Introduction
Recent developments in nanotechnology have witnessed the rapidly evolving power of this interdisciplinary field with myriad of applications in medical sciences, in the development of smart electronic materials, in alternative energy generation, in environmental restoration and in various allied fields1–14. All of these advancements require the production of a large variety of nanoparticles, including both the metallic and non metallic, in large scales. As the nanorevolution continues to unfold, it is imperative that the manufacturing processes, for both nanoparticle production and nanoparticle embedded finished products, incorporate environmentally sound and non polluting technologies. Several of the currently used nanoparticle production processes utilize toxic chemicals either in the form of reducing agents to reduce various metal salts to their corresponding nanoparticles or as stabilizing agents to stop nanoparticles from agglomeration15–17. For example, hydrazine and sodium borohydride are powerful reducing agents which are currently used in the reduction reactions of gold (and metal compounds) to produce gold and various metallic nanoparticles15,16. Both hydrazine and sodium borohydride are highly toxic to living organism and the environment. If certain chemical ingredients used in the nanoparticles production processes are non toxic, the chemical trail that is left behind in the course of production of such chemicals may lead to environmental pollution upon sustained use of such processes for a long time. If alternative processes are not available, due care must be exercised in proper handling and disposal of toxic chemicals and various reducing and stabilizing agents in manufacturing processes.
It is important to recognize that various herbs, spices and plant sources occlude powerful antioxidants as photochemical constituents in seeds, stems, fruits and in leaves.18–22 These naturally occurring antioxidants are already within the human food chain and have been proven to be non toxic to living organisms and to the environment for thousands of years.23–26 The utility of plant based phytochemicals in the overall synthesis and architecture of nanoparticles and various nanoparticle embedded products is highly attractive as it brings an important symbiosis between natural/plant sciences and nanotechnology.27–29 This connection between plant sciences and nanotechnology provides an inherently green approach to nanotechnology referred to as green nanotechnology.30–32 We have recently reported the application of phytochemicals available within Soy and Tea as dual reducing and stabilizing agents for the synthesis of gold nanoparticles.33, 34 We herein report the utility of phytochemicals occluded within cumin as reducing agents for the reduction of gold salts to the corresponding gold nanoparticles. Phytochemical constitutents of cumin include: volatile oils, fats, numerous alcohols and aldehydes.35–41 The volatile oil has been characterized as primarily aldehydes (up to 60%) including cuminaldehyde. The primary phytochemicals that provide characteristic aroma of unheated whole seeds are 3p-menthen-7al and cuminaldehyde in combination with other related aldehydes. Cumin also contains safrole, a natural mutagenic compound, which is degraded by cooking.42 The powerful antioxidant properties of Cumin seeds have been attributed to cocktail of occluded phytochemicals. Antioxidant phytochemicals in cumin promote several important health benefits. Studies in mice have revealed the inhibition of the induction of gastric squamous cell carcinomas.43 In vivo studies in rats fed with cumin, have demonstrated a protective effect against induced colonic cancer.44 Cumin seeds have been shown to be non toxic and non carcinogenic when tested by the reverse mutation Salmonella typhimurium (TA100) test.45
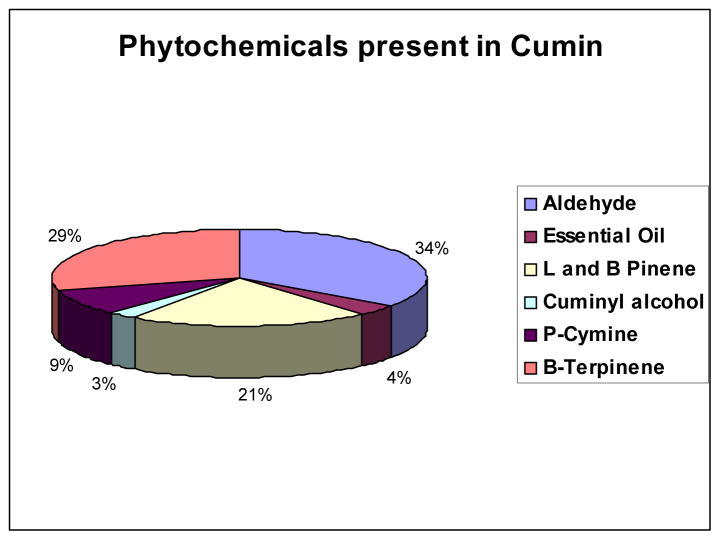
The powerful antioxidant characteristics of various phytochemicals within cumin prompted us to test their efficacy in reducing sodium tetrachloroaurate to corresponding gold nanoparticles. We hypothesized that the effective utilization of various phytochemicals that contain functional groups such as carboxyl, amino, thiol and hydroxyl units present within the multitudes of phytochemicals frameworks, including cumin aldehyde, α-and β-Pinene, cuminyl alcohol, p-Cymine, and β-Terpinene within cumin (Figure 1) will provide synergistic chemical reduction power for the reduction of gold salts into their corresponding nanoparticles. We further hypothesized that the cumin aldehyde along with a host of alcohols and terpinenes, cymines and pinenes of cumin will provide a coating of phytochemicals on the gold nanoparticles thus, paving an unprecedented process for the production and stabilization of gold nanoparticles in a singular green process. The rationale behind this hypothesis is based on the reduction capabilities of cocktail of phytochemicals present in cumin and their ability to chemically reduce gold (III) salts to nanoparticles with consequent coating of phytochemicals, and a host of other phytochemicals present in cumin on the freshly generated gold nanoparticles. We argued that validation of this hypothesis would result in a versatile ‘Green Nanotechnology’ with consequent applications of gold nanoparticles in a myriad of applications in nanomedicine and technology. On the technology front, large scale production of nanoparticles through plant species and non toxic seeds will minimize/eliminate chemical interventions thus, resulting in true green and non-polluting industrial processes for the production of nanoparticle-based smart materials.46–48 We herein, report an unprecedented synthetic route that involves the production of well-defined spherical gold nanoparticles by simple mixing of cumin to an aqueous solution of sodium tetrachloro aurate. Production of gold nanoparticles in this cumin–mediated Green Nanotechnological process is achieved within 30 minutes. The gold nanoparticles generated through cumin-mediated process were further stabilized by another naturally available plant source glyco protein, Gum Arabic. Gum Arabic stabilized and cumin-initiated gold nanoparticles exhibited long term stability (over a period of 4 weeks) suggesting that the cocktail of phytochemicals in cumin, juxtaposed by glyco proteins of Gum Arabic, serve as excellent coatings on nanoparticles and thus, provide robust shielding from aggregations. In addition, the phytochemical coatings on nanoparticles have rendered non-toxic features to these ‘Green Gold Nanoparticles’ as demonstrated through detailed MTT assays performed on normal fibroblast cells. Results of our studies presenting a new ‘Nano-Naturo’ connection for the production and utility of gold nanoparticles for potential applications in Nanomedicine and technology are discussed in the following sections.
