Abstract
Interaction protein for cytohesin exchange factors 1 (IPCEF1) is a recently identified protein that binds to cytohesin 2 and might participate in membrane receptor trafficking by enhancing the activity of cytohesin 2 in cultured cells. However, whether IPCEF1 is involved in this process in vivo is unknown. Here, we report that IPCEF1 mRNA is expressed in the dorsal root ganglions (DRGs) of adult rats. Moreover, peripheral nerve injury induced by fifth spinal nerve ligation and transection or sciatic nerve transection significantly up-regulated expression of IPCEF1 mRNA in the injured DRGs. Because peripheral nerve injury leads to changes in membrane receptor trafficking in the injured DRG, the correlation of IPCEF1 mRNA expression with nerve injury input in the injured DRG suggests that IPCEF1 might participate in the mechanisms that underlie nerve injury-induced membrane receptor trafficking in the DRG under neuropathic pain conditions.
Short report
Clinically, nerve injury-induced neuropathic pain is characterized by spontaneous pain as well as mechanical and thermal hyperalgesia and allodynia. Despite considerable research into the neurobiological mechanisms of neuropathic pain during the past decades, our understanding of this disorder is still incomplete. Recent studies showed that peripheral nerve injury might lead to changes in plasma membrane receptor trafficking in major pain-related regions in the nervous system [e.g., dorsal root ganglion (DRG)]; such changes are thought to contribute to the central mechanisms that underlie neuropathic pain [1–4]. However, how peripheral nerve injury alters plasma membrane receptor trafficking in these regions is unknown.
Cytohesin 2 is a protein in the family of guanine-nucleotide exchange factors (GEFs) and plays critical roles in membrane receptor trafficking events, such as receptor-mediated endocytosis and regulated exocytosis, through activation of the ADP-ribosylation factor 6 small GTPases [5–7]. Recently, in a yeast two-hybrid screen of a rat brain cDNA library in which cytohesin 2 was used as bait, a protein known as interaction protein for cytohesin exchange factor 1 (IPCEF1) was identified [5]. IPCEF1 interacts and co-localizes with cytohesin 2 in the cells [5]. Previous in vitro studies showed that IPCEF1 participated in membrane receptor trafficking by increasing the activity of cytohesin 2 [5–6]. This finding suggests that IPCEF1 may be involved in nerve injury-induced changes in membrane receptor trafficking in DRG. If so, the expression of IPCEF1 would be expected to increase in this region after peripheral nerve injury in adult animals.
