Abstract
The article describes the study of targeted chemotherapeutic intervention on solid tumors by means of ultrasound and doxorubicin- or paclitaxel-loaded perfluoropentane nanoemulsions. Nanodroplets of the emulsions accumulated in a tumor by passive targeting. Under the action of a tumor-directed therapeutic ultrasound, the nanodroplets converted into vapor microbubbles. In vivo, the nanodroplets strongly retained the loaded drugs; yet, under ultrasound-mediated vaporization they released the drugs into the tumor tissue, thereby implementing effective targeting into the tumor. The tumors subjected to this treatment regressed effectively; however, after some time they recurred. The recurring tumors were more resistant to the repeated therapy than the primary ones. At present, the causes of of the resistance development and methods for its elimination are unclear and they are under investigation.
Introduction
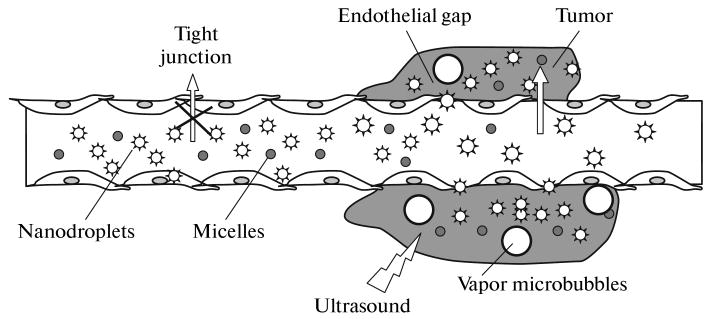
Present-day chemotherapy is associated with severe side effects caused by the drug-induced action on healthy tissues. In addition, in the process of chemotherapy, cells often become resistant to an active chemotherapeutic agent or multiple resistant to a plethora of chemotherapeutic agents. The use of nanomedicine methods opens new lines of attack on the problem of systemic toxicity of anticancer drugs. The Achilles' heel of malignant tumors is the enhanced permeability of their blood vessels [1]. In blood vessels of healthy tissues, the endothelial cells form a dense packing and do not let objects larger than 7 nm pass. In contrast to them, blood vessels in tumor tissues have sizeable gaps between the endothelial cells; they allow extravasation (i.e., transfer from vessels into surrounding tissues) of particles with sizes up to 750 nm and even larger [2]. This property is fundamental for the targeting of chemotherapeutic agents to a tumor, or so-called passive targeting. Drugs are encapsulated in nanoparticles (Fig. 1) that cannot penetrate blood vessels of healthy tissues; however, they penetrate through inter-endothelial gaps and accumulate in a tumor. Liposomes, micelles, nanoemulsions, and nanobubbles are examples of such nanocarriers of drugs. The dosage forms shown in Fig. 1 comprise polymeric micelles (small circles), nanodroplets (stars), and microbubbles (large circles); the micelles are formed by biodegradable block copolymers (for example, poly(ethylene glycol)-block-poly(L-lactide) (PEG-PLLA) or poly(ethylene glycol)-block-poly(caprolactone) (PEG-PCL)); the droplets and bubbles are formed by perfluorocarbon (for example, perfluoropentane (PFP) and are stabilized by the same (or another) block copolymer. A lipophilic drug, for example, doxorubicin (DOX), is localized in micelle cores and on the surface of the nanodroplets or microbubbles. The inter-endothelial junctions in blood vessels of healthy tissues do not allow extravasation of drug-loaded micelles and nanodroplets (marked with a cross). To the contrary, tumors are characterized by a defective vasculature with great gaps between the endothelial cells, which leads to the extravasation of drug-loaded micelles and nanodroplets resulting in their accumulation in the tumor interstitium. If microbubbles are formed during injection, they remain in the blood circulation.
Fig. 1.
