Abstract
Definitive treatment of cancer has eluded scientists for decades. Current therapeutic modalities like surgery, chemotherapy, radiotherapy and receptor-targeted antibodies have varied degree of success and generally have moderate to severe side effects. Gene therapy is one of the novel and promising approaches for therapeutic intervention of cancer. Viral vectors in general and adenoviral (Ad) vectors in particular are efficient natural gene delivery systems and are one of the obvious choices for cancer gene therapy. Clinical and preclinical findings with a wide variety of approaches like tumor suppressor and suicide gene therapy, oncolysis, immunotherapy, anti-angiogenesis and RNA interference using Ad vectors have been quite promising, but there are still many hurdles to overcome. Shortcomings like increased immunogenicity, prevalence of preexisting anti-Ad immunity in human population and lack of specific targeting limit the clinical usefulness of Ad vectors. In recent years, extensive research efforts have been made to overcome these limitations through a variety of approaches including the use of conditionally-replicating Ad and specific targeting of tumor cells. In this review, we discuss the potential strengths and limitations of Ad vectors for cancer therapy.
INTRODUCTION
Cancer ranks high amongst the causes of disease-related deaths [1]. Conventional therapies including, but not limited to, chemotherapy, radiotherapy, antibody therapy and surgical intervention, have only been partially successful in treating most malignancies [2]. Therefore, there is an urgent need for the development of novel therapeutic strategies, not only to completely cure cancer, but also to prevent it from occurring/reoccurring. Cancer gene therapy is one such promising approach, which is rapidly evolving as a possible therapeutic intervention for cancers. Application of viral vectors (viruses that have been genetically modified to deliver foreign genes) in general and adenovirus (Ad) vectors in particular, has already generated widespread expectations for improved cancer treatment and prevention.
Soon after Ad isolation in 1953 [3], its anti-tumor potential was evident from the fact that tumor regression was observed in clinical cases of cervical carcinoma following Ad inoculation [4]. However, it was only after significant developments in recombinant DNA technology that Ad emerged as a potential therapeutic agent for cancers. During the last decade Ad vectors have evolved as an efficient tool for cancer treatment; till date many clinical trials with variable but encouraging results have already been conducted or are currently in progress (Table 1). This is because of several advantages of Ad vectors such as efficient transgene delivery and expression, transduction of both dividing and non-dividing cells, ease of propagation to high titers, episomal persistence within the nucleus with minimal risk of genomic insertional mutagenesis, relative stability in blood following systemic administration, easy maneuverability of Ad genome, high capacity to accommodate foreign gene inserts, lytic life cycle and significant progress in our understanding of the biology of Ad. Importantly, Ad therapeutic applications have also been demonstrated to be safe to human beings in several clinical trials [5, 6].
Table 1.
Examples of Ad vectors for cancer gene therapy
| Ad vector | Tumor targeting modification |
E3 status |
Effector gene |
Basis of tumor selectivity/antitumor action |
Tumor type | Route of inoculation |
Combination therapy |
Success rate/response |
Clinical phase |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| ONYX-015 | E1B 55 kDa deletion | ✘ | None | Defect in p53/late mRNA export | Head and neck cancer | i.t. | None | Objective response rate of 21% | II | [306] |
| ONYX-015 | E1B 55 kDa deletion | ✘ | None | Defect in p53/late mRNA export | Head and neck cancer | i.t. | 5-FU + Cisplatin | Objective response rate of 63% | II | [57] |
| ONYX-015 | E1B 55 kDa deletion | ✘ | None | Defect in p53/late mRNA export | Solid tumor/advanced carcinoma | i.v. | Enbrel* | 4/9 patients showed stable disease | I | [307] |
| H101 | E1B 55 kDa deletion | ✘ | None | Defect in p53/late mRNA export | Head and neck or esophagus squamous cell cancer | i.t. | Cisplatin + 5- FU or adriamycin +5-FU | Objective response rate of 72.7% with chemotherapy | III | [61] |
| CG5757 |
|
✓ | None |
|
Bladder transitional cell carcinoma, lung or prostate cancer xenograft mouse models | i.t.or i.v. | None | Regression in tumor growth rate by ~72% | PC | [308] |
| CG7870 |
|
✓ | None | Upregulated probasin/PSA promoter activity | LNCaP xenografts mouse model | i.t. | Radiation | Drop in serum PSA Synergistic effect or radiation in tumor regression | PC | [287] |
| AdE4PSES E1a | E1A and E4 under PSES bidirectional promoter | ✓ | None | Upregulated PSA/PSMA activity | CWR22rv prostate tumor cells in xenograft mouse model | i.t. or i.v. | None | Tumor growth regression | PC | [114] |
| HILMI | E1A and E1B under TCF response elements | ✓ | None | Activated Wnt pathway | Anaplastic thyroid cancer xenograft mouse model | i.t. | None | Delay in tumor growth | PC | [309] |
| Ad5- CD/TKrep | E1B 55 kDa deletion | ✘ | CD/HSV- TK |
|
Prostate cancer | i.pr. | Radiation therapy, 5-FC+ vGCV | Significant decline in PSA levels in all patients | I | [143] |
| Ad5CMV- p53 (INGN201; Advexin) | None (Replication incompetent) | ✘ | p53 | Restoration of p53 function | Esophageal squamous cell carcinoma | i.t. | None | 6/10 patients had stable disease til l1yr | I/II | [20] |
| Ad-mda7 (INGN241) | None (Replication incompetent) | ✘ | mda7/IL24 | Mda7-mediated cytotoxicity | Advanced cancer(multiple cancer types) | i.t. | None | Tumor cell apoptosis, immuneactivation | I | [310] |
| E10A | None (Replication | ✘ | Endostatin | Anti-angiogenesis | Solid tumors | i.t. | None | Mild antitumor | I | [311] |
| OC- CAVE1(Canine Ad-2) | E1A under osteocalcin promotor | ✓ | None | Upregulated osteocalcin activity | D22 canine osteosarcoma cells in xenograft mouse model | i.t. | None | Tumor growth regression | PC | [286] |
| (Ad5Luc1- CK1) | Ad5 containing CAV-1 knob | ✓ | None | Enhanced transduction | Cancer cell lines/ovarian cancer/liver primary tissue slices | In vitro | None | Superior transduction than Ad5 | PC | [261] |
| Ad5.pk7-Δ24 |
|
✓ | None |
|
Orthotopic breast cancer mouse model | i.t. or i.v. | None | Regression in tumor growth | PC | [312] |
| Ad5/3- RGD | Ad5 with Ad3 knob having RGD motif | ✓ | None | Enhanced transduction | Human glioma xenograft mouse model | i.t. | None | 1000 fold increased infectivity than Ad5 | PC | [313] |
| Internavec |
|
✘ | siRNA targeting k-ras |
|
|
|
None |
|
PC | [158] |
| Ad.IFN-β | None (Replication incompetent) | ✘ | IFN-β | IFN-β mediated cytotoxicity and immune stimulation | Malignant pleural mesothelioma and metastatic pleural effusions | i.p. | None | 7/10 – antitumor immune response4/10 – Objective response | I | [314] |
| YKL- IL12/B7 | E1B 55 kDa deletion | ✘ | IL-12 and B7–1 |
|
Murine melanoma B16-F10 tumor model | i.t. | None | Delayed tumor growth, increased survival rate, enhanced T-cell response | PC | [201] |
| TNFerade | None (Replication incompetent) | ✘ | TNF-α under radio- induciblepromoter | TNF- α mediated cytotoxicity and immunostimulation | Soft tissue sarcoma | i.t. | Radiation therapy | Objective response rate of 85% | I | [302] |
| Ad-E1A- COX | E1A gene ligation to COX2 3′UTR | ✘ | None | RAS/P-MAPK specific increased E1A mRNA stabilization |
|
|
None | Increased oncolysis in tumor cells with high P-MAPK activity | PC | [136] |
| Ad5- CXCR4- UTR-E1A |
|
✘ | None |
|
|
|
None | Improvement in tumor selectivity | PC | [137] |
✓, intact;
recombinant dimer of human TNF- α receptor;
, partial or complete deletion; CAV, canine adenovirus; CD, cytosine deaminase; COX, cyclooxygenase; CXCR4, CXC chemokine receptor; E1A, E1B, E3, E4, Ad early genes; E2F, elongation factor; FGF, fibroblast growth factor; FU, fluorouracil; GCV, ganciclovir; HSV-TK, herpes simplex virus-thymidine kinase; hTERT, human telomerase reverse transcriptase; i.p., intrapleural; i.pr., intraproastatic; i.t., intratumoral; i.v., intravenous; IFN, interferon; IL, interleukin; MAPK, mitogen-activated protein kinase; MDA, melanoma-differentiation associated gene; PC, Pre-clinical; PSA, prostate-specific antigen; PSES, prostate-specific chimeric promoter-enhancer; PSMA, prostate-specific membrane antigen; Rb, retinoblastoma; siRNA, small interfering RNA; TCF, T-cell factor; TNF, tumor necrosis factor; UTR, untranslated region
Ad vectors based on human Ad serotype 5 (Ad5) and 2 (Ad2) are most frequently used in several types of cancer gene therapy. Attachment of Ad5 and Ad2 to a susceptible cell is mediated by high-affinity binding of the Ad fiber knob to the primary receptor, coxsackievirus and Ad receptor (CAR), followed by a secondary interaction of the penton base with integrins resulting in virus internalization into the cell [7, 8]. CAR is expressed in a variety of normal tissues contributing to promiscuous Ad tropism and lack of specific targeting; on the contrary many tumor cells express lower levels of CAR thus are refractory to transduction by Ad vectors [9]. Additional limitations include the predominant tropism of Ad to the liver resulting in low therapeutic index at target tissues, and Ad vector neutralization by preexisting antibodies resulting in a rapid vector clearance [10]. Because of these limitations, extensive use of Ad vectors in clinical cases of cancer has been hampered. Some of these attributes of Ad, which are otherwise considered as limitations in long-term gene therapy for genetic diseases, are often beneficial in case of cancer gene therapy. For instance, strong induction of immune response by Ad can act as an adjuvant to activate/enhance the otherwise diminished immunity against tumor cells. Similarly, a rapid clearance of Ad is also beneficial to cancer gene therapy to produce desirable anti-cancer effect within a short period and protect the healthy cells from long-term exposure to toxic products.
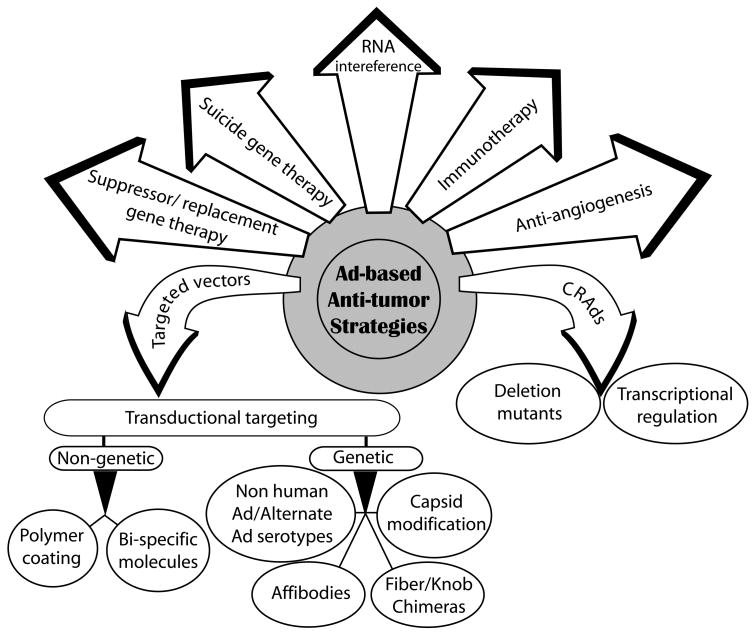
During the last decade, substantial progress has been made to counteract the limitations of Ad vectors in cancer therapy. Innovative strategies like the use of conditional replicating adenoviruses (CRAds), incorporation of tumor suppressor or suicide genes into Ad, targeted vector delivery to tumor cells or targeted expression of therapeutic genes, either used alone or in novel combinations have resulted in improved safety and efficacy of Ad vectors (Fig. 1). Despite promising preclinical data with Ad vectors, complete therapeutic efficacy is yet to be attained. Rapid developments in Ad research and combinations of Ad-mediated gene therapy with additional therapeutic interventions have been proposed to increase cancer treatment efficacies. This review discusses the potential and limitations of Ad vectors for cancer therapy with focus on various strategies that are being employed to improve the safety and potency of Ad-mediated cancer gene therapy.
Figure 1. Strategies utilizing Ad vectors for cancer gene therapy.
TUMOR SUPPRESSOR GENE/ANTI-TUMOR RECEPTOR THERAPY
A large numbers of tumor suppressor genes control cell proliferation by regulating cell cycle progression and apoptotic pathways. Mutations in tumor suppressor genes resulting in their loss-of-function lead to uncontrolled cell proliferation and development of tumors. Most of the tumors have been shown to have one or more inactivated tumor suppressor genes that attribute to their aggressive behavior. Restoration of tumor suppressor gene function through gene delivery can correct the tumor phenotype. For example, p53, one of the most widely studied tumor suppressor genes, is mutated in more than 50% of all human tumors and serves as an ideal target for gene replacement therapy [11]. Until now, more that 60 cancer gene therapy clinical trials (phase I to III) using Ad vectors expressing p53 have been approved for treatment of a vast variety of cancers (Journal of Gene Medicine, http://www.wiley.co.uk/genmed/clinical/). Restoration of wild-type p53 by replication-defective Ad-mediated gene transfer exhibited excellent safety profile and resulted in significant anti-tumor effects [12]. Restoration of p53 has also demonstrated significant bystander effect (defined as the growth inhibition/killing of non-transduced cells) by inhibiting angiogenesis and promoting immune response towards tumor cells [13–16]. Furthermore, p53 replacement increases the sensitivity of tumor cells towards chemotherapy and radiotherapy by restoration of apoptotic pathways, and when used together, a significant increase in anti-tumor efficacy has been achieved [17]. An Ad vector (trademarked as Gendicine) with p53 expression cassette in the deleted E1 region, has been approved by the State Food and Drug Administration (SFDA) of China for commercial use, thus becoming the first approved gene therapy product for treatment of cancer [18]. During the phase II/III clinical trials, Gendicine demonstrated significant synergistic effects in combination with radiotherapy, chemotherapy, surgery or hyperthermia for the treatment of cancers [18]. A similar vector developed by Introgen Therapeutics (INGN 201, ADVEXIN) is in clinical development for the treatment of various cancers in North America and Europe [19, 20].
In tumor cells with aberrant retinoblastoma (Rb) function, Ad vectors have been used to inhibit tumor growth by restoring normal Rb function in tumor cells [21, 22]. To further enhance the anti-tumor efficacy, various Rb mutants and splice variants have been identified that showed more potent anti-tumor effect as compared to wild type Rb [23, 24]. Similarly, Ad vector-mediated delivery of other tumor suppressor genes like p14ARF, p16INK4a, p21, p27, breast cancer 1 (BRCA1), phosphatase tensin homologue (PTEN), Ras homolog gene family member B (RhoB), fragile histidine triad (FHIT) or p53 upregulated modulator of apoptosis (PUMA) etc. have been investigated for treatment of different tumors [25–32]. Defect in cellular apoptosis is considered a crucial factor in the development of cancer as well as resistance to traditional anti-cancer therapies [33]. Ad vectors based strategies to induce selective apoptosis of tumor cells by delivery of proapoptotic molecules like B-cell lymphoma/leukemia-2 (Bcl-2) family proteins (Bax, Bcl-XS, Bcl-XAK, Bik/Nbk etc.) or death ligands like tumor necrosis factor (TNF)-α, CD95/Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) have been described that enhanced the cytotoxicity and sensitized the tumor cells to chemotherapy or radiotherapy [34]. Significant anti-tumor responses were observed in these studies thus underscoring the potential of Ad vectors expressing tumor suppressor genes in cancer gene therapy.