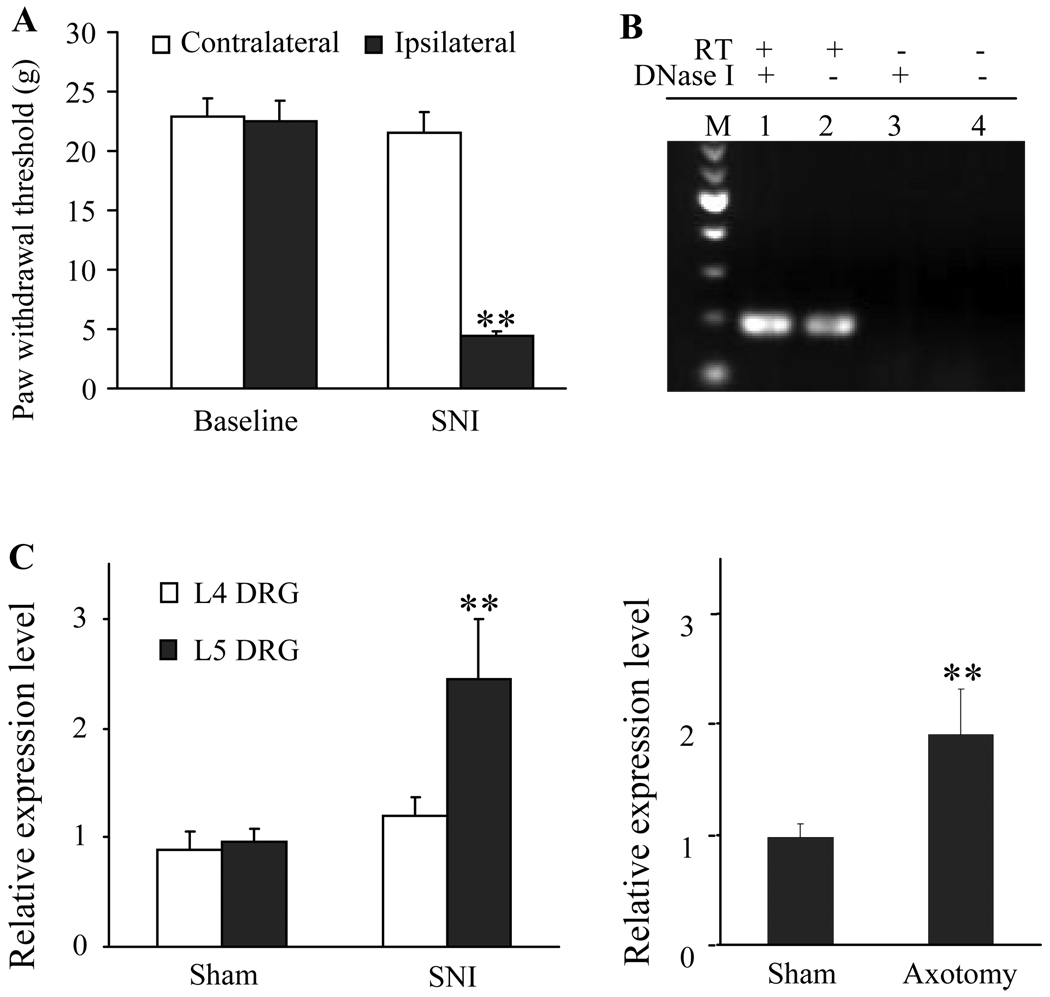
To test our hypothesis, we used two peripheral nerve injury models, L5 spinal nerve injury (SNI) and sciatic nerve transection (axotomy), to examine the effect of peripheral nerve injury on IPCEF1 mRNA expression in DRG. For the SNI model, nociceptive hypersensitivity was monitored by measuring paw withdrawal thresholds (PWTs) in response to mechanical stimuli. SNI induced mechanical hypersensitivity, as indicated by a significant decrease from baseline in PWTs on the ipsilateral side (but not on the contralateral side) on day 14 post-surgery (Figure 1A). After axotomy, the rats displayed no responses to mechanical stimuli on the ipsilateral side, but basal responses to stimuli of the contralateral hindpaw were intact during the observation period (data not shown). Sham surgery did not cause ipsilateral or contralateral mechanical hypersensitivity in either model (data not shown). On day 14 post-surgery, ipsilateral and contralateral L4 and L5 DRGs were collected from SNI-treated and corresponding sham-operated groups; ipsilateral and contralateral L5 DRGs were harvested from axotomy-treated and corresponding sham-operated groups.
Figure 1. IPCEF1 mRNA is expressed and up-regulated in the injured DRGs after peripheral nerve injury.
(A) L5 spinal nerve injury (SNI)-induced mechanical allodynia. SNI led to a significant decrease in paw withdrawal threshold (PWT) in response to mechanical stimuli on the ipsilateral, but not contralateral, side on day 14. ** p < 0.01, compared with the corresponding baseline. (B) IPCEF1 mRNA expression in rat DRG demonstrated by RT-PCR with a strand-specific primer. Lane 1 displays a 194-bp band amplified from the cDNA reverse-transcribed from DRG RNA. Lane 2 shows the same band amplified from the genomic DNA and cDNA reverse-transcribed from DRG RNA. Lane 3 is a negative control without reverse transcription (RT) but with DNase I treatment. Lane 4 is a control without RT and DNase I treatment. M: 100 bp DNA ladder maker. (C) Relative expression levels of IPCEF1 mRNA in the DRGs from SNI group, axotomy group, and their corresponding sham groups. ** p < 0.01 compared to sham group.