Schematic representation of the passive drug targeting through the defective vasculature of a tumor. The dosage forms comprise polymeric micelles (small circles), nanodroplets (stars), and vapor PFP microbubbles (large circles). The micelles are formed by biodegradable block copolymers (e.g., PEG-PLLA or PEG-PCL), and the nanodroplets and bubbles are formed by perfluorocarbon (e.g., PFP) stabilized by the same (or another) block copolymer. The nanodroplets are formed after the introduction of PFP into a copolymer solution and emulsification of this system. Primary microbubbles (with micron diameters) are formed from nanodroplets under the action of hyperthermia or ultrasound, and large microbubbles appear due to coalescence of the primary microbubbles. A lipophilic chemotherapeutic drug, e.g., DOX or paclitaxel, is localized in the micelle cores and (or) on the surface of nanodroplets/microbubbles. The endothelial lining of the blood vessels in normal tissues does not allow extravasation of drug-loaded micelles or nano/microbubbles (marked by cross). On the contrary, tumors are characterized by a defective vasculature with large gaps between the endothelial cells, which leads to extravasation of drug-loaded micelles and nanobubbles and results in their accumulation in the tumor interstitium. If large microbubbles are formed upon injection, they remain in blood circulation. The figure is taken from [5].
An alternative approach to targeting is to form carriers that respond to pH variations or physical stimuli and release drugs under the action of light or sound. The development of ultrasound-responsive carriers is particularly attractive, since ultrasound has a number of significant advantages over other types of treatment: it can be directed to deep-seated tumors, it has many controlled parameters that allow optimization of treatment, and it can be used simultaneously for imaging and therapy.
A combination of the approaches described allows implementing double targeting—the passive one due to predominant carrier accumulation in the tumor and the active one due to a tumor-directed physical stimulus that releases the drug locally in the tumor. It is this approach that is used in works of the laboratory of the first author of this paper [3–6].
Materials and Methods
Block Copolymers
The block copolymers used in this study were bought from Polymer Source Inc. (Montreal, Quebec, Canada). In the majority of the experiments, the PEG-PLLA block copolymer had a total molecular weight of 9.700 dalton (Da), whereas the blocks weighed 5.000 Da and 4.700 Da, respectively. This copolymer was used in the in vitro experiments (unless otherwise specified). In the animal experiments presented below, we used the PEG-PLLA copolymer with a total molecular weight of 4.000 Da; the weight of each respective block was 2.000 Da. In the in vitro experiments, we also used a PEG-PCL copolymer with a total molecular weight of 4.600 Da; the blocks had a weight of 2.000 Da and 2600 Da, respectively.
Micellar Solutions and Loading of Chemotherapeutic Drugs into Micelles
Micellar solutions of the PEG-PLLA and PEG-PCL block copolymers were prepared by a solvent exchange technique, which is described in detail in previous articles [5, 6]; the DOX was loaded into micelles in the course of preparation of the micelles themselves. The chemotherapeutic drug Genexol (Genexol-PM, hereafter GPM) being a paclitaxel encapsulated in the PEG-PLLA micelles was bought from the Samyang Corp. (Taejon, South Korea). A desired weight of the powder was dissolved in the PEG-PLLA or PEG-PCL micellar solution.
Formulations
The paclitaxel-loaded nanoemulsions were prepared as follows. The micellar-encapsulated paclitaxel (GEN) was dissolved in the micelles formed by a 0.25% PEG-PLLA solution in a phosphate buffer, and 1 vol % PFP was added to this solution. Next, the samples were sonicated by 20-kHz ultrasound in ice-cold water until the entire amount of PFP was transferred into the emulsion. Hereafter, this formulation is called nbGEN.
The DOX-loaded nanoemulsions were prepared by the technique described earlier [6, 7]. Briefly, the drug was first loaded into the PEG-PLLA or PEG-PCL micelles; after that, the PFP was added to the micellar solution and the mixture was sonicated as described above.