Figure 1.
Composition of constituents of various phytochemicals in cumin.
EXPERIMENTAL
Materials and Methods
Chemicals and cumin precursors for the synthesis of gold nanoparticles (AuNPs) were procured from standard vendors: NaAuCl4 (Alfa-Aesar) and Cumin from organic grocery sources. Transmission Electron Microscope (TEM) images were obtained on JEOL 1400 Transmission Electron Microscope (TEM), JEOL LTD., Tokyo, Japan. TEM samples were prepared by placing 5 μL of gold nanoparticle solution on the 300 mesh carbon coated copper grid and allowed to sit for five minutes; excess solution was removed carefully and the grid was allowed to dry for an additional ten minutes. The average size and size distribution of gold nanoparticles synthesized were determined by processing the TEM image using image processing software such as Adobe Photoshop (with Fovea plug-ins). The absorption measurements were recorded using Varian Cary 50 UV-Vis Spectrophotometers with 1 mL of gold nanoparticle solution in disposable cuvettes of 10 mm path length.
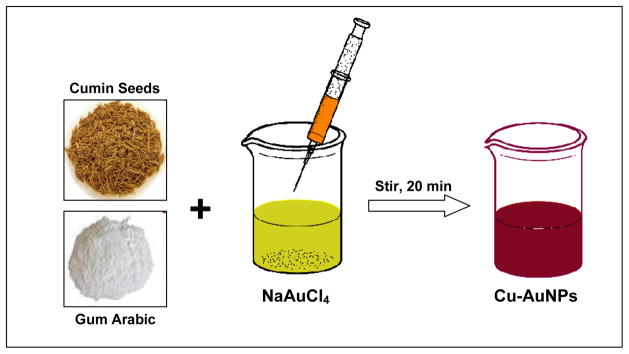
Cumin Initiated and Gum Arabic Stabilized Gold Nanoparticles (Cu-AuNP)
To a 20 mL vial was added 12 mg of gum arabic, 6 mL of doubly ionized water (DI). The reaction mixture was stirred continuously at 45 °C for 10 minutes. To the stirring mixture was added 100 μL of 0.1M NaAuCl4 (in DI water) followed by 300 mg of cumin seeds. The color of the mixture turned purple-red from pale yellow within 5 minutes indicating the formation of gold nanoparticles. The reaction mixture was stirred for an additional 15 minutes at RT. The gold nanoparticles thus formed were separated from residual cumin seeds immediately using a 5 micron filter and were characterized by UV-Vis absorption spectroscopy and TEM.
In vitro Stability Studies of Cu-AuNPs
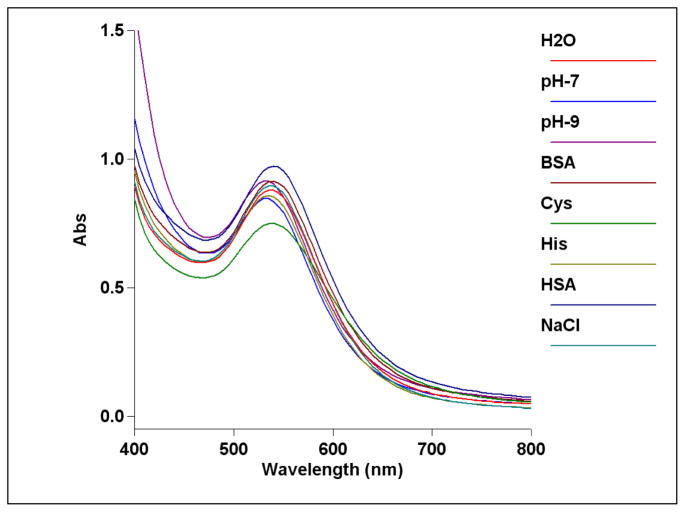
In vitro stabilities of cumin-mediated gold nanoparticles (Cu-AuNPs) were tested in the presence of NaCl, cysteine, histidine, HSA and BSA solutions. Typically, 1 mL of gold nanoparticle solution was added to glass vials containing 0.5 mL of each 5 % NaCl, 0.5 % cysteine, 0.2 M histidine, 0.5 % HSA, 0.5 % BSA solutions respectively and incubated for 30 min. The stability and the identity of gold nanoparticles were measured by recording UV absorbance after 30 min (Fig 3). The plasmon resonance band at ~535 nm confirmed the retention of nanoparticulates in all the above mixtures. TEM measurements also inferred the retention of the nanoparticulate compositions of gold nanoconstructs in each medium signifying robust nature of these nanoparticles under in vitro conditions.
Figure 3.
UV-visible spectra of Cu-AuNPs showing in vitro stability of the nanoparticles in different media, e.g. 5 % NaCl, 0.5 % cysteine, 0.2M Histidine, 0.5 % HSA, 0.5 % BSA and at different pH.
