections including those caused by parasites, the intense proinflammatory potential of this tissue indicates that it may play a major role in the innate immune response.
In addition to adipocytes, adipose tissue is composed of other cell types including fibroblasts, endothelial cells, skeletal, and smooth muscle cells. During inflammation adipose tissue is infiltrated by leukocytes and macrophages. Thus questions have been raised regarding the specific contribution of the adipocyte to the inflammatory process. Previously, we reported that infection of cultured adipocytes resulted in the synthesis of inflammatory mediators (6). Herein we extend our previous studies demonstrating that infection of adipocytes by T. cruzi results in increased expression of cytokines and chemokines and the data provide a possible mechanistic basis for these observations.
Methods And Procedures
Parasite
The Tulahuen strain of T. cruzi was maintained in A/J mice (Jackson Laboratories, Bar Harbor, ME) and in cultured L6E9 myoblasts (11).
Adipocyte differentiation in cell culture and infection
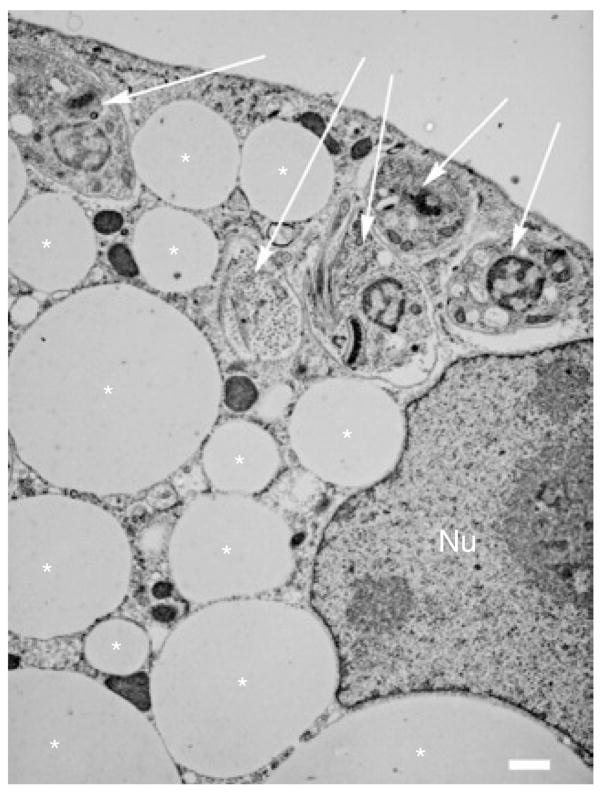
3T3-L1 murine fibroblasts were propagated and differentiated to adipocytes in culture plates as previously described (6). Cells were used between day 8 and 12 postinduction of differentiation. The cultured adipocytes were infected by trypomastigotes at a multiplicity of infection of 2:1 to 5:1 (Figure 1).
Figure 1.
Representative electron micrograph of a Trypanosoma cruzi–infected cultured adipocyte. Asterisks indicate lipid droplet. Arrows indicate parasites. Nu indicates adipocyte nucleus. Bar = 1 μm.