Particle Size Distribution
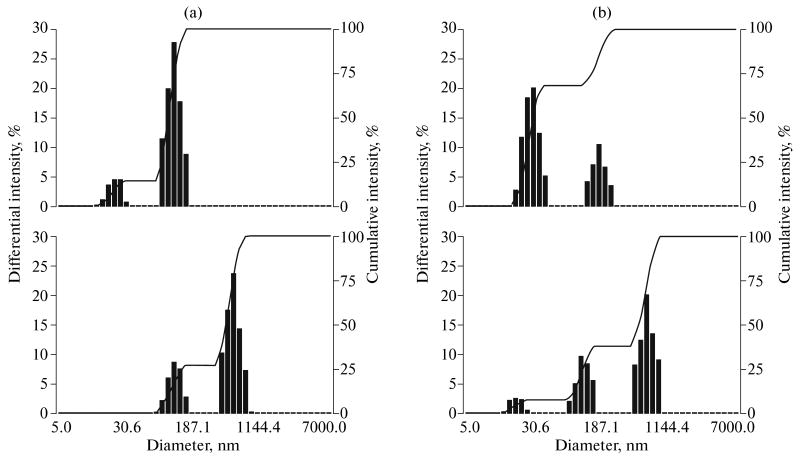
Size distribution of nanoparticles was measured by dynamic light scattering at a scattering angle of 165° using a Delsa Nano S instrument (Beckman Coulter, Osaka, Japan) equipped with a 658 nm laser and a temperature controller. The size distributions of the nanoparticles were typically bimodal as shown in Fig. 2 (the upper row), where the size of the unloaded micelles (the upper row, a) was somewhat lower than that of the drug-loaded micelles (the upper row, b).
Fig. 2.
Particle size distribution: (a) empty micelles (0.25% PEG-PLLA solution) (at the top) and nanodroplets formed by 1 vol % PFP introduced into 0.25% PEG-PLLA solution (at the bottom, right peak); (b) paclitaxel-loaded micelles (0.25% PEG-PLLA solution) (at the top) and paclitaxel-loaded nanodroplets (at the bottom, right peak).
Upon the introduction of PFP into the micellar solution and sonication (the bottom row), the micelles (22 nm) disappeared, and the nanodroplets (500–600 nm, the right peak in the distributions) were formed.
Therapeutic Sonication
An unfocused ultrasound with a frequency of 1 or 3 MHz was generated by an Omnisound 3000 instrument (Accelerated Care Plus Inc, Sparks, Nevada, United States) equipped with a 5-cm2 ultrasonic head. According to the manufacturer, the space-average intensity was 3.4 W/cm2 at a frequency of 1 MHz and 2 W/cm2 at 3 MHz. Due to inhomogeneity of the close field, the intensity peaks significantly exceeded these values: in the location of the sample (or the tumor) the values measured at frequencies of 1 and 3 MHz were 12 and 23 W/cm2, respectively. The acoustic pressure was measured using a HNR-0500 needle hydrophone (Onda, Sunnyvale, CA, United States).
Ultrasound Imaging
The experiments on imaging were carried out in vitro and in vivo with the use of a 14-MHz clinical diagnostic transducer (Acuson Sequoia 512). With the purpose to simulate the bioacoustic effects that take place in the tumor interstitium after extravasation of nanodroplets, an agarose gel was used.
Cells
The A2780 ovarian cancer and MDA MB231 breast cancer cells were bought from the American Type Culture Collection (Manassas, VA, United States). The cells were incubated in an RPMI-1640 medium with addition of 10% calf blood serum in a humid atmosphere containing 5% CO2
The In Vivo Experiments
Athymic nude mice at the age of 4–6 weeks were bought from the Charles River Laboratories (Wilmington, MA, United States). All the experiments were carried out in accordance with recommendations of the USA National Institute of Health, and they were approved by the respective committee of the University of Utah. For the inoculation, 100 μl of the cell suspension in the RPMI-1640 medium with no calf blood serum was used; the cells (1 × 106 cells per mouse) were inoculated subcutaneously to the right and left flank of the mouse. The treatment was started when at least one of the tumors achieved a volume of 150–200 mm3 measured as V = L × W2/2, where L is the tumor length, and W is the tumor width in two mutually perpendicular directions.