An alternative approach to suppress tumor growth is the ligand-receptor interaction-mediated tumor inhibition. Ad vectors have been utilized to successfully deliver ligands to oncogenic receptors either in the form of anti-receptor antibodies or ligands (natural or recombinant). The association of ligands to their receptors often results in their down regulation or activation of downregulatory signal transduction pathways, which ultimately lead to inhibition of tumor growth. Elevated levels of EphA2, a receptor tyrosine kinase protein, were observed in breast [35], prostate [36], urinary bladder [37] and many other cancer cell lines and clinical specimens when compared to normal epithelium, and therefore, EphA2 is an attractive candidate for anti- cancer therapy. In normal epithelium, the localization of EphA2 at intercellular junctions favors binding with its membrane-anchored ligand, EphrinA1 and phophosphorylation of the receptor. However, in cancer cells, over-expressed EphA2 is defective in ligand binding due to its altered localization [38, 39]. Furthermore, in cancer cells functioning of cell adhesion molecule, E-cadherin, also regulates phophorylation of EphA2 [38]. Thus, EphA2 in cancer cells is largely unphosphorylated and binding of EphrinA1 causes EphA2 phosphorylation and degradation, and consequently negatively regulate tumor growth and metastasis. In order to mimic ligand-induced downregulation of EphA2, monoclonal antibodies targeting EphA2 have been utilized. These antibodies increased EphA2 phosphorylation and promoted its degradation [40]. It was also demonstrated that Ad-mediated delivery of secretory form of EphrinA1, resulted in autophosphorylation and increased turnover of EphA2, which negatively regulated breast cancer cell growth and survival in vitro and in vivo [41, 42]. Similarly, anti-ErbB2 antibody delivered via Ad vector exhibited ErbB2 down regulation, increased apoptosis, cytotoxicity and overall anti-tumor effect in cell culture as well as in animal models [43, 44].
CONDITIONALLY REPLICATING ADENOVIRUSES (CRAds)
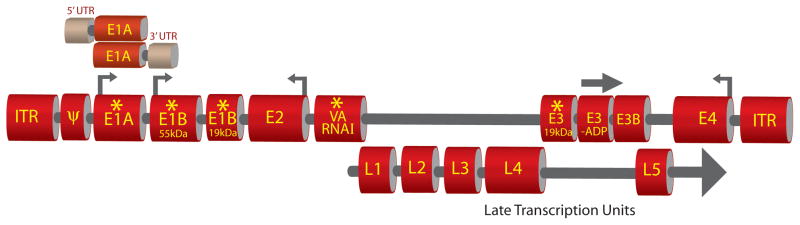
Lysis of tumor cells by replication-deficient Ad vectors is limited because of the lack of efficient penetration and spread to solid tumor mass. Application of replication-competent Ad vectors that selectively replicate in and kill tumor cells while sparing the normal cells is a promising approach to counter such limitations. Self-perpetuating CRAds (also referred to as oncolytic Ad) cause lysis of infected cancer cells and subsequent transduction of surrounding tumor cells could lead to several fold increase in their therapeutic indices. CRAds could be generated mainly by the following strategies; 1) by introducing mutation/s in selected viral essential genes whose functions could be complemented only in tumor cells but not in normal cells (deletion mutants), 2) by regulating expression of essential viral genes by placing them under tissue or tumor-specific transcriptional regulatory elements (TREs) (transcriptional regulation) (Fig. 2).
Figure 2. Strategies for development of CRAds.
Various deletions can be introduced at different locations (indicated by asterisk) to enable selective replication of Ad vectors in tumor cells [52, 74, 84, 86, 94]. Usually the function of protein encoded by the mutated gene is complemented in tumor cells but not in the normal cells. Expression of some of the critical genes of Ad (indicated with arrows) can be exogenously controlled by tumor-specific regulatory elements [109–112]. Ligation of defined 3′UTR or 5′UTR to the E1A gene can result in tumor selective stabilization or translation of E1A mRNA respectively [136, 137]. Such modifications allow tumor-specific replication of Ad vectors. Retention of E3 genes (E3B and E3-ADP) or overexpression of ADP (indicated by bold arrow) usually potentiates the efficacy of Ad vectors [91, 97]. The late transcription units that encode Ad structural proteins remain unmodified. ITR, Inverted terminal repeat. UTR, Untranslated region. E1A, E1B, E2, E3 and E4 are Ad early genes. L1, L2, L3, L4 and L5 are Ad late genes. Ψ, Ad packaging signal. VA, Virus-associated. ADP, Ad death protein.
Deletion mutant CRAds
Development of CRAds that could selectively replicate in tumor cells has benefited from our understanding of the interactions of Ad proteins with cellular proteins. Ad E1 proteins modulate cell cycle control to favor virus replication. The E1A gene product binds to wild type retinoblastoma (Rb) protein leading to the release and activation of transcription factor E2F [45]. Activated E2F-mediated transcription of cell cycle S-phase entry genes enable Ad to take advantage of the cellular replication machinery [46]. E2F also transactivates the p14ARF gene and increases cellular levels of p53 by preventing it from murine double minute (MDM2)-mediated degradation [47]. However, the higher level of p53 causes cellular apoptosis or cell cycle arrest and consequently prevents viral replication. To overcome this p53 action, Ad E1B 55 kDa protein together with E4 open reading frame (ORF) 6 encoded protein, binds to and inactivate p53, thus preventing cellular apoptosis and allowing virus replication and spread [48, 49]. E1B 19 kDa protein, a bcl-2-related protein, also inhibits E1A-induced apoptosis [50, 51]. Defects in p53 and/or Rb proteins in a variety of tumors is well documented [11]. This knowledge together with aforementioned understanding of virus interaction with host cell machinery has provided the opportunity to develop CRAds. ONYX-015 (also known as dl1520) is an E1B 55 kDa mutant vector that selectively replicates in cancer cells with defective p53 but not in normal cells with functional p53 [52]. ONYX-015 was the first CRAd to enter the clinical trials [53] and since then has undergone numerous clinical trials (phase I to III) for a variety of cancers, including head and neck squamous cell carcinoma, glioblastoma, hepatocellular carcinoma, colorectal carcinoma, sarcomas, ovarian, pancreatic and hepatobiliary cancers, with variable efficacies [54, 55]. Therapeutic efficacy of ONYX-015 as a single therapeutic agent was relatively limited, however, when used in combination with chemotherapy, encouraging anti-tumor effects were observed [55–57]. Additional oncolyitc vectors with defect in E1B 55 kDa have also been generated that demonstrated similar anti-tumor efficacies [58, 59]. H101 is one such vector that has been investigated through extensive clinical trials (phase I to III) in China against variety of tumors [60]. A phase III trial utilizing H101, with or without chemotherapy combination, was conducted with patients of head and neck or esophagus squamous cell cancer (n=123) [60, 61]. An objective response rate of 72.7% with combination therapy in contrast to 40.4% with chemotherapy alone was obtained. H101 was subsequently approved by the SFDA of China for commercial use for treatment of nasopharyngeal carcinoma in combination with chemotherapy, making it the first commercial CRAd product for cancer gene therapy.
Despite the encouraging results, the correlation between p53 status and susceptibility to E1B 55 kDa mutant CRAds, has not been consistent [62–64]. On the contrary, some experiments have demonstrated that E1B 55 kDa - p53 binding is required for Ad-mediated cell death or productive infection [65, 66]. It has been argued that the preferential killing of some tumor types by ONYX-015 may be due to differences in vector infectivity, permissiveness, expression of early Ad proteins [67], loss of p14ARF [68], nature of p53 mutation [69], or late viral RNA export [70] rather than p53 status alone. Moreover, the efficacy of replication of ONYX-015 in tumors is attenuated as compared to that of wild-type virus [71]. This is probably because other functions of E1B 55 kDa protein, such as translation and nuclear export of late viral mRNA and inhibition of host cell protein synthesis are compromised [72]. Additional mutations in E1B 55 kDa protein that resulted in tumor selectivity without compromising viral replication efficacy have also been identified [73].
In an alternative approach, Ad E1A mutant vectors (for example - Δ24 and dl922–947) with deletion in Rb-binding domain (CR-2), which predominantly target cancer cells with abnormal Rb pathway have been generated [74, 75]. Unlike E1B 55 kDa mutation, CR-2 deletions do not inhibit the vector replication in tumor cells; therefore, these vectors showed better anti-tumor efficacy both in vitro as well as in vivo compared to E1B 55 kDa mutant vectors [75]. However, replication of this virus was not entirely tumor-restricted as rapidly dividing normal cells may also favor virus replication, thus raising safety concerns [74]. Further, modifications like incorporation of tumor-specific promoters to control critical viral gene expression (as in ONYX-411) or addition of targeting ligands, such as Arg-Gly-Asp (RGD) were introduced that successfully enhanced the tumor cell selectivity as well as safety of E1A mutant vectors [76, 77].
Many of the natural tumor-selective viruses like reovirus and vesicular stomatitis virus (VSV) depend on upregulation of Ras in tumors for their replication [78, 79]. Ad vectors have also been genetically modified to selectively replicate in tumor cells with upregulated Ras levels. During infection of normal cells, exposure of viral double-stranded RNA (dsRNA) induces cellular interferon (IFN) that leads to the activation of protein kinase R (PKR) [80]. Activated PKR in turn phosphorylates the translation initiation factor eIF-2α resulting in inhibition of viral protein translation and virus replication [81]. Ad have evolved a mechanism to counter PKR-mediated inhibition by virtue of virus-associated RNAs (VA RNAs) that bind to and inactivate PKR [82, 83]. Activity of PKR in tumor cells is additionally inhibited by activated Ras pathway, allowing virus replication to proceed. This observation provided the opportunity to generate a VA RNA I mutant CRAd vector (dl331) that showed attenuated replication in normal cells while replicating efficiently in tumor cells with active Ras [84]. Similarly, in Epstein-Barr virus (EBV)-associated tumors, EBV-encoded small RNA I complements for Ad VA RNA I function, thus allowing selective replication of VA RNA I mutant Ad [85].
In another strategy, deletion of Ad E1B-19 kDa ORF resulted in profound cellular apoptosis [86]. This strategy, therefore, enhanced viral release and cell-to-cell spread with better anti-tumor effects while maintaining efficient viral replication compared to wild type or other E1B mutant vectors [86–88]. Furthermore, E1B-19 kDa blocks TNF-α-mediated cell death by various mechanisms [89]. As a result, in the presence of TNF-α, E1B-19 kDa ORF-deleted Ad vectors replicated selectively in tumor cells with frequently disregulated apoptotic pathways, but not in normal cells because of premature apoptosis [90, 91]. E3 region has been considered dispensable for replication of Ad in cell culture and has been removed from most of the first generation Ad vectors [10]. However, the subsequent studies have suggested important role of E3 proteins in evasion from host immune response, cell lysis, viral release and spread [92]. E3B proteins [receptor internalization and degradation (RID) complex (10.4/14.5 kDa) and 14.7 kDa] are known to inhibit Fas-, TRAIL- or TNF-induced apoptosis of host cells [92] and deletion of E3B region resulted in decreased virus replication, rapid virus clearance from tumor cells and reduction in overall anti-tumoral potency [93]. Therefore, retention of E3B in Ad-based oncolytic vectors seems important [91]. E3 gp19 kDa protein binds to and inhibits MHC I expression on cell surface, thus evading detection by cytotoxic T lymphocytes (CTL) [92]. As tumor cells have evolved to evade CTL, the function of gp19 kDa is redundant in tumor cells. Therefore, the deletion of gp19 kDa ORF should allow Ad to selectively propagate in tumor cells, whereas Ad-infected normal cells will be detected by CTL and eliminated [94].
Ad death protein (ADP, E3–11.6 kDa) is another protein that is required for efficient lysis of Ad-infected cells, virus release and spread. [95]. Deletion of ADP resulted in delayed and slow cell lysis [95], while overexpression of ADP in replication-competent Ad vectors (e.g. KD-1, KD-3, VRX-007) demonstrated increase in anti-tumor efficacy [96, 97]. On the other hand, some investigators have analyzed the utility of selective removal of E3 genes (such as ADP, gp19 kDa, E3B) and substituting them with therapeutic transgenes under endogenous Ad promoters [94, 98, 99]. For example, replacement of ADP with a therapeutic transgene resulted in delayed cell lysis and prolonged expression of therapeutic transgene due to extended survival of infected cells and consequent improved tumor cell killing by the therapeutic gene [98, 100]. Similarly, high levels of transgene expression, restricted to the late phase of infection, have been achieved by placing the transgene in the E3B region under the control of viral endogenous regulatory elements [98]. Another Ad protein encoded by E4ORF4 is shown to be highly toxic and induces p53-independent apoptosis selectively in broad range of cancer cells [101]. Subramanian et al., [102] employed genetic screening of random mutants and identified several viral early and late genes that influence the spread of Ad vectors and may be of importance in developing new CRAd.
To further enhance the safety of these approaches, an E1A and E1B-55 kDa double-restricted Ad vector (AxdAdB-3) has been generated that replicates less efficiently in normal cells while retaining potent oncolytic properties against variety of cancers [103–106]. Likewise, an Ad vector with triple mutations (in E1A, E1B-55 kDa and E1B-19 kDa genes) demonstrated significantly improved therapeutic profile [107]. Clearly, development of mutant Ad vectors with tumor-selective replication favors investigation for further genetic mutations that could allow better tumor specificity without compromising virus replication.
Transcriptionally regulated CRAds
Another method for the development of the CRAds is to replace viral promoters controlling critical transcription units by TREs, also referred to as transcriptional targeting [108]. As a result of transcriptional targeting, vector gene expression and replication is expected to occur selectively or preferentially in target/tumor cells. Evidently, the E1A gene is the obvious choice to be controlled by TREs as it is the most vital gene responsible for viral replication together with modification of the host cell environment to favor viral replication. The first TRE-regulated Ad vector (CN706), with E1A expression controlled by prostate-specific antigen (PSA)-derived minimal enhancer/promoter, selectively replicated in prostate cancer cells expressing PSA and demonstrated potent anti-tumor effects [109]. Subsequently, a wide variety of TREs such as those regulating expression of human telomerase reverse transcriptase (hTERT), transcription factor E2F, α-fetoprotein (AFP) and others, have been investigated for various cancers that guided tumor-selective vector replication with encouraging results [108].
Although exogenous control of a single viral essential gene resulted in tissue/tumor-specific replication, leaky replication has also been observed in normal cells. Further improvement in tumor specificity and safety of CRAds could be achieved by exogenously controlling additional essential vector genes (e.g., E1B, E2 and E4) [110–114]. Along these lines, telomelysin (OBP-301), a CRAd in which the hTERT promoter element drives the expression of E1A and E1B genes linked together with an internal ribosome entry site (IRES), has been developed, which selectively replicated in variety of cancer cells with high telomerase activity [115]. Similarly, simultaneous control of E1A and E4 genes under the E2F-1 promoter or the hTERT promoter, respectively (OAS403) resulted in tumor-selective cell killing and reduced hepatotoxicity [116]. Further, use of regulatory elements such as hTERT, E2F and survivin that are universally active in most cancer cells, offers additional advantage to target a variety of tumors [77, 117, 118]. Wnt signaling pathway is constitutively upregulated in certain cancer types, most notably in colon cancer [119]. Upregulation of Wnt pathway ultimately leads to nuclear translocation and accumulation of β-catenin and co-activation of transcription factors like T-cell factor (TCF) and lymphoid enhancer-binding factor (LEF) [119]. Oncolytic Ad vectors, with TCF/LEF binding sites engineered in multiple early gene promoters, have been designed that preferentially replicated in cancer cells with activated Wnt pathway [112, 120]. Transcriptionally-regulated CRAds targeting hypoxic tumor microenvironment have also been generated. Hypoxia-inducible factor (HIF) mediates transcriptional responses by binding to hypoxia-responsive elements (HRE) of target genes. HRE-driven CRAds showed specific destruction of hypoxic tumor cells that are otherwise resistant to traditional therapies [121, 122]. However, some of the studies have also demonstrated adverse effects of hypoxia on Ad replication necessitating further modifications for improvement in HRE-driven CRAds [123, 124].