Immunoblot analysis
After infection, the adipocytes were washed four times with phosphate buffered saline (pH 7.4) and lysed in 1 ml lysis buffer containing 50 mmol/l Tris pH 7.5, 1% NP-40, and 150 mmol/l sodium chloride plus protease inhibitor cocktail. Protein lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% acrylamide gels and transferred to BA85 nitrocellulose (Schleicher & Schuell, Florham Park, NJ). Blots were probed with various antibodies as indicated. Primary and secondary antibodies were diluted in phosphate-buffered saline with 0.1% Tween 20 and 1% bovine serum albumin. Bound antibodies were detected by enhanced chemiluminescence according to the manufacturer’s instructions (Amersham, Piscataway, NJ). Antibodies to the components of the Notch pathway were obtained from Abcam (Cambridge, MA). All other antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Real time PCR quantification
RNA was isolated from control and infected fat cells using the Trizol reagent following the manufacturer’s protocol (Invitrogen, Carlsbad, CA). RNA was reverse transcribed from 100 ng of total RNA in a final volume of 20 μl using Superscript II reverse transcriptase according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). The reverse transcription mixture consisted of 0.5 mmol/l dNTPs, 20 mmol/l dithiothritol, 30 mmol/l Tris HCl pH 8.3, 75 mmol/l KCl, 3 mmol/l MgCl2, 500 ng oligo dT, and 200 U of superscript RT RNase H-reverse transcriptase (Invitrogen). The reaction was incubated for 50 min at 42 °C. The primers used for the amplification of quantitative PCR (qPCR) of Toll-like receptors (TLRs), chemokines, chemokine receptors; cyclins, and glyceraldehyde-3-phosphate dehydrogenase are listed in Supplementary Table S1 online.
The qPCR was run using PCR Sybr Green Master Mix (Roche Applied Science, Indianapolis, IN) and magnesium chloride in the Light Cycler (Roche Applied Science). To normalize gene expression, mRNA expression of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase was measured. For each sample, both the housekeeping and target genes were amplified in triplicate using the reaction condition and analytic parameters described previously (6).
RESULTS
Electron microscopy
Trypomastigotes of T. cruzi readily infected adipocytes. The intracellular amastigotes resided in the cytoplasm for >96 h postinfection (PI).
Effect of T. cruzi infection on mitogen activated protein kinases and caveolin-1
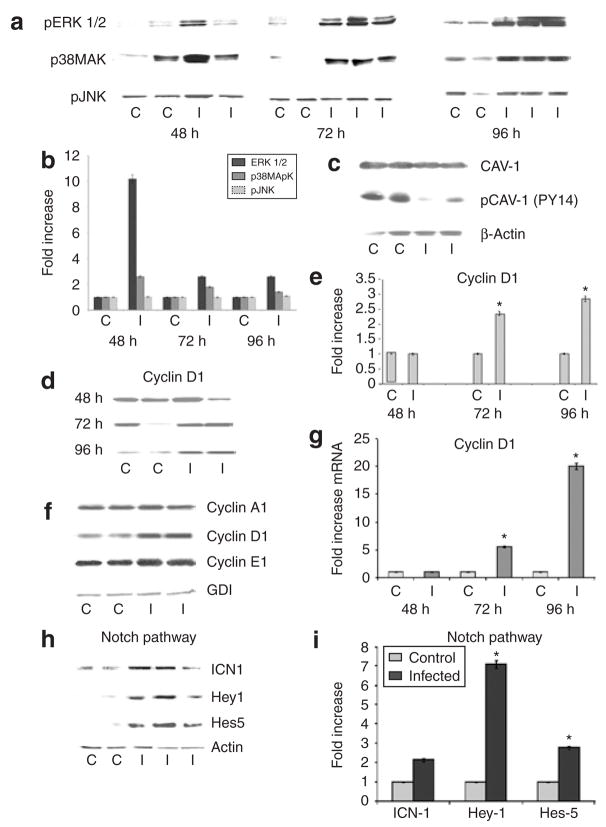
Adipocytes are rich in caveolin-1. This is an important structural and regulatory protein of plasma membrane raft structures (12), which have been previously implicated in the negative regulation of the expression of extracellular signal-regulated kinases 1 and 2 (ERK 1/2) and cyclin D1 (13). Immunoblot analysis revealed that infection resulted in a reduction in the expression of phosphorylated caveolin-1 and increased abundance of pERK (consistent with activation of ERK) (Figure 2a–c). Interestingly, infection also resulted in increased p38MAPK but not pJNK abundance (Figure 2a,b).
Figure 2.