The dosage forms were injected systemically through the tail vein. The ovarian carcinoma was treated twice a week for two weeks (the dosage form was the paclitaxel encapsulated in 1% PFP/0.25% PEG-PLLA); for the treatment of breast cancer, two injections were made with a week interval (the dosage form was the DOX encapsulated in 2% PFP/0.375% PEG-PCL). In the experiments presented below, the DOX dose was 2.64 mg/kg, and the paclitaxel dose amounted to 20 mg/kg. The DOX dose was less than the one used traditionally (6–10 mg/kg), because selective drug accumulation in the tumor was anticipated. The volume injected was 100 μl (DOX) and 200 μl (paclitaxel). For every mouse, one of the two tumors (the one that was larger before the treatment) was sonicated by an unfocused ultrasound in 4–5 h after the drug injection; the contact between the source and the mouse skin was achieved with the use of Aquasonic Coupling Gel. The ultrasound frequency was 3 MHz, and the duty cycle was 20% (breast cancer) or 1 MHz in the persistent sonication mode (ovarian cancer). Despite the significant effect observed upon sonication with a frequency of 3 MHz, in the trial experiments with ovarian cancer, the ultrasound frequency was decreased down to 1 MHz with the purpose of ensuring deeper penetration of ultrasound, which is essential for the experiments with internal tumors that are carried out at present. The upper limit of the injected dose of paclitaxel-loaded nanodroplets was estimated on the basis of their size distribution and concentration of the PFP introduced. With the purpose of estimating the number of nanodroplets of the given size per mouse, the following formula was used: Nm = (v × f)/(4/3πr3 × 10−18), where v = 2 μl is the PFP volume introduced into 200 μl of emulsion, f is the volume fraction of droplets of the given size, and r is the droplet radius in the maximum of the respective distribution peak expressed in nanometers. The dose estimated in this fashion was 3 × 1011 nanodroplets per mouse for the droplets with a size less than 200 nm and 1 × 1010 per mouse for the droplets with a size of 500–700 nm.
Results and Discussion
We have recently developed nanoemulsions based on PFP stabilized by amphiphilic block copolymers, such as PEG-PLLA or PEG-PCL [5, 6]. Upon the introduction of PFP into drug-loaded micelles and the sonication of the system obtained by a low-frequency ultrasound in ice-cold water, the block copolymer and the drug transferred from the micelles to the nanodroplet surface, which is confirmed by the decrease in the micelle size down to the size of empty micelles and by the increase in the droplet size (see Figs. 2a, 2b).
Since PFP has a boiling point of 29°C, at physiologic temperatures it either evaporates inside a copolymer shell or forms a superheated liquid phase inside the shell. As a rule, at physiologic temperatures inside the shells mentioned, PFP remains in the form of a superheated liquid inside nanodroplets that have a bimodal size distribution (Fig. 2). The size of the nanodroplets (less than 750 nm) contributes to their extravasation (i.e., transfer from a blood vessel into a surrounding tissue) and accumulation in the tumor interstitium, which is confirmed by ultrasound imaging, since not only bubbles but also droplets of PFP are echogenic. Under the action of a therapeutic ultrasound, nanodroplets are converted into vapor microbubbles (Fig. 3). At present, this effect, being called acoustic droplet vaporization, attracts much attention owing to its potential application in nanomedicine [7–9]. Microbubbles exhibit much higher echogenicity than droplets (Fig. 4), and it is possible to observe the process of droplet conversion into vapor bubbles by the ultrasound imaging. It should be noted that the droplet-to-bubble conversion can occur not only as a result of the ultrasound treatment but also due to mechanisms of another nature; for example, due to the action of shear stresses that are generated during nanoemulsion injection through a thin needle (Fig. 4); this should be taken into account for the clinical use of the technique. The DOX fluorescence confirmed its localization in the bubble shell (Fig. 5); paclitaxel is anticipated to have the same localization.