We first used reverse transcriptase-polymerase chain reaction (RT-PCR) with a strand-specific primer (see Methods) to examine whether rat DRG expressed IPCEF1 mRNA. To exclude the genomic DNA contamination in RNA extracts from the DRGs, DNase I was used. As indicated in Figure 1B, a 194-bp band amplified from the cDNA reverse-transcribed from rat DRG RNA was detected in the DRG. PCR products were further verified by automatic DNA sequencing and found to be completely complementary to IPCEF1 mRNA. GADPH mRNA as a loading control was also detected in the DRG (data not shown). Then we used a quantitative real-time RT-PCR approach to measure the changes of IPCEF1 mRNA in DRGs after nerve injury. The ratios of ipsilateral to corresponding contralateral DRG IPCEF1 mRNA were calculated. We found that both SNI and axotomy significantly increased IPCEF1 mRNA expression in the injured (ipsilateral L5) DRGs (Figure 1C). Compared with sham groups (0.961 ± 0.123 for SNL; 0.976 ± 0.112 for axotomy), the ratios of ipsilateral to contralateral IPCEF1 mRNA in L5 DRGs were increased by 1.94 fold after axotomy (p < 0.01; Figure 1C) and by 2.54 fold after SNI (p < 0.01; Figure 1C). Interestingly, L5 SNI did not alter IPCEF1 mRNA expression in the intact (ipsilateral L4) DRG (0.887 ± 0.170 for SNI group and 1.189 ± 0.182 for sham group) (p > 0.05; Figure 1C).
Emerging evidence indicates that membrane receptor trafficking on DRG cells could be changed following peripheral nerve injury [1–3]. For example, peripheral nerve injury down-regulates µ-opioid peptide receptors and up-regulates P2X3 receptors on the injured DRG surface membranes [1–3]. These changes are considered to be involved in the mechanisms underlying the induction and expression of neuropathic pain. Understanding how peripheral nerve injury alters membrane receptor trafficking in DRG neurons may provide further insight into the mechanisms of neuropathic pain and allow development of novel therapeutic strategies to treat this condition.
In the present study, we provide the first evidence that the expression of IPCEF1 mRNA is significantly increased in the injured DRG on day 14 after peripheral nerve injury. This increase is expected to up-regulate IPCEF1 protein expression in the injured DRG, which will be further confirmed when a specific antibody against IPCEF1 becomes available. IPCEF1 has been reported to interact with cytohesin 2 and regulate its activity in vitro [5]. Because cytohesin 2 is critical for membrane receptor trafficking [5–7], it is very likely that the nerve injury-induced increase in IPCEF1 expression may enhance cytohesin 2 activity and contribute to membrane receptor trafficking in the injured DRG under neuropathic pain conditions. IPCEF1 is a newly identified protein and its function in vivo is unknown. To our knowledge, this study is the first to show that peripheral nerve injury leads to an up-regulation of IPCEF1 mRNA expression in the DRG. Additional studies are required to determine which type of DRG cells express this up-regulation, how nerve injury input increases IPCEF1 mRNA expression in the injured DRG, and whether IPCEF1 deletion affects DRG membrane receptor trafficking and the development of the neuropathic pain.
In summary, we found that IPCEF1 mRNA was significantly increased in the injured DRGs following peripheral nerve injury. This up-regulation of IPCEF1 mRNA expression might underlie the mechanisms of nerve injury-induced changes in DRG membrane receptor trafficking under neuropathic pain conditions.
Materials and methods
Animals
Male Sprague-Dawley rats (250–350 g) were used in accordance with the regulations of the Institutional Animal Care and Use Committee at the Johns Hopkins University. All efforts were made to minimize the number of animals used and their suffering.