As an additional safety feature, external regulation of replication of oncolytic Ad by utilizing elements of “Tet-On gene regulatory system” to control E1A gene expression has been reported [125]. With this approach, tight pharmacological control (via addition or withdrawal of doxycycline) of Ad replication and oncolysis was observed. Additional systems to externally regulate vector replication by controlling E1A expression by the glucocorticoid-responsive mouse mammary tumor virus (MMTV) promoter (through addition of dexamethasone) or by rapamycin dimerization system have also been successfully employed [126, 127]. Alternatively, tissue-specific promoters were incorporated with drug responsive elements to allow control over vector replication [128, 129]. Heat-or-radiation inducible promoters have also been employed to regulate transgene expression [130, 131].
To restrict the size of TREs while maintaining or enhancing the stringent control of vector replication, designer artificial promoters have been developed by fusing various regulatory elements [132, 133]. For example, bidirectional promoters to simultaneously control expression of two key viral genes, such as E1A and E1B or E1A and E4 have been employed [114, 129]. Dual-specific hybrid promoter constituting the regulatory elements that responds to hypoxia and estrogen has been engineered to impart greater selectivity to oncolytic Ad [134]. The field of transcriptionally-regulated CRAds is challenging and presents several limitations. Various viral and non-viral factors can alter the anticipated response of the heterologous promoter. The presence of cis-acting enhancer elements or cryptic transcription initiation sites located upstream of the E1A region, particularly in the left ITR or the packaging signal, are sometimes responsible for transcription read-through of viral E1A or transgene, regardless of the presence of TREs [135], thus raising safety concerns. Various innovative strategies like insertion of additional transcription terminators or insulators upstream of heterologous promoters, change in the orientation of E1A expression cassette, or relocation of the packaging signal from the left to the right ITR could be employed to address these limitations [133].
Recently, post transcriptional processes that control the stability or translation of mRNA, have been investigated for their application in the development of CRAds (Fig. 2). In the tumor environment where proliferative signals predominate, expression of certain tumor-associated proteins is enhanced partly by stabilization of mRNA by 3′UTR, regulated via activated P-MAPK (mitogen activated protein kinase) pathway. By ligating 3′UTR from gene encoding prostaglandin-endoperoxide synthase 2 (PTGS2, frequently upregulated in cancer cells) to the E1A gene, selective stabilization of E1A mRNA and expression in tumor cells with activated Ras/MAPK pathway was observed [136]. Stability of mRNA for a wide variety of genes in various physiological conditions is regulated by 3′UTR; therefore, 3′UTR ligation to critical Ad genes could be used as generalized strategy to impart tumor selectivity to Ad vectors. In a recent study, tumor-specific control of mRNA translation initiation, in combination with transcriptional regulation, was exploited to restrict non-specific replication of CRAds [137]. Addition of the long and complex 5′UTR preceding the E1A gene restricted its translation in the breast cancer cells expressing high levels of eukaryotic initiation factor 4E (eIF4E) [137].
Though no CRAd has yet been developed that exhibits absolute lack of replication and toxicity to normal cells, the level of selectivity and safety that have been achieved is remarkable. Further developments to enhance tumor-selective replication and potency together with multiple layers of protection to healthy tissues would result in the development of safe and effective Ad vectors.
SUICIDE GENE THERAPY
Suicide gene therapy involves the selective targeting of chemotherapeutic agents to tumors by gene-directed enzyme prodrug therapy (GDEPT). A nontoxic prodrug can be converted into a toxin within tumor cells by prodrug converting enzyme delivered by Ad. Traditional chemotherapeutic agents are often toxic to normal cells and therefore difficult to administer in high doses to maintain appropriate therapeutic index within the tumor tissue; whereas, non-toxic prodrugs can be administered at high doses without significant negative effects to the normal cells. Two of the best characterized suicide genes are the herpes simplex virus type I thymidine kinase (HSV-TK) and the Escherichia coli cytosine deaminase (CD) genes. HSV-TK phosphorylates ganciclovir (GCV), which in turn interacts with cellular DNA polymerase and interferes with DNA synthesis to cause death of rapidly dividing cells, while CD converts 5-fluorocytosine (5-FC) to a highly toxic metabolite 5-fluorouracil (5-FU), commonly used for cancer chemotherapy [138]. Apart from the direct cytotoxicity, GDEPT-mediated cell death is largely attributed to the potent bystander effect on the uninfected neighboring tumor cells and non-cancer stromal cells because of transfer of toxic drug via gap junctions or diffusion and activation of anti-tumor immune response against lysed tumor cell byproducts [139].
Initially, replication-defective Ad vectors were used for GDEPT but better anti-tumor efficacy was observed when used with replication competent vectors [140]. Ad vector-mediated concomitant delivery of the HSV-TK and the CD as a double suicide gene therapy with subsequent administration of respective prodrugs resulted in a synergistic anti-tumor response [141, 142]. Moreover, potent increase in radiosensitivity of tumor cells was also observed. A phase I clinical trial for treatment of prostate cancer that employed a novel three-pronged strategy including a oncolytic Ad expressing double suicide genes HSV-TK and CD together with prodrug therapy and radiotherapy has demonstrated encouraging results [143].
In order to spare normal cells from cytotoxicity, the prodrug converting enzyme should be selectively expressed in tumor cells; therefore, Ad vectors are often delivered locally. To further improve the anti-tumor potency while minimizing the deleterious effects to normal cells, transductional targeting to tumor cells, targeted expression of suicide genes, or vector essential genes by tumor selective promoters or combination of both, has been tried. For example in Ad.HE1HCD3 vector, expression of E1A as well as of CD is controlled by the melanoma-specific human tyrosinase enhancer (HTE)/promoter while incorporation of the RGD peptide to the fiber knob enhanced its transduction to tumor cells. In another approach, the CD gene was strategically placed in the E3B region of CRAd (ONYX-411) under the control of viral late gene regulatory elements [144]. E1A mutation and regulation of E1A and E4 expression by E2F1 gene promoter restricted viral replication and CD expression mainly in tumor cells. Coupled with tumor-specific virus replication, robust expression of CD was observed in tumor cells, both in vitro and in vivo. Subsequent administration of 5-FC further enhanced the anti-tumor efficacy [144]. To target the localized expression of therapeutic gene, the HSV-TK/CD fusion gene controlled by heat-inducible heat shock protein (HSP)-70 promoter was delivered via E1B 55 kDa mutant CRAd [130]. Heat-inducible expression of the fusion gene together with potent cytotoxicity in human prostate carcinoma cells in the presence of prodrugs was observed.
Several other suicide gene/prodrug combinations are being investigated in clinical or preclinical studies [140]. Formulation of better dosage regimen and protocols, considering kinetics of prodrug and vector transgene expression, should further improve the therapeutic value of this therapy. The timing of administration of prodrug is critical to maximize the benefits from GDEPT. Boucher et al [145] proposed a novel method of sequential administration of prodrugs 5-FC followed by GCV. This approach further enhanced the synergistic cytotoxicity of double suicide gene therapy. Additional care should be taken to avoid drug-induced premature cell death resulting in impaired viral replication. Reduced virus proliferation and yield can significantly lower the benefits of Ad-mediated suicide gene therapy.
RNA INTERFERENCE
RNA interference (RNAi) is a mechanism of sequence-specific inhibition of gene expression by small interfering double-stranded RNA [146]. It is not only an endogenous regulatory mechanism of cellular gene expression but also a tool to therapeutically suppress over-expressing genes [147]. RNAi has been successfully used as a method to silence gene expression in several in vitro and in vivo research models and emerging data clearly implicate its potential and usefulness in clinical treatment of diseases. Accordingly, a large collection of RNAi resources has also been generated for cancer biology research [148]. RNAi can be carried out by different approaches; for example, transfection of chemically synthesized double-stranded RNA to cells or delivery of short hairpin RNA (shRNA) via viral vectors. Inefficiency in transfection, cell type specificity and high cost limit the use of chemically synthesized dsRNA. Viral vector-based approaches for RNAi have certain advantages like higher transfection efficiency, continuous production of siRNA in cells and sustained protein suppression [147, 148].
RNAi-mediated by replication-incompetent Ad vectors
Ad vector expressing shRNA under the control of cytomegalovirus (CMV) promoter were initially shown to successfully suppress the expression of enhanced green fluorescent protein (EGFP) reporter gene in vitro as well as in vivo [149]. This report also demonstrated the usefulness of Ad-mediated delivery of RNAi to the brain and liver. Ad vector with the U6 promoter-driven expression of shRNA was demonstrated to successfully infect primary cells and induce gene suppression [150]. In this study, efficient and prolonged knockdown of the cellular targets in keratinocytes, synoviocytes and human umbilical vein endothelial cells (HUVECs) was reported. Other recombinant Ad vectors with a polymerase III or H-1 RNA promoter expressing siRNA molecules targeting p53 were effective in suppressing p53 expression in mammalian cells [151, 152]. A replication-incompetent Ad expressing shRNA for IL-8, a proangiogenic cytokine, reduced migration and tube formation of endothelial cells and invasion of the liver (Hep3B) or the lung (A549) cancer cells [153]. In a multidrug-resistant cell line, Ad-mediated shRNA delivery was associated with reversal of drug-resistant phenotype [154]. In colon cancer cells, Ad-mediated delivery of shRNA against anti-apoptotic protein Bcl-XL inhibited in vitro cell viability and in vivo tumor growth [155]. Delivery of shRNA against matrix metalloproteinase-2 (MMP-2) inhibited migration of A549 human lung cancer cells [156].
RNAi-mediated by CRAds
As described previously, CRAd with replication restricted to cancer cells are oncolytic. These vectors are not only therapeutic on their own might but can also enhance the efficacy by oncogene knockdown via RNAi. CRAd vector with shRNA for a firefly luciferase reporter gene was demonstrated to be effective in gene suppression [157]. In another study, the effect of siRNAs against mutant Ras was augmented when deliverd via ONYX-411 vector and showed a potent anti-tumor effect in a xenograft model with minimal cytotoxicity to normal cells [158]. Oncolytic Ad expressing shRNA against vascular endothelial growth factor (VEGF) prolonged gene silencing compared to replication-incompetent Ad and exhibited anti-angiogenic effects [159]. In a recent study, Ad-mediated knockdown of Apollon, an apoptosis inhibitor protein, induced apoptosis and inhibited cell proliferation in vitro and tumorigenicity in vivo [160].
These reports clearly demonstrate the usefulness of Ad-mediated RNAi in various cancer research models. Further research is needed to evaluate the efficacy and cytotoxicity of this strategy in clinical trials. Besides this, VA RNA may inhibit RNA interference machinery. In one study, Ad infection in 293 cells suppressed RNAi-mediated silencing of GFP expression [161]. It was found that both VA RNA I and VA RNA II interfere with the activity of dicer enzyme as well as RNA-induced silencing complex (RISC), and suppress RNAi by acting as competitive substrates [161, 162]. Thus, these results indicate that future strategies for efficient RNAi using Ad also need to take into account the suppression of RNAi by VA RNAs.
ANTI-ANGIOGENSIS THERAPY
Tumors are dependent on angiogenesis for their growth and metastasis [163]. Angiogenesis in tumors is a multi-step process, which is regulated by several angiogenesis stimulatory and inhibitory molecules. By delivering anti-angiogenic drugs systemically or intratumorally, suppression of angiogenesis is expected to ablate tumor growth and metastasis. Therefore, development of anti-angiogenic therapy alone or in combination with other conventional anti-cancer therapies is regarded as a promising approach for the treatment of cancers [164]. Ad vectors have been utilized in anti-angiogenesis gene therapy by delivering diverse anti-angiogenic proteins to tumor tissues.
Ad vector-mediated transfer of the secretable form of anti-angiogenic protein platelet factor 4 (sPF4) resulted in prolonged survival and reduced angiogenesis in tumors in a mouse model of intracerebral gliomas [165]. Endothelium-associated receptors in tumor blood vessels are another attractive target for anti-angiogenesis gene therapy. Tie2, an endothelium-specific receptor tyrosine kinase, is known to play a role in tumor angiogenesis. The soluble Tie2 receptor, capable of blocking the Tie2 pathway, when delivered intravenously via replication-incompetent Ad, significantly inhibited growth of implanted tumors and reduced lung metastasis in mice [166]. In another approach, Ad-mediated gene transfer of angiopoietin-2 (Ang-2), a conditional antagonist and agonist for the endothelium Tie-2 receptor, significantly inhibited tumor angiogenesis, promoted tumor apoptosis, and suppressed tumor growth.[167]. The amino terminal fragment (ATF) of urokinase type plasminogen activator (uPA) is an inhibitor of cell invasion and uPA/uPAR signaling [168]. Local or systemic administration of Ad expressing murine ATF (AdmATF) resulted in growth arrest of tumor and inhibition of angiogenesis as well as metastases in xenograft and syngenic mouse models. Another inhibitor of angiogenesis, endostatin, was exogenously generated in vivo by Ad vector-mediated gene delivery [169]. Higher systemic levels of endostatin were shown to be associated with 40% reduction in tumor growth in mouse models [169]. Similarly, Ad vector-mediated delivery of kallistatin, a modulator of angiogenesis, suppressed angiogenesis in a rat model and human breast cancer cell xenografts in nude mice [170]. Ad vector expressing anti-angiogenic protein thrombospondin-2 (NfTSp2) reduced tumor growth rate and tumor-associated angiogenesis when injected intratumorally [171]. In human prostate cancer cells (PC-3MM2), Ad-mediated expression of IFN-β, an immunostimulatory and anti-angiogenic cytokine, inhibited growth of xenografts in nude mice when delivered intra-tumorally. Furthermore, downregulation of expression of pro-angiogenic proteins, IL-8 and VEGF-A, was also observed [172]. NK4 (amino terminal kringle-domain peptide of HGF) functions as an inhibitor of angiogenesis by competing with hepatocyte growth factor (HGF) for its receptor, cMet. Systemic or intratumoral administration of Ad encoding NK4 (AdCMV.NK4) reduced angiogenesis and enhanced apoptosis resulting in growth inhibition of subcutaneously implanted tumors [173]. However, despite encouraging results, the efficacy of anti-angiogenic therapy is limited, may be because of the presence of redundant signaling pathways regulating angiogenesis in tumor cells to maintain their proliferation and survival. Future efforts must be directed towards multiple targets as well as with combination of other anti-cancer approaches.
CANCER IMMUNOTHERAPY
Immune system is capable of identifying and removing tumor cells in the body, however, cancer cells are remarkable in their abilities to evade immune responses. Some of the strategies by which cancer cells escape the host immune response include secretion of immunosuppressive or anti-inflammatory proteins, defect in antigen processing and presentation due to mutations in antigen presentation machinery or reduced expression of major histocompatibility complex (MHC) molecules and Fas-mediated apoptosis in tumor-specific lymphocytes etc. [174–176]. The ineffectiveness of immune response (either humoral or cell-mediated) and the development of immunosuppression during oncogenesis indicate that the immune system needs to be boosted but with exquisite specificity towards cancer cells to concur oncogenesis [2, 177]. The ultimate goal of cancer immunotherapy is to generate endogenous immune response to defend against developing or well-established tumors. Ad vectors have been utilized to boost anti-cancer immunity; for example, by delivering immunostimulatory molecules or by transducing dendritc cells (DC) for appropriate presentation of tumor associated antigen (TAA) and efficient induction of anti-tumor immune responses.
Ad-mediated cytokine/costimulatory proteins delivery
Exogenous delivery of cytokines modifies the intratumoral cytokine milieu and activates the responder cells for anti-tumor action. Many studies have investigated the administration of recombinant cytokines such as interleukin (IL)-2, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, IFN-α, and IFN-γ to induce immunostimulatory effect for cancer therapy [178–180]. However, because of their systemic toxicity, a short in vivo half-life and a subtherapeautic levels at tumor site, their use is limited. To counter these problems, Ad-mediated local (intratumoral) delivery of cytokines has been investigated that resulted in increased survival and reduction in tumor growth. IL-2 is known to possess anti-tumor activities through stimulation of cell-mediated killing activity of CTLs, lymphokine-activated killer (LAK) cells, or tumor infiltrating lymphocytes (TILs) [181]. Subcutaneous inoculation of tumor cells treated with replication-deficient Ad vector expressing human IL-2 (AdCAIL-2) delayed the tumor growth, while intratumoral inoculation of AdCAIL-2 led to tumor regression in syngenic mouse model [182]. Furthermore, establishment of systemic immunity was observed as tumor regressed mice displayed resistance to tumor growth when challenged with tumorogenic cells. Another cytokine, IL-12, has demonstrated its anti-tumor activity through its ability to promote proliferation and cytolytic potential of CTLs and natural killer (NK) cells [183]. In a mouse model, the intratumoral injection of Ad expressing IL-12 resulted in potent regression of established tumors and treated animals resisted the tumor development following subsequent tumor challenge [184, 185]. Similarly, many studies have investigated Ad-mediated delivery of cytokines such as IL-18 [186], IL-6 [187], IFN-α, β or γ [188–190], and TNF-α [191] that activated anti-tumor immune responses through diverse mechanisms with substantial anti-tumor effect. Moreover, when delivered via Ad in selected combinations, such as IL-12 and IL-2 or IL-12 and lymphotactin, these cytokines synergized with each other to facilitate tumor regression while minimizing cytokine-mediated side-effects [192–195].
