Abstract
Retinal Müller glial cells, in addition to providing homeostatic support to retinal neurons, have been shown to engage in modulation of neuronal activity and regulate vasomotor responses in the retina, among other functions. Calcium-mediated signaling in Müller cells has been implicated to play a significant role in the intracellular and intercellular interactions necessary to carry out these functions. Although the basic molecular mechanisms of calcium signaling in Müller cells have been described, the dynamics of calcium responses in Müller cells have not been fully explored. Here, we provide a quantitative characterization of calcium signaling in an in vitro model of Müller cell signaling using the rMC-1 cell line, a well-established line developed from rat Müller cells. rMC-1 cells displayed robust intracellular calcium transients and the capacity to support calcium transient-mediated intercellular calcium waves with signaling dynamics similar to that reported for Müller cells in in situ retinal preparations. Furthermore, pharmacological perturbations of intracellular calcium transients with thapsigargin and intercellular calcium waves with purinergic receptor antagonists and gap junction blockers (PPADS and FFA, respectively) suggest that the molecular mechanisms that underlie calcium signaling in rMC-1 cells has been conserved with those of Müller cells. This model provides a robust in vitro system for investigating specific mechanistic hypotheses of intra- and intercellular calcium signaling in Müller cells.
Keywords: Glial cells, Müller cells, Calcium signaling, Calcium dynamics, Retina
Introduction
Müller cells are the primary macroglial cell type of the neural sensory retina and have diverse functions in both health and disease. The classical role of these cells is providing homeostatic support to retinal neurons,4 although a number of other functions including modulating neuronal activity via bi-directional communication with neurons in the inner nuclear layer,2,4,35–37 regulating vasomotor responses in the retina,15,29,50 and contributing to degenerative retinal pathologies through reactive gliosis have been proposed.3,18 Underlying the bi-directional signaling of Müller glia with neurons and vascular regulation are calcium changes that mediate intra- and intercellular signaling processes.29 Müller cells in in situ retinal and eye cup preparations have been shown to generate transient increases in intracellular calcium both spontaneously1,45 and in response to light stimulation,37 with frequencies and durations comparable to those observed for astrocytes in brain slices33,40 and in vivo.16 Activation of metabotropic purinergic P2Y receptors by extracellular adenosine 5′-triphosphate (ATP) leading to the formation of inosital triphosphate (IP3) by phospholipase C (PLC), and IP3-dependent calcium release from intracellular stores have been implicated as key steps in the generation of calcium transients in vitro20,25 and in situ,23,25,34,37,55 although there is also evidence for activation of ionotropic purinergic receptors (i.e., P2X7) that augment the calcium increase via influx from the extracellular milieu.5,39 Stimulation of in situ preparations with 488-nm light flashes has been shown to increase neuronal activity that correlates with an increased frequency of calcium transients in Müller cells.37 Light-evoked calcium responses have been shown to be blocked by suramin, a purinergic antagonist, and apyrase, which hydrolyzes ATP, providing support for an ATP-dependent mechanism. Interestingly, tetrodotoxin (TTX) is also able to block light-induced calcium responses in Müller cells, suggesting that amacrine and ganglion cells (retinal neurons that generate action potentials) may be necessary for light-evoked signaling to Müller cells.37 Conversely, signaling from Müller cells to neurons may also be mediated by calcium changes. It has been suggested that calcium increases in Müller cells mediate the release of ATP that, once hydrolyzed to adenosine by ecto-nucleotidases, induce hyperpolarizations in retinal ganglion cells by activating A1 adenosine receptors that open potassium channels.35 However, the details of this calcium-dependent ATP release require further investigation.
Calcium signaling in Müller cells has also been implicated to play an important role in pathology. Specifically, upregulated intracellular calcium responses have been associated with gliosis of Müller cells in retinal detachment and proliferative vitreoretinopathy (PVR).3,18,56 Müller cell endfeet in acutely isolated porcine retinal wholemounts display increased calcium responses to ATP stimulation one to three days following experimental rhegmatogenous detachment, along with increased expression of P2Y1 and P2Y2 receptors. Furthermore, this increase in calcium sensitivity extended to Müller cells beyond the region of detachment; potentially caused by alterations in the functional state of P2 receptors or resensitization of receptors by soluble growth factors released during pathology.18,58 Intracellular calcium increases via activation of metabotropic and ionotropic P2 receptors by extracellular ATP (as observed in retinal pathology) have also been shown to stimulate DNA synthesis and cell proliferation in primary Müller cells30,31,39 and are implicated as a potential mechanism for increased glial mitogenic activity in PVR.5,18 Finally, adenosine, the degradation product of ATP, has also been found at elevated concentrations under pathological conditions such as retinal hypoxia,43 and has been shown to potentiate increased Müller calcium transients in response to light stimulation.37
An intriguing but controversial observation made in in situ and in vitro experiments is the capability of Müller cells to support intercellular calcium waves mediated by intracellular calcium transients.1,34,37,38 Stimulation of Müller cells and retinal astrocytes in acutely isolated retina and eye cups using ATP ejection, mechanical, or electrical stimulation evoke calcium transients that propagate outward to adjacent glial cells as intercellular calcium waves.34,38 Intercellular signaling between Müller cells is mediated by ATP signaling via purinergic receptors, since waves have been shown to be blocked by the purinergic receptor antagonist, suramin.34 In addition, gap junction channels formed by homeotypic and heterotypic coupling of hemichannels involving connexin 43 have also been implicated in calcium transient-mediated intercellular signaling, presumably by allowing exchange of small secondary messengers (i.e., Ca and IP3).17,21,32,51,59 Although experimentally evoked calcium waves have been shown to participate in the modulation of neuronal activity and vasomotor responses,29 these observations are controversial because intercellular calcium waves in Müller cells have not been observed under physiological conditions in vivo, raising the issue of whether the in vitro and in situ observations may be an experimental artifact.37 Calcium transients observed in individual Müller cells of acutely isolated eye cups in response to 488-nm light stimulation were not seen to propagate through networks,37 although neither the physiological range of luminous stimuli nor physiologically realistic stimulation patterns have been fully explored, thus necessitating more detailed follow-up studies. However, the same paper did report intercellular calcium signaling between Müller cells under conditions that mimic neuropathology.37 In the presence of elevated adenosine, light stimulation sometimes produced a delayed calcium response in Müller cells that propagated into neighboring cells,37 suggesting a potential contribution of intercellular calcium waves to disease states in the neural retina.