Representative immunoblot/quantitative PCR analyses of the effect of Trypanosoma cruzi infection on expression of mitogen activated protein kinases, cell cycle regulatory proteins, and proteins of the notch signaling pathway in cultured adipocytes. (a) Immunoblot analysis of pERK (ERK42/44), p38 MAPK, and pJNK 48–96 h postinfection (PI). (b) Quantitative analysis of immunoblot (a) normalized to guanine nucleotide dissociation inhibitor (GDI). (c) Immunoblot analysis of pCaveolin-1 96 h PI. (d) Immunoblot analysis of cyclin D1 48, 72, and 96 h PI. (e) Quantitative analysis of immunoblots (d) demonstrating a significant increase in cyclin D1 protein levels normalized to GDI. (f) Immunoblot analysis of cyclin A1, cyclin D1, and cyclin E1 in infected adipocytes 96 h PI. (g) Fold-increase in the expression of cyclin D1 mRNA level as analyzed by quantitative reverse transcriptase–PCR 48–96 h PI. (h) Representative immunoblot analysis of levels of ICN, Hey-1, and Hes-5 in infected adipocytes. (i) Quantitative representation of data shown in h. C, control; I, infected. (n = 3, asterisk denotes significance, P < 0.05).
Effect of T. cruzi infection on cyclins and the notch pathway
There was a dramatic increase in the mRNA level of cyclin D1 (Figure 2g). Immunoblot analysis demonstrated an increase in cyclin A1 and cyclin E1 (Figure 2f) compared with uninfected adipocytes. Immunoblot analysis also showed an increased expression of cyclin D1 at 72 and 96 h PI (Figure 2d–f). Since the notch signaling pathway increases the expression of cyclin D1, immunoblotting was used to analyze intracellular notch (ICN) and the downstream proteins Hey-1 and Hes-5. A significant increase in the expression of ICN, Hey-1, and Hes-5 was observed in infected adipocytes compared with uninfected control cells (Figure 2h,i).
T. cruzi infection of adipocytes upregulates the inflammatory response
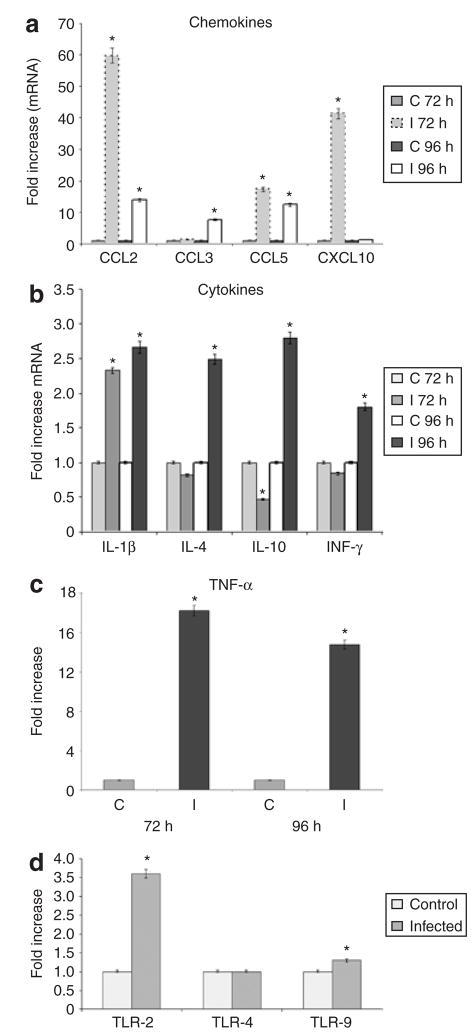
Infection of fat cells resulted in an increase in the expression of chemokines as determined by qPCR. At 72 h significant fold-increases in mRNA levels of CCL2, CCL5, and CXCL10 (60, 17.4, and 41.4, respectively) compared with uninfected fat cells were observed (Figure 3a). In addition, at 96 h there were significant fold-increases in CCL2, CCL3, and CCL5 (13.8, 7.7, and 12.4, respectively). The mRNA levels for CXCL5 and the receptors CCR5 and CCR3 were not increased (data not shown). There was a significant upregulation of mRNA expression of cytokines including tumor necrosis factor-α, interleukin (IL)-1β, interferon-γ, and IL-10 (Figure 3b,c).