Fig. 3.
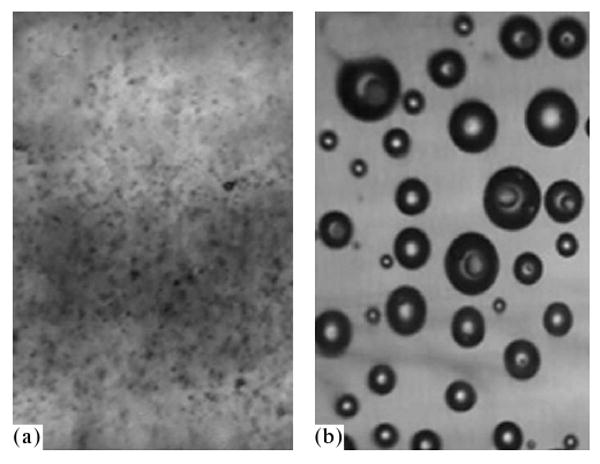
Microphotographs of the agarose gel with introduced nanodroplets (1 vol % PFP encapsulated into 0.25% PEG-PLLA) before (a) and after (b) sonication by the ultrasound with a frequency of 90 kHz. The agarose gel is used here as a model of the tumor interstitium, the viscosity of which considerably exceeds the blood viscosity.
Fig. 4.
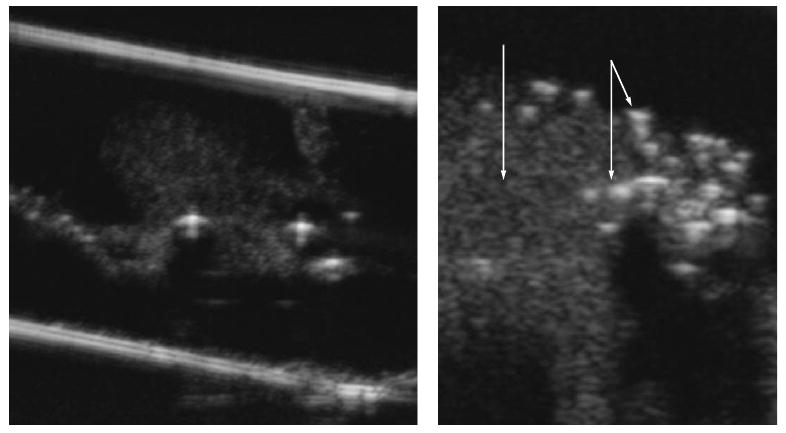
Ultrasound imaging of droplets (darker spots) and bubbles (brighter spots) upon the injection of nanodroplets (1 vol % PFP/0.25% PEG-PLLA) into the agarose gel (on the left). The enlarged part of the image is given on the right for the illustration of differences in the echogenicity of the droplets and bubbles; the single arrow indicates the accumulation of droplets; the double arrow, bubbles.
Fig. 5.