Although the basic molecular mechanisms of calcium signaling in Müller cells have been described, the dynamics of calcium responses in Müller cells have not been fully explored. This is critical for investigating any physiological or pathophysiological roles calcium signaling may be playing. Here we provide a quantitative characterization of calcium signaling in an in vitro model using the rMC-1 cell line, a well-established line developed from rat Müller cells46 that has been used in numerous studies to investigate the cell biology of Müller cells and their contributions to pathology.10–12,19,26,46,48 The dynamics of individual intracellular calcium transients and intercellular calcium waves were analyzed in individual cells and networks of rMC-1-cells. Very similar to data from primary Müller cells in vitro and in situ, rMC-1 cells displayed robust intracellular calcium transients and the capacity to support calcium transient-mediated intercellular calcium waves. Furthermore, pharmacological experiments suggest that the molecular mechanisms that underlie calcium signaling in rMC-1 cells are dependent on activation of purinergic receptors by extracellular ATP and, to a lesser extent IP3-mediated gap junctional signaling, similar to that described for Müller cells. Lastly, the dynamics of calcium signaling in rMC-1 cells are quantitatively very similar to in situ Müller cells in intact retinal preparations that preserve the local cytoarchitecture. Although in vitro systems are simplified representations of physiological conditions, the calcium signaling mechanisms in rMC-1 cells seem to have been conserved with respect to the known physiological mechanisms in Müller cells. And although rMC-1 cells differ significantly from their relatives in the retina in some respects (e.g., morphology, functional polarization, etc.), the results we provide in the present work as well as the data from others10–12,19,26,46,48 suggest that, on a molecular and cellular level, rMC-1 cells are a good model of Müller cells and can provide an opportunity to study these fundamental processes under controlled experimental conditions where the complexity of the physiology or pathophysiology may confound direct measurements.
Materials and Methods
Reagents and Cell Cultures
rMC-1 Müller cells (originally obtained courtesy of Dr. Vijay Sarthy, Northwestern University, Chicago, IL) were passaged four to five times to expand them from frozen stocks. All experiments were performed one day after recovered cells were seeded on P35 glass-bottom Petri dishes (MatTek Corp., Ashland, MA) at ∼200,000 cells/cm2 incubated in culture media (high glucose Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 1% (v/v) Pen/Strep) at 37°C and 5% CO2. Cell cultures reached workable confluency (>80%) overnight. Media changes, in which all media were replaced, were performed every two days. Unless otherwise stated, all reagents were obtained from Sigma (St. Louis, MO).
Calcium Imaging
rMC-1 cultures of ∼80% confluency were washed twice with Kreb-HEPES buffer (KHB) solution (10 mM HEPES, 4.2 mM NaHCO3, 10 mM glucose, 1.18 mM MgSO4, 1.18 mM KH2PO4, 4.69 mM KCl, 118 mM NaCl, 1.29 mM CaCl2, pH 7.4) and incubated with 5 μM Fluo-4AM in KHB for 1 h at room temperature. Excess dye was removed by washing twice with KHB and an additional incubation of 30 min at room temperature was done to equilibrate intracellular dye concentration and ensure complete intracellular esterification. Intracellular calcium transients were induced by the application of adenosine 5′-triphosphate (ATP) at a final concentration of 50 μM. A range of ATP stimulation concentrations were tested (from 1 to 500 μM); 50 μM was the lowest concentration of ATP capable of inducing intracellular calcium responses in rMC-1 cells. Additionally, this concentration of ATP (50 μM) has been used extensively in published studies on calcium transients in primary Müller glia cultures and acute retinal preparations as an effective dose that does not seem to negatively affect the cells.34,38,58 The use of a similar stimulation condition allowed the direct comparison of data in other published work. Treatment of rMC-1 cultures with thapsigargin (1 μM) were done at room temperature for 20 min prior to addition of ATP. Intercellular calcium waves were initiated by a single mechanical stimulation delivered to a localized region of 1–3 cells using a 0.5 μm i.d. micropipette tip (WPI Inc., Sarasota, FL) mounted on a M325 Micrometer Slide Micromanipulator (WPI Inc., Sarasota, FL). Comparable data were obtained using pharmacological ATP stimulation, although it was harder to ensure that only a localized region was initially stimulated; therefore, only data and results for mechanical stimulations are presented. Treatment of rMC-1 cultures with pyridoxal phosphate-6-azophenyl-2′, 4′-disulfonic acid (PPADS; 100 μM), flufenamic acid (FFA; 100 μM), MRS2179 (100 μM), and apyrase (50 U/mL) was done by incubation with the respective compounds during the de-esterification phase. We analyzed seven FFA-treated cultures; three PPADS-treated cultures; three MRS2179-treated cultures; three apyrase-treated cultures; and compared them to the five untreated rMC-1 cultures.
Visualization of calcium indicator dye fluorescence was done using a 488-nm (FITC) filter on an Olympus IX81 inverted fluorescence confocal microscope (Olympus Optical, Tokyo, Japan) that included epifluoresence, confocal, phase, brightfield, and Hoffman differential interference contrast (DIC) modalities. Real-time movie recordings of intracellular calcium transients were acquired at 5 Hz for 500 s while intercellular calcium transient propagation was acquired at 16.3 Hz until dissipation of the waves using a Hamamatsu ORCA-ER digital camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan) and Image-Pro Plus data acquisition and morphometric software (version 5.1.0.20, Media Cybernetics, Inc., Silver Spring, MD).
Quantification of Calcium Transients
Using ImageJ, an open source morphometric application (http://rsbweb.nih.gov/ij/), the circle-select tool was modified to allow manual selection of individual cells on the xy-plane of each movie using circles of 4 pixels (∼5 μm) in diameter. Each cell was considered as an individual region of interest (ROI). In building the ROI list for each movie, we traced cells in the frame in which they appeared brightest as a result of an activation event. By going through all the frames in a movie, we were able to catalog every cell in the field of view that participated in the propagation of signaling waves in the network for a given movie. An ImageJ plugin was used to calculate the average intensity for each ROI in each frame as well as the x–y coordinates of its area centroids. All of this data was organized in matrix format for postprocessing analyses. Since the fluorescence intensity of Fluo-4 AM was proportional to calcium concentrations, changes in cytosolic calcium concentrations could be inferred from the fluorescence profile of individual cells.
For analysis of individual intracellular calcium transients, the data was processed to identify periods of sustained increases in fluorescence intensity. Due to the highly dynamic and cyclic nature of the intracellular calcium transients, an averaging filter of 15 frames was applied to reduce noise and a first-derivative filter was then used to identify significant and sustained increases in calcium (i.e., 15 or more consecutive frames with positive derivative values). We defined this as the rise phase of the intracellular calcium transients. For analysis of intercellular calcium waves, an averaging filter of five frames was applied to the data to reduce noise in the fluorescence signals. The change in fluorescence intensity normalized to the level of baseline fluorescence (ΔF/F) was taken to indicate the magnitude of calcium changes within rMC-1 cells. ΔF/F greater than two standard deviations from baseline and a decrease of fluorescence intensity to 10% of its peak value were used as criteria for fluorescence profiles of completed calcium transients (i.e., that had experienced both full activation and deactivation; see Fig. 3). Real-time recordings of calcium signaling in response to mechanical stimulations were assessed at both the network and individual cell level. All calculations and graphs were done using Matlab (Mathworks, Natick, MA).