Cell Culture
Minimum essential medium (MEM with nonessential amino acids, powdered), HEPES, bovine insulin, streptomycin sulfate, penicillin-G, were obtained from Sigma Chemical Company (St. Louis, MO); all were “cell culture tested” when available. Bovine calf serum, phenol red (sodium salt), and lyophilized trypsin were obtained from Gibco BRL (Grand Island, NY). PC-3 cells obtained from ATCC were maintained in RPMI medium supplemented with 4.5 g/L D-glucose, 25 mM HEPES, 0.11 g/L sodium pyruvate, 1.5 g/L sodium bicarbonate, 2 mM L-glutamine and 10 % FBS and antibiotics. For MTT, human fibroblasts primary cultures were used and obtained from Prof. Cris Lorson, Bond Life Science Centre at University of Missouri-Columbia. The fibroblast cells were maintained in DMEM with 10 pgmL−1 phenol red, 10 mM HEPES, 100 units mL−1 penicillin, 100 pgmL−1 streptomycin, and 10% donor bovine serum (maintenance medium).
In Vitro Cytotoxicity measurements (MTT Assay)
The in vitro cytotoxicity evaluation of Cu-AuNPs was performed as described by the supplier (ATCC, USA). Briefly, 1 × 104 fibroblasts cells at the exponential growth phase were seeded in each well of a flat-bottomed 96-well polystyrene-coated plate and were incubated at 37 °C for 24 h in CO2 incubator at 5% CO2 environment. Series of dilutions like 10, 50, 100, 150 and 200 μM (gold atoms) of these nanoparticles were made in the medium. Each concentration was added to the plate in pentaplet manner. After 24 h incubation, 10 μL per well MTT (stock solution 5mg mL−1 PBS) (ATCC, USA) was added for 6 h and formazan crystals so formed were dissolved in 100 μL detergent. The plates were kept for 18 h in dark at 25 °C to dissolve all the crystals and the intensity of developed color was measured by micro plate reader (Dynastic MR 5000, USA) operating at 570 nm wavelength. Wells with complete medium, nanoparticles, and MTT, but without cells were used as blanks. Untreated cells were considered 100 % viable.
Cellular Uptake of Cu-AuNPs
PC-3 prostate cancer cells obtained from ATCC were used for the in-vitro cell internalization analysis. PC-3 cells were maintained in RPMI medium supplemented with 4.5 g/L D-glucose, 25 mM HEPES, 0.11 g/L sodium pyruvate, 1.5 g/L sodium bicarbonate, 2 mM L-glutamine and 10 % FBS and antibiotics. Known concentration of Cu-AuNPs (100 μg/mL) were added to each type of cells (~10000 cells) and incubated for 4 h at 37°C. Following incubation, cells were washed three times with PBS, centrifuged into small pellets, and fixed with 2% glutaraldehyde, 2% paraformaldehyde in sodium cacodylate buffer (0.1 M). The cells were further fixed with 1% buffered osmium tetraoxide and dehydrated in an ethanol series before embedding in Epon-Spurr epoxy resin. Sections (75–85 nm) were cut using Leica Ultracut UCT ultramicrotome and placed on a TEM grid. The sections were post-stained with uranyl acetate and lead citrate for organelle visualization. The prepared samples were viewed with JEOL 1400 Transmission Electron Microscope.
Results and discussion
Our overall long term objectives toward the design and development of biocompatible gold nanomaterials for medical applications has prompted us to pursue the application of phytochemicals in plants/seeds and various vectors from the plant kingdom for the synthesis of gold nanoparticles. Our new effort for the production of gold nanoparticles uses direct interaction of sodium tetrachloaurate with cumin seeds and gum arabic (Scheme 1) without intervention of any toxic chemical reducing agents or additional chemicals. This rationale of using 100% green resources for conducting chemical reactions thus qualifies the condition of a true 100% green chemical process. The important constituents of various phytochemicals in cumin are outlined in Figure 1.
Scheme 1.
Synthesis of Cu-AuNPs from cumin seeds and gum Arabic.
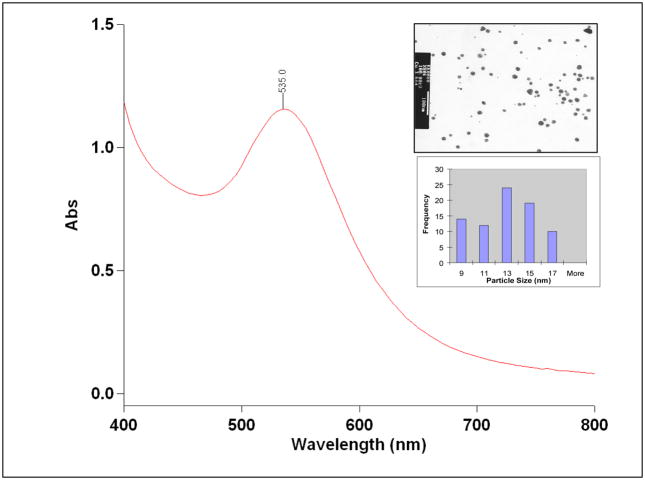
The UV absorption measurements showed that the plasmon resonance wavelength, λmax of Cu-AuNPs is ~535 nm (Figure 2). The sizes of Cu-AuNPs are in the range of 10–15 nm as measured from TEM techniques. The current discovery on the unique chemical power of phytochemicals in cumin in initiating nanoparticle formation is of paramount importance in the context of the production of gold nanoparticles for medical and technological applications under non toxic conditions. One of the paramount prerequisites of utilizing AuNPs for in vivo imaging and therapy applications is that the nanoparticles should be produced and stabilized in biologically benign media. 27,49,50 With the available methods of producing AuNPs, it is often necessary to remove unreacted toxic chemicals and byproducts. Typical known methods of making gold nanoparticles utilize harsh conditions, such as the application of sodium borohydride to reduce AuCl4−.15,16,51, 52 Although such processes lead to efficient production of gold nanoparticles, the presence of sodium borohydride, even in trace amounts, may be unsuitable for use in biomedical applications of gold nanoparticles. The high reduction capabilities of sodium borohydride result in reduction of biogenic chemical functionalities present on peptide backbones, thus either reducing or eliminating the biospecificity of biomolecules. Normally, thiol containing organic compounds are employed to stabilize AuNPs from agglomeration.51 Thiol-gold nanoparticle interaction is strong and makes gold nanoparticles highly stable.53, 54 Therefore, such AuNPs once stabilized by thiols cannot be further conjugated to useful drug moieties including peptides, proteins and various biochemical vectors that are normally used to target diagnostic and therapeutic gold nanoparticles on to tumor and various disease sites in the body. This means that the thiol-stabilized AuNPs will have limited applicability in the development of AuNP-labeled biomolecules for use in the design of target specific nanoscale imaging or therapeutic agents. Other methods that have been described in the literature utilize cocktail of chemicals in their production protocols. Such techniques are not environmentally friendly and have many drawbacks that impede the efficient utilization of AuNPs in biomedicine applications.