Figure 3.
Effects of Trypanosoma cruzi infection on expression of cytokines, chemokines, and Toll-like receptors (TLRs) in adipocytes. (a) Quantitative PCR (qPCR) analysis of CCL2, CCL3, CCL5, and CXCL10 represented as fold-increase in mRNA levels 72 and 96 h postinfection (PI). (b) qPCR analyses demonstrate fold-increases in mRNA expression levels of interleukin (IL)-1β, IL-4, IL-10, and IFN-γ 72 and 96 h PI. (c) Fold-increase in the mRNA levels of TNF-α determined by qPCR 72 and 96 h PI. (C, control; I, infected). (n = 3). (d) qPCR analysis of TLR-2, TLR-4, and TLR-9 in fat cells 96 h PI. The data are shown as fold change normalized to glyceraldehyde-3-phosphate dehydrogenase. The increases in TLR-2 and TLR-9 are significant (n = 3, asterisk denotes significance, P < 0.05).
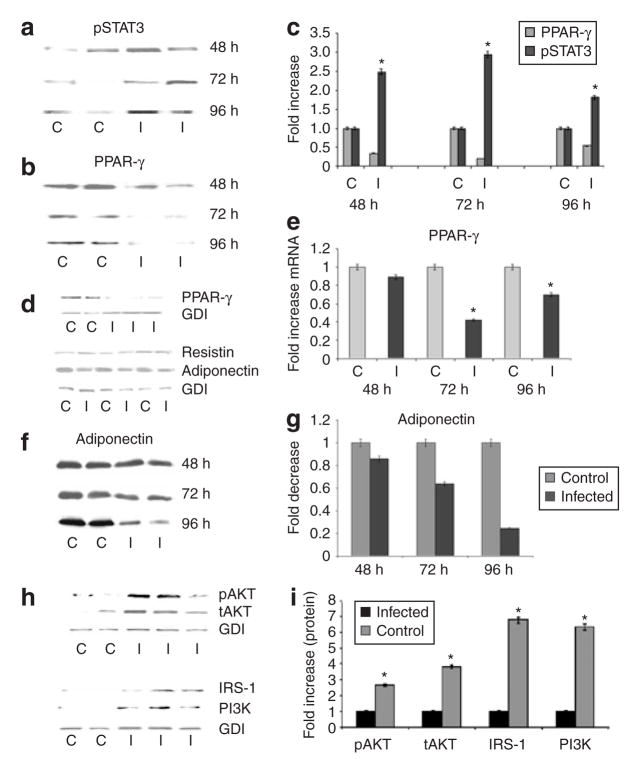
The expression of TLRs (TLR-2 and TLR-9) mRNA was significantly increased as a result of infection whereas there was no significant change in the mRNA level of TLR-4 (Figure 3d). Signal Transducer and Activator of Transcription-3 (STAT3) is an important component of cytokine signaling in adipocytes. Immunoblot analysis demonstrated that STAT3 was activated from 48 to 96 h PI (Figure 4a,c).
Figure 4.