Apart from cytokines and chemokines, certain membrane bound receptors are also required for efficient activation of immune response. T-cells, in addition to engaging their receptors to MHCs, require interaction of CD28 with costimulatory molecules such as B7–1 (CD80) and B7–2 (CD86), typically present on professional antigen-presenting cells, for their activation [196]. Unfortunately, most of the tumor cells do not express B7 receptors on their surface that results in T-cell anergy. Replication-deficient Ad vector have been investigated to deliver B7–1 costimulatory molecules to the cancer cells, which resulted in efficient activation of anti-tumor T-cells [197, 198]. In a combinatorial approach, replication-incompetent Ad-mediated expression of B7–1 together with IL-2 elicited more potent anti-tumor response following intratumoral inoculation in breast adenocarcinoma mouse model when compared to Ad vector expressing either gene alone [199]. Recently, co-expression of B7–1 with cytokines like GM-CSF or IL-12 by E1B 55 kDa mutant CRAd showed robust anti-tumor efficacy in immunocompetent mice with melanoma B16-F10 tumors [200, 201]. Massive infiltration of CD8+/CD4+ T-cells in the tumor tissue was also observed. Ad-mediated gene transfer of additional costimulatory molecules, such as 4–1BB ligand or CD40 ligand, either alone or in combination with other cytokines or costimulatory molecules, to the tumor cells also elicited strong anti-tumor immunity to suppress tumor growth [202–207]. Altogether, Ad-mediated delivery of cytokines or costimulatory molecules is a feasible and potent approach for cancer therapy.
AD VECTORS IN DENDRITIC (DC)-MEDIATED CANCER IMMUNOTHERAPY
DCs are highly efficient and specialized antigen-presenting cells that present MHC-labeled epitopes to T-cells to generate antigen-specific immune responses. Ad vectors have been used to modify DCs, either to effectively present tumor-specific antigens to effector immune cells or to enhance their ability to generate antigen-specific immune responses. Many studies have demonstrated that ex vivo transduction of DCs with Ad vectors expressing TAA followed by its inoculation in tumor-bearing animal resulted in induction of anti-tumor immunity [208]. Ad transduced DCs-based cancer gene therapy offers several advantages. For example, apart from transferring immune activating genes, the Ad vector, on its own, exhibits adjuvant effects and has ability to induce DC activation and maturation, which further assist in induction of stronger anti-tumor immune responses [209, 210]. Ad vector-delivered TAA is expressed intracellularly, processed and loaded onto both MHC I and MHC II molecules, thus allowing appropriate and relatively persistent induction of CD8+ as well as CD4+ responses [208]. Furthermore, ex vivo treatment of DCs by Ad vectors also circumvent preexisting antiviral immunity and induce better anti-tumor responses [211]. Mice vaccinated with DCs transduced by Ad vector expressing truncated form of Her2 resulted in a significantly delayed onset of breast cancer in mice [212]. Vaccinated mice demonstrated strong anti-tumor immunity and delayed tumor development. Similarly, DC transduced with an Ad expressing hTERT effectively induced hTERT-specific CTLs against various tumor cell lines [213]. Similar studies have investigated various TAA delivered to DCs via Ad that boosted immune responses to effectively target tumor cells [208, 214].
Alternatively, efficiency of DCs to generate anti-tumor immune responses can be potentiated by transducing them with Ad vectors carrying various immunomodulatory genes such as cytokines or costimulatory receptors. For example, intratumoral inoculation of bone marrow-derived DCs transduced with Ad vector expressing IL-12 successfully eradicated 50–100% malignant nodules in murine model for colon cancer [215]. Another DC-based approach involves Ad-mediated expression of CD40 ligand on its surface [216, 217]. In these studies, activation of DCs with subsequent induction of CTLs and cytokines production resulted in suppression of tumor growth. Cotransduction of DCs with the TAA gene and a gene encoding immunomodulatory molecule [such as AFP and IL-18 or carcinoembryonic antigen (CEA) and GM-CSF] elicited a stronger TAA-specific immune response and could be an effective strategy for cancer immunotherapy [218, 219].
Despite encouraging results, efficacy of Ad-based DC vaccines for cancer immunotherapy is limited because of relatively low Ad transduction in CAR-deficient DCs [220]. Several strategies have been investigated to improve the efficiency of Ad transduction to DCs. Since DCs express high levels of integrins on their surface, modified Ad vectors with RGD sequences on their fiber knob, demonstrated increased transduction of DCs [221]. Additional targeting strategies to direct Ad vector to DCs also resulted in improved transduction of DCs [214].
TARGETED Ad VECTORS/TRANSDUCTIONAL TARGETING
Widespread distribution of CAR restricts the Ad vectors’ availability at the target site/s. Poor transduction of many tumor types because of low levels of CAR expression further worsens this scenario [222]. To circumvent these limitations, several targeting and retargeting approaches have been applied to improve the transduction of tumor cells by Ad (Fig. 3). The broadly applicable strategy for targeting Ad vectors to tumor cells is to render them CAR-blind and redirect them to the target tumor cells by incorporation of tumor-specific ligands on the virus surface. Since Ad internalization is fiber/knob-dependent, majority of the strategies are directed towards the modification of the fiber/knob to change its tropism. Some important targeting strategies with relevant examples of cancer models are discussed below.
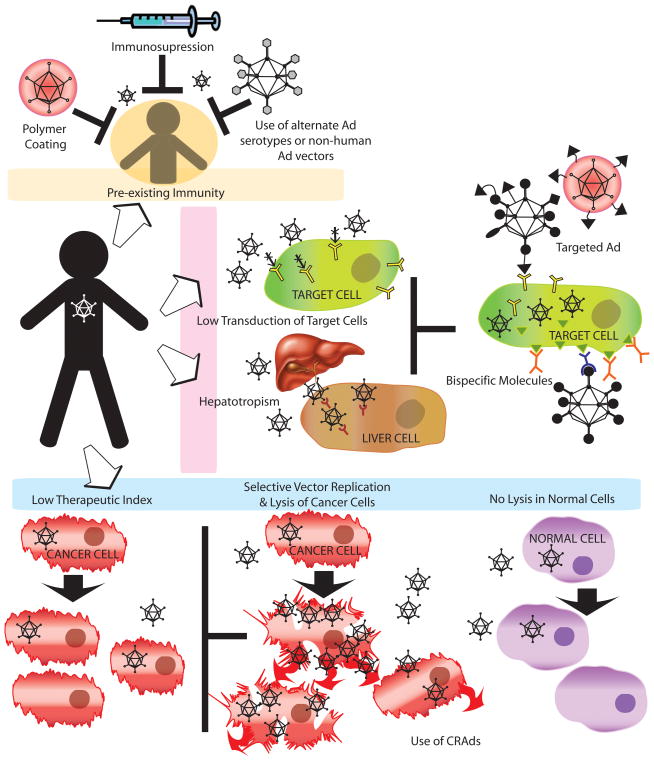
Figure 3. Strategies for improving Ad vectors for cancer gene therapy.
In spite of the several advantages of Ad vectors in cancer gene therapy there are many limitations like presence of preexisting immunity, lower transduction of target cells, hepatotropism and lower therapeutic index in tumor environment that limit its widespread use. In order to overcome preexisting vector immunity, polymer coating of Ad, immunosuppression and use of less prevalent human Ad serotypes or nonhuman Ad can be used. Poor Ad transduction of many cancer cells and inhibition of predominant hepatotropism can be attained by using bispecific molecules, incorporation of specific ligands on viral capsids, or use of fiber-knob chimeras. Furthermore, CRAds can be used to increase vector penetration and spread within the tumor mass while sparing the normal cells. (See text for details).
Polymer coating
One of the non-genetic, simple and effective strategies of changing Ad tropism is modification of the virion surface. A polymeric coating of poly-[N-(2-hydroxypropyl)methacrylamide] (pHPMA, a hydrophilic multivalent polymer) or biodegradable alginate microparticle coating to Ad has been achieved [223–225]. This strategy also allows retargeting of Ad vectors by incorporation of additional targeting ligands like fibroblast growth factor (FGF) or VEGF onto the polymer-coated virus, thereby allowing CAR-independent and ligand-mediated internalization [223]. Incorporation of EGF[226] or laminin-derived peptide (SIKVAV) (an alpha6-integrin binding peptide) ligand [227] on polymer-modified Ad has been successfully achieved to improve tumor cells-specific transduction. Chemical conjugation of Ad vectors with polyethylene glycol (PEG) (PEGylation) has also been reported [228]. Selective targeting could be achieved by attachment of variety of targeting ligands (peptides, proteins or antibodies) to the ends of PEG chains [229]. Such modifications also shield the vector from Ad-specific neutralizing antibodies.
Bispecific molecules
Ad vector retargeting could also be achieved by complexing Ad with bispecific molecules that ablate CAR binding while directing the vector to a novel receptor. Such bispecific molecule consists of two components – one that binds with high affinity to the fiber knob and the other that binds with high specificity with a tumor-specific receptor. The bispecific molecule can either be created by chemically linking the two components or by expressing them together as a fusion protein. For example, bispecific antibodies that bridge the vector knob or fiber to tumor-specific receptors like epidermal growth factor receptors (EGFR), tumor-associated antigen 72 (TAG-72), human epithelial cell adhesion molecule (EpCAM), carcinoembryonic antigen (CEA), human endoglin (CD105) and others have been utilized to successfully achieve CAR-independent retargeting of Ad vectors to cancer cell types [230–236]. Besides bispecific antibodies, anti-fiber antibody chemically conjugated to a tumor-specific ligand such as folate, showed efficient vector targeting to cancer cells via cell surface folate receptors [237]. In another approach, bispecific fusion proteins consisting of soluble-form of CAR (sCAR) together with targeting ligand like human EGF were used for targeted delivery of the Ad vector to cancer cells expressing EGFR [238]. High affinity binding between avidin and biotin has also been investigated for Ad targeting [239]. A biotin acceptor protein was genetically incorporated to the Ad vector fiber, thus enabling it to be retargeted to new receptors when conjugated to biotinylated antibodies [239]. Apart from targeting, this vector system could be useful in ligand screening and vector purification.
Capsid protein modifications
The two-component nature of bispecific molecule approaches adds complications in manufacturing and maintaining batch-to-batch homogeneity. Moreover, progeny virions, in case of CRAds, would be devoid of these modifications. Therefore, emphasis is given to targeted vectors that can be generated by genetic modifications. The fiber is the capsid protein of choice to incorporate foreign ligands and two locations have been identified (C terminus and HI loop of fiber knob) that accept such modifications [240–242]. On these locations, the integrin-binding RGD ligand has been added with promising results [240, 243]. Similarly, a polylysine (pK7) ligand, either alone or in combination with other ligand like RGD, has also been introduced into the fiber to enhance Ad infectivity in a wide variety of tumor cells that overexpress heparin sulphate proteoglycans (HSPGs) [244–246]. Recently, Hesse et al., demonstrated three functional insertion sites (namely EG, HI, and IJ loops) for incorporation of peptide ligands in the Ad serotype 41 (Ad41) short fiber knob without effecting fiber trimerization [247]. This knowledge could be further exploited to design targeted vectors with modified tropism by incorporating a wide repertoire of ligands.
Besides fiber/knob, other capsid proteins such as hexon and penton have also been investigated to alter vector tropism by ligand incorporation. Vigne et al. successfully demonstrated incorporation of RGD ligand at the hypervariable region (HVR) 5 of Ad5 hexon leading to increase in transduction of integrin-rich cells [248]. Subsequently, additional locations in the hexon HVRs that can accept ligand incorporation while retaining vector stability and infectivity were identified [249]. Similarly, Ad vector-bearing a hemagglutinin (HA) peptide at the penton base exhibited tropism towards HA receptor-expressing cell lines [250]. Besides hexon and penton, capsid polypeptide IX (pIX) has also been demonstrated to tolerate ligand incorporation (e.g. polylysine or RGD) and subsequent retargeting to specific receptors [251, 252]. Moreover, the pIX location has also been used for expressing large imaging molecules such as eGFP for ease in vector detection [253]. Recently, minor capsid protein pIIIa, has also been suggested as a site for incorporation of a targeting ligand or imaging molecule [254].
Affibody
Despite significant progress in targeting strategies for Ad vectors, there is still a shortage of naturally available appropriate and viable targeting ligands and all targeting ligands cannot be easily incorporated into the Ad capsid to form a stable virion. One of the alternative approaches to resolve this limitation is the incorporation of rationally designed protein ligand, an affibody, to the viral capsid [255, 256]. Affibodies are the proteins designed from three-helix bundle domain Z of Staphylococcus protein A. The Z domain has been used as a scaffold for construction of combinatorial phagemid libraries from which affibody variants that target desired molecules could be selected using phage display technology [257]. Natural tropism of Ad was successfully altered by modifying Ad fiber to incorporate affibody to Her2 [255]. The trimeric structure of affibody-knob chimera was facilitated by incorporation of trimerization domain of phage T4 fibritin protein [255]. These results are encouraging and indicate that natural tropism of Ad can be modified and utilized to target wide range of cancer cells.
Fiber/Knob Chimeras
As discussed earlier, the knob domain of the Ad fiber mainly recognizes the primary cellular receptor, CAR, for HAd5 internalization. Therfore an insightful scheme to confer novel tropism to Ad vectors is the substitution of fiber/knob with that of other Ad serotypes that utilize non-CAR receptors for their internalization. For example, mosaic Ad vectors having Ad5 and Ad3 knobs demonstrated expanded tropism in various cancer cell lines [258]. Ad5 chimeric vectors carrying fiber/knob from several other human Ad serotypes or non-human Ad such as Ad35 [259], Ad41 [260], canine adenovirus (CAV) type 1 [261], CAV2 [262], and ovine adenovirus (OAV) type 8 [263] have also been generated. These recombinant vectors showed efficient transduction of CAR-deficient cells, reduced liver tropism and better transduction of cancer cells including ovarian cancer, malignant glioma, head and neck cancer etc. [264–269]. On similar lines, a novel mosaic fiber Ad5 vector (Ad5/3-sigma 1) encoding reovirus sigma 1 and chimeric Ad5 fiber with Ad3 knob has been developed that showed enhanced infectivity of ovarian cancer cell lines as well as primary slice cultures of human ovarian cancer via CAR-independent pathways [270]. Ad5 fiber has also been implicated to be responsible for hepatic sequestration of the virus and strong induction of innate immune response as compared to that by fiber from some other serotypes [271, 272]. Therefore, swapping of Ad5 fiber with that from other serotypes might additionally contribute to reduce hepatotropism and vector immunogenicity. However, some of the recent studies have demonstrated that interaction of Ad5 hexon protein with blood coagulation factor X (FX) mediate the vector uptake by the liver [273, 274]. Significant variations among Ad serotypes to transduce hepatocytes that correlated with their ability to bind FX were also observed. Further studies investigating interaction of FX and/or other blood factors with capsid proteins of alternate Ad vectors would further clarify their role in vector tropism and biodistribution.