Figure 2.
UV-visible spectrum of Cu-AuNps synthesized by cumin seeds upon reduction of NaAuCl4 in gum Arabic. The inset shows the TEM and size distribution histogram of the gold nanoparticle solution.
Nanoparticle Characterization and Size Distribution
Physicochemical properties, such as size, charge, and morphology of gold nanoparticles generated using cumin seeds in aqueous solutions, were determined by three independent techniques; Transmission Electron microscopy (TEM), Differential Centrifugal Sedimentation (DCS, Disc Centrifuge, CPS Instruments), and Dynamic light scattering (DLS). TEM and CPS were used to determine the core size of gold nanoparticles and DLS was used to evaluate the size of cumin initiated and gum arabic coated gold.
Size and Morphology
TEM measurements on Cu-AuNPs show that the particles are spherical in shape within the size range of 10–15 nm (Table 1). Size distribution analysis of Cu-AuNPs confirms that particles are well dispersed (Figure 2 and Table 1). DCS technique measures sizes of nanoparticles by determining the time required for nanoparticles to traverse a sucrose density gradient created in a disc centrifuge.55 Both the techniques, TEM and DCS, provide sizes of metallic-gold cores. Gold nanoparticulate sizes measured by TEM and DCS are 13±4 and 12±2 nm respectively. (Figure 2 and Table 1). Dynamic light scattering method was employed to calculate the sizes of gold nanoparticles coated with phytochemicals of cumin and gum arabic (hydrodynamic radius). The phytochemical coatings on nano-gold surfaces are expected to cause substantial changes in the hydrodynamic radius of Cu-AuNPs. Hydrodynamic diameter of Cu-AuNP as determined from DLS measurements is 77±1 nm, suggesting that cumin phytochemicals (essential oils, free amino acids, variety of flavonoid glycosides) are capped on gold nanoparticles. The measurement of charge on nanoparticles and Zeta Potential (ζ) provides crucial information on the stability of nanoparticle dispersion. The magnitude of measured zeta potential is an indication of repulsive forces that are present and can be used to predict the long-term stability of the nanoparticulate dispersion. The stability of nanoparticulate dispersion depends upon the balance of the repulsive and attractive forces that exist between nanoparticles as they approach one another. If all the particles have a mutual repulsion then the dispersion will remain stable. However, little or no repulsion between particles, lead to aggregation. The negative zeta potential of −15±1 mV for Cu-AuNP indicates that the particles repel each other and there is no tendency for the particles to aggregate (Table 1 and Figure 2).
Table 1.
Physicochemical data parameters of Cu-AuNPs.
| Size [nm] |
Zeta Potential [mV] |
In Vitro Stability | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | TEM | DCS | DLS | NaCl (5 %) |
Cysteine (0.5 %) |
Histidine (0.2 M) |
HSA (0.5%) |
BSA (0.5%) |
pH 9 (PBS) |
||
| Cu-AuNP | 13±4 | 12±2 | 77±1a | −15.0 | S | S | S | S | S | S | |
Hydrodynamic Diameter.
S: Stable.
Role of Cumin Phytochemicals
Synthetic conditions have been optimized for the quantitative large scale conversions of NaAuCl4 to the corresponding AuNPs using cumine seeds and gum arabic as a stabilizer. The chemical roles of different phytochemicals in cumin responsible for the production of Cu-AuNPs are still not fully understood but we believe that water soluble constituents of cumin may be playing a major role in the overall reduction process of NaAuCl4.
The main phytochemicals present in cumin seeds consist of volatile essential oils (5 %), numerous free amino acids and a variety of flavonoid glycosides, including derivatives of apigenin and luteolin.35–41 In order to understand the critical roles of the various phytochemicals present in cumin seeds on the overall reduction of NaAuCl4 to the corresponding gold nanoparticles, we have performed a series of independent experiments using commercially available chemicals which are present in cumin seeds. Results of our experiments using those chemical compounds have unambiguously confirmed that none of the individual constituents are potentially reducing and stabilizing the gold nanoparticles, instead the cocktails of all the chemicals along with gum arabic are responsible for the synthesis of gold nanoparticles in aqueous medium. However, each individual phytochemicals failed to provide effective coating to shield the nanoparticles from agglomeration. In order to capitalize on the reduction powers of the components present in cumin, we have utilized gum Arabic (a glycoprotein) as a naturally available stabilizing agent in our reactions. Results from these experiments have revealed that the synergistic effect of all the constituents (essential oil and flavonoid derivatives) present in cumin seeds act as an excellent reducing agents to reduce the Au(III) to the corresponding gold nanoparticles which are stabilized by gum arabic. These experiments have unambiguously confirmed that cumin constituents can serve the important role in reducing gold salts to gold nanoparticles and the gum arabic provides the required stabilization to the system.
It is remarkable to note that the use of gum arabic, in the above reactions provides additional advantages. The use of gum arabic with cumin has increased the stability of the nanoparticles.56 This observation demonstrates that gum arabic may be presumably serving as a biochemical platform to drive such reactions to completion with consequent production of well defined and uniform spherical gold nanoparticles by cumin constituents.