Effect of Trypanosoma cruzi infection on expression of pSTAT3, PPAR-γ, adipocyte-specific proteins and insulin/IGF-1 receptor signaling proteins in adipocytes. (a) Representative immunoblot analysis of pSTAT3 expression 48, 72, and 96 h postinfection (PI). (b) Immunoblot analysis of PPAR-γ abundance 48, 72, and 96 h PI. (c) Quantitative analysis of protein abundance presented as fold-increase. PPAR-γ is significantly reduced and pSTAT3 is significantly increased at all time-points. (d) Representative immunoblot analysis of PPAR-γ, resistin, and adiponectin in fat cells 96 h postinfection. (e) Quantitative PCR showing fold decrease in the expression of PPAR-γ 48, 72, and 96 h PI (n = 3). (f) Representative immunoblot analysis of adiponectin (MW = 30 kDa) abundance 48, 72, and 96 h PI. There is a significant reduction in abundance 72 and 96 h PI (asterisk denotes significance, P < 0.05). (g) Quantitative representation of data shown in f normalized to GDI. (h) Representative immunoblot analysis of pAKT/IRS-1 and PI3K abundance in control (C) and infected (I) adipocytes 96 h PI. (i) Quantitative representation of data shown in h. (n = 3). All changes in the infected cells are significant (P < 0.05).
Adiponectin and PPAr-γ, AKt, and PI3kinase
Adiponectin and peroxisome proliferator–activated receptor-γ (PPAR-γ) negatively regulate the inflammatory response in adipocytes. There was a significant reduction in the abundance of adiponectin as a result of infection. In addition, there was a significant reduction in PPAR-γ both by immunoblot analysis and by qPCR (Figure 4b,d,e). The reduction in adiponectin levels 96 h PI was consistent with our earlier observation that adiponectin expression was reduced at 48 h PI (Figure 4d,f,g). T. cruzi infection of adipocytes resulted in increased expression of resistin and IRS-1 as determined by immunoblotting. Immunoblot analysis also demonstrated increases in the abundance of PI3kinase (subunit 85) and pAKT as a result of infection (Figure 4h).
DISCUSSION
Adipose tissue can influence multiple biochemical and physiological processes including energy homeostasis, insulin resistance, and inflammation. Although adipose tissue is composed of many cell types, the adipocyte remains the main focus of attention. The major signaling pathways of the adipocyte are not dramatically different than those which are found in other mammalian cells. However, under proper conditions, activation of these signaling pathways results in the synthesis of proteins that are unique to the adipocyte, including adipsin, leptin, resistin, and adiponectin (7,10).
The synthesis of inflammatory mediators by adipose tissue is now appreciated to be an important aspect of the total inflammatory process (14), including inflammation induced by microbial agents and endotoxin, as confirmed by observations of inflammation in the fatless mouse model (15). The parasite T. cruzi readily infects and resides in cultured adipocytes (6). The current study was performed to evaluate the role of the adipocyte, in T. cruzi infection, in a model system devoid of many other cell types ordinarily found in adipose tissue such as endothelial cells, vascular smooth muscle cells, and macrophages. The culture system used depends on the differentiation of fibroblasts into adipocytes, and it is noted that residual fibroblasts may be observed (10–15% in a typical cell culture). This system, however, is the standard in vitro adipocyte culture method on which much of the literature is based. Adipocytes infected for 96 h maintain their integrity and intracellular amastigotes are observed (as monitored by electron microscopy and Giemsa staining).
It was determined that T. cruzi infection of cultured adipocytes induces an inflammatory phenotype. For example, there was increased expression of chemokines (CCL2, CCL3, CCL5, and CXCL10) and proinflammatory cytokines (tumor necrosis factor-α, IL-10, and interferon-γ). There were increased levels of IL-10, an anti-inflammatory cytokine. In addition, STAT3 is an important downstream mediator of cytokine signaling (16) that has been reported to be induced in this infection (17).
TLRs have been reported to be activated during T. cruzi infection of other cell types (18,19). In infected cultured adipocytes, TLR-2 and -9 are upregulated, whereas TLR-4 is not. In adipose tissue TLR-2, -4, and -9 are upregulated as a consequence of infection (E.N., P.E.S., H.B.T., unpublished data). The reason for this discrepancy between in vitro and in vivo data is not known. However, in vivo, the TLR-4 upregulation may be contributed by other cell types, such as macrophages.