FIGURE 3.
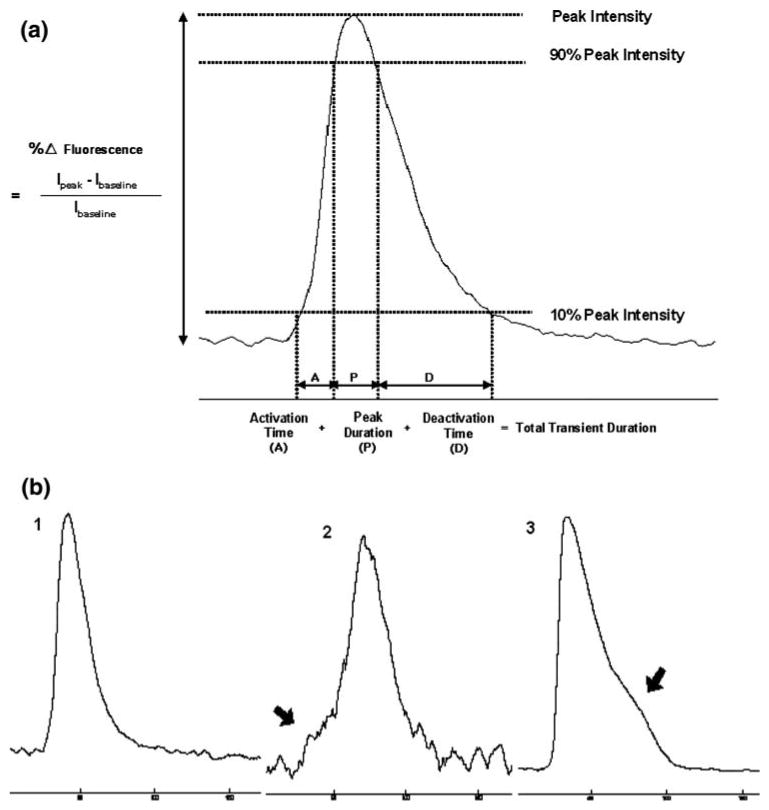
(a) Definition of characterization parameters for the analyses of calcium transients. (b) Examples of different observed fluorescent responses (see text for details).
Immunocytochemistry
Immunocytochemistry (ICC) was performed on rMC-1 cultures prepared identically to those used for calcium imaging. Cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 15 min and washed twice with physiologically buffered saline (PBS; Invitrogen, Carlsbad, CA). Cultures labeled for glial fibrillary acidic protein (GFAP) were permeabilized in 1% FBS and 0.1% Triton X-100 (Fisher Scientific International, Hampton, NH) for 30 min before incubating with anti-GFAP primary antibody (1:50; Sigma, St. Louis, MO) while cultures labeled for P2Y1 receptor, P2X7 receptor, and connexin 43 were incubated with their respective primary antibodies at 1:25 (Invitrogen, San Francisco, CA), 1:10 (Sigma, St. Louis, MI), and 1:50 (Chemicon, Temecula, CA) dilutions, respectively, with 10% FBS in PBS for 2 h. Routine negative controls for all conditions included the omission of the primary antibody and incubation with 10% FBS in PBS during the primary incubation step. For secondary antibody labeling, cells labeled for GFAP were incubated with tetramethylrhodamine isothiocyanate (TRITC) conjugated anti-mouse IgG (1:50; Sigma, St. Louis, MO) while cells labeled for P2Y1R, P2X7R, and Cx43 were incubated with fluorescein isothiocyanate (FITC) conjugated anti-rabbit IgG (1:50; Sigma St. Louis, MO). Following the ICC, all slides were mounted using Molecular Probes Prolong® Gold antifade reagent with DAPI (Eugene, OR).
Results
rMC-1 Cells Express Specific Markers for Glial Cells and Metabotropic Purinergic Receptors
Qualitatively, rMC-1 cells show similar expression patterns to Müller cells for key proteins, both in our hands and in previous work. Immunocytochemical characterization of glial fibrillary acidic protein (GFAP) showed low levels of baseline expression (Fig. 1a, e), consistent with in vivo Müller cells which only express high levels of GFAP when they become reactive following injury.10,26 Antibody labeling of P2Y1R, a metabotropic purinergic receptor that is G-protein coupled to induce release of calcium from intracellular stores through the formation of inosital triphosphate (IP3) by PLC, displayed a strong expression profile (Fig. 1b, f). In addition, we also obtained positive labeling for P2X7 receptors (Fig. 1c, g), demonstrating the presence of both ionotropic and metabotropic forms of purinergic receptors on rMC-1 cells, consistent with known mechanisms for the role of ATP in calcium signaling by Müller cells.5,39 Finally, there was also positive expression for the gap junctional protein connexin 43 (CX43; Fig. 1d, h), a subtype commonly expressed on primary Müller cells.21,59
FIGURE 1.
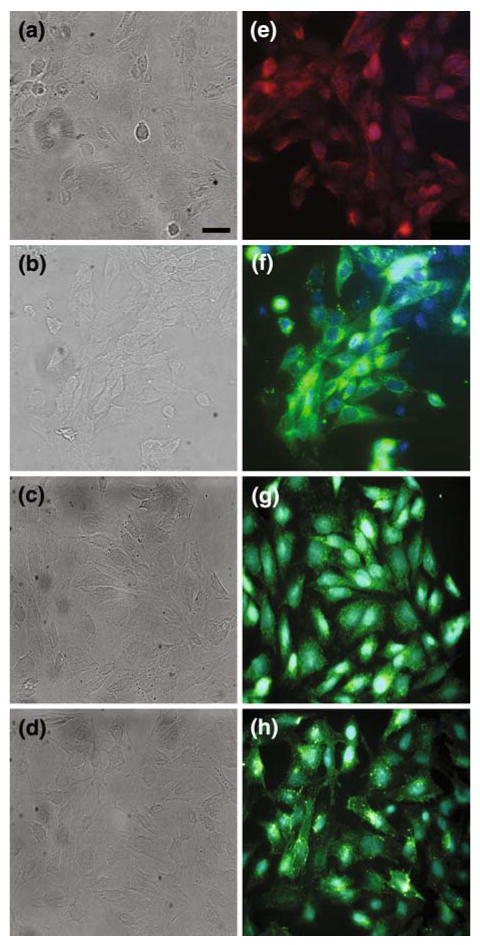
Immunocytochemistry of cultured rMC-1 Müller cells for GFAP, P2Y1R, P2X7R, and Cx43. (a–d) Phase-contrast micrographs of fluorescence images in (e–h), respectively. (e–h) GFAP (e), P2Y1R (e), P2X7R (G), and Cx43 (H) immunoreactivity. All images were taken at ×400, scale bar = 25 μm.