USE OF ALTERNATE Ad VECTORS
Even though, the fiber/knob chimeric Ad vectors have been successfully used to achieve alternate tropism, they can still be neutralized by antibodies against hexon epitopes [275, 276]. Therefore, some of the rare human Ad serotypes and nonhuman Ads are being developed and investigated as alternate vectors for gene delivery [277, 278]. For example, replication-defective vectors derived from Ad serotypes 3, 7, 11, 35 and others have been developed. Most of these serotypes are less prevalent in human population, have different immunologic epitopes and exhibit novel tropism, thus offering potential advantage over Ad5-based vectors [278]. Vectors based on nonhuman Ads originally derived from pig (porcine adenovirus type 3; PAd3) [279, 280] or cattle (bovine adenovirus type 3; BAd3) [281, 282] have been developed. It has been demonstrated that there are no preexisting virus neutralizing antibodies against PAd3 or BAd3 in humans, and importantly, Ad5-neutralizing antibodies did not cross-neutralize PAd3 or BAd3 [224, 283]. PAd3 and BAd3 vectors can efficiently transduce several types of human and murine cells in culture [283] and cellular internalization of these vectors was CAR- and integrin-independent [284, 285]. In vivo studies in mice also indicated the altered biodistribution patterns for BAd3 and PAd3 vectors as compared to Ad5 vector (Sharma et al., unpublished results). Similarly other vectors based on nonhuman Ad such as CAV, OAV, chimpanzee Ad, and fowl Ad are also being developed [277]. Some of these vectors are already under investigation for use in cancer gene therapy. A transcriptionally regulated CAV-2 vector (OC-CAVE1) in which E1A expression is under the control of the osteocalcin promoter has been developed [286]. Besides replicating in and killing canine osteosarcoma cells in vitro, it provides an excellent immune-competent syngenic animal tumor model to better understand the role of immune system in therapeutic efficacy of CRAds.
COMBINATION OF Ad WITH CHEMOTHERAPY AND RADIOTHERAPY
Conventional therapies like chemotherapy or radiotherapy, when used alone, have not been absolutely effective in treatment of cancer, and therefore, are often used in combinations. Likewise, after numerous preclinical and clinical trials, it is now evident that Ad-based therapy have been more effective when appropriately used in combination with other therapeutic modalities [54]. Because of lack of cross-resistance and divergent anti-tumor mechanism of Ad-based therapy with that of chemotherapy or radiotherapy, additive or synergistic responses have been observed when used in combination. The goal of combination therapy is to attack the cancer cells at multiple fronts and thus giving them minimum chance to survive or develop resistance. Significant increase in anti-tumor efficacy of Ad vectors such as ONYX-015, H101, and CG7870 was observed when used in combination with chemotherapy or radiotherapy [55, 60, 287]. As discussed earlier, Ad-mediated GDEPT has also been used to selectively direct the toxicity of chemotherapeutic drugs to the tumor cells, with potent increase in radiosensitivity [140, 288]. A combination of replication-competent Ad-mediated suicide gene therapy and radiotherapy provided long-term benefit to patients of prostate cancer [289]. In another approach, Ad vectors delivered genes (MDA7/IL-24, PUMA, PTEN or p53) effectively reversed the resistance to various chemotherapeutic drugs or radiotherapy by diverse mechanisms [17, 32, 290, 291]. Alternatively, silencing of genes responsible for drug resistance such as multiple drug resistance 1 (MDR-1) or MDR-1 transporters, and prohibitin via Ad delivered shRNA, antisense RNA or ribozymes also resulted in improved chemosensitization of cancer cells [154, 292, 293]. Many studies have indicated that the dose of vector can also be significantly reduced when used in combination with radiotherapy thus reducing dose-dependent toxicities [287, 294]. Though the mechanism of additive or synergistic effect of combination therapy is not known, several hypotheses have been proposed such as increase in Ad transduction of tumor cells because of increased CAR expression [295], E1A-mediated cellular chemosensitization or radiosensitization [296, 297], or increase in virus replication following radiotherapy or chemotherapy [294, 298]. Ad vectors that utilize chemo- and radio-inducible early growth response (Egr-1) promoter to express TNF-α have been generated [299, 300]. In phase I clinical trials, a combination of such vectors and radiation treatment demonstrated encouraging anti-tumoral response for the treatment of soft tissue sarcoma or solid tumors [301, 302].
CONCLUSIONS AND FUTURE DIRECTIONS
After several years of extensive research it is clearly evident that Ad vectors possess attributes of a suitable vector for anti-cancer therapy. To date numerous modifications have already been made in the original wild-type Ad to meet the diverse anti-cancer therapeutic needs. The ability of replication-incompetant Ad to deliver and overexpress transgenes and that of CRAd to selectively replicate in and lyse tumor cells are attractive attributes of Ad-mediated anti-cancer therapy. To further potentiate the selective oncolysis by Ad vectors, cytotoxic genes such as tumor suppressors, proapoptotic genes, prodrug-converting enzymes, antiangiogenic molecules or immunostimulatory molecules under the control of cancer-specific regulatory elements have been applied. In addition, detargeting and retargeting of Ad vectors by capsid modifications or incorporation of tumor-specific ligands have added to the specificity of oncolytic Ad vectors. These approaches are leading towards novel anticancer therapeutics for clinical applications. Indeed, SFDA approval of Gendicine as well as oncolytic H101 in China has laid the foundation for future development of Ad-based anti-cancer therapy.
Although Ad vector-mediated gene delivery has yielded promising results in a number of cancer models, overall therapeutic success has been below expectation. Limited success of Ad in many anti-cancer therapies may be due to inefficient viral delivery to the tumor site because of vector neutralization by host immune system, increased uptake by the liver or poor infectivity of the tumor cells. Preexisting vector immunity together with vector interactions with blood cells or complement proteins also pose critical obstacles to the systemic delivery of Ad vectors to target disseminated metastases. Several approaches, including the use of alternate Ad serotypes or nonhuman Ad, nanoparticle coating of Ad, and capsid modifications are potential alternatives to overcome such limitations. Cell-based delivery of oncolytic viruses is emerging as a novel alternative delivery approach in which in vitro infected cells hide and carry the oncolytic virus to the cancer tissue [303]. Another hurdle for systemically administered vector is endothelial cell layer, which the vector has to cross to reach the target tissue. Vector modification to allow transcytosis (transport of molecule from one side of cell to the other side) is a potential approach to enable Ad vector transport across the endothelium [304, 305]. Application of novel strategies such as regulation of stability or translation of mRNA for development of CRAds and recently demonstrated feasibility of fibritin-affibody-based approaches to Ad vector targeting provides new opportunities for further improvement in Ad-mediated cancer gene therapy. Overall, the prominent concerns in the application of Ad vectors need to be continually addressed and improvised. A better understanding of the synergistic interaction between the chemotherapy/radiotherapy with Ad vectors should also potentiate clinical application of multimodal cancer therapy. In conclusion, Ad-based cancer gene therapy, either as a single agent or in combinations, offer great promise for cancer treatment.
Acknowledgments
This work was supported by Public Health Service grant CA110176 from the National Cancer Institute. We are thankful to Jane Kovach for her excellent secretarial assistance. We apologize to all the authors whose excellent work we failed to include in this review mainly because of unintentional oversight on our part.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1:138–47. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- 3.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84:570–3. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 4.Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–8. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q, Moyana T, Xiang J. Cancer gene therapy by adenovirus-mediated gene transfer. Curr Gene Ther. 2001;1:101–22. doi: 10.2174/1566523013349002. [DOI] [PubMed] [Google Scholar]
- 6.Douglas JT. Adenoviral vectors for gene therapy. Mol Biotechnol. 2007;36:71–80. doi: 10.1007/s12033-007-0021-5. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 8.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–19. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Kuroki M, Hamada H, et al. Cancer-targeting gene therapy using tropism-modified adenovirus. Anticancer Res. 2007;27:3679–84. [PubMed] [Google Scholar]
- 10.Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–12. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 12.Roth JA. Adenovirus p53 gene therapy. Expert Opin Biol Ther. 2006;6:55–61. doi: 10.1517/14712598.6.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Van Meir EG, Polverini PJ, Chazin VR, et al. Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat Genet. 1994;8:171–6. doi: 10.1038/ng1094-171. [DOI] [PubMed] [Google Scholar]
- 14.Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–23. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 15.Carroll JL, Nielsen LL, Pruett SB, Mathis JM. The role of natural killer cells in adenovirus-mediated p53 gene therapy. Mol Cancer Ther. 2001;1:49–60. [PubMed] [Google Scholar]
- 16.Bouvet M, Ellis LM, Nishizaki M, et al. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998;58:2288–92. [PubMed] [Google Scholar]
- 17.El-Deiry WS. The role of p53 in chemosensitivity and radiosensitivity. Oncogene. 2003;22:7486–95. doi: 10.1038/sj.onc.1206949. [DOI] [PubMed] [Google Scholar]
- 18.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–27. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 19.INGN 201: Ad-p53, Ad5CMV-p53 adenoviral p53, p53 gene therapy--introgen, RPR/INGN 201. Drugs R D. 2007;8:176–87. doi: 10.2165/00126839-200708030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Shimada H, Matsubara H, Shiratori T, et al. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci. 2006;97:554–61. doi: 10.1111/j.1349-7006.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley DJ, Nikitin AY, Lee WH. Adenovirus-mediated retinoblastoma gene therapy suppresses spontaneous pituitary melanotroph tumors in Rb+/− mice. Nat Med. 1996;2:1316–21. doi: 10.1038/nm1296-1316. [DOI] [PubMed] [Google Scholar]
- 22.Fueyo J, Gomez-Manzano C, Yung WK, et al. Suppression of human glioma growth by adenovirus-mediated Rb gene transfer. Neurology. 1998;50:1307–15. doi: 10.1212/wnl.50.5.1307. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Multani AS, Zhou JH, et al. Adenoviral-mediated retinoblastoma 94 produces rapid telomere erosion, chromosomal crisis, and caspase-dependent apoptosis in bladder cancer and immortalized human urothelial cells but not in normal urothelial cells. Cancer Res. 2003;63:760–5. [PubMed] [Google Scholar]
- 24.Wills KN, Mano T, Avanzini JB, et al. Tissue-specific expression of an anti-proliferative hybrid transgene from the human smooth muscle alpha-actin promoter suppresses smooth muscle cell proliferation and neointima formation. Gene Ther. 2001;8:1847–54. doi: 10.1038/sj.gt.3301603. [DOI] [PubMed] [Google Scholar]
- 25.Deng WG, Nishizaki M, Fang B, Roth JA, Ji L. Induction of apoptosis by tumor suppressor FHIT via death receptor signaling pathway in human lung cancer cells. Biochem Biophys Res Commun. 2007;355:993–9. doi: 10.1016/j.bbrc.2007.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeish IA, Bell SJ, Lemoine NR. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Ther. 2004;11:497–503. doi: 10.1038/sj.gt.3302238. [DOI] [PubMed] [Google Scholar]
- 27.Qiu Z, Cui FL, Xu CL, Gu ZQ, Sun YH. Suppression of invasion and angiogenesis in human prostate cancer PC-3 cells by adenovirus-mediated co-transfer of PTEN and P27. Zhonghua Nan Ke Xue. 2007;13:201–5. [PubMed] [Google Scholar]
- 28.Chen F, Li Y, Lu Z, Gao J, Chen J. Adenovirus-mediated Ink4a/ARF gene transfer significantly suppressed the growth of pancreatic carcinoma cells. Cancer Biol Ther. 2005;4:1348–54. doi: 10.4161/cbt.4.12.2183. [DOI] [PubMed] [Google Scholar]
- 29.Couderc B, Pradines A, Rafii A, et al. In vivo restoration of RhoB expression leads to ovarian tumor regression. Cancer Gene Ther. 2008;15:456–64. doi: 10.1038/cgt.2008.12. [DOI] [PubMed] [Google Scholar]
- 30.Marot D, Opolon P, Brailly-Tabard S, et al. The tumor suppressor activity induced by adenovirus-mediated BRCA1 overexpression is not restricted to breast cancers. Gene Ther. 2006;13:235–44. doi: 10.1038/sj.gt.3302637. [DOI] [PubMed] [Google Scholar]
- 31.Hall MC, Li Y, Pong RC, et al. The growth inhibitory effect of p21 adenovirus on human bladder cancer cells. J Urol. 2000;163:1033–8. [PubMed] [Google Scholar]
- 32.Chen Y, Qian H, Wang H, et al. Ad-PUMA sensitizes drug-resistant choriocarcinoma cells to chemotherapeutic agents. Gynecol Oncol. 2007;107:505–12. doi: 10.1016/j.ygyno.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Kabore AF, Johnston JB, Gibson SB. Changes in the apoptotic and survival signaling in cancer cells and their potential therapeutic implications. Curr Cancer Drug Targets. 2004;4:147–63. doi: 10.2174/1568009043481551. [DOI] [PubMed] [Google Scholar]
- 34.Eberle J, Fecker LF, Hossini AM, Kurbanov BM, Fechner H. Apoptosis pathways and oncolytic adenoviral vectors: promising targets and tools to overcome therapy resistance of malignant melanoma. Exp Dermatol. 2008;17:1–11. doi: 10.1111/j.1600-0625.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 35.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6. [PubMed] [Google Scholar]
- 36.Walker-Daniels J, Coffman K, Azimi M, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Abraham S, Knapp DW, Cheng L, et al. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–60. doi: 10.1158/1078-0432.CCR-05-1505. [DOI] [PubMed] [Google Scholar]
- 38.Zantek ND, Azimi M, Fedor-Chaiken M, et al. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–38. [PubMed] [Google Scholar]
- 39.Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis. 2003;20:59–68. doi: 10.1023/a:1022546620495. [DOI] [PubMed] [Google Scholar]
- 40.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–7. [PubMed] [Google Scholar]
- 41.Noblitt LW, Bangari DS, Shukla S, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–66. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 42.Noblitt LW, Bangari DS, Shukla S, Mohammed S, Mittal SK. Immunocompetent mouse model of breast cancer for preclinical testing of EphA2-targeted therapy. Cancer Gene Ther. 2005;12:46–53. doi: 10.1038/sj.cgt.7700763. [DOI] [PubMed] [Google Scholar]
- 43.Jiang M, Shi W, Zhang Q, et al. Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin Cancer Res. 2006;12:6179–85. doi: 10.1158/1078-0432.CCR-06-0746. [DOI] [PubMed] [Google Scholar]
- 44.Arafat WO, Gomez-Navarro J, Buchsbaum DJ, et al. Effective single chain antibody (scFv) concentrations in vivo via adenoviral vector mediated expression of secretory scFv. Gene Ther. 2002;9:256–62. doi: 10.1038/sj.gt.3301639. [DOI] [PubMed] [Google Scholar]
- 45.Whyte P, Buchkovich KJ, Horowitz JM, et al. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–9. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 46.Flint J, Shenk T. Viral transactivating proteins. Annu Rev Genet. 1997;31:177–212. doi: 10.1146/annurev.genet.31.1.177. [DOI] [PubMed] [Google Scholar]