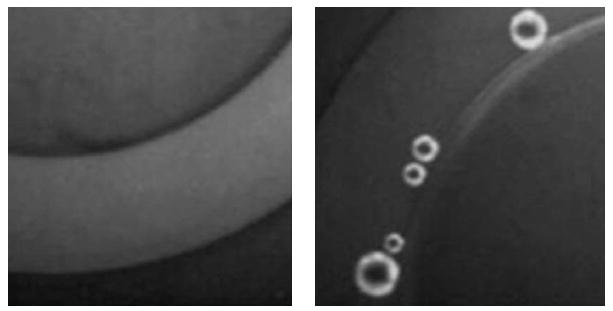
Localization of fluorescent DOX in microbubble walls (fluorescent microphotograph). On the left, there is the image of the microcapillary with a diameter of 340 μm (the light arc in the middle of the image) filled with the DOX-loaded nanoemulsion. The image is obtained at room temperature; the fluorescent nanodroplets are not resolved under the ×100 optical magnification, being maximal for the instrument setting used in the work. On the right, there are microbubbles formed under heating of the vessel, under vaporization of superheated nanodroplets and coalescence of formed vapor bubbles.
In our approach, the strategy of therapy of malignant tumors consists in systemic injection of a drug-loaded nanoemulsion; thereafter, in the few hours that are required for the carrier accumulation in the tumor, the latter is sonicated by a therapeutic ultrasound; under its action, the drug is released from the carrier locally in the tumor site and is internalized by the tumor cells as shown in Fig. 6. Cancer cells introduced into a DOX-loaded nanoemulsion do not fluoresce before the application of an ultrasound, and only microbubbles can be seen (Fig. 6a). After the sonication, the fluorescence of the microbubbles shown in Fig. 6a decreases or disappears, whereas the cells start fluorescing (Figs. 6b, 6c). These data show that under the action of the ultrasound, DOX is released from the microbubbles and is internalized by the cells. The bubbles can be seen in Fig. 6b; however, there are no visible bubbles in Fig. 6c that shows another site of the same sample. The survival of visible bubbles, although of lesser intensity (Fig. 6b), shows that the drug transfer from the bubbles into the cells does not require collapse of the bubbles.
Fig. 6.
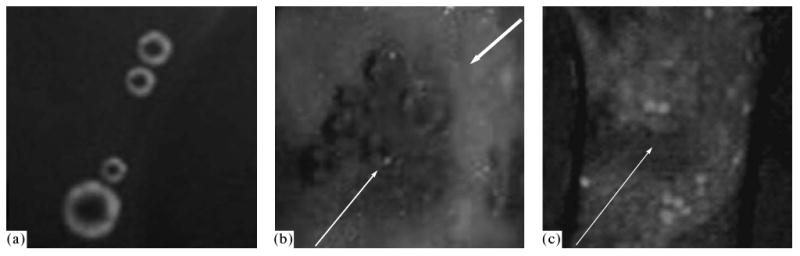
Fluorescence of microbubbles in the presence of the MBA MB231 breast cancer cells in the capillary with a diameter of 340 μm. Before the cell sonication, fluorescence of the microbubbles is observed; the cells do not exhibit fluorescence (a). After the sonication, the microbubble fluorescence decreases, whereas the cells, on the contrary, start fluorescing (b, c). Thus, under the action of ultrasound, DOX is released from the microbubbles and is internalized by the cells. The bubbles can be seen in some sites (b) and cannot be seen in the other, as in the image (c) that presents another site of the same sample. Survival of visible bubbles, although of lesser intensity (b), shows that the drug transfer from the bubbles into the cells does not require collapse of the bubbles. The figure is taken from [6].
The in vivo trial experiments were carried out with the purpose of answering the questions of how strongly nanodroplets and microbubbles retain the drug in vivo and what is the role of ultrasound: is the cell death caused by the action of ultrasound in combination with bubbles that concentrate ultrasound energy or is it caused by the cytotoxic action of the drug released from the carrier under the action of ultrasound? To answer these questions, the following experiments were carried out. Athymic nu/nu mice were inoculated with breast cancer cells (Fig. 7) or ovarian cancer cells (Fig. 8) to the right and left flanks as described in the Materials and Methods Section. Nanoemulsions loaded with DOX (Fig. 7) or paclitaxel (Fig. 8) were systemically injected through the tail vein, so that in circulation they get into both tumors. However, only the right tumor was sonicated (3 MHz, 20%, 150 s for breast cancer and 1 MHz, 3.4 W/cm2, 1 min for ovarian cancer); ultrasound was applied 4 h after the injection; this time is required for the nanoemulsion accumulation in the tumor. With the purpose of verifying the generality of the method proposed, the types of the copolymers and ultrasound parameters were different in the experiments, and two different models of tumors were used. The nonsonicated left tumor grew with the same rate as the control tumors, whereas the sonicated tumor regressed rapidly and appeared to be completely resolved after two treatments (however, a recurrence of the tumor growth was observed after the completion of the therapy, see below). These experiments showed that without ultrasound the drug was retained strongly by nanodroplets in vivo, since the nonsonicated tumor grew with the rate of the control tumors. Ultrasound effectively triggered the drug release from the carrier.