rMC-1 Cells Exhibited ATP-Induced Intracellular Calcium Transients
Using real-time calcium-sensitive fluorescence imaging, we recorded intracellular calcium transients in rMC-1 cells at a low frequency in the absence of applied stimulation. Five 500-s real-time recordings were analyzed and showed that approximately 2% of the cells in culture produced intracellular calcium transients in buffer in the absence of applied stimulation; an average of 23.3 ± 3.0 calcium transients were observed in each movie at a rate of 1.67 ± 0.71 transients/signaling cell/500 s, with calcium elevations averaging 5.9 ± 0.5 s in duration. Application of 50 μM ATP significantly increased the number of intracellular calcium transients in rMC-1 cells to 482.8 ± 241.5 per movie (p<0.01, Fig. 2a). Furthermore, ATP-induced increases in calcium signaling were due to significant increases in the number of cells exhibiting intracellular calcium transients (from 2% to 31%; p<0.01, Fig. 2b) rather than an increase in the frequency of calcium transients per signaling cell; which were 1.67 ± 0.71 and 1.74 ± 0.25 transients/signaling cell/500 s for controls and 50 μM ATP, respectively, with no significant differences detected via the Student t-test. Interestingly, along with the increase in the number of intracellular calcium transients in ATP-treated cultures, we also observed a significant decrease in the average duration of calcium elevations to 3.4 ± 0.6 s (Fig. 2c) following application of ATP. Quantification of the amplitude change (ΔF/F) of spontaneous vs. ATP-stimulated calcium transients showed an amplitude change of 31.8 ± 5.7% for control cultures. Corresponding to the shorter duration of calcium elevations with the application of ATP, we observed a decrease in the average amplitude of calcium transients in comparison to controls (14.3 ± 2.9%, p<0.01, Fig. 2d). Taken together, these results suggest that elevated extracellular ATP induced a higher number of faster calcium transients with smaller amplitudes vs. those of control conditions.
FIGURE 2.
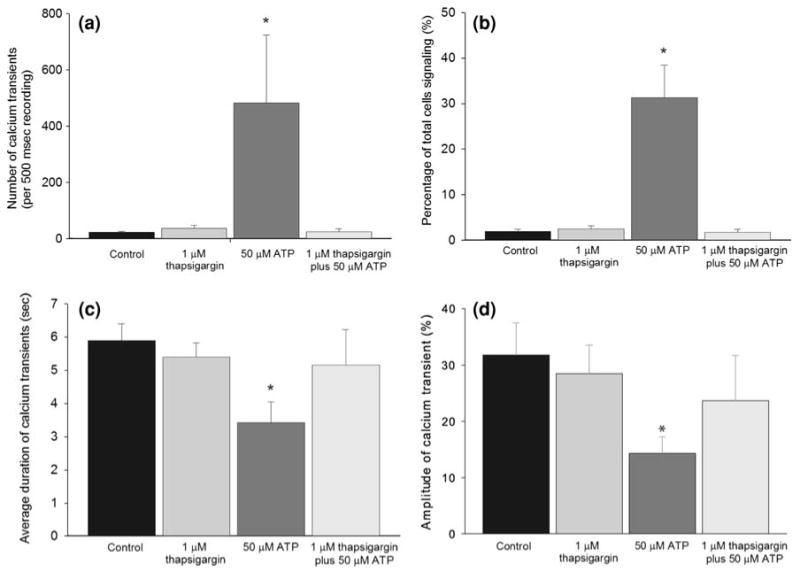
Intracellular calcium transients in untreated rMC-1 vs. cells treated with 50 μM ATP, 1 μM Thapsigargin, and 50 μM ATP with 1 μM Thapsigargin. Two-tailed Student t-tests were used to test for statistical significance. (a) The total number of calcium transients recorded during 500-s movies. (b) Percentage of cells that exhibited intracellular calcium transients during 500-s movies. (c) The average duration of calcium transients. (d) The average amplitude change of calcium transients calculated as ΔF/F. *p<0.01, **p<0.001, n = 7.
To probe the molecular mechanisms responsible for ATP-induced intracellular calcium transients in rMC-1 cells, we analyzed the effects of applying thapsigargin, a drug that depletes intracellular calcium stores via inhibition of the calcium ATPase pump on the endoplasmic reticulum (ER).53 Thapsigargin applied at a concentration of 1 μM effectively blocked the increase in the number of intracellular calcium transients previously measured with 50 μM extracellular ATP and rendered it comparable to that of control cultures (Fig. 2a). As before, our results indicated that this decreased incidence of calcium transients in the presence of elevated ATP (to 25.2 ± 10.0 transients per movie) was due to a significantly lower number of signaling rMC-1 cells in thapsigargin-treated cultures rather than changes in the frequency of transients per cell (which are 1.51 ± 0.39, 1.74 ± 0.25, and 1.51 ± 0.57 transients/signaling cell/500 s under 1 μM thapsigargin, 50 μM ATP, and 50 μM ATP plus 1 μM thapsigargin, respectively, with no significant differences detected by ANOVA). Furthermore, in the presence of thapsigargin, the amplitude and duration of calcium elevation in ATP-stimulated calcium transients were comparable to that of the control cultures (23.7 ± 8.0% and 5.15 ± 1.1 s).
Finally, to explore the possible ionotropic as well as the metabotropic components of the purinergic response, we tested ATP-enhanced responses in the presence of zero extracellular calcium and in the presence of specific inhibitors. In our hands, rMC-1 cells demonstrated an increase in intracellular calcium rather than calcium oscillations due to zero-extracellular calcium, thus implicating a role for extracellular calcium, potentially acting through ionotropic purinergic receptors; a return to calcium-containing buffer restored the ATP response. To explore the role of metabotropic purinergic receptor signaling, specifically the P2Y1 receptor, on ATP-potentiated intracellular calcium transients, we applied MRS2179 to specifically block this receptor. 100 μM MRS2179 reduced the percentage of signaling cells from 31% to 11% in the presence of ATP as well as decreased the number of intracellular calcium transients approximately 50% (to 246.2 ± 182.8), with no significant changes to the duration or amplitude of oscillations. Finally, application of 5 mM PPADS, a broad-spectrum purinergic receptor antagonist, completely but reversibly inhibited ATP-stimulated calcium transients. The removal of PPADS restored the response of these cells to extracellular ATP.