In Vitro Stability Studies
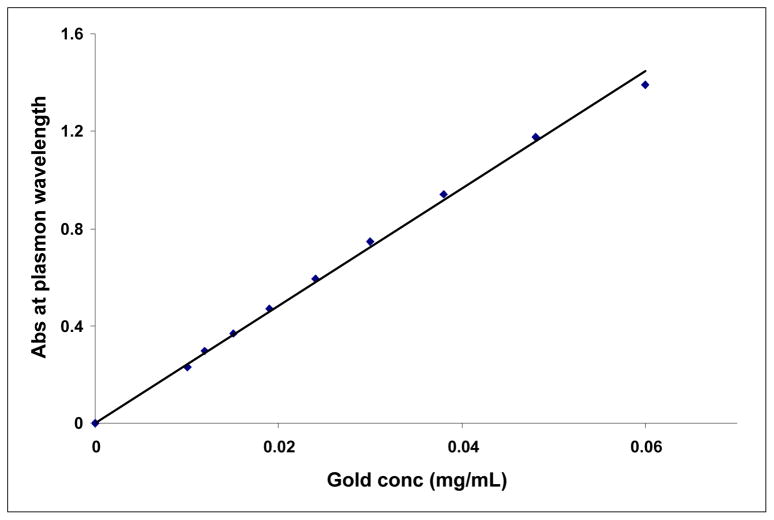
The most important criteria for in vivo molecular imaging applications is the stability of gold nanoparticles over a reasonable time period. The stability of Cu-AuNPs was evaluated by monitoring the plasmon (λmax) in 0.5 % Cysteine, 0.2 M Histidine, 0.5 % Human Serum Albumin (HSA), 0.5 % Bovine Serum Albumin (BSA) or 5 % NaCl solutions over 30 min. (Figure 3) The stability of Cu-AuNPs has also been checked at pH 5, 7 and pH 9 phosphate buffer solutions. The plasmon wavelength in all the above formulations showed minimal shifts of ~1–5 nm. Our results from these in vitro stability studies have confirmed that the gold nanoparticles are intact and thus, demonstrate excellent in vitro stability of Cu-AuNPs in biological fluids at physiological pH (Figure 3). For various biomedical applications which require lower concentrations of gold, it is vitally important that dilutions of nanoparticle solutions do not alter their characteristic chemical and photophysical properties. We have undertaken a detailed investigation to ascertain the effect of dilution on the stability of Cu-AuNP. In order to establish the stability of Cu-AuNPs under dilution, the plasmon resonance wavelength (λmax) was monitored after every successive addition of 0.1 mL of doubly ionized (DI) water to 1 mL of Cu-AuNP solutions. The absorption intensity at λmax is found to be linearly dependent on the concentration of Cu-AuNPs, in accordance with Beer Lambert’s law as shown in Figure 4. It is important to recognize that λmax of gold nanoparticles did not change at very dilute conditions. These are typical concentrations encountered when working at cellular levels.
Figure 4.
Change in plasmon absorption maximum (λmax) of Cu-AuNP as a function of dilution.
It is thus conceivable that the cocktail of phytochemicals in cumin along with non-toxic phytochemical gum arabic (Figure 1) are acting synergistically in stabilizing gold nanoparticles from any agglomerations in solution. It is also remarkable that this biocompatible Cu-AuNPs remains stable in aqueous media for over a month. These results suggest that the green nanotechnological process reported herein provides both the production and stabilization processes under mild conditions without the intervention of any man made harsh chemicals.
Cytotoxicity Studies
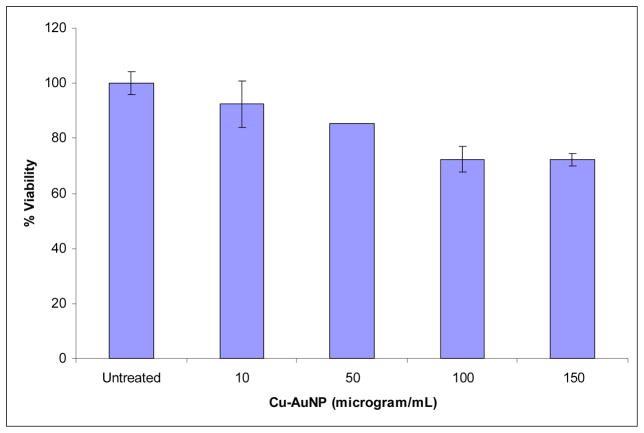
The cytotoxicity of Cu-AuNP under in vitro conditions in human fibroblast cells was examined in terms of the effect of gold nanoparticles on cell proliferation by the MTT assay. Untreated cells as well as cells treated with 10, 50, 100, 150 and 200 μM concentrations of gold nanoparticles for 24 h were subjected to the MTT assay for cell viability determination. In this assay, only cells that are viable after 24 h exposure to the sample are capable to metabolize a dye (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) efficiently and produce a purple coloured crystals which is dissolved in a detergent and analyzed spectrophotometrically. After 24 h of post treatment, fibroblast cells showed excellent viability even up to 150μM concentrations of Cu-AuNP (Figure 5). These results clearly demonstrate that the phytochemicals within cumin and gum arabic provide a non toxic coating on gold nanoparticles. It is also important to recognize that a vast majority of Gold (I) and Gold (III) compounds exhibit varying degrees of cytotoxicity to a variety of cells.57 The lack of any noticeable toxicity of Cu-AuNP provides new opportunities for the safe delivery and applications of such nanoparticles in molecular imaging and therapy.
Figure 5.
Cell viability of fibroblast cells after 24 h post incubation with increasing amounts of CuAuNPs.