There was also activation of components of the MAPK pathway during T. cruzi infection. Specifically ERK and p38 MAPK were activated whereas JNK was not. We have observed in a variety of cell types infected with T. cruzi activation of ERK, some of p38MAPK but not of JNK. This is true of smooth muscle cells and endothelial cells. Here, we also demonstrate a similar observation in cultured adipocytes. Although we do not yet understand the underlying mechanistic reasons, the data for the adipocytes is fully consistent with the observations in these other cell types (20,21).
T. cruzi infection of adipocytes resulted in increased expression of cyclin D1. We have reported that infection has a similar result in other cell types such as endothelial and vascular smooth muscle cells (20,21). Cyclin D1 is generally associated with cell proliferation; however, cultured adipocytes are usually terminally differentiated. The increased expression of cyclin D1 is of interest because it is upregulated by ERK and inversely regulated by caveolin-1 (13). Indeed, here we have demonstrated that infection resulted in a reduction in the expression of caveolin-1 and activation of ERK. Both of these events increase the expression of cyclin D1. The reduction in caveolin-1 expression has also been demonstrated to be associated with an increased proinflammatory cytokine response (12,22). Interestingly, infection activates the notch pathway which regulates, in part, the expression of cyclin D1 (23). The increase in cyclin D1 expression after infection may also, in part, represent a component of the underlying fibroblasts.
PPAR-γ is highly expressed in adipose tissue (24) and, in addition to the adipokine adiponectin, represses the inflammatory process (25,26). The mechanism by which adiponectin exerts an anti-inflammatory effect is unclear. There is evidence that it may be mediated by one of its principal targets, the AMP-activated protein kinase (27). In addition, there are reports that a truncated bacterial form of adiponectin has anti-inflammatory effects on the endothelium as a result of AMPK-mediated modulation of NF-κB and Akt/PKB (28,29). It is clear that a reduction in the level of adiponectin is associated with an increase in inflammation (7). Importantly, there is an inverse relationship between PPAR-γ and inflammation as well as between PPAR-γ and cyclin D1 (30). The cyclin D1/PPAR-γ relationship has been demonstrated in the human breast cancer model where an increased expression of cyclin D1 tracked with a reduction in PPAR-γ (31). Recent evidence suggests a similar relationship between adiponectin and PPAR-γ (25,26). In our current experiments, we have clearly demonstrated that T. cruzi infection resulted in a reduction in the expression of adiponectin and PPAR-γ as well as an increase in the expression of cyclin D1.
As stated previously, adipose tissue is composed of different cell types (7). Many of these cells including inflammatory cells synthesize the 21 amino acid peptide endothelin-1 (ET-1). Adipocytes do not synthesize ET-1 but they have both ETA and ETB receptors (32) and it has been reported that ET-1 downregulates adiponectin via multiple pathways (33–35). T. cruzi infection is associated with an increase in ET-1 plasma levels as well as an increase in the expression of ET-1 mRNA and protein in tissues. The sources of ET-1 in adipose tissue includes endothelial cells, fibroblasts, and inflammatory cells. It is, therefore, possible that the ET-1 synthesized by the cells present in the infected adipose tissue acts on adipocytes, resulting in the decrease in adiponectin observed. ET-1 itself is regarded by some as a proinflammatory cytokine as it can cause an increase in cytokine synthesis and can in turn be activated by cytokines and ERK (36). ET-1 can also increase the expression of leukocyte adhesion molecules (37). The addition of ET-1 to cultured adipocytes causes an increase in the synthesis of leptin which may regulate inflammation (38).