Intercellular Calcium Waves were Mediated by ATP and IP3 and Displayed Specific Signaling Dynamics
Localized stimulation of rMC-1 cells induced a propagating wave of calcium transients that spread radially outward from an initial activated cell. For each recording, all cells participating in calcium waves were individually analyzed to quantitatively study the spatial and temporal properties of calcium transient propagation. To characterize the dynamics of intracellular calcium transient responses that underlie calcium waves, we measured: (1) activation time, the rise time from 10% to 90% of the peak amplitude, (2) deactivation time, the decay time from 90% to 10% of the peak amplitude, and (3) ΔF/F, the percent change of maximum Fluo-4 fluorescence intensity with respect to the baseline resting state (Fig. 3a). We observed kinetic variations in calcium transient responses (Fig. 3b). Some cells displayed a rapid increase in cytosolic calcium (Fig. 3b, responses 1 and 3) while others had a gradual increase from baseline that preceded the main calcium rise (Fig. 3b, response 2). Additionally, some cells presented a smooth and rapid decrease (Fig. 3b, response 1), while others had a kinetically slower decrease (Fig. 3b, response 2) and displayed a secondary calcium hump (Fig. 3b, response 3). Combining five data sets (100 ± 20 cells were analyzed per recording), the averaged values for activation and deactivation times were 3.87 ± 0.62 s and 18.91 ± 2.60 s, respectively. The average change in the amplitude of the fluorescence signal of the calcium indicator (ΔF/F) was 67.4 ± 16.5%. It was interesting to note that intracellular calcium transients in the context of a calcium wave were significantly longer in duration than spontaneous or ATP-induced transients in individual cells, although it is not clear mechanistically why.
We then investigated the population dynamics of calcium wave events in intact networks by plotting the calcium transient parameters described above as a function of radial distance from the stimulation source for all individual cells that contributed to a wave. We were interested in assessing statistical trends associated with changes in individual calcium transients as a function of radial distance. Box plots show spatiotemporal data binned in 50 μm radial sections (Fig. 4). The centers of the boxes denote the median value while the upper and lower edges are the 75th and 25th percentiles, respectively. The whiskers show the range of the data and extreme outliers (+; defined by >1.5 of interquartile distance). The plots suggest that cells located closer to the stimulation site preferentially displayed shorter activation times (Fig. 4a; ∼2 s at radius, r<50 μm, and >6 s at r>150 μm), longer deactivation times (Fig. 4b; ∼37 s at r<50 μm, and <25 s at r>150 μm), and larger calcium transient amplitudes (Fig. 4c; ΔF/F = 80% at r<50 μm, and ΔF/F<30% at r>150 μm). We note that these observations may be due to higher extracellular ATP concentrations near the center of a calcium wave34 possibly due to the clustering of the initially activated cells following mechanical stimulation. Furthermore, cells located closer to the stimulation site exhibited a larger range of amplitude changes (i.e., 20–200% at r<50 μm, and 10–75% at r>150 μm) and deactivation times (i.e., 10–100 s at r<50 μm, and 15–35 s at r>150 μm), while a large range of activation times were observed in cells irrespective of their location (e.g., 0.5–14 s at r<50 μm, and 4.7–17 s at r>150 μm).
FIGURE 4.
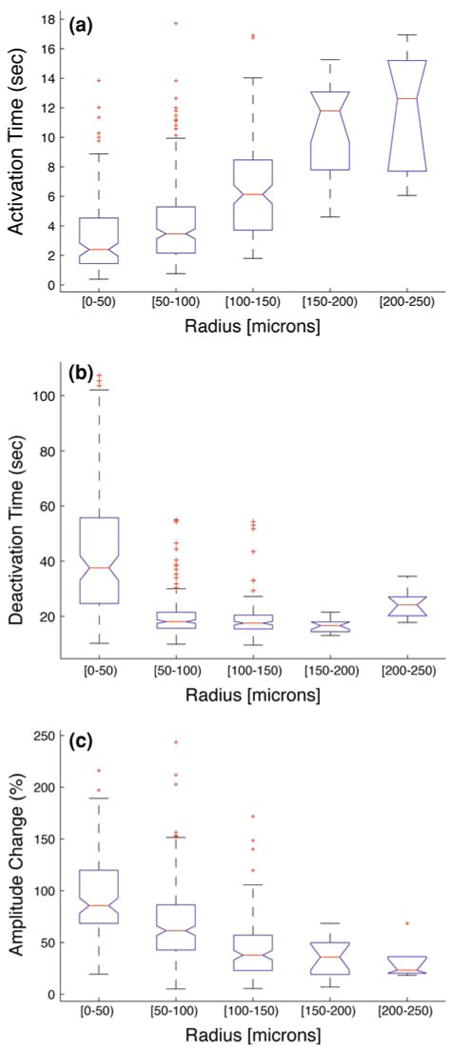
Box plots of descriptive calcium transient parameters vs. radial distance from the site of stimulation. The centers of the boxes denote the median value while the upper and lower edges are the 75th and 25th percentiles, respectively. The whiskers show the range of the data, and extreme outliers (defined by >1.5 of the interquartile distance) are represented by +. (a) Activation time vs. radial distance. (b) Deactivation time vs. radial distance. (c) Amplitude vs. radial distance.
Finally, we calculated the velocity of signal propagation between rMC-1 cells, by plotting the radial distance of each activated cell from the stimulation site against its time to activation from the time of simulation. The distance of signal propagation as a function of time followed a logarithmic trend that was fitted well with a power function (Fig. 5, R2 = 0.86 ± 0.06). To give a sense of the signaling speed of the wave, the calculated average propagation velocities at 0.2, 0.4, 0.6, 0.8, and 1.0 s following stimulations were 23.8 ± 3.7, 18.8 ± 2.2, 16.8 ± 1.5, 15.5 ± 1.0, 14.6 ± 0.8 μm/s, respectively. The average signaling speed decreased to 10.5 ± 3.3 after 5 s, and 7.8 ± 1.0 μm/s after 10 s. This data suggests that decreases in the signaling speed and response amplitudes along the radial direction of the wave may be associated with mechanisms responsible for the cessation of signal propagation, perhaps by reducing the regenerative component of the calcium wave.
FIGURE 5.