- 47.Bates S, Phillips AC, Clark PA, et al. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–5. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 48.Yew PR, Berk AJ. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–5. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 49.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–3. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 50.Boyd JM, Malstrom S, Subramanian T, et al. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994;79:341–51. doi: 10.1016/0092-8674(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 51.Rao L, Debbas M, Sabbatini P, et al. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992;89:7742–6. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 53.Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 54.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 55.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 56.Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–45. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 57.Khuri FR, Nemunaitis J, Ganly I, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 58.Lu W, Zheng S, Li XF, et al. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial. World J Gastroenterol. 2004;10:3634–8. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, Kim J, Lee B, et al. Oncolytic potential of E1B 55 kDa-deleted YKL-1 recombinant adenovirus: correlation with p53 functional status. Int J Cancer. 2000;88:454–63. doi: 10.1002/1097-0215(20001101)88:3<454::aid-ijc19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 60.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–8. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 61.Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23:1666–70. [PubMed] [Google Scholar]
- 62.Rothmann T, Hengstermann A, Whitaker NJ, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–8. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hay JG, Shapiro N, Sauthoff H, et al. Targeting the replication of adenoviral gene therapy vectors to lung cancer cells: the importance of the adenoviral E1b-55kD gene. Hum Gene Ther. 1999;10:579–90. doi: 10.1089/10430349950018652. [DOI] [PubMed] [Google Scholar]
- 64.Geoerger B, Grill J, Opolon P, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–72. [PubMed] [Google Scholar]
- 65.Dix BR, O’Carroll SJ, Myers CJ, Edwards SJ, Braithwaite AW. Efficient induction of cell death by adenoviruses requires binding of E1B55k and p53. Cancer Res. 2000;60:2666–72. [PubMed] [Google Scholar]
- 66.Hall AR, Dix BR, O’Carroll SJ, Braithwaite AW. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat Med. 1998;4:1068–72. doi: 10.1038/2057. [DOI] [PubMed] [Google Scholar]
- 67.Steegenga WT, Riteco N, Bos JL. Infectivity and expression of the early adenovirus proteins are important regulators of wild-type and DeltaE1B adenovirus replication in human cells. Oncogene. 1999;18:5032–43. doi: 10.1038/sj.onc.1202886. [DOI] [PubMed] [Google Scholar]
- 68.Ries SJ, Brandts CH, Chung AS, et al. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015) Nat Med. 2000;6:1128–33. doi: 10.1038/80466. [DOI] [PubMed] [Google Scholar]
- 69.Hann B, Balmain A. Replication of an E1B 55-kilodalton protein-deficient adenovirus (ONYX-015) is restored by gain-of-function rather than loss-of-function p53 mutants. J Virol. 2003;77:11588–95. doi: 10.1128/JVI.77.21.11588-11595.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–23. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Dix BR, Edwards SJ, Braithwaite AW. Does the antitumor adenovirus ONYX-015/dl1520 selectively target cells defective in the p53 pathway? J Virol. 2001;75:5443–7. doi: 10.1128/JVI.75.12.5443-5447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babiss LE, Ginsberg HS. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J Virol. 1984;50:202–12. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen Y, Kitzes G, Nye JA, Fattaey A, Hermiston T. Analyses of single-aminoacid substitution mutants of adenovirus type 5 E1B-55K protein. J Virol. 2001;75:4297–307. doi: 10.1128/JVI.75.9.4297-4307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heise C, Hermiston T, Johnson L, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–9. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 75.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 76.Page JG, Tian B, Schweikart K, et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-delta24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. Am J Obstet Gynecol. 2007;196:389.e1–389.e10. doi: 10.1016/j.ajog.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Johnson L, Shen A, Boyle L, et al. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–37. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 78.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. Embo J. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol. 2001;75:3474–9. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Der SD, Lau AS. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci U S A. 1995;92:8841–5. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Kitajewski J, Schneider RJ, Safer B, et al. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986;45:195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- 83.Schneider RJ, Safer B, Munemitsu SM, Samuel CE, Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985;82:4321–5. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cascallo M, Capella G, Mazo A, Alemany R. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 2003;63:5544–50. [PubMed] [Google Scholar]
- 85.Wang Y, Xue SA, Hallden G, et al. Virus-associated RNA I-deleted adenovirus, a potential oncolytic agent targeting EBV-associated tumors. Cancer Res. 2005;65:1523–31. doi: 10.1158/0008-5472.CAN-04-3113. [DOI] [PubMed] [Google Scholar]
- 86.Sauthoff H, Heitner S, Rom WN, Hay JG. Deletion of the adenoviral E1b-19kD gene enhances tumor cell killing of a replicating adenoviral vector. Hum Gene Ther. 2000;11:379–88. doi: 10.1089/10430340050015851. [DOI] [PubMed] [Google Scholar]
- 87.Harrison D, Sauthoff H, Heitner S, et al. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved--deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum Gene Ther. 2001;12:1323–32. doi: 10.1089/104303401750270977. [DOI] [PubMed] [Google Scholar]
- 88.Kim J, Cho JY, Kim JH, Jung KC, Yun CO. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther. 2002;9:725–36. doi: 10.1038/sj.cgt.7700494. [DOI] [PubMed] [Google Scholar]
- 89.Degenhardt K, Perez D, White E. Pathways used by adenovirus E1B 19K to inhibit apoptosis. Symp Soc Exp Biol. 2000;52:241–51. [PubMed] [Google Scholar]
- 90.Liu TC, Hallden G, Wang Y, et al. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol Ther. 2004;9:786–803. doi: 10.1016/j.ymthe.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 91.Liu TC, Wang Y, Hallden G, et al. Functional interactions of antiapoptotic proteins and tumor necrosis factor in the context of a replication-competent adenovirus. Gene Ther. 2005;12:1333–46. doi: 10.1038/sj.gt.3302555. [DOI] [PubMed] [Google Scholar]
- 92.Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Hallden G, Hill R, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–35. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 94.Hawkins LK, Johnson L, Bauzon M, et al. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the 6.7 K/gp19 K region. Gene Ther. 2001;8:1123–31. doi: 10.1038/sj.gt.3301507. [DOI] [PubMed] [Google Scholar]
- 95.Tollefson AE, Scaria A, Hermiston TW, et al. The adenovirus death protein (E3–11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doronin K, Toth K, Kuppuswamy M, et al. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74:6147–55. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doronin K, Toth K, Kuppuswamy M, et al. Overexpression of the ADP (E3–11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–87. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 98.Hawkins LK, Hermiston T. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the E3B region. Gene Ther. 2001;8:1142–8. doi: 10.1038/sj.gt.3301509. [DOI] [PubMed] [Google Scholar]
- 99.Hawkins LK, Hermiston TW. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the ADP region. Gene Ther. 2001;8:1132–41. doi: 10.1038/sj.gt.3301508. [DOI] [PubMed] [Google Scholar]
- 100.Sauthoff H, Pipiya T, Heitner S, et al. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum Gene Ther. 2002;13:1859–71. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]
- 101.Branton PE, Roopchand DE. The role of adenovirus E4orf4 protein in viral replication and cell killing. Oncogene. 2001;20:7855–65. doi: 10.1038/sj.onc.1204862. [DOI] [PubMed] [Google Scholar]
- 102.Subramanian T, Vijayalingam S, Chinnadurai G. Genetic identification of adenovirus type 5 genes that influence viral spread. J Virol. 2006;80:2000–12. doi: 10.1128/JVI.80.4.2000-2012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fukuda K, Abei M, Ugai H, et al. E1A, E1B double-restricted adenovirus for oncolytic gene therapy of gallbladder cancer. Cancer Res. 2003;63:4434–40. [PubMed] [Google Scholar]
- 104.Satoh M, Wang H, Ishidoya S, et al. Oncolytic virotherapy for prostate cancer by E1A, E1B mutant adenovirus. Urology. 2007;70:1243–8. doi: 10.1016/j.urology.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 105.Wakayama M, Abei M, Kawashima R, et al. E1A, E1B double-restricted adenovirus with RGD-fiber modification exhibits enhanced oncolysis for CAR-deficient biliary cancers. Clin Cancer Res. 2007;13:3043–50. doi: 10.1158/1078-0432.CCR-06-2103. [DOI] [PubMed] [Google Scholar]
- 106.Wang H, Satoh M, Abe H, et al. Oncolytic viral therapy by bladder instillation using an E1A, E1B double-restricted adenovirus in an orthotopic bladder cancer model. Urology. 2006;68:674–81. doi: 10.1016/j.urology.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 107.Kim J, Kim JH, Choi KJ, Kim PH, Yun CO. E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Hum Gene Ther. 2007;18:773–86. doi: 10.1089/hum.2006.167. [DOI] [PubMed] [Google Scholar]
- 108.Ko D, Hawkins L, Yu DC. Development of transcriptionally regulated oncolytic adenoviruses. Oncogene. 2005;24:7763–74. doi: 10.1038/sj.onc.1209048. [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez R, Schuur ER, Lim HY, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–63. [PubMed] [Google Scholar]
- 110.Kuppuswamy M, Spencer JF, Doronin K, et al. Oncolytic adenovirus that overproduces ADP and replicates selectively in tumors due to hTERT promoter-regulated E4 gene expression. Gene Ther. 2005;12:1608–17. doi: 10.1038/sj.gt.3302581. [DOI] [PubMed] [Google Scholar]
- 111.Kawashima T, Kagawa S, Kobayashi N, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285–92. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 112.Brunori M, Malerba M, Kashiwazaki H, Iggo R. Replicating adenoviruses that target tumors with constitutive activation of the wnt signaling pathway. J Virol. 2001;75:2857–65. doi: 10.1128/JVI.75.6.2857-2865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Doronin K, Kuppuswamy M, Toth K, et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol. 2001;75:3314–24. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li X, Zhang YP, Kim HS, et al. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65:1941–51. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- 115.Fujiwara T, Urata Y, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr Cancer Drug Targets. 2007;7:191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- 116.Ryan PC, Jakubczak JL, Stewart DA, et al. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 2004;11:555–69. doi: 10.1038/sj.cgt.7700735. [DOI] [PubMed] [Google Scholar]
- 117.Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SL. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003;10:1241–7. doi: 10.1038/sj.gt.3301987. [DOI] [PubMed] [Google Scholar]
- 118.Kamizono J, Nagano S, Murofushi Y, et al. Survivin-responsive conditionally replicating adenovirus exhibits cancer-specific and efficient viral replication. Cancer Res. 2005;65:5284–91. doi: 10.1158/0008-5472.CAN-04-2657. [DOI] [PubMed] [Google Scholar]
- 119.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 120.Fuerer C, Iggo R. Adenoviruses with Tcf binding sites in multiple early promoters show enhanced selectivity for tumour cells with constitutive activation of the wnt signalling pathway. Gene Ther. 2002;9:270–81. doi: 10.1038/sj.gt.3301651. [DOI] [PubMed] [Google Scholar]
- 121.Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–72. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 122.Cho WK, Seong YR, Lee YH, et al. Oncolytic effects of adenovirus mutant capable of replicating in hypoxic and normoxic regions of solid tumor. Mol Ther. 2004;10:938–49. doi: 10.1016/j.ymthe.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 123.Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12:902–10. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- 124.Pipiya T, Sauthoff H, Huang YQ, et al. Hypoxia reduces adenoviral replication in cancer cells by downregulation of viral protein expression. Gene Ther. 2005;12:911–7. doi: 10.1038/sj.gt.3302459. [DOI] [PubMed] [Google Scholar]
- 125.Fechner H, Wang X, Srour M, et al. A novel tetracycline-controlled transactivator-transrepressor system enables external control of oncolytic adenovirus replication. Gene Ther. 2003;10:1680–90. doi: 10.1038/sj.gt.3302051. [DOI] [PubMed] [Google Scholar]
- 126.Avvakumov N, Mymryk JS. New tools for the construction of replication-competent adenoviral vectors with altered E1A regulation. J Virol Methods. 2002;103:41–9. doi: 10.1016/s0166-0934(01)00440-2. [DOI] [PubMed] [Google Scholar]
- 127.Chong H, Ruchatz A, Clackson T, Rivera VM, Vile RG. A system for small-molecule control of conditionally replication-competent adenoviral vectors. Mol Ther. 2002;5:195–203. doi: 10.1006/mthe.2002.0531. [DOI] [PubMed] [Google Scholar]
- 128.Hernandez-Alcoceba R, Pihalja M, Wicha MS, Clarke MF. A novel, conditionally replicative adenovirus for the treatment of breast cancer that allows controlled replication of E1a-deleted adenoviral vectors. Hum Gene Ther. 2000;11:2009–24. doi: 10.1089/10430340050143435. [DOI] [PubMed] [Google Scholar]
- 129.Hsieh CL, Yang L, Miao L, et al. A novel targeting modality to enhance adenoviral replication by vitamin D(3) in androgen-independent human prostate cancer cells and tumors. Cancer Res. 2002;62:3084–92. [PubMed] [Google Scholar]
- 130.Lee YJ, Galoforo SS, Battle P, et al. Replicating adenoviral vector-mediated transfer of a heat-inducible double suicide gene for gene therapy. Cancer Gene Ther. 2001;8:397–404. doi: 10.1038/sj.cgt.7700310. [DOI] [PubMed] [Google Scholar]
- 131.Rasmussen H, Rasmussen C, Lempicki M, et al. TNFerade Biologic: preclinical toxicology of a novel adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene. Cancer Gene Ther. 2002;9:951–7. doi: 10.1038/sj.cgt.7700518. [DOI] [PubMed] [Google Scholar]
- 132.Nettelbeck DM, Jerome V, Muller R. Gene therapy: designer promoters for tumour targeting. Trends Genet. 2000;16:174–81. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- 133.Nettelbeck DM. Cellular genetic tools to control oncolytic adenoviruses for virotherapy of cancer. J Mol Med. 2007 doi: 10.1007/s00109-007-0291-1. [DOI] [PubMed] [Google Scholar]
- 134.Hernandez-Alcoceba R, Pihalja M, Qian D, Clarke MF. New oncolytic adenoviruses with hypoxia- and estrogen receptor-regulated replication. Hum Gene Ther. 2002;13:1737–50. doi: 10.1089/104303402760293574. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto M, Davydova J, Takayama K, Alemany R, Curiel DT. Transcription initiation activity of adenovirus left-end sequence in adenovirus vectors with e1 deleted. J Virol. 2003;77:1633–7. doi: 10.1128/JVI.77.2.1633-1637.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ahmed A, Thompson J, Emiliusen L, et al. A conditionally replicating adenovirus targeted to tumor cells through activated RAS/P-MAPK-selective mRNA stabilization. Nat Biotechnol. 2003;21:771–7. doi: 10.1038/nbt835. [DOI] [PubMed] [Google Scholar]
- 137.Stoff-Khalili MA, Rivera AA, Nedeljkovic-Kurepa A, et al. Cancer-specific targeting of a conditionally replicative adenovirus using mRNA translational control. Breast Cancer Res Treat. 2008;108:43–55. doi: 10.1007/s10549-007-9587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aghi M, Kramm CM, Chou TC, Breakefield XO, Chiocca EA. Synergistic anticancer effects of ganciclovir/thymidine kinase and 5-fluorocytosine/cytosine deaminase gene therapies. J Natl Cancer Inst. 1998;90:370–80. doi: 10.1093/jnci/90.5.370. [DOI] [PubMed] [Google Scholar]
- 139.Lumniczky K, Safrany G. Cancer gene therapy: combination with radiation therapy and the role of bystander cell killing in the anti-tumor effect. Pathol Oncol Res. 2006;12:118–24. doi: 10.1007/BF02893457. [DOI] [PubMed] [Google Scholar]
- 140.Schepelmann S, Springer CJ. Viral vectors for gene-directed enzyme prodrug therapy. Curr Gene Ther. 2006;6:647–70. doi: 10.2174/156652306779010679. [DOI] [PubMed] [Google Scholar]
- 141.Wu DH, Liu L, Chen LH. Antitumor effects and radiosensitization of cytosine deaminase and thymidine kinase fusion suicide gene on colorectal carcinoma cells. World J Gastroenterol. 2005;11:3051–5. doi: 10.3748/wjg.v11.i20.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rogulski KR, Zhang K, Kolozsvary A, Kim JH, Freytag SO. Pronounced antitumor effects and tumor radiosensitization of double suicide gene therapy. Clin Cancer Res. 1997;3:2081–8. [PubMed] [Google Scholar]