Infection also results in increased expression PI3kinase and activation of AKT, suggesting that this infection may induce components of the insulin/IGF-1 receptor cascade. This would be surprising as the upregulation of proinflammatory pathways is generally associated with a downregulation of the insulin signal transduction pathway (39,40). It is not clear what is responsible for this phenomenon, but it can be observed with a high degree of reproducibility in these cells. Despite the upregulation of some of the components of the pathway, there were no differences with respect to a dose–response to insulin in infected cells (not shown). It remains to be determined whether other pathways influenced by insulin may be affected, such as events leading to differences in the rate of lipid accumulation or lipolysis. T. cruzi is likely to have an impact on lipid pathways in vivo, yet these issues have not been examined to date.
We consider these findings as very significant. There is generally a positive correlation between inflammation and insulin resistance. However, the infection of adipocytes with a parasite that resides intracellularly is rather different from exposing adipocytes to conventional proinflammatory stimuli such as endotoxin. The continued intracellular presence of the parasites clearly has a differential effect on insulin sensitivity, perhaps by lowering the levels of one of the critical lipid mediators of insulin resistance. Although IRS-1 levels are not necessarily indicative of activity, it is generally accepted that pAkt levels downstream are an excellent reflection of the cellular insulin signaling activity in 3T3-L1 adipocytes. These interesting observations currently form the basis for an ongoing project in the laboratory.
Our studies and those of others (6) indicate that adipose tissue is a target of infection. In addition, adipose tissue and adipocytes probably represent a reservoir from which chronic T. cruzi infection may be reactivated during periods of immunosuppression. The observations in this paper demonstrate that T. cruzi infection results in an alteration of signaling pathways in adipocytes that likely influence systemic inflammation and energy homeostasis.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI068538 (H.B.T.) and AI052739 (H.B.T.), DK055758, DK075887, DK071030 (P.E.S.), Fogarty International Center Grant TW006857 (H.B.T.), C.J. de A. was supported by Fogarty Training Grant D43TW007129, and the Burroughs-Welcome Funds Career Awards for medical scientists (M.S.D).
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Kirchhoff LV, Pearson RD. The emergence of Chagas disease in the United States and Canada. Curr Infect Dis Rep. 2007;9:347–350. doi: 10.1007/s11908-007-0053-9. [DOI] [PubMed] [Google Scholar]
- 2.Vaidian AK, Weiss LM, Tanowitz HB. Chagas’ disease and AIDS. Kinetoplastid Biol Dis. 2004;3:2. doi: 10.1186/1475-9292-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H, Chan J, Wittner M, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 4.Huang H, Petkova SB, Cohen AW, et al. Activation of transcription factors (AP-1 and NF-κB) in murine chagasic myocarditis. Activation of transcription factors AP-1 and NF-κB in murine Chagasic myocarditis. Infect Immun. 2003;71:2859–2567. doi: 10.1128/IAI.71.5.2859-2867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 6.Combs TP, Nagajyothi, Mukherjee S, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 7.Desruisseaux MS, Nagajyothi, Trujillo ME, Tanowitz HB, Scherer PE. Adipocyte, adipose tissue, and infectious disease. Infect Immun. 2007;75:1066–1078. doi: 10.1128/IAI.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazan U, Romero IA, Cancello R, et al. Human adipose cells express CD4, CXCR4, CCR5 and receptors: a new target cell type for the immunodeficiency virus-1? FASEB J. 2002;16:1254–1256. doi: 10.1096/fj.01-0947fje. [DOI] [PubMed] [Google Scholar]