Fig. 7.
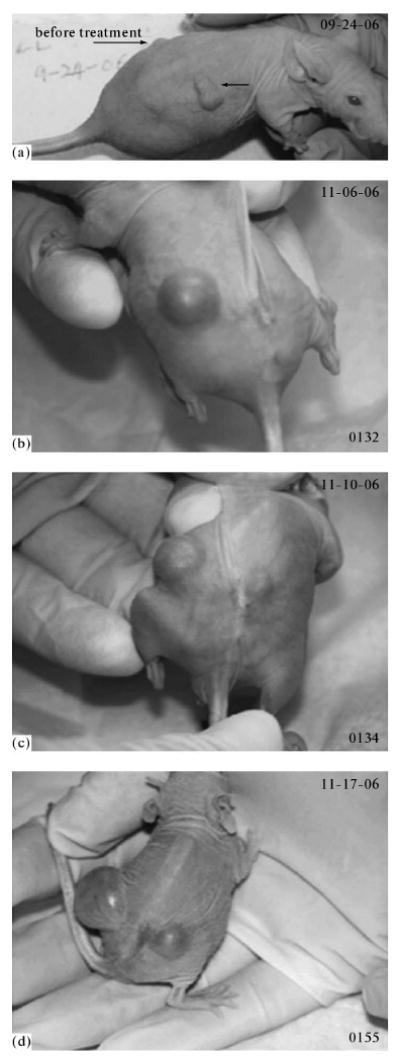
Photographs of the mouse with two MDA MB231 breast cancer tumors; (a) the tumors (indicated by arrows) in the left and right mouse flanks before the treatment. The mouse was treated by two intravenous injections (with a week interval) of 100 μl of microbubbles containing 0.75 mg/ml DOX / 2%PFP / 0.5% PEG-PLLA; the right tumor was sonicated for 150 s by the 3-MHz ultrasound at a nominal power density of 2 W/cm2 and a duty cycle of 20%, which corresponds to ultrasound exposure for 30 s; the left tumor was not sonicated. The sonication was executed in 4 h after the drug injection. Before the treatment, the right tumor was larger than the left one. (b) The photograph of the mouse was taken 36 days after the completion of the treatment. The nonsonicated left tumor grew with the same rate as the control tumors, which shows the absence of the drug release from the microbubbles in the absence of ultrasound. The right tumor appeared completely resolved in a week after the completion of the treatment. (c, d) The recurrence of the right tumor was observed in a month and a half after the completion of the treatment; the last photograph (d) was taken in 47 days after the completion of the treatment. The figure is taken from [6].
Fig. 8.