Cellular Interactions
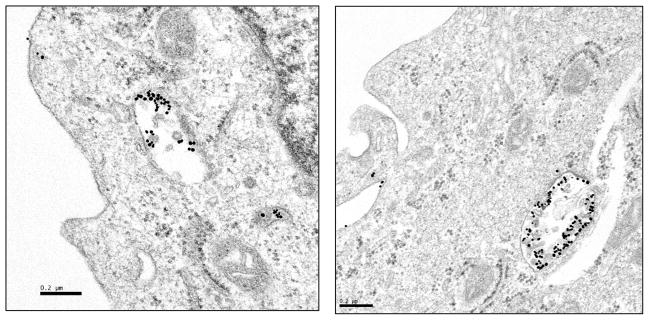
Cellular internalization studies of gold nanoparticles solutions provide insights into cellular uptake and such information will enhance the scope of gold nanoparticles in biomedicine. Gold nanoparticles are currently investigated for their potential applications as diagnostic/therapeutic agents, therapeutic delivery vectors, and intracellular imaging agents.58–63 Selective cell and nuclear targeting of gold nanoparticles will provide new pathways for the site specific delivery of gold nanoparticles as diagnostic/therapeutic agents. A number of studies have demonstrated that phytochemicals in soybeans and tea have an ability to penetrate the cell membrane and internalize within the cellular matrix.33, 34, 64, 65 Cancer cells are highly metabolic and porous in nature and are known to internalize solutes rapidly compared to normal cells.66, 67 Therefore, we hypothesized that cumin-derived gold nanoparticles will also show internalization within cancer cells. We undertook the cellular interactions and uptake studies via incubation of aliquots of Cu-AuNP with prostate (PC-3) cancer cells. TEM images of prostate tumor cells post treated with Cu-AuNP unequivocally validated our hypothesis. Significant internalization of nanoparticles via endocytosis within the PC-3 cells was observed (Figure 6). The internalization of nanoparticles within cells could occur via processes including phagocytosis, fluid-phase endocytosis, and receptor-mediated endocytosis. The viability of PC-3 cells internalized with Cu-AuNP suggests that the phytochemical coating renders the nanoparticles to be non-toxic to cells and corroborate the results as seen in the cytotoxicity studies discussed above. This internalization of gold nanoparticles, keeping the cellular machinery intact, will provide new opportunities for probing cellular processes via nanoparticulate-mediated imaging.
Figure 6.
TEM images showing endocytosis of Cu-AuNP in Prostate Cancer (PC-3) cells.
Conclusions
The unique chemical, biological, photophysical and magnetic properties of gold nanoparticles will continue to unravel a rich applied and commercially viable products within the medical, civilian, defence, environmental and space exploration sectors. Over the next decade, advances in nanotechnology as they relate to nanomedicine and technological applications will lightly impact all of us. Although there is no question on the scientific power and the positive impact of nanoscience and nanotechnology in transforming medical diagnosis and therapy, the potential toxic side effects of nanoparticles administered via intravenous or oral pathways or when nanoparticles are used in a myriad of finished products cannot be discounted. Therefore, concerted efforts must be invested in the development of non-toxic nanoparticles for utility in a wide spectrum of applications. The studies reported in this paper provide the power of plant sciences to bring about a paradigms shift on future developments in nanotechnology. Our results have demonstrated the unique kinetic propensity of Phytochemicals, present in cumin, to reduce the gold metal at the micro or in picomolar/subnanomolar concentrations, to the corresponding gold nanoparticles. The versatile phytochemical mediated green nanotechnological process has been shown to be effective in both the generation and stabilization of non-toxic gold nanoparticles for direct applications in a myriad of diagnostic and therapeutic applications. Occlusion of cancer fighting phytochemicals in various plant species and their future utility in the development of tumor specific gold nanoparticles will provide unprecedented opportunities toward the design and development of functional gold nanoparticles that can be safely produced, stored and shipped world wide. The connection between plant sciences and nanotechnology has the potential to develop an attractive symbiosis between green revolution and nanotechnology with realistic prospects for minimizing/eliminating the application and generation of toxic chemicals that destroy living organisms and our environment.
Acknowledgments
This work has been supported by the generous support from the National Institutes of Health/National Cancer Institute under the Cancer Nanotechnology Platform program (grant number: 5R01CA119412-01), NIH - 1R21CA128460-01 and University of Missouri-Research Board - Program C8761 RB 06-030.
References
- 1.Azzazy HM, Mansour MM, Kazmierczak SC. Nanodiagnostics: a new frontier for clinical laboratory medicine. Clin Chem. 2006;52(7):1238–46. doi: 10.1373/clinchem.2006.066654. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 2008;60(11):1289–306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Han G, Ghosh P, Rotello VM. Multi-functional gold nanoparticles for drug delivery. Adv Exp Med Biol. 2007;620:48–56. doi: 10.1007/978-0-387-76713-0_4. [DOI] [PubMed] [Google Scholar]
- 4.Han G, Ghosh P, Rotello VM. Functionalized gold nanoparticles for drug delivery. Nanomed. 2007;2(1):113–23. doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- 5.Jain KK. Role of nanobiotechnology in developing personalized medicine for cancer. Technol Cancer Res Treat. 2005;4(6):645–50. doi: 10.1177/153303460500400608. [DOI] [PubMed] [Google Scholar]
- 6.Jain KK. Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta. 2005;358(1–2):37–54. doi: 10.1016/j.cccn.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clin Chem. 2007;53(11):2002–9. doi: 10.1373/clinchem.2007.090795. [DOI] [PubMed] [Google Scholar]
- 8.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomed. 2008;3(5):703–17. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonvico F, Dubernet C, Colombo P, Couvreur P. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr Pharm Des. 2005;11(16):2095–105. doi: 10.2174/1381612054065738. [DOI] [PubMed] [Google Scholar]