Spinal nerve injury (SNI)
After the rats (n = 18) were anesthetized with isoflurane, a dorsolateral skin incision was made on the lower back. The fifth lumbar transverse process was identified and freed of its muscle attachment and then removed. The underlying fifth lumbar nerve root was isolated, ligated with a 3-0 silk suture, and transected just distal to the ligature according to the method described previously [8–9]. After appropriate hemostasis, the skin and muscle layers were closed with a silk suture. In the sham group, the surgical procedure was identical to that described above, except that the spinal nerve was not transected or ligated.
Sciatic nerve axotomy
After the rats (n = 16) were anesthetized with isoflurane, a 0.5-cm incision was made above the knee parallel to the femur. The left sciatic nerve was exposed, and the nerve was cut at a point approximately 1 cm distal from the exit point of spinal nerve roots. Proximal and distal stumps were separated to ensure full transection. The wound was closed with a silk suture. In the sham group, the surgical procedure was identical to that described above, except that the sciatic nerve was not transected.
Behavioral testing
Mechanical paw withdrawal thresholds (PWTs) were measured with the up-down testing paradigm 1 day before surgery and on day 14 after nerve injury or sham surgery [4]. Behavioral testing was performed by experimenters blinded to the rats’ surgical group. Each animal was placed in a Plexiglas chamber on an elevated mesh screen. At least 30 minutes were allotted for behavioral accommodation before testing was begun. Von Frey hairs in log increments of force (3.61, 3.84, 4.08, 4.31, 4.56, 4.74, 4.93, 5.18 g) were applied to the plantar surface of the rat left and right hindpaws. The 4.31-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next higher von Frey hair was used. The test was ended when (i) a negative response was obtained with the 5.18-g hair, (ii) four stimuli were applied after the first positive response, or (iii) nine stimuli were applied to one hind paw.
Total RNA preparation and RT-PCR
Naive animals (n = 10) were decapitated following behavioral testing. L4 and/or L5 DRGs were collected immediately. Total RNA was extracted by the Trizol method (Invitrogen, Carlsbad, CA), precipitated with isopropanol, and treated with DNase I (New England Biolabs, Ipswich, MA). The quality and quantity of RNA samples were assessed by using a NanoDrop 1000 UV-Vis spectrophotometer (Midland, ON, Canada). Using Omniscript RT kit (QIAGEN, Valencia, CA) with specific primers (IPCEF1: 5’–AGC AGG GTG TTC ATG AC–3’ or GAPDH: 5’–GAG GGT GCA GCG AAC TTT ATT GAT G–3’), we reversely transcribed a single strand cDNA for IPCEF1 or GAPDH from RNA (10 µg). Template (1 µl) was amplified by PCR with platinum Taq polymerase (1 unit) in 20 µl total reaction volume containing 0.5 µmol of each specific PCR primer. The IPCEF1 PCR primer sequences were 5’–GCT AGC CTG TCT CCT CTT GG–3’ (forward) and 5’–GGC TGT CAG CTT TGG GTC AT–3’ (reverse). As a loading control, GAPDH cDNA was also amplified with primer sequences 5’–ACC ACA GTC CAT GCC ATC AC–3’ (forward) and 5’–TCC ACC ACC CTG TTG CTG TA–3’ (reverse). Each PCR cycle consisted of 20 seconds at 94°C, 20 seconds at 55°C, and 30 seconds at 72°C. PCR amplification was carried out for 30 cycles. After amplification, the products were separated on a 1.2% agarose gel containing 0.025% ethidium bromide. Bands were then visualized under UV illumination, and gels were photographed with the BioDoc-It imaging system (Ultra-Violet Products Ltd, Upland, CA).