- 143.Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–506. [PubMed] [Google Scholar]
- 144.Zhan J, Gao Y, Wang W, et al. Tumor-specific intravenous gene delivery using oncolytic adenoviruses. Cancer Gene Ther. 2005;12:19–25. doi: 10.1038/sj.cgt.7700730. [DOI] [PubMed] [Google Scholar]
- 145.Boucher PD, Im MM, Freytag SO, Shewach DS. A novel mechanism of synergistic cytotoxicity with 5-fluorocytosine and ganciclovir in double suicide gene therapy. Cancer Res. 2006;66:3230–7. doi: 10.1158/0008-5472.CAN-05-3033. [DOI] [PubMed] [Google Scholar]
- 146.Hannon GJ. RNA interference. Nature. 2002;418:244–51. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 147.Milhavet O, Gary DS, Mattson MP. RNA interference in biology and medicine. Pharmacol Rev. 2003;55:629–48. doi: 10.1124/pr.55.4.1. [DOI] [PubMed] [Google Scholar]
- 148.Iorns E, Lord CJ, Turner N, Ashworth A. Utilizing RNA interference to enhance cancer drug discovery. Nat Rev Drug Discov. 2007;6:556–68. doi: 10.1038/nrd2355. [DOI] [PubMed] [Google Scholar]
- 149.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–10. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 150.Arts GJ, Langemeijer E, Tissingh R, et al. Adenoviral vectors expressing siRNAs for discovery and validation of gene function. Genome Res. 2003;13:2325–32. doi: 10.1101/gr.1332603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao LJ, Jian H, Zhu H. Specific gene inhibition by adenovirus-mediated expression of small interfering RNA. Gene. 2003;316:137–41. doi: 10.1016/s0378-1119(03)00750-9. [DOI] [PubMed] [Google Scholar]
- 152.Shen C, Buck AK, Liu X, Winkler M, Reske SN. Gene silencing by adenovirus-delivered siRNA. FEBS Lett. 2003;539:111–4. doi: 10.1016/s0014-5793(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 153.Yoo JY, Kim JH, Kim J, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15:635–51. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 154.Kaszubiak A, Holm PS, Lage H. Overcoming the classical multidrug resistance phenotype by adenoviral delivery of anti-MDR1 short hairpin RNAs and ribozymes. Int J Oncol. 2007;31:419–30. [PubMed] [Google Scholar]
- 155.Zhu H, Zhu Y, Hu J, et al. Adenovirus-mediated small hairpin RNA targeting Bcl-XL as therapy for colon cancer. Int J Cancer. 2007;121:1366–72. doi: 10.1002/ijc.22856. [DOI] [PubMed] [Google Scholar]
- 156.Chetty C, Bhoopathi P, Joseph P, et al. Adenovirus-mediated small interfering RNA against matrix metalloproteinase-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5:2289–99. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- 157.Carette JE, Overmeer RM, Schagen FH, et al. Conditionally replicating adenoviruses expressing short hairpin RNAs silence the expression of a target gene in cancer cells. Cancer Res. 2004;64:2663–7. doi: 10.1158/0008-5472.can-03-3530. [DOI] [PubMed] [Google Scholar]
- 158.Zhang YA, Nemunaitis J, Samuel SK, et al. Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA. Cancer Res. 2006;66:9736–43. doi: 10.1158/0008-5472.CAN-06-1617. [DOI] [PubMed] [Google Scholar]
- 159.Yoo JY, Kim JH, Kwon YG, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15:295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 160.Chu L, Gu J, Sun L, et al. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene Ther. 2008;15:484–94. doi: 10.1038/gt.2008.6. [DOI] [PubMed] [Google Scholar]
- 161.Andersson MG, Haasnoot PC, Xu N, et al. Suppression of RNA interference by adenovirus virus-associated RNA. J Virol. 2005;79:9556–65. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aparicio O, Razquin N, Zaratiegui M, Narvaiza I, Fortes P. Adenovirus virus-associated RNA is processed to functional interfering RNAs involved in virus production. J Virol. 2006;80:1376–84. doi: 10.1128/JVI.80.3.1376-1384.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 164.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 165.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–42. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 166.Lin P, Buxton JA, Acheson A, et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc Natl Acad Sci U S A. 1998;95:8829–34. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cao Y, Sonveaux P, Liu S, et al. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:3835–44. doi: 10.1158/0008-5472.CAN-06-4056. [DOI] [PubMed] [Google Scholar]
- 168.Li H, Lu H, Griscelli F, et al. Adenovirus-mediated delivery of a uPA/uPAR antagonist suppresses angiogenesis-dependent tumor growth and dissemination in mice. Gene Ther. 1998;5:1105–13. doi: 10.1038/sj.gt.3300742. [DOI] [PubMed] [Google Scholar]
- 169.Feldman AL, Restifo NP, Alexander HR, et al. Antiangiogenic gene therapy of cancer utilizing a recombinant adenovirus to elevate systemic endostatin levels in mice. Cancer Res. 2000;60:1503–6. [PMC free article] [PubMed] [Google Scholar]
- 170.Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–52. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 171.Hahn W, Ho SH, Jeong JG, et al. Viral vector-mediated transduction of a modified thrombospondin-2 cDNA inhibits tumor growth and angiogenesis. Gene Ther. 2004;11:739–45. doi: 10.1038/sj.gt.3302219. [DOI] [PubMed] [Google Scholar]
- 172.Lee J, Wang A, Hu Q, Lu S, Dong Z. Adenovirus-mediated interferon-beta gene transfer inhibits angiogenesis in and progression of orthotopic tumors of human prostate cancer cells in nude mice. Int J Oncol. 2006;29:1405–12. [PubMed] [Google Scholar]
- 173.Maemondo M, Narumi K, Saijo Y, et al. Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther. 2002;5:177–85. doi: 10.1006/mthe.2002.0533. [DOI] [PubMed] [Google Scholar]
- 174.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res. 2000;20:2665–76. [PubMed] [Google Scholar]
- 175.Yannelli JR, Wroblewski JM. On the road to a tumor cell vaccine: 20 years of cellular immunotherapy. Vaccine. 2004;23:97–113. doi: 10.1016/j.vaccine.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 176.Abrahams VM, Kamsteeg M, Mor G. The Fas/Fas ligand system and cancer: immune privilege and apoptosis. Mol Biotechnol. 2003;25:19–30. doi: 10.1385/MB:25:1:19. [DOI] [PubMed] [Google Scholar]
- 177.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–5. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 178.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 179.Windbichler GH, Hausmaninger H, Stummvoll W, et al. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer. 2000;82:1138–44. doi: 10.1054/bjoc.1999.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Porta C, Paglino C, Imarisio I, Bonomi L. Cytokine-based immunotherapy for advanced kidney cancer: past results and future perspectives in the era of molecularly targeted agents. ScientificWorldJournal. 2007;7:837–49. doi: 10.1100/tsw.2007.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eberlein TJ, Schoof DD. The role of interleukin-2 in cancer immunotherapy. Compr Ther. 1991;17:49–56. [PubMed] [Google Scholar]
- 182.Addison CL, Braciak T, Ralston R, et al. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci U S A. 1995;92:8522–6. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Robertson MJ, Ritz J. Interleukin 12: Basic Biology and Potential Applications in Cancer Treatment. Oncologist. 1996;1:88–97. [PubMed] [Google Scholar]
- 184.Bramson JL, Hitt M, Addison CL, et al. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- 185.Gambotto A, Tuting T, McVey DL, et al. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Cancer Gene Ther. 1999;6:45–53. doi: 10.1038/sj.cgt.7700013. [DOI] [PubMed] [Google Scholar]
- 186.Hwang KS, Cho WK, Yoo J, et al. Adenovirus-mediated interleukin-18 mutant in vivo gene transfer inhibits tumor growth through the induction of T cell immunity and activation of natural killer cell cytotoxicity. Cancer Gene Ther. 2004;11:397–407. doi: 10.1038/sj.cgt.7700711. [DOI] [PubMed] [Google Scholar]
- 187.Tanaka F, Abe M, Akiyoshi T, et al. The anti-human tumor effect and generation of human cytotoxic T cells in SCID mice given human peripheral blood lymphocytes by the in vivo transfer of the Interleukin-6 gene using adenovirus vector. Cancer Res. 1997;57:1335–43. [PubMed] [Google Scholar]
- 188.Santodonato L, Ferrantini M, Palombo F, et al. Antitumor activity of recombinant adenoviral vectors expressing murine IFN-alpha in mice injected with metastatic IFN-resistant tumor cells. Cancer Gene Ther. 2001;8:63–72. doi: 10.1038/sj.cgt.7700274. [DOI] [PubMed] [Google Scholar]
- 189.Odaka M, Wiewrodt R, DeLong P, et al. Analysis of the immunologic response generated by Ad. IFN-beta during successful intraperitoneal tumor gene therapy. Mol Ther. 2002;6:210–8. doi: 10.1006/mthe.2002.0656. [DOI] [PubMed] [Google Scholar]
- 190.Khorana AA, Rosenblatt JD, Sahasrabudhe DM, et al. A phase I trial of immunotherapy with intratumoral adenovirus-interferon-gamma (TG1041) in patients with malignant melanoma. Cancer Gene Ther. 2003;10:251–9. doi: 10.1038/sj.cgt.7700568. [DOI] [PubMed] [Google Scholar]
- 191.Wright P, Braun R, Babiuk L, et al. Adenovirus-mediated TNF-alpha gene transfer induces significant tumor regression in mice. Cancer Biother Radiopharm. 1999;14:49–57. doi: 10.1089/cbr.1999.14.49. [DOI] [PubMed] [Google Scholar]
- 192.Addison CL, Bramson JL, Hitt MM, et al. Intratumoral coinjection of adenoviral vectors expressing IL-2 and IL-12 results in enhanced frequency of regression of injected and untreated distal tumors. Gene Ther. 1998;5:1400–9. doi: 10.1038/sj.gt.3300731. [DOI] [PubMed] [Google Scholar]
- 193.Emtage PC, Wan Y, Hitt M, et al. Adenoviral vectors expressing lymphotactin and interleukin 2 or lymphotactin and interleukin 12 synergize to facilitate tumor regression in murine breast cancer models. Hum Gene Ther. 1999;10:697–709. doi: 10.1089/10430349950018463. [DOI] [PubMed] [Google Scholar]
- 194.Liu Y, Huang H, Saxena A, Xiang J. Intratumoral coinjection of two adenoviral vectors expressing functional interleukin-18 and inducible protein-10, respectively, synergizes to facilitate regression of established tumors. Cancer Gene Ther. 2002;9:533–42. doi: 10.1038/sj.cgt.7700466. [DOI] [PubMed] [Google Scholar]
- 195.Narvaiza I, Mazzolini G, Barajas M, et al. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol. 2000;164:3112–22. doi: 10.4049/jimmunol.164.6.3112. [DOI] [PubMed] [Google Scholar]
- 196.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 197.Iwakami SI, Setoguchi Y, Saijo Y, Azuma M, Fukuchi Y. Replication-deficient adenovirus-mediated transfer of B7–1 (CD80) cDNA induces anti-tumour immunity in isolated human lung cancer. Respirology. 2001;6:135–44. doi: 10.1046/j.1440-1843.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 198.Gilligan MG, Knox P, Weedon S, et al. Adenoviral delivery of B7–1 (CD80) increases the immunogenicity of human ovarian and cervical carcinoma cells. Gene Ther. 1998;5:965–74. doi: 10.1038/sj.gt.3300672. [DOI] [PubMed] [Google Scholar]
- 199.Putzer BM, Hitt M, Muller WJ, et al. Interleukin 12 and B7–1 costimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression. Proc Natl Acad Sci U S A. 1997;94:10889–94. doi: 10.1073/pnas.94.20.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Choi KJ, Kim JH, Lee YS, et al. Concurrent delivery of GM-CSF and B7–1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13:1010–20. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- 201.Lee YS, Kim JH, Choi KJ, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7–1 in an immunocompetent murine model. Clin Cancer Res. 2006;12:5859–68. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 202.Kikuchi T, Crystal RG. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum Gene Ther. 1999;10:1375–87. doi: 10.1089/10430349950018049. [DOI] [PubMed] [Google Scholar]
- 203.Loskog A, Dzojic H, Vikman S, et al. Adenovirus CD40 ligand gene therapy counteracts immune escape mechanisms in the tumor Microenvironment. J Immunol. 2004;172:7200–5. doi: 10.4049/jimmunol.172.11.7200. [DOI] [PubMed] [Google Scholar]
- 204.Habib-Agahi M, Phan TT, Searle PF. Co-stimulation with 4–1BB ligand allows extended T-cell proliferation, synergizes with CD80/CD86 and can reactivate anergic T cells. Int Immunol. 2007;19:1383–94. doi: 10.1093/intimm/dxm106. [DOI] [PubMed] [Google Scholar]
- 205.Xu DP, Sauter BV, Huang TG, et al. The systemic administration of Ig-4–1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther. 2005;12:1526–33. doi: 10.1038/sj.gt.3302556. [DOI] [PubMed] [Google Scholar]
- 206.Martinet O, Ermekova V, Qiao JQ, et al. Immunomodulatory gene therapy with interleukin 12 and 4–1BB ligand: long- term remission of liver metastases in a mouse model. J Natl Cancer Inst. 2000;92:931–6. doi: 10.1093/jnci/92.11.931. [DOI] [PubMed] [Google Scholar]
- 207.Yoshida H, Katayose Y, Unno M, et al. A novel adenovirus expressing human 4–1BB ligand enhances antitumor immunity. Cancer Immunol Immunother. 2003;52:97–106. doi: 10.1007/s00262-002-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xia D, Moyana T, Xiang J. Combinational adenovirus-mediated gene therapy and dendritic cell vaccine in combating well-established tumors. Cell Res. 2006;16:241–59. doi: 10.1038/sj.cr.7310032. [DOI] [PubMed] [Google Scholar]
- 209.Kanagawa N, Koretomo R, Murakami S, et al. Factors involved in the maturation of murine dendritic cells transduced with adenoviral vector variants. Virology. 2008;374:411–20. doi: 10.1016/j.virol.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 210.Geutskens SB, van der Eb MM, Plomp AC, et al. Recombinant adenoviral vectors have adjuvant activity and stimulate T cell responses against tumor cells. Gene Ther. 2000;7:1410–6. doi: 10.1038/sj.gt.3301251. [DOI] [PubMed] [Google Scholar]
- 211.Wan Y, Emtage P, Foley R, Carter R, Gauldie J. Murine dendritic cells transduced with an adenoviral vector expressing a defined tumor antigen can overcome anti-adenovirus neutralizing immunity and induce effective tumor regression. Int J Oncol. 1999;14:771–6. doi: 10.3892/ijo.14.4.771. [DOI] [PubMed] [Google Scholar]
- 212.Sakai Y, Morrison BJ, Burke JD, et al. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res. 2004;64:8022–8. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- 213.Chen L, Liang GP, Tang XD, et al. In vitro anti-tumor immune response induced by dendritic cells transfected with hTERT recombinant adenovirus. Biochem Biophys Res Commun. 2006;351:927–34. doi: 10.1016/j.bbrc.2006.10.165. [DOI] [PubMed] [Google Scholar]
- 214.Gallo P, Dharmapuri S, Cipriani B, Monaci P. Adenovirus as vehicle for anticancer genetic immunotherapy. Gene Ther. 2005;12 Suppl 1:S84–91. doi: 10.1038/sj.gt.3302619. [DOI] [PubMed] [Google Scholar]
- 215.Melero I, Duarte M, Ruiz J, et al. Intratumoral injection of bone-marrow derived dendritic cells engineered to produce interleukin-12 induces complete regression of established murine transplantable colon adenocarcinomas. Gene Ther. 1999;6:1779–84. doi: 10.1038/sj.gt.3301010. [DOI] [PubMed] [Google Scholar]
- 216.Liu Y, Zhang X, Zhang W, et al. Adenovirus-mediated CD40 ligand gene-engineered dendritic cells elicit enhanced CD8(+) cytotoxic T-cell activation and antitumor immunity. Cancer Gene Ther. 2002;9:202–8. doi: 10.1038/sj.cgt.7700429. [DOI] [PubMed] [Google Scholar]
- 217.Tomihara K, Kato K, Masuta Y, et al. Gene transfer of CD40-ligand to dendritic cells stimulates interferon-gamma production to induce growth arrest and apoptosis of tumor cells. Gene Ther. 2008;15:203–13. doi: 10.1038/sj.gt.3303056. [DOI] [PubMed] [Google Scholar]
- 218.Ojima T, Iwahashi M, Nakamura M, et al. Benefits of gene transduction of granulocyte macrophage colony-stimulating factor in cancer vaccine using genetically modified dendritic cells. Int J Oncol. 2007;31:931–9. [PubMed] [Google Scholar]
- 219.Cao DY, Yang JY, Dou KF, Ma LY, Teng ZH. alpha-fetoprotein and interleukin-18 gene-modified dendritic cells effectively stimulate specific type-1 CD4- and CD8-mediated T-Cell response from hepatocellular carcinoma patients in Vitro. Hum Immunol. 2007;68:334–41. doi: 10.1016/j.humimm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 220.Okada N, Tsukada Y, Nakagawa S, et al. Efficient gene delivery into dendritic cells by fiber-mutant adenovirus vectors. Biochem Biophys Res Commun. 2001;282:173–9. doi: 10.1006/bbrc.2001.4527. [DOI] [PubMed] [Google Scholar]
- 221.Okada N, Masunaga Y, Okada Y, et al. Gene transduction efficiency and maturation status in mouse bone marrow-derived dendritic cells infected with conventional or RGD fiber-mutant adenovirus vectors. Cancer Gene Ther. 2003;10:421–31. doi: 10.1038/sj.cgt.7700586. [DOI] [PubMed] [Google Scholar]