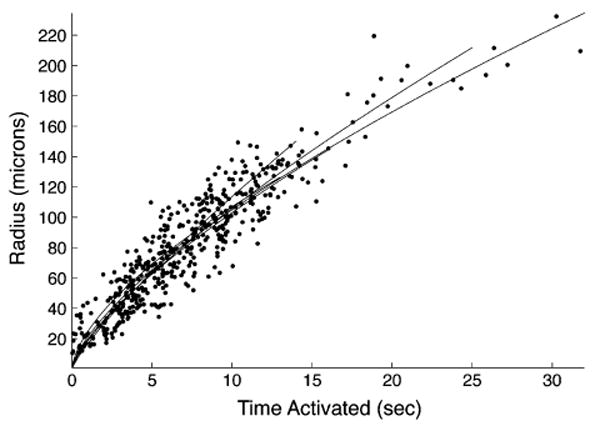
Calculation of the velocity of intercellular calcium transient propagation between rMC-1 cells by plotting the distance of activated rMC-1 cells from the stimulation site against its time to activation (defined at 10% of its measured peak amplitude) in relation to the time of simulation. The resultant plot followed a logarithmic trend that fitted well with a power function to estimate the calcium signaling propagation velocity. Each dataset (n = 5) fitted with a power function, 0.77<R2<0.91.
To examine the molecular mechanisms underlying intercellular calcium waves, we pharmacologically perturbed calcium-mediated signaling by applying the purinergic receptor antagonist PPADS24 and the gap junction blocker, FFA.41 Cultures treated with FFA and PPADS resulted in a 70% and 86% decrease in the number of responsive cells, respectively, as compared to control cultures (Fig. 6a). PPADS also affected the duration of individual responses. There was a significant increase in the average duration of intracellular calcium transients in cultures treated with PPADS (86.71 ± 46.6 s, mean ± s.e.) as compared to FFA (29.1 ± 3.3 s, mean ± s.e) or nontreated controls (31.1 ± 3.1 s, mean ± s.e.; Fig. 6b). These results suggest that the underlying molecular mechanisms responsible for intercellular calcium waves in rMC-1 cells are similar to those described for Müller cells and astrocytes.34 Finally, we examined the role of ATP as the extracellular signaling factor in by using the ATPase apyrase at 50 U/mL. Apyrase completely blocked the propagation of calcium transients from initially activated cells to neighboring cells. Application of apyrase at 10 U/mL also reduced calcium transient propagation to neighboring cells, although some responses were sometimes seen to travel to adjacent cells in immediate cell–cell contact. These results provided further evidence for the involvement of ATP in the molecular mechanism underlying intercellular calcium waves in rMC-1 cells.
FIGURE 6.
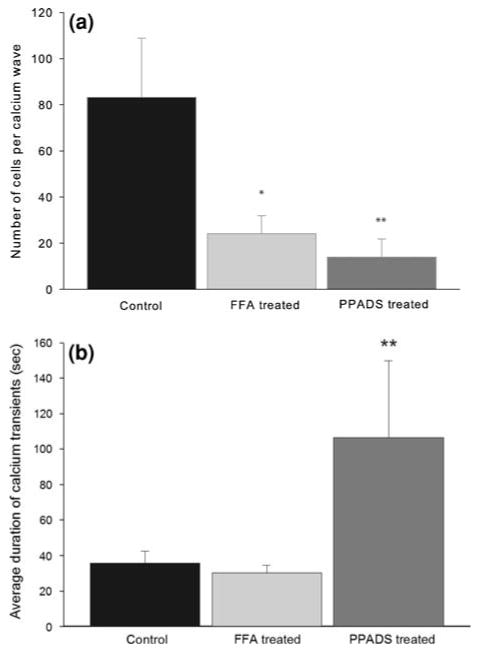
FFA-treated and PPADS-treated rMC-1 cultures blocked IP3- and ATP-mediated calcium signaling, respectively. (a) Average number of activated cells per calcium wave. *p<0.01, n = 17; **p<0.01, n = 12. (b) Average duration of the calcium transients per calcium wave. **p<0.01, n = 12.
Discussion
We introduced and characterized the dynamics of an in vitro model for studying intracellular and intercellular calcium signaling using the rMC-1 cell line derived from primary rat Müller cells.46 Although in vitro systems are simplified representations of physiological conditions, culture systems provide an opportunity to manipulate and investigate molecular and cellular processes in isolation. If the fundamental molecular mechanisms under investigation in the in vitro system are conserved with respect to known physiological processes, then the former provides an opportunity to study elements of these processes at a fundamental level under controlled experimental conditions. The molecular mechanisms that underlie calcium signaling in rMC-1 cells and the dynamics of intracellular calcium transients and intercellular calcium waves are similar to those reported for primary Müller cells and in situ retinal preparations, and thereby provide a molecular model of calcium signaling in Müller cells.
Immunocytochemically, rMC-1 cells exhibited low baseline levels of GFAP, a specific marker for astrocytic and related macroglial cells,13,54 similar to non-reactive Müller cells. In addition, they showed positive expression for P2Y1R, a G-protein-coupled metabotropic purinergic receptor involved in calcium mobilization from intracellular stores,23,29,55 as well as for the P2X7 ionotropic purinergic receptor and connexin 43, which have been respectively shown to augment cytosolic calcium via influx of extracellular calcium in response to ATP5,39 and coordinate intercellular coupling of calcium transients via the formation of gap junction channels.17,51 Functionally, rMC-1 cells exhibited intracellular calcium transients that were significantly increased in the presence of ATP, suggesting that these cells express functionally intact purinergic receptors. The effect of ATP was blocked by the application of thapsigargin, which has been previously shown to deplete intracellular calcium stores by specifically inhibiting endoplasmic reticulum calcium-ATPases.53 Our results suggest that ATP-evoked calcium transients in rMC-1 cells were initiated by the release of calcium from intracellular stores similar to that reported for primary Müller cells.31 To further elucidate signaling via metabotropic purinergic receptors, we applied 2′-Deoxy-N6-methyladenosine-3′, 5′-bisphosphate (MRS2179), a specific antagonist of the P2Y1 receptor,7,22 and showed that it significantly reduced the percentage of signaling cells as well as decreased the number of intracellular calcium transients in the presence of ATP by approximately 50%. The inability of MRS2179 to completely abolish the ATP-induced increase in intracellular calcium transients in the presence of thapsigargin suggests the possibility of additional ATP sensitive metabotropic purinergic receptors subtypes on rMC-1 cells, some of which have been shown to be expressed by primary Müller cells (i.e., P2Y2, P2Y4, P2Y6).14,42 Application of pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), a nonspecific P2Y receptor antagonist that blocks intracellular calcium mobilization by inhibiting IP3 channels57 that has been used against P2Y2, P2Y4, P2Y6, P2Y138,9,22,28,44,52 and P2Y1 receptors.6,27,47 was able to completely but reversibly inhibit ATP-stimulated calcium transients. In addition, the results point to the potential involvement of ionotropic purinergic receptors, since the removal of extracellular calcium impeded the ability to these cells to exhibit calcium transients in the presence of ATP. However, additional pharmacological characterization is needed to further elucidate a potential ionotropic receptor role in rMC-1 calcium signaling.