Quantitative real-time RT-PCR
Total RNA was extracted from the ipsilateral and contralateral L4 and/or L5 DRGs of the rats subjected to SNI, axotomy, or sham surgery. Two DRGs were pooled together to achieve enough RNA. RNA was precipitated, treated, and transcribed as described above. cDNA was amplified by real-time PCR by using the probe for IPCEF1 (5’ - /56 - FAM/AGC TGA GAA AGT CTT TTG TGA AC GAT GC/3BHQ_1/ - 3’) (Integrated DNA Technologies, Coralville, IA), and GADPH (Ref: Rn99999916_s1) (Applied Biosystems, Foster City, CA) as an internal control for normalization. Real-time PCR primers used were the same as those described above. Each sample was run in quadruplicate in a 20-µl reaction with TaqMan Universal PCR master mix kit (Applied Biosystems). Reactions were performed in an ABI 7500 Fast real-time PCR system (Applied Biosystems). Ratios of ipsilateral-side mRNA levels to contralateral-side mRNA levels were calculated by using the ΔCt method (2−ΔΔCt) at a threshold of 0.02 [10]. All data were normalized to GAPDH, which has been demonstrated to be stable even in the face of peripheral nerve injury insult [11].
Statistical analysis
All results are expressed as mean ± SEM. Differences between sham and nerve-injured groups were analyzed by Student’s t-test. The significance was set at p < 0.05.
Acknowledgements
This work was supported by National Institutes of Health Grants NS 058886 and NS 057343, and Johns Hopkins University Blaustein Pain Research Fund (Y.-X. T). The authors thank Claire Levine, MS, for her editorial assistance.
Footnotes
Authors’ contributions
XG participated in design and interpretation of the study, carried out behavioral testing, RT-PCR, and real-time RT-PCR experiments, and wrote a draft of the manuscript. XZ was involved in design of the study and contributed to RT-PCR and real-time RT-PCR experiments. YXT contributed to the surgery, the concept and design of the study, the data analysis and interpretation, and critical review the manuscript.
Contributor Information
Xiaowei Guan, Email: xguan3@jhmi.edu.
Xuguang Zhu, Email: xuguangz@yahoo.com.
Yuan-Xiang Tao, Email: ytao1@jhmi.edu.
References
- 1.Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Foulkes T, Nassar MA, Lane T, Matthews EA, Baker MD, Gerke V, Okuse K, Dickenson AH, Wood JN. Deletion of annexin 2 light chain p11 in nociceptors causes deficits in somatosensory coding and pain behavior. J Neurosci. 2006;26:10499–10507. doi: 10.1523/JNEUROSCI.1997-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Bao Lan, Guan JS. Role of delivery and trafficking of δ-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Singh OV, Yaster M, Xu JT, Guan Y, Guan X, Dharmarajan AM, Raja SN, Zeitlin PL, Tao YX. Proteome of synaptosome-associated proteins in spinal cord dorsal horn after peripheral nerve injury. Proteomics. 2009;9:1241–1253. doi: 10.1002/pmic.200800636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkateswarlu K. Interaction protein for cytohesin exchange factors 1 (IPCEF1) binds cytohensin 2 and modifies its activity. J Biol Chem. 2003;278:43460–43469. doi: 10.1074/jbc.M304078200. [DOI] [PubMed] [Google Scholar]
- 6.Venkateswarlu K. Analysis of the interaction between cytohesin 2 and IPCEF1. Methods Enzymol. 2005;404:252–266. doi: 10.1016/S0076-6879(05)04024-3. [DOI] [PubMed] [Google Scholar]
- 7.Theis MG, Knorre A, Kellersch B, Moelleken J, Wieland F, Kolanus W, Famulok M. Discriminatory aptamer reveals serum response element transcription regulated by cytohensin-2. Proc Natl Acad Sci USA. 2004;101:11221–11226. doi: 10.1073/pnas.0402901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide sythase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Tao F, Liaw WJ, Bredt DS, Johns RA, Tao YX. Effect of knock down of spinal cord PSD-93/chapsin-110 on persistent pain induced by complete Freund's adjuvant and peripheral nerve injury. Pain. 2003;106:187–196. doi: 10.1016/j.pain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]