- 10.Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37(9):1896–908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 11.Yang DP, Cui DX. Advances and prospects of gold nanorods. Chem Asian J. 2008;3(12):2010–22. doi: 10.1002/asia.200800195. [DOI] [PubMed] [Google Scholar]
- 12.Walsh D, Arcelli L, Ikoma T, Tanaka J, Mann S. Dextran templating for the synthesis of metallic and metal oxide sponges. Nat Mater. 2003;2(6):386–90. doi: 10.1038/nmat903. [DOI] [PubMed] [Google Scholar]
- 13.Yang MD, Liu YK, Shen JL, Wu CH, Lin CA, Chang WH, Wang HH, Yeh HI, Chan WH, Parak WJ. Improvement of conversion efficiency for multi-junction solar cells by incorporation of Au nanoclusters. Opt Express. 2008;16(20):15754–8. doi: 10.1364/oe.16.015754. [DOI] [PubMed] [Google Scholar]
- 14.Won Bae Kim TVGJ, Rodriguez-Rivera JA. Dumesic, Powering Fuel Cells with CO via Aqueous Polyoxometalates and Gold Catalysts. Science. 2004;305:1280–1283. doi: 10.1126/science.1100860. [DOI] [PubMed] [Google Scholar]
- 15.Esumi K, Suzuki KA, K Torigoe. Preparation of gold nanoparticles in formamide and N,N-dimethylformamide in the presence of poly(amidoamine) dendrimers with surface methyl ester groups. Colloids and Surfaces, A: Physicochemical and Engineering Aspects. 2001;189(1–3):155–61. [Google Scholar]
- 16.Feitz AGJ, Waite D. Process for producing a nanoscale zero-valent metal by reduction of inorg. salts with dithionite or borohydride Application: WO. CRC for Waste Management & Pollution Control Limited; Australia: 2004. p. 36. [Google Scholar]
- 17.Lin J, O’Connor ZWCJ. Formation of ordered arrays of gold nanoparticles from CTAB reverse micelles. Materials Letters. 2001;49(5):282–86. [Google Scholar]
- 18.Deorukhkar A, Sethi SKG, Aggarwal BB. Back to basics: how natural products can provide the basis for new therapeutics. Expert Opinion on Investigational Drugs. 2007;16(11):1753–1773. doi: 10.1517/13543784.16.11.1753. [DOI] [PubMed] [Google Scholar]
- 19.Nishino H, Tokuda YSH, Masuda M. Cancer Control by Phytochemicals. Current Pharmaceutical Design. 2007;13(33):3394–3399. [PubMed] [Google Scholar]
- 20.Johnson IT. Phytochemicals and Cancer. Proceedings of the Nutrition Society. 2007;66(2):207–215. doi: 10.1017/S0029665107005459. [DOI] [PubMed] [Google Scholar]
- 21.Kwon KH, Yu ABS, Huang MT, Kong ANT. Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models. Acta Pharmacologica Sinica. 2007;28(09):1409–1421. doi: 10.1111/j.1745-7254.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaur MRA. Transcription Factors: Molecular Targets for Prostate Cancer Intervention by Phytochemicals. Current Cancer Drug Targets. 2007;7(13):355–367. doi: 10.2174/156800907780809732. [DOI] [PubMed] [Google Scholar]
- 23.Dekker MVR. Dealing with variability in food production chains: a tool to enhance the sensitivity of epidemiological studies on phytochemicals. Eur J Nutr. 2003;42(1):67–72. doi: 10.1007/s00394-003-0412-8. [DOI] [PubMed] [Google Scholar]
- 24.Holst BWG. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19(2):73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Record IRMJ, Dreosti IE. Black tea, green tea, and tea polyphenols. Effects on trace element status in weanling rats. Biol Trace Elem Res. 1996;53(1–3):27–43. doi: 10.1007/BF02784542. [DOI] [PubMed] [Google Scholar]
- 26.Rufer CEKS. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem. 2006;54(8):2926–2931. doi: 10.1021/jf053112o. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, Ahmad N, Cui H, Mousa SA, Mukhtar H. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69(5):1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthew A, Albrecht CWEaCLR. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. [Google Scholar]
- 29.Roy KLS. A green method for synthesis of radioactive gold nanoparticles. Green Chem. 2006;8:1063–1066. [Google Scholar]
- 30.Hutchison JE. Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano. 2008;2(3):395–402. doi: 10.1021/nn800131j. [DOI] [PubMed] [Google Scholar]
- 31.Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145(1–2):83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan PV. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res. 2006;341(12):2012–8. doi: 10.1016/j.carres.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Shukla R, Nune SK, Chanda N, Katti K, Mekapothula S, Kulkarni RR, Welshons WV, Kannan R, Katti KV. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small. 2008;4(9):1425–36. doi: 10.1002/smll.200800525. [DOI] [PubMed] [Google Scholar]
- 34.Nune SKCN, Shukla R, Katti K, Kulkarni RR, Subramanian T, Mekapothula S, Kannan R, Katti KV. Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J Mater Chem. 2009 doi: 10.1039/b822015h. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa T, Takayanagi T, Kitajima J. Water-soluble constituents of cumin: monoterpenoid glucosides. Chem Pharm Bull (Tokyo) 2002;50(11):1471–8. doi: 10.1248/cpb.50.1471. [DOI] [PubMed] [Google Scholar]
- 36.Behera SNS, Mohan Rao LJ. Microwave heating and conventional roasting of cumin seeds (Cuminum cyminum L.) and effect on chemical composition of volatiles. Food Chemistry. 2004;87(1):25–29. [Google Scholar]
- 37.Nagi A, Alhaj NASMN, Zamri HF, Abdullah R. Extraction of Essential Oil from Nigella sativa Using Supercritical Carbon Dioxide: Study of Antibacterial Activity. American Journal of Pharmacology and Toxicology. 2008;3(4):225–228. [Google Scholar]
- 38.Parlatan A, Saricoban C, Ozcan MM. Chemical composition and antimicrobial activity of the extracts of Kefe cumin (Laser trilobum L.) fruits from different regions. Int J Food Sci Nutr. 2008:1–12. doi: 10.3109/09637480801993938. [DOI] [PubMed] [Google Scholar]
- 39.Khan MA. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology. 1999;7(1):15–35. doi: 10.1007/s10787-999-0023-y. [DOI] [PubMed] [Google Scholar]