The average duration of ATP-induced intracellular calcium transients in rMC-1 cells was within the range of response durations reported for Müller cells in intact retinal preparations, which were measured to be between 2.5 and 6 s.37 Surprisingly, along with the increase in the number of intracellular calcium transients in ATP-treated cultures, we also observed a decrease in the average duration and amplitude of calcium elevations in the presence of ATP. The exact mechanism for this is unclear. However, since ATP is a ligand for a number purinergic receptors, it is likely that at elevated extracellular concentrations ATP also activates a number of ionotropic purinergic receptors, such as the P2X7 receptor we showed to be present on rMC-1 cells (see Fig. 1). This would drastically increase the permeability of the plasma membrane to monovalent and divalent ions, thereby potentially reducing the duration and amplitude of calcium transient responses triggered in individual cells. In addition, it has been suggested that light induces Müller cell calcium transients in the retina via the release of ATP by amacrine and/or retinal ganglion cell neurons.37 The exceptional similarity between the duration of calcium transients in ATP-stimulated rMC-1 cells (3.4 ± 0.6 s) and that previously reported for Müller cells in light-stimulated intact retinal preparations (3.84 ± 0.82 s) further supports that the rMC-1 cell line has retained the principle mechanisms associated with physiological intracellular calcium mobilization and signaling pathways in Müller cells.
Since in situ experiments have also shown the ability of Müller cells to support intercellular calcium waves38 and similar events have been observed under conditions that mimic pathology,37 we also characterized the dynamics of calcium waves in rMC-1 cultures and tested whether their propagation necessitated an ATP and/or IP3-dependent signaling mechanism. The ability of rMC-1 cell networks to support intercellular calcium waves was demonstrated by the radial propagation of signaling events following a stimulation. Published studies by several groups have implicated extracellular ATP as the primary facilitator of calcium waves in Müller cell networks of healthy and diseased retina.4,29,55,56 There is evidence for a similar mechanism in rMC-1 networks based on measurements of calcium waves following the application of the purinergic receptor antagonist PPADS,24 which resulted in a significant (86%) reduction in the size of the calcium wave as measured based on the number of participating cells. Apyrase, an ATP diphosphohydrolase, inhibited the propagation of calcium transients to secondary cells adjacent to the stimulation site, further implicating a key role of extracellular ATP in intercellular calcium waves in rMC-1 network signaling. However, there were also some differences between rMC-1 cells and Müller cells. Specifically the inhibition of gap junctions using flufenamic acid (FFA), a pharmacological agent that has been shown to reduce Cx32, Cx43, Cx46, and Cx40 currents by 85% to 95%,41,49 caused a decrease in intercellular calcium signaling by 70% in rMC-1 cells, which has only been previously reported as the primary mechanism for intercellular calcium waves between retinal astrocytes.34 There was also a significant increase in the averaged duration of the intracellular calcium transients in cultures treated with PPADS.
Our calculated value of relative calcium transient amplitudes (ΔF/F), activation, and deactivation times (67.4 ± 16.5%, 3.87 ± 0.62 s, and 18.91 ± 2.60 s, respectively) for intercellular calcium waves in rMC-1 cell networks were comparable to published results for Müller calcium transients in mechanically stimulated calcium waves in situ.34,38 The calculated speed of the initial signal propagation in rMC-1 networks was 23.8 ± 3.7 μm/s along the radial direction, which was similar to the reported value of 23.1 ± 6.7 μm/s for mechanically stimulated calcium waves in situ. Combining the results of our kinetics analysis and pharmacological studies, the data suggests that intercellular calcium transient propagation in rMC-1 networks is both qualitatively and quantitatively similar to that reported for Müller cells in in situ retinal preparations.
Acknowledgments
This work was funded by NIH grant R01 NS054736-01 and a Walter Coulter Foundation for Biomedical Engineering Early Career Award to G.S.
Footnotes
Publisher's Disclaimer: OPEN ACCESS: This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Agulhon C, Platel JC, Kolomiets B, Forster V, Picaud S, Brocard J, Faure P, Brulet P. Bioluminescent imaging of Ca2+ activity reveals spatiotemporal dynamics in glial networks of dark-adapted mouse retina. J Physiol. 2007;583:945–958. doi: 10.1113/jphysiol.2007.135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biedermann B, Bringmann A, Franze K, Faude F, Wiedemann P, Reichenbach A. GABA(A) receptors in Muller glial cells of the human retina. Glia. 2004;46:302–310. doi: 10.1002/glia.20004. [DOI] [PubMed] [Google Scholar]
- 3.Bringmann A, Iandiev I, Pannicke T, Wurm A, Buhner E, Reichenbach A, Wiedemann P, Uhlmann S. Porcine Muller glial cells increase expression of BKCa channels in retinal detachment. Curr Eye Res. 2007;32:143–151. doi: 10.1080/02713680601139333. [DOI] [PubMed] [Google Scholar]
- 4.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Bringmann A, Pannicke T, Moll V, Milenkovic I, Faude F, Enzmann V, Wolf S, Reichenbach A. Upregulation of P2X(7) receptor currents in Muller glial cells during proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2001;42:860–867. [PubMed] [Google Scholar]
- 6.Brown CA, Charlton SJ, Boarder MR. Enhancement of the response to purinergic agonists in P2Y1 transfected 1321N1 cells by antagonists suramin and PPADS. Br J Pharmacol. 1997;120:1049–1052. doi: 10.1038/sj.bjp.0701010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. Cloned and transfected P2Y4 receptors: characterization of a suramin and PPADS-insensitive response to UTP. Br J Pharmacol. 1996;119:1301–1303. doi: 10.1111/j.1476-5381.1996.tb16038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br J Pharmacol. 1996;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Sarthy VP, Kern TS. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R735–741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 12.Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984;25:1321–1328. [PubMed] [Google Scholar]
- 14.Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T. Identification of P2Y receptor subtypes in human Muller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest Ophthalmol Vis Sci. 2005;46:3000–3007. doi: 10.1167/iovs.05-0043. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson EC, Stevens ER, Wolosker H, Miller RF. Endogenous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol. 2007;98:122–130. doi: 10.1152/jn.00057.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacobas DA, Suadicani SO, Iacobas S, Chrisman C, Cohen MA, Spray DC, Scemes E. Gap junction and purinergic P2 receptor proteins as a functional unit: insights from transcriptomics. J Membr Biol. 2007;217:83–91. doi: 10.1007/s00232-007-9039-7. [DOI] [PubMed] [Google Scholar]