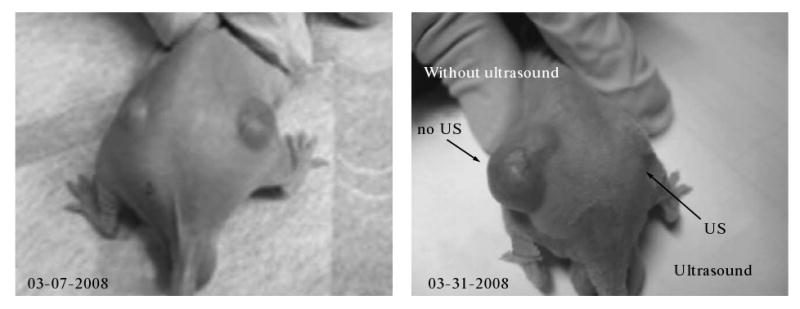
Results of chemotherapy of a mouse with two ovarian carcinoma tumors; the mouse was treated by systemic injections of paclitaxel encapsulated into nanodroplets (nbGEN); the paclitaxel dose was 20 mg/kg; the right tumor was sonicated by the 1 MHz ultrasound at a nominal power density of 3.4 W/cm2 in the persistent sonication mode; the exposure duration was 1 min. In order to prevent superheating of skin, a water-filled bag lubricated with an Aquasonic gel on either side was placed between the source and the mouse skin for ensuring acoustic contact; ultrasound was applied in 4.5 h after the drug injection. On the left, there is a photograph of the mouse before the treatment; the photograph on the right was taken in three weeks after the beginning of the treatment. The left (nonsonicated) tumor grew with the rate of the control tumors, whereas the right tumor (sonicated) regressed effectively. These results demonstrate that in the absence of ultrasound, paclitaxel is strongly retained by nanodroplets in vivo; however, it is effectively released under the action of ultrasound.
The second series of trial experiments was carried out with empty (i.e., not drug-loaded) bubbles with the same design as the series described above. In the absence of drugs, the sonicated tumors grew with the rate of the control ones. This showed that the death of cancer cells under the therapy applied was due to the cytotoxic action of the drug released by ultrasound rather than due to the effect of the ultrasound itself.
The trial tests showed that the therapy proposed is promising. However, as one can see from Fig. 7, the tumor recurred in a month and a half after the completion of the treatment; a repeated course of treatment by the previous scheme appeared noneffective. This means that in the first course of treatment, the cancer cells either became DOX-resistant or a selection of drug resistant cells occurred. At present, the causes and methods for solution of this problem are under investigation.
Conclusions
Targeted nanotherapy of malignant tumors by means of ultrasound-responsive nanocarriers leads to effective regression of tumors, and it can find common use in oncology after solution of the problem of the tumor recurrence after the completion of the treatment. Search for the mechanism of the resistance development and of methods for its prevention is currently in progress.
Acknowledgments
We thank the USA National Institute of Health (grant no. NIHR01 EB1033) for the support of the research.
References
- 1.Iyer A, Khaled G, Fang J, Maeda H. Drug Discov Today. 2006;11:812. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Campbel RB. Anti-Cancer Agents Med Chem. 2006;6:503. doi: 10.2174/187152006778699077. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport N. Nanotechnology in Cancer Therapy. CRC Press; Boca Raton: 2006. pp. 417–437. [Google Scholar]
- 4.Gao ZG, Fain HD, Rapoport N. J Control Release. 2005;102:203. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport N, Gao Z, Kennedy A. J Natl Cancer Inst. 2007;99:1095. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 6.Gao Z, Kennedy AM, Christensen DA, Rapoport N. Ultrasonics. 2008;48:260. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kripfgans O, Fowlkes J, Miller D, Eldevik O, Carson P. Ultrasound Med Biol. 2000;26:1177. doi: 10.1016/s0301-5629(00)00262-3. [DOI] [PubMed] [Google Scholar]
- 8.Kripfgans O, Fabiilli M, Carson P, Fowlkes J. J Acoust Soc Am. 2004;116:272. doi: 10.1121/1.1755236. [DOI] [PubMed] [Google Scholar]
- 9.Lo A, Kripfgans O, Carson P, Fowlkes J. Ultrasound Med Biol. 2006;32:95. doi: 10.1016/j.ultrasmedbio.2005.09.009. [DOI] [PubMed] [Google Scholar]