- 222.Kim M, Zinn KR, Barnett BG, et al. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur J Cancer. 2002;38:1917–26. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 223.Fisher KD, Stallwood Y, Green NK, et al. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–8. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 224.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–67. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- 225.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–9. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morrison J, Briggs SS, Green N, et al. Virotherapy of Ovarian Cancer With Polymer-cloaked Adenovirus Retargeted to the Epidermal Growth Factor Receptor. Mol Ther. 2008;16:244–51. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 227.Stevenson M, Hale AB, Hale SJ, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007;14:335–45. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- 228.Croyle MA, Yu QC, Wilson JM. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther. 2000;11:1713–22. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- 229.Eto Y, Yoshioka Y, Mukai Y, Okada N, Nakagawa S. Development of PEGylated adenovirus vector with targeting ligand. Int J Pharm. 2008;354:3–8. doi: 10.1016/j.ijpharm.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 230.Haisma HJ, Grill J, Curiel DT, et al. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 2000;7:901–4. doi: 10.1038/sj.cgt.7700198. [DOI] [PubMed] [Google Scholar]
- 231.Haisma HJ, Pinedo HM, Rijswijk A, et al. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Ther. 1999;6:1469–74. doi: 10.1038/sj.gt.3300969. [DOI] [PubMed] [Google Scholar]
- 232.Heideman DA, Snijders PJ, Craanen ME, et al. Selective gene delivery toward gastric and esophageal adenocarcinoma cells via EpCAM-targeted adenoviral vectors. Cancer Gene Ther. 2001;8:342–51. doi: 10.1038/sj.cgt.7700313. [DOI] [PubMed] [Google Scholar]
- 233.Nettelbeck DM, Miller DW, Jerome V, et al. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105) Mol Ther. 2001;3:882–91. doi: 10.1006/mthe.2001.0342. [DOI] [PubMed] [Google Scholar]
- 234.Korn T, Nettelbeck DM, Volkel T, Muller R, Kontermann RE. Recombinant bispecific antibodies for the targeting of adenoviruses to CEA-expressing tumour cells: a comparative analysis of bacterially expressed single-chain diabody and tandem scFv. J Gene Med. 2004;6:642–51. doi: 10.1002/jgm.555. [DOI] [PubMed] [Google Scholar]
- 235.Nettelbeck DM, Rivera AA, Kupsch J, et al. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int J Cancer. 2004;108:136–45. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- 236.Kelly FJ, Miller CR, Buchsbaum DJ, et al. Selectivity of TAG-72-targeted adenovirus gene transfer to primary ovarian carcinoma cells versus autologous mesothelial cells in vitro. Clin Cancer Res. 2000;6:4323–33. [PubMed] [Google Scholar]
- 237.Douglas JT, Rogers BE, Rosenfeld ME, et al. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–8. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 238.Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–84. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Parrott MB, Adams KE, Mercier GT, et al. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther. 2003;8:688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 240.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Krasnykh V, Dmitriev I, Mikheeva G, et al. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–52. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Michael SI, Hong JS, Curiel DT, Engler JA. Addition of a short peptide ligand to the adenovirus fiber protein. Gene Ther. 1995;2:660–8. [PubMed] [Google Scholar]
- 243.Wickham TJ, Tzeng E, Shears LL, 2nd, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–9. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–3. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 245.Wu H, Seki T, Dmitriev I, et al. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647–53. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- 246.Koizumi N, Mizuguchi H, Utoguchi N, Watanabe Y, Hayakawa T. Generation of fiber-modified adenovirus vectors containing heterologous peptides in both the HI loop and C terminus of the fiber knob. J Gene Med. 2003;5:267–76. doi: 10.1002/jgm.348. [DOI] [PubMed] [Google Scholar]
- 247.Hesse A, Kosmides D, Kontermann RE, Nettelbeck DM. Tropism modification of adenovirus vectors by peptide ligand insertion into various positions of the adenovirus serotype 41 short-fiber knob domain. J Virol. 2007;81:2688–99. doi: 10.1128/JVI.02722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vigne E, Mahfouz I, Dedieu JF, et al. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J Virol. 1999;73:5156–61. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wu H, Han T, Belousova N, et al. Identification of sites in adenovirus hexon for foreign peptide incorporation. J Virol. 2005;79:3382–90. doi: 10.1128/JVI.79.6.3382-3390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Einfeld DA, Brough DE, Roelvink PW, Kovesdi I, Wickham TJ. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base. J Virol. 1999;73:9130–6. doi: 10.1128/jvi.73.11.9130-9136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J Virol. 2002;76:6893–9. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Vellinga J, Rabelink MJ, Cramer SJ, et al. Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX. J Virol. 2004;78:3470–9. doi: 10.1128/JVI.78.7.3470-3479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Meulenbroek RA, Sargent KL, Lunde J, Jasmin BJ, Parks RJ. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion--generation of fluorescent virus through the incorporation of pIX-GFP. Mol Ther. 2004;9:617–24. doi: 10.1016/j.ymthe.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 254.Glasgow JN, Everts M, Curiel DT. Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther. 2006;13:830–44. doi: 10.1038/sj.cgt.7700928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Belousova N, Mikheeva G, Gelovani J, Krasnykh V. Modification of adenovirus capsid with a designed protein ligand yields a gene vector targeted to a major molecular marker of cancer. J Virol. 2008;82:630–7. doi: 10.1128/JVI.01896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Henning P, Magnusson MK, Gunneriusson E, et al. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum Gene Ther. 2002;13:1427–39. doi: 10.1089/10430340260185067. [DOI] [PubMed] [Google Scholar]
- 257.Nord K, Gunneriusson E, Ringdahl J, et al. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol. 1997;15:772–7. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 258.Takayama K, Reynolds PN, Short JJ, et al. A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism. Virology. 2003;309:282–93. doi: 10.1016/s0042-6822(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 259.Rea D, Havenga MJ, van Den Assem M, et al. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J Immunol. 2001;166:5236–44. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- 260.Nicol CG, Graham D, Miller WH, et al. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol Ther. 2004;10:344–54. doi: 10.1016/j.ymthe.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 261.Stoff-Khalili MA, Rivera AA, Glasgow JN, et al. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005;12:1696–706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- 262.Glasgow JN, Kremer EJ, Hemminki A, et al. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology. 2004;324:103–16. doi: 10.1016/j.virol.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 263.Nakayama M, Both GW, Banizs B, et al. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology. 2006;350:103–15. doi: 10.1016/j.virol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 264.Breidenbach M, Rein DT, Wang M, et al. Genetic replacement of the adenovirus shaft fiber reduces liver tropism in ovarian cancer gene therapy. Hum Gene Ther. 2004;15:509–18. doi: 10.1089/10430340460745829. [DOI] [PubMed] [Google Scholar]
- 265.Kanerva A, Mikheeva GV, Krasnykh V, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–80. [PubMed] [Google Scholar]
- 266.Ulasov IV, Tyler MA, Zheng S, Han Y, Lesniak MS. CD46 represents a target for adenoviral gene therapy of malignant glioma. Hum Gene Ther. 2006;17:556–64. doi: 10.1089/hum.2006.17.556. [DOI] [PubMed] [Google Scholar]
- 267.Zheng S, Ulasov IV, Han Y, et al. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–60. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 268.Ni S, Gaggar A, Di Paolo N, et al. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer Gene Ther. 2006;13:1072–81. doi: 10.1038/sj.cgt.7700981. [DOI] [PubMed] [Google Scholar]
- 269.Suominen E, Toivonen R, Grenman R, Savontaus M. Head and neck cancer cells are efficiently infected by Ad5/35 hybrid virus. J Gene Med. 2006;8:1223–31. doi: 10.1002/jgm.957. [DOI] [PubMed] [Google Scholar]
- 270.Tsuruta Y, Pereboeva L, Glasgow JN, et al. A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clin Cancer Res. 2007;13:2777–83. doi: 10.1158/1078-0432.CCR-06-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schoggins JW, Nociari M, Philpott N, Falck-Pedersen E. Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J Virol. 2005;79:11627–37. doi: 10.1128/JVI.79.18.11627-11637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Molinier-Frenkel V, Prevost-Blondel A, Hong SS, et al. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J Biol Chem. 2003;278:37175–82. doi: 10.1074/jbc.M303496200. [DOI] [PubMed] [Google Scholar]
- 273.Kalyuzhniy O, Di Paolo NC, Silvestry M, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A. 2008;105:5483–8. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 275.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–23. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–8. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–62. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Stone D, Lieber A. New serotypes of adenoviral vectors. Curr Opin Mol Ther. 2006;8:423–31. [PubMed] [Google Scholar]
- 279.Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–36. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 280.Reddy PS, Idamakanti N, Babiuk LA, Mehtali M, Tikoo SK. Porcine adenovirus-3 as a helper-dependent expression vector. J Gen Virol. 1999;80:2909–16. doi: 10.1099/0022-1317-80-11-2909. [DOI] [PubMed] [Google Scholar]
- 281.Mittal SK, Prevec L, Graham FL, Babiuk LA. Development of a bovine adenovirus type 3-based expression vector. J Gen Virol. 1995;76:93–102. doi: 10.1099/0022-1317-76-1-93. [DOI] [PubMed] [Google Scholar]
- 282.Reddy PS, Idamakanti N, Chen Y, et al. Replication-defective bovine adenovirus type 3 as an expression vector. J Virol. 1999;73:9137–44. doi: 10.1128/jvi.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005;327:960–6. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 284.Bangari DS, Sharma A, Mittal SK. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochem Biophys Res Commun. 2005;331:1478–84. doi: 10.1016/j.bbrc.2005.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bangari DS, Mittal SK. Porcine adenovirus serotype 3 internalization is independent of CAR and alphavbeta3 or alphavbeta5 integrin. Virology. 2005;332:157–66. doi: 10.1016/j.virol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 286.Hemminki A, Kanerva A, Kremer EJ, et al. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–73. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 287.Dilley J, Reddy S, Ko D, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–22. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- 288.Freytag SO, Paielli D, Wing M, et al. Efficacy and toxicity of replication-competent adenovirus-mediated double suicide gene therapy in combination with radiation therapy in an orthotopic mouse prostate cancer model. Int J Radiat Oncol Biol Phys. 2002;54:873–85. doi: 10.1016/s0360-3016(02)03005-5. [DOI] [PubMed] [Google Scholar]
- 289.Freytag SO, Stricker H, Peabody J, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–42. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 290.Emdad L, Lebedeva IV, Su ZZ, et al. Melanoma differentiation associated gene-7/interleukin-24 reverses multidrug resistance in human colorectal cancer cells. Mol Cancer Ther. 2007;6:2985–94. doi: 10.1158/1535-7163.MCT-07-0399. [DOI] [PubMed] [Google Scholar]
- 291.Pappas G, Zumstein LA, Munshi A, Hobbs M, Meyn RE. Adenoviral-mediated PTEN expression radiosensitizes non-small cell lung cancer cells by suppressing DNA repair capacity. Cancer Gene Ther. 2007;14:543–9. doi: 10.1038/sj.cgt.7701050. [DOI] [PubMed] [Google Scholar]
- 292.Folmer Y, Schneider M, Blum HE, Hafkemeyer P. Reversal of drug resistance of hepatocellular carcinoma cells by adenoviral delivery of anti-ABCC2 antisense constructs. Cancer Gene Ther. 2007;14:875–84. doi: 10.1038/sj.cgt.7701082. [DOI] [PubMed] [Google Scholar]
- 293.Gregory-Bass RC, Olatinwo M, Xu W, et al. Prohibitin silencing reverses stabilization of mitochondrial integrity and chemoresistance in ovarian cancer cells by increasing their sensitivity to apoptosis. Int J Cancer. 2008;122:1923–30. doi: 10.1002/ijc.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chen Y, DeWeese T, Dilley J, et al. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453–60. [PubMed] [Google Scholar]
- 295.Hemminki A, Kanerva A, Liu B, et al. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res. 2003;63:847–53. [PubMed] [Google Scholar]
- 296.Liao Y, Yu D, Hung MC. Novel approaches for chemosensitization of breast cancer cells: the E1A story. Adv Exp Med Biol. 2007;608:144–69. doi: 10.1007/978-0-387-74039-3_11. [DOI] [PubMed] [Google Scholar]
- 297.Martin-Duque P, Sanchez-Prieto R, Romero J, et al. In vivo radiosensitizing effect of the adenovirus E1A gene in murine and human malignant tumors. Int J Oncol. 1999;15:1163–8. doi: 10.3892/ijo.15.6.1163. [DOI] [PubMed] [Google Scholar]
- 298.Yu DC, Chen Y, Dilley J, et al. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–25. [PubMed] [Google Scholar]
- 299.Kircheis R, Wagner E. Technology evaluation: TNFerade, GenVec. Curr Opin Mol Ther. 2003;5:437–47. [PubMed] [Google Scholar]
- 300.Lopez CA, Kimchi ET, Mauceri HJ, et al. Chemoinducible gene therapy: a strategy to enhance doxorubicin antitumor activity. Mol Cancer Ther. 2004;3:1167–75. [PubMed] [Google Scholar]
- 301.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 302.Mundt AJ, Vijayakumar S, Nemunaitis J, et al. A Phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res. 2004;10:5747–53. doi: 10.1158/1078-0432.CCR-04-0296. [DOI] [PubMed] [Google Scholar]
- 303.Power AT, Wang J, Falls TJ, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–30. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 304.Tang Y, Han T, Everts M, et al. Directing adenovirus across the blood-brain barrier via melanotransferrin (P97) transcytosis pathway in an in vitro model. Gene Ther. 2007;14:523–32. doi: 10.1038/sj.gt.3302888. [DOI] [PubMed] [Google Scholar]
- 305.Zhu ZB, Makhija SK, Lu B, et al. Transport across a polarized monolayer of Caco-2 cells by transferrin receptor-mediated adenovirus transcytosis. Virology. 2004;325:116–28. doi: 10.1016/j.virol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 306.Nemunaitis J, Ganly I, Khuri F, et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–66. [PubMed] [Google Scholar]
- 307.Nemunaitis J, Senzer N, Sarmiento S, et al. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14:885–93. doi: 10.1038/sj.cgt.7701080. [DOI] [PubMed] [Google Scholar]
- 308.Li Y, Idamakanti N, Arroyo T, et al. Dual promoter-controlled oncolytic adenovirus CG5757 has strong tumor selectivity and significant antitumor efficacy in preclinical models. Clin Cancer Res. 2005;11:8845–55. doi: 10.1158/1078-0432.CCR-05-1757. [DOI] [PubMed] [Google Scholar]
- 309.Abbosh PH, Li X, Li L, et al. A conditionally replicative, Wnt/beta-catenin pathway-based adenovirus therapy for anaplastic thyroid cancer. Cancer Gene Ther. 2007;14:399–408. doi: 10.1038/sj.cgt.7701024. [DOI] [PubMed] [Google Scholar]
- 310.Tong AW, Nemunaitis J, Su D, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–72. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 311.Lin X, Huang H, Li S, et al. A phase I clinical trial of an adenovirus-mediated endostatin gene (E10A) in patients with solid tumors. Cancer Biol Ther. 2007;6:648–53. doi: 10.4161/cbt.6.5.4004. [DOI] [PubMed] [Google Scholar]
- 312.Ranki T, Kanerva A, Ristimaki A, et al. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5. pk7-Delta24, for the treatment of advanced breast cancer. Gene Ther. 2007;14:58–67. doi: 10.1038/sj.gt.3302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tyler MA, Ulasov IV, Borovjagin A, et al. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol Cancer Ther. 2006;5:2408–16. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- 314.Sterman DH, Recio A, Carroll RG, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456–66. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]