- 18.Iandiev I, Uckermann O, Pannicke T, Wurm A, Tenckhoff S, Pietsch UC, Reichenbach A, Wiedemann P, Bringmann A, Uhlmann S. Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47:2161–2171. doi: 10.1167/iovs.05-0595. [DOI] [PubMed] [Google Scholar]
- 19.Kannan R, Bao Y, Wang Y, Sarthy VP, Kaplowitz N. Protection from oxidant injury by sodium-dependent GSH uptake in retinal Muller cells. Exp Eye Res. 1999;68:609–616. doi: 10.1006/exer.1998.0639. [DOI] [PubMed] [Google Scholar]
- 20.Keirstead SA, Miller RF. Calcium waves in dissociated retinal glial (Muller) cells are evoked by release of calcium from intracellular stores. Glia. 1995;14:14–22. doi: 10.1002/glia.440140104. [DOI] [PubMed] [Google Scholar]
- 21.Kihara AH, Mantovani de Castro L, Belmonte MA, Yan CY, Moriscot AS, Hamassaki DE. Expression of connexins 36, 43, and 45 during postnatal development of the mouse retina. J Neurobiol. 2006;66:1397–1410. doi: 10.1002/neu.20299. [DOI] [PubMed] [Google Scholar]
- 22.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Holtzclaw LA, Russell JT. Muller cell Ca2+ waves evoked by purinergic receptor agonists in slices of rat retina. J Neurophysiol. 2001;85:986–994. doi: 10.1152/jn.2001.85.2.986. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Wakakura M. P1-/P2-purinergic receptors on cultured rabbit retinal Muller cells. Jpn J Ophthalmol. 1998;42:33–40. doi: 10.1016/s0021-5155(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Colome AM, Lee I. Pharmacological characterization of inositol-1,4,5,-trisphosphate binding to membranes from retina and retinal cultures. J Neurosci Res. 1996;44:149–156. doi: 10.1002/(SICI)1097-4547(19960415)44:2<149::AID-JNR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Lu SC, Bao Y, Huang ZZ, Sarthy VP, Kannan R. Regulation of gamma-glutamylcysteine synthetase subunit gene expression in retinal Muller cells by oxidative stress. Invest Ophthalmol Vis Sci. 1999;40:1776–1782. [PubMed] [Google Scholar]
- 27.Marcet B, Chappe V, Delmas P, Verrier B. Pharmacological and signaling properties of endogenous P2Y1 receptors in cystic fibrosis transmembrane conductance regulator-expressing Chinese hamster ovary cells. J Pharmacol Exp Ther. 2004;309:533–539. doi: 10.1124/jpet.103.063396. [DOI] [PubMed] [Google Scholar]
- 28.Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 29.Metea MR, Newman EA. Calcium signaling in specialized glial cells. Glia. 2006;54:650–655. doi: 10.1002/glia.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A. P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci. 2003;44:1211–1220. doi: 10.1167/iovs.02-0260. [DOI] [PubMed] [Google Scholar]
- 31.Moll V, Weick M, Milenkovic I, Kodal H, Reichenbach A, Bringmann A. P2Y receptor-mediated stimulation of Muller glial DNA synthesis. Invest Ophthalmol Vis Sci. 2002;43:766–773. [PubMed] [Google Scholar]
- 32.Nedergaard M, Cooper AJ, Goldman SA. Gap junctions are required for the propagation of spreading depression. J Neurobiol. 1995;28:433–444. doi: 10.1002/neu.480280404. [DOI] [PubMed] [Google Scholar]
- 33.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 34.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25:5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pannicke T, Fischer W, Biedermann B, Schadlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Muller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 41.Rana S, Dringen R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci Lett. 2007;415:45–48. doi: 10.1016/j.neulet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 42.Reifel Saltzberg JM, Garvey KA, Keirstead SA. Pharmacological characterization of P2Y receptor subtypes on isolated tiger salamander Muller cells. Glia. 2003;42:149–159. doi: 10.1002/glia.10198. [DOI] [PubMed] [Google Scholar]
- 43.Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–222. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- 45.Rogers KL, Stinnakre J, Agulhon C, Jublot D, Shorte SL, Kremer EJ, Brulet P. Visualization of local Ca2+ dynamics with genetically encoded bioluminescent reporters. Eur J Neurosci. 2005;21:597–610. doi: 10.1111/j.1460-9568.2005.03871.x. [DOI] [PubMed] [Google Scholar]
- 46.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- 47.Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shelton MD, Kern TS, Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Muller cells: model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 49.Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- 50.Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suadicani SO, Flores CE, Urban-Maldonado M, Beelitz M, Scemes E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia. 2004;48:217–229. doi: 10.1002/glia.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suarez-Huerta N, Pouillon V, Boeynaems J, Robaye B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur J Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- 53.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trimmer PA, Reier PJ, Oh TH, Eng LF. An ultrastructural and immunocytochemical study of astrocytic differentiation in vitro: changes in the composition and distribution of the cellular cytoskeleton. J Neuroimmunol. 1982;2:235–260. doi: 10.1016/0165-5728(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 55.Uckermann O, Grosche J, Reichenbach A, Bringmann A. ATP-evoked calcium responses of radial glial (Muller) cells in the postnatal rabbit retina. J Neurosci Res. 2002;70:209–218. doi: 10.1002/jnr.10406. [DOI] [PubMed] [Google Scholar]
- 56.Uckermann O, Uhlmann S, Weick M, Pannicke T, Francke M, Reichenbach A, Wiedemann P, Bringmann A. Upregulation of purinergic P2Y receptor-mediated calcium responses in glial cells during experimental detachment of the rabbit retina. Neurosci Lett. 2003;338:131–134. doi: 10.1016/s0304-3940(02)01402-7. [DOI] [PubMed] [Google Scholar]
- 57.Vigne P, Pacaud P, Urbach V, Feolde E, Breittmayer JP, Frelin C. The effect of PPADS as an antagonist of inositol (1,4,5)trisphosphate induced intracellular calcium mobilization. Br J Pharmacol. 1996;119:360–364. doi: 10.1111/j.1476-5381.1996.tb15994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weick M, Wiedemann P, Reichenbach A, Bringmann A. Resensitization of P2Y receptors by growth factor-mediated activation of the phosphatidylinositol-3 kinase in retinal glial cells. Invest Ophthalmol Vis Sci. 2005;46:1525–1532. doi: 10.1167/iovs.04-0417. [DOI] [PubMed] [Google Scholar]
- 59.Zahs KR, Kofuji P, Meier C, Dermietzel R. Connexin immunoreactivity in glial cells of the rat retina. J Comp Neurol. 2003;455:531–546. doi: 10.1002/cne.10524. [DOI] [PubMed] [Google Scholar]