- 40.Ramadan MF, Morsel JT. Characterization of phospholipid composition of black cumin (Nigella sativa L.) seed oil. Nahrung. 2002;46(4):240–4. doi: 10.1002/1521-3803(20020701)46:4<240::AID-FOOD240>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.al-Gaby AM. Amino acid composition and biological effects of supplementing broad bean and corn proteins with Nigella sativa (black cumin) cake protein. Nahrung. 1998;42(5):290–4. doi: 10.1002/(sici)1521-3803(199810)42:05<290::aid-food290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 42.Farag SE, Abo-Zeid M. Degradation of the natural mutagenic compound safrole in spices by cooking and irradiation. Nahrung. 1997;41(6):359–61. doi: 10.1002/food.19970410609. [DOI] [PubMed] [Google Scholar]
- 43.Aruna K, Sivaramakrishnan VM. Anticarcinogenic effects of some Indian plant products. Food Chem Toxicol. 1992;30(11):953–6. doi: 10.1016/0278-6915(92)90180-s. [DOI] [PubMed] [Google Scholar]
- 44.Salim EI, Fukushima S. Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. Nutr Cancer. 2003;45(2):195–202. doi: 10.1207/S15327914NC4502_09. [DOI] [PubMed] [Google Scholar]
- 45.Hanafy MS, Hatem ME. Studies on the antimicrobial activity of Nigella sativa seed (black cumin) J Ethnopharmacol. 1991;34(2–3):275–8. doi: 10.1016/0378-8741(91)90047-h. [DOI] [PubMed] [Google Scholar]
- 46.Poliakoff M, Fitzpatrick JM, Farren TR, Anastas PT. Green chemistry: science and politics of change. Science. 2002;297(5582):807–10. doi: 10.1126/science.297.5582.807. [DOI] [PubMed] [Google Scholar]
- 47.Poliakoff M, Licence P. Sustainable technology: green chemistry. Nature. 2007;450(7171):810–2. doi: 10.1038/450810a. [DOI] [PubMed] [Google Scholar]
- 48.Tang SLYSRL, Poliakoff M. Principles of green chemistry: PRODUCTIVELY. Green Chem. 2005;7:761–762. [Google Scholar]
- 49.Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M. Biological synthesis of triangular gold nanoprisms. Nat Mater. 2004;3(7):482–8. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- 50.Mohanpuria PRNK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. Journal of Nanoparticle Research. 2007;10:507–517. [Google Scholar]
- 51.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 52.Brust MWM, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid Liquid system. J Chem Soc Chem Commun. 1994:801–802. [Google Scholar]
- 53.Abad JM, Mertens SF, Pita M, Fernandez VM, Schiffrin DJ. Functionalization of thioctic acid-capped gold nanoparticles for specific immobilization of histidine-tagged proteins. J Am Chem Soc. 2005;127(15):5689–94. doi: 10.1021/ja042717i. [DOI] [PubMed] [Google Scholar]
- 54.Roux S, Garcia B, Bridot JL, Salome M, Marquette C, Lemelle L, Gillet P, Blum L, Perriat P, Tillement O. Synthesis, characterization of dihydrolipoic acid capped gold nanoparticles, and functionalization by the electroluminescent luminol. Langmuir. 2005;21(6):2526–36. doi: 10.1021/la048082i. [DOI] [PubMed] [Google Scholar]
- 55.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–83. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 56.Kattumuri V, Katti K, Bhaskaran S, Boote EJ, Casteel SW, Fent GM, Robertson DJ, Chandrasekhar M, Kannan R, Katti KV. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: in vivo pharmacokinetics and X-ray-contrast-imaging studies. Small. 2007;3(2):333–41. doi: 10.1002/smll.200600427. [DOI] [PubMed] [Google Scholar]
- 57.Shaw IC. Gold-based therapeutic agents. Chem Rev. 1999;99(9):2589–600. doi: 10.1021/cr980431o. [DOI] [PubMed] [Google Scholar]
- 58.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7(6):1542–50. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 59.Hong R, Han G, Fernandez JM, Kim BJ, Forbes NS, Rotello VM. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc. 2006;128(4):1078–9. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 60.Nakai T, Kanamori T, Sando S, Aoyama Y. Remarkably size-regulated cell invasion by artificial viruses. Saccharide-dependent self-aggregation of glycoviruses and its consequences in glycoviral gene delivery. J Am Chem Soc. 2003;125(28):8465–75. doi: 10.1021/ja035636f. [DOI] [PubMed] [Google Scholar]
- 61.Osaki F, Kanamori T, Sando S, Sera T, Aoyama Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J Am Chem Soc. 2004;126(21):6520–1. doi: 10.1021/ja048792a. [DOI] [PubMed] [Google Scholar]
- 62.Rensen PC, Sliedregt LA, Ferns M, Kieviet E, van Rossenberg SM, van Leeuwen SH, van Berkel TJ, Biessen EA. Determination of the upper size limit for uptake and processing of ligands by the asialoglycoprotein receptor on hepatocytes in vitro and in vivo. J Biol Chem. 2001;276(40):37577–84. doi: 10.1074/jbc.M101786200. [DOI] [PubMed] [Google Scholar]
- 63.Yang PH, Sun X, Chiu JF, Sun H, He QY. Transferrin-mediated gold nanoparticle cellular uptake. Bioconjug Chem. 2005;16(3):494–6. doi: 10.1021/bc049775d. [DOI] [PubMed] [Google Scholar]
- 64.Na HK, Surh YJ. Intracellular signaling network as a prime chemopreventive target of (−)-epigallocatechin gallate. Mol Nutr Food Res. 2006;50(2):152–9. doi: 10.1002/mnfr.200500154. [DOI] [PubMed] [Google Scholar]
- 65.Sun de J, Liu Y, Lu DC, Kim W, Lee JH, Maynard J, Deisseroth A. Endothelin-3 growth factor levels decreased in cervical cancer compared with normal cervical epithelial cells. Hum Pathol. 2007;38(7):1047–56. doi: 10.1016/j.humpath.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol. 2001;21(20):6913–26. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liebman MA, Williams BR, Daley KM, Sharon J. Generation and preliminary characterization of an antibody library with preferential reactivity to human colorectal cancer cells as compared to normal human blood cells. Immunol Lett. 2004;91(2–3):179–88. doi: 10.1016/j.imlet.2003.12.002. [DOI] [PubMed] [Google Scholar]