- 9.Pasarica M, Dhurandhar NV. Infectobesity: obesity of infectious origin. Adv Food Nutr Res. 2007;52:61–102. doi: 10.1016/S1043-4526(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 10.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 11.Rowin KS, Tanowitz HB, Wittner M, Nyguen HT, Nadal-Ginard B. Inhibition of Muscle differentiation by Trypanosoma cruzi. Proc Natl Acad Sci USA. 1983;80:6390–6394. doi: 10.1073/pnas.80.20.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2005;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hulit J, Bash T, Fu M, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 200;275:21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 14.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 15.Pajvani UB, Trujillo ME, Combs TP, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Hua K, Caveney EJ, Takahashi N, Harp JB. Protein inhibitor of activated STAT3 inhibits adipogenic gene expression. Biochem Biophys Res Commun. 2006;339:923–931. doi: 10.1016/j.bbrc.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 17.Corrêa-de-Santana E, Paez-Pereda M, Theodoropoulou M, et al. Hypothalamus-pituitary-adrenal axis during Trypanosoma cruzi acute infection in mice. J Neuroimmunol. 2006;173:12–22. doi: 10.1016/j.jneuroim.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Ropert C, Gazzinelli RT. Regulatory role of Toll-like receptor 2 during infection with Trypanosoma cruzi. J Endotoxin Res. 2004;10:425–430. doi: 10.1179/096805104225006507. [DOI] [PubMed] [Google Scholar]
- 19.Campos MA, Gazzinelli RT. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Mediators Inflamm. 2004;13:139–143. doi: 10.1080/09511920410001713565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan GS, Mukherjee S, Nagajyothi F, et al. Trypanosoma cruzi infection induces proliferation of vascular smooth muscle cells. Infect Immun. 2006;74:152–159. doi: 10.1128/IAI.74.1.152-159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee S, Huang H, Petkova SB, et al. Trypanosoma cruzi infection activates extracellular signal-regulated kinase in cultured endothelial and smooth muscle cells. Infect Immun. 2004;72:5274–5282. doi: 10.1128/IAI.72.9.5274-5282.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen AW, Park DS, Woodman SE, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–C474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 23.Stahl M, Ge C, Shi S, Pestell RG, Stanley P. Notch1-induced transformation of RKE-1 cells requires up-regulation of cyclin D1. Cancer Res. 2006;66:7562–7570. doi: 10.1158/0008-5472.CAN-06-0974. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AM, Staels B. Review: peroxisome proliferators-activated receptor-γ and adipose tissue-understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrin Metabol. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto Y, Arita Y, Nishida M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Pattabiraman N, Zhou J-F, et al. Cyclin D1 repression of peroxisome proliferator-activated receptor gamma expression and transactivation. Mol Cell Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Pattabiraman N, Zhou JN, et al. Cyclin D1 repression of peroxisome proliferator-activated receptor-γ expression and transactivation. Mol Cell Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 33.Clarke KJ, Zhong Z, Schwartz DD, et al. Regulation of adiponectin secretion by endothelin-1. Biochem Biophys Res Commun. 2003;312:945–949. doi: 10.1016/j.bbrc.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Bedi D, Clarke KJ, Dennis JC, et al. Endothelin-1 inhibits adiponectin secretion through a phosphatidylinositol 4, 5-bisphosphate/actin-dependent-mechanism. Biochem Biophys Res Commun. 2006;345:332–339. doi: 10.1016/j.bbrc.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 35.Juan C-C, Chuang T-Y, Chang C-L, Huang S-W, Ho LT. Endothelin-1 regulates adiponectin gene expression and secretion in 3T3-L1 adipocytes via distinct signaling pathways. Endocrinology. 2007;148:1835–1842. doi: 10.1210/en.2006-0654. [DOI] [PubMed] [Google Scholar]
- 36.Teder P, Noble PW. A Cytokine reborn? Endothelin-1 in pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol. 2000;23:7–10. doi: 10.1165/ajrcmb.23.1.f192. [DOI] [PubMed] [Google Scholar]
- 37.McCarron RM, Wang L, Stanimirovic DB, Spatz M. Endothelin induction of adhesion molecule expression on human brain microvascular endothelial cells. Neurosci Lett. 1993;156:31–34. doi: 10.1016/0304-3940(93)90432-k. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Tanaka H, Richardson JA, et al. Endothelin-1 stimulates leptin production in adipocytes. J Biol Chem. 2001;276:28471–28477. doi: 10.1074/jbc.M103478200. [DOI] [PubMed] [Google Scholar]
- 